Exploring Variability in Rifampicin Plasma Exposure and Development of Anti-Tuberculosis Drug-Induced Liver Injury among Patients with Pulmonary Tuberculosis from the Pharmacogenetic Perspective
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Sample Collection and Pretreatment Procedure
2.3. Determination of RIF Pharmacokinetic Parameters
2.4. SNP Detection and Analysis
2.5. Assessment of DILI
2.6. Statistical Data Analysis
3. Results
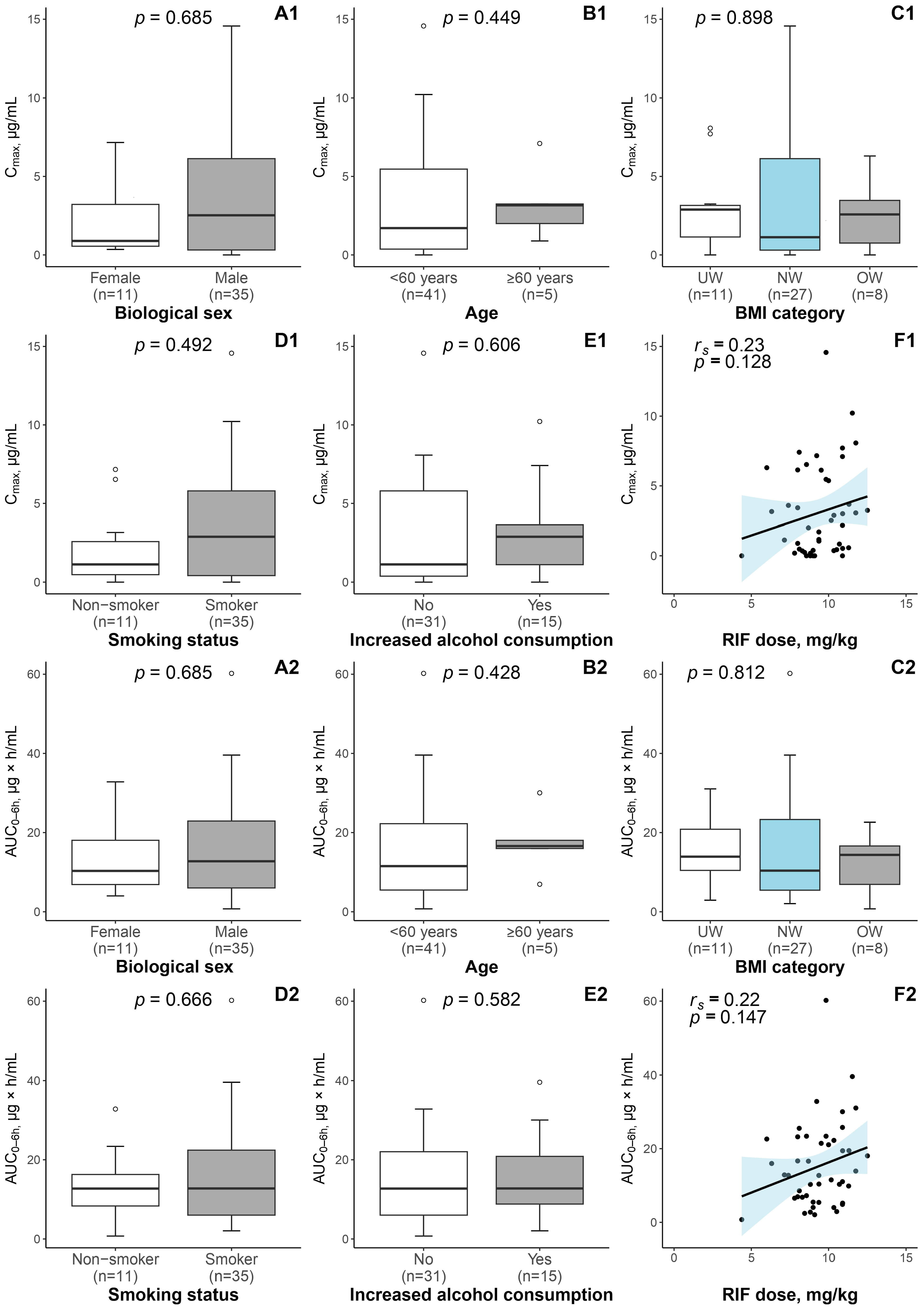
3.1. Patient Characteristics and RIF Exposure
3.2. Results of DILI Assessment
3.3. RIF-Associated Pharmacogene SNP Detection
3.4. Relationship between the Detected SNPs and the RIF Pharmacokinetic Parameters
3.5. Relationship between the Selected SNPs and the Development of DILI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Tuberculosis Report 2022; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2022.
- WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment—Drug-Susceptible Tuberculosis Treatment; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2022.
- Prideaux, B.; Via, L.E.; Zimmerman, M.D.; Eum, S.; Sarathy, J.; O’Brien, P.; Chen, C.; Kaya, F.; Weiner, D.M.; Chen, P.-Y.; et al. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat. Med. 2015, 21, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Rifat, D.; Prideaux, B.; Savic, R.M.; Urbanowski, M.E.; Parsons, T.L.; Luna, B.; Marzinke, M.A.; Ordonez, A.A.; DeMarco, V.P.; Jain, S.K.; et al. Pharmacokinetics of rifapentine and rifampin in a rabbit model of tuberculosis and correlation with clinical trial data. Sci. Transl. Med. 2018, 10, eaai7786. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.M.; Mitchison, D.A. Experimental models to explain the high sterilizing activity of rifampin in the chemotherapy of tuberculosis. Am. Rev. Respir. Dis. 1981, 123 Pt 1, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, E.H.; Dooley, K.E. Chemotherapy of Tuberculosis and Nontuberculous Mycobacteria, Including Leprosy. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 14th ed.; Brunton, L.L., Knollmann, B.C., Eds.; McGraw Hill: New York, NY, USA, 2023; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=3191§ionid=269172224 (accessed on 8 May 2023).
- Gumbo, T.; Louie, A.; Deziel, M.R.; Liu, W.; Parsons, L.M.; Salfinger, M.; Drusano, G.L. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob. Agents Chemother. 2007, 51, 3781–3788. [Google Scholar] [CrossRef]
- Walubo, A.; Chan, K.; Woo, J.; Chan, H.S.; Wong, C.L. The disposition of antituberculous drugs in plasma of elderly patients. II. Isoniazid, rifampicin and pyrazinamide. Methods Find. Exp. Clin. Pharmacol. 1991, 13, 551–556. [Google Scholar] [PubMed]
- McIlleron, H.; Wash, P.; Burger, A.; Norman, J.; Folb, P.I.; Smith, P. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob. Agents Chemother. 2006, 50, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Nijland, H.M.J.; Ruslami, R.; Stalenhoef, J.E.; Nelwan, E.J.; Alisjahbana, B.; Nelwan, R.H.H.; van der Ven, A.J.A.M.; Danusantoso, H.; Aarnoutse, R.E.; van Crevel, R. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin. Infect. Dis. 2006, 43, 848–854. [Google Scholar] [CrossRef]
- Um, S.-W.; Lee, S.W.; Kwon, S.Y.; Yoon, H.I.; Park, K.U.; Song, J.; Lee, C.-T.; Lee, J.-H. Low serum concentrations of anti-tuberculosis drugs and determinants of their serum levels. Int. J. Tuberc. Lung Dis. 2007, 11, 972–978. [Google Scholar]
- Requena-Méndez, A.; Davies, G.; Ardrey, A.; Jave, O.; López-Romero, S.L.; Ward, S.A.; Moore, D.A.J. Pharmacokinetics of rifampin in Peruvian tuberculosis patients with and without comorbid diabetes or HIV. Antimicrob. Agents Chemother. 2012, 56, 2357–2363. [Google Scholar] [CrossRef]
- Segovia, R.C.M.; Ramírez, A.M.D.; Cook, H.J.; Aquino, M.M.; Pérez, M.V.; Brundage, R.C.; Moreno, S.R. Population pharmacokinetics of rifampicin in Mexican patients with tuberculosis. J. Clin. Pharm. Ther. 2013, 38, 56–61. [Google Scholar] [CrossRef]
- Saktiawati, A.M.I.; Sturkenboom, M.G.G.; Stienstra, Y.; Subronto, Y.W.; Sumardi; Kosterink, J.G.W.; van der Werf, T.S.; Alffenaar, J.-W.C. Impact of food on the pharmacokinetics of first-line anti-TB drugs in treatment-naive TB patients: A randomized cross-over trial. J. Antimicrob. Chemother. 2016, 71, 703–710. [Google Scholar] [CrossRef]
- Alsultan, A.; Peloquin, C.A. Therapeutic drug monitoring in the treatment of tuberculosis: An update. Drugs 2014, 74, 839–854, Erratum in Drugs 2014, 74, 2061. [Google Scholar] [CrossRef]
- Fahimi, F.; Tabarsi, P.; Kobarfard, F.; Bozorg, B.D.; Goodarzi, A.; Dastan, F.; Shahsavari, N.; Emami, S.; Habibi, M.; Salamzadeh, J. Isoniazid, rifampicin and pyrazinamide plasma concentrations 2 and 6 h post dose in patients with pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2013, 17, 1602–1606. [Google Scholar] [CrossRef]
- Niward, K.; Forsman, L.D.; Bruchfeld, J.; Chryssanthou, E.; Carlström, O.; Alomari, T.; Carlsson, B.; Pohanka, A.; Mansjö, M.; Nordvall, M.J.; et al. Distribution of plasma concentrations of first-line anti-TB drugs and individual MICs: A prospective cohort study in a low endemic setting. J. Antimicrob. Chemother. 2018, 73, 2838–2845. [Google Scholar] [CrossRef]
- Ramachandran, G.; Chandrasekaran, P.; Gaikwad, S.; Kupparam, H.K.A.; Thiruvengadam, K.; Gupte, N.; Paradkar, M.; Dhanasekaran, K.; Sivaramakrishnan, G.N.; Kagal, A.; et al. Subtherapeutic Rifampicin Concentration Is Associated with Unfavorable Tuberculosis Treatment Outcomes. Clin. Infect. Dis. 2020, 70, 1463–1470. [Google Scholar] [CrossRef]
- Pasipanodya, J.G.; Srivastava, S.; Gumbo, T. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin. Infect. Dis. 2012, 55, 169–177. [Google Scholar] [CrossRef]
- Dizaji, S.P.; Taala, A.; Masoumi, M.; Ebrahimzadeh, N.; Fateh, A.; Siadat, S.D.; Vaziri, F. Sub-minimum inhibitory concentration of rifampin: A potential risk factor for resuscitation of Mycobacterium tuberculosis. Antimicrob. Resist. Infect. Control. 2017, 6, 116. [Google Scholar] [CrossRef]
- Sekaggya-Wiltshire, C.; von Braun, A.; Lamorde, M.; Ledergerber, B.; Buzibye, A.; Henning, L.; Musaazi, J.; Gutteck, U.; Denti, P.; de Kock, M.; et al. Delayed Sputum Culture Conversion in Tuberculosis-Human Immunodeficiency Virus-Coinfected Patients with Low Isoniazid and Rifampicin Concentrations. Clin. Infect. Dis. 2018, 67, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Bao, Z.; Forsman, L.D.; Hu, Y.; Ren, W.; Gao, Y.; Li, X.; Hoffner, S.; Bruchfeld, J.; Alffenaar, J.-W. Drug Exposure and Minimum Inhibitory Concentration Predict Pulmonary Tuberculosis Treatment Response. Clin. Infect. Dis. 2021, 73, e3520–e3528. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, J.; Zhang, Y.; Tang, S.; Zhan, S. Key factors of susceptibility to anti-tuberculosis drug-induced hepatotoxicity. Arch. Toxicol. 2015, 89, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Devarbhavi, H.; Singh, R.; Patil, M.; Sheth, K.; Adarsh, C.K.; Balaraju, G. Outcome and determinants of mortality in 269 patients with combination anti-tuberculosis drug-induced liver injury. J. Gastroenterol. Hepatol. 2013, 28, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Yimer, G.; Gry, M.; Amogne, W.; Makonnen, E.; Habtewold, A.; Petros, Z.; Aderaye, G.; Schuppe-Koistinen, I.; Lindquist, L.; Aklillu, E. Evaluation of patterns of liver toxicity in patients on antiretroviral and anti-tuberculosis drugs: A prospective four arm observational study in ethiopian patients. PLoS ONE 2014, 9, e94271. [Google Scholar] [CrossRef] [PubMed]
- Satyaraddi, A.; Velpandian, T.; Sharma, S.K.; Vishnubhatla, S.; Sharma, A.; Sirohiwal, A.; Makharia, G.K.; Sinha, S.; Biswas, A.; Singh, S. Correlation of plasma anti-tuberculosis drug levels with subsequent development of hepatotoxicity. Int. J. Tuberc. Lung Dis. 2014, 18, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kalow, W.; Tang, B.K.; Endrenyi, L. Hypothesis: Comparisons of inter- and intra-individual variations can substitute for twin studies in drug research. Pharmacogenetics 1998, 8, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Metushi, I.G.; Cai, P.; Zhu, X.; Nakagawa, T.; Uetrecht, J.P. A fresh look at the mechanism of isoniazid-induced hepatotoxicity. Clin. Pharmacol. Ther. 2011, 89, 911–914. [Google Scholar] [CrossRef]
- McDonagh, E.M.; Boukouvala, S.; Aklillu, E.; Hein, D.W.; Altman, R.B.; Klein, T.E. PharmGKB summary: Very important pharmacogene information for N-acetyltransferase 2. Pharmacogenet. Genom. 2014, 24, 409–425. [Google Scholar] [CrossRef]
- Weiner, M.; Peloquin, C.; Burman, W.; Luo, C.-C.; Engle, M.; Prihoda, T.J.; Mac Kenzie, W.R.; Bliven-Sizemore, E.; Johnson, J.L.; Vernon, A. Effects of tuberculosis, race, and human gene SLCO1B1 polymorphisms on rifampin concentrations. Antimicrob. Agents Chemother. 2010, 54, 4192–4200. [Google Scholar] [CrossRef]
- Chigutsa, E.; Visser, M.E.; Swart, E.C.; Denti, P.; Pushpakom, S.; Egan, D.; Holford, N.H.G.; Smith, P.J.; Maartens, G.; Owen, A.; et al. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: Dosing implications. Antimicrob. Agents Chemother. 2011, 55, 4122–4127. [Google Scholar] [CrossRef]
- Yimer, G.; Ueda, N.; Habtewold, A.; Amogne, W.; Suda, A.; Riedel, K.-D.; Burhenne, J.; Aderaye, G.; Lindquist, L.; Makonnen, E.; et al. Pharmacogenetic & pharmacokinetic biomarker for efavirenz based ARV and rifampicin based anti-TB drug induced liver injury in TB-HIV infected patients. PLoS ONE 2011, 6, e27810. [Google Scholar] [CrossRef]
- Li, L.M.; Chen, L.; Deng, G.H.; Tan, W.T.; Dan, Y.J.; Wang, R.Q.; Chen, W.S. SLCO1B1 *15 haplotype is associated with rifampin-induced liver injury. Mol. Med. Rep. 2012, 6, 75–82. [Google Scholar] [CrossRef]
- Kwara, A.; Cao, L.; Yang, H.; Poethke, P.; Kurpewski, J.; Tashima, K.T.; Mahjoub, B.D.; Court, M.H.; Peloquin, C.A. Factors associated with variability in rifampin plasma pharmacokinetics and the relationship between rifampin concentrations and induction of efavirenz clearance. Pharmacotherapy 2014, 34, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, J.; Tang, S.; Zhang, Y.; Lv, X.; Wu, S.; Xia, Y.; Deng, P.; Ma, Y.; Tu, D.; et al. Association of polymorphisms in drug transporter genes (SLCO1B1 and SLC10A1) and anti-tuberculosis drug-induced hepatotoxicity in a Chinese cohort. Tuberculosis 2015, 95, 68–74. [Google Scholar] [CrossRef]
- Zazuli, Z.; Barliana, M.I.; Mulyani, U.A.; Perwitasari, D.A.; Ng, H.; Abdulah, R. Polymorphism of PXR gene associated with the increased risk of drug-induced liver injury in Indonesian pulmonary tuberculosis patients. J. Clin. Pharm. Ther. 2015, 40, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Allegra, S.; Fatiguso, G.; Calcagno, A.; Baietto, L.; Motta, I.; Favata, F.; Cusato, J.; Bonora, S.; Di Perri, G.; D’avolio, A. Role of vitamin D pathway gene polymorphisms on rifampicin plasma and intracellular pharmacokinetics. Pharmacogenomics 2017, 18, 865–880. [Google Scholar] [CrossRef] [PubMed]
- Dompreh, A.; Tang, X.; Zhou, J.; Yang, H.; Topletz, A.; Ahwireng, E.A.; Antwi, S.; Enimil, A.; Langaee, T.; Peloquin, C.A.; et al. Effect of Genetic Variation of NAT2 on Isoniazid and SLCO1B1 and CES2 on Rifampin Pharmacokinetics in Ghanaian Children with Tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e02099-17. [Google Scholar] [CrossRef]
- Huerta-García, A.P.; Medellín-Garibay, S.E.; Salazar-González, R.A.; Ortiz-Álvarez, A.; Magaña-Aquino, M.; Rodríguez-Pinal, C.J.; Portales-Pérez, D.P.; Romano-Moreno, S.; Milán-Segovia, R.d.C. Anthropometric and Genetic Factors Associated with the Exposure of Rifampicin and Isoniazid in Mexican Patients with Tuberculosis. Ther. Drug Monit. 2019, 41, 648–656. [Google Scholar] [CrossRef]
- Naidoo, A.; Chirehwa, M.; Ramsuran, V.; McIlleron, H.; Naidoo, K.; Yende-Zuma, N.; Singh, R.; Ncgapu, S.; Adamson, J.; Govender, K.; et al. Effects of genetic variability on rifampicin and isoniazid pharmacokinetics in South African patients with recurrent tuberculosis. Pharmacogenomics 2019, 20, 225–240. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Bai, H.; Wang, M.; Jiao, L.; Peng, W.; Wu, T.; Liu, T.; Chen, H.; Song, X.; et al. Genetic polymorphisms in PXR and NF-κB1 influence susceptibility to anti-tuberculosis drug-induced liver injury. PLoS ONE 2019, 14, e0222033. [Google Scholar] [CrossRef]
- Francis, J.; Zvada, S.P.; Denti, P.; Hatherill, M.; Charalambous, S.; Mungofa, S.; Dawson, R.; Dorman, S.; Gupte, N.; Wiesner, L.; et al. A Population Pharmacokinetic Analysis Shows that Arylacetamide Deacetylase (AADAC) Gene Polymorphism and HIV Infection Affect the Exposure of Rifapentine. Antimicrob. Agents Chemother. 2019, 63, e01964-18. [Google Scholar] [CrossRef]
- Medellin-Garibay, S.E.; Huerta-García, A.P.; Rodríguez-Báez, A.S.; Magaña-Aquino, M.; Ortiz-Álvarez, A.; Portales-Pérez, D.P.; Milán-Segovia, R.d.C.; Romano-Moreno, S. A population approach of rifampicin pharmacogenetics and pharmacokinetics in Mexican patients with tuberculosis. Tuberculosis 2020, 124, 101982. [Google Scholar] [CrossRef]
- Yang, M.; Pan, H.; Chen, H.; Liu, W.; Lu, L.; He, X.; Yi, H.; Tang, S. Association between NR1I2 polymorphisms and susceptibility to anti-tuberculosis drug-induced hepatotoxicity in an Eastern Chinese Han population: A case-control study. Infect. Genet. Evol. 2020, 83, 104349. [Google Scholar] [CrossRef]
- Weiner, M.; Gelfond, J.; Johnson-Pais, T.L.; Engle, M.; Johnson, J.L.; Whitworth, W.C.; Bliven-Sizemore, E.; Nsubuga, P.; Dorman, S.E.; Savic, R.; et al. Decreased plasma rifapentine concentrations associated with AADAC single nucleotide polymorphism in adults with tuberculosis. J. Antimicrob. Chemother. 2021, 76, 582–586. [Google Scholar] [CrossRef]
- Perumal, R.; Arodola-Oladoyinbo, O.; Naidoo, A.; Kawuma, A.N.; Naidoo, K.; Gengiah, T.N.; Chirehwa, M.; Padayatchi, N.; Denti, P. Altered drug exposures of first-line TB drugs in a moxifloxacin-containing treatment regimen. Int. J. Tuberc. Lung Dis. 2022, 26, 766–774. [Google Scholar] [CrossRef]
- Wang, N.; Guo, S.; Liu, H.; Ding, Y.; Yao, R.; Liu, Z.; Zhu, H.; Chen, X.; Yang, X.; Chen, X.; et al. Relevance of gene polymorphisms of NAT2 and NR1I2 to anti-tuberculosis drug-induced hepatotoxicity. Xenobiotica 2022, 52, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; Volume 3. [Google Scholar]
- Kivrane, A.; Grinberga, S.; Sevostjanovs, E.; Igumnova, V.; Pole, I.; Viksna, A.; Bandere, D.; Krams, A.; Cirule, A.; Pugovics, O.; et al. LC-MS/MS method for simultaneous quantification of the first-line anti-tuberculosis drugs and six primary metabolites in patient plasma: Implications for therapeutic drug monitoring. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1185, 122986. [Google Scholar] [CrossRef]
- Igumnova, V.; Kivrane, A.; Viksna, A.; Norvaisa, I.; Ranka, R. Next-Generation Sequencing and Bioinformatics-Based Protocol for the Full-Length CYP2E1 Gene Polymorphism Analysis. Pharmgenom. Pers. Med. 2022, 15, 959–965. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Yang, H.; Wang, K. Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat. Protoc. 2015, 10, 1556–1566. [Google Scholar] [CrossRef]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef]
- The Jamovi Project. jamovi (Version 2.3) [Computer Software]. 2022. Available online: https://www.jamovi.org (accessed on 8 May 2023).
- World Health Organization. A Healthy Lifestyle—WHO Recommendations. 6 May 2010. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle—Who-recommendations (accessed on 8 May 2023).
- Durand, F.; Jebrak, G.; Pessayre, D.; Fournier, M.; Bernuau, J. Hepatotoxicity of antitubercular treatments: Rationale for monitoring liver status. Drug Saf. 1996, 15, 394–405. [Google Scholar] [CrossRef]
- Abbara, A.; Chitty, S.; Roe, J.K.; Ghani, R.; Collin, S.M.; Ritchie, A.; Kon, O.M.; Dzvova, J.; Davidson, H.; Edwards, T.E.; et al. Drug-induced liver injury from antituberculous treatment: A retrospective study from a large TB centre in the UK. BMC Infect. Dis. 2017, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Fukami, T.; Kobayashi, Y.; Watanabe, A.; Nakajima, M.; Yokoi, T. Human arylacetamide deacetylase is responsible for deacetylation of rifamycins: Rifampicin, rifabutin, and rifapentine. Biochem. Pharmacol. 2011, 82, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Fukami, T.; Kobayashi, Y.; Takamiya, M.; Aoki, Y.; Nakajima, M.; Yokoi, T. A novel polymorphic allele of human arylacetamide deacetylase leads to decreased enzyme activity. Drug Metab. Dispos. 2012, 40, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Yang, J.; Liu, S.; Sun, R.; Chen, H.; Long, N.; Jiang, R.; Gui, C. Genetic polymorphisms of human hepatic OATPs: Functional consequences and effect on drug pharmacokinetics. Xenobiotica 2020, 50, 297–317. [Google Scholar] [CrossRef]
- Schwarz, U.I.; zu Schwabedissen, H.E.M.; Tirona, R.G.; Suzuki, A.; Leake, B.F.; Mokrab, Y.; Mizuguchi, K.; Ho, R.H.; Kim, R.B. Identification of novel functional organic anion-transporting polypeptide 1B3 polymorphisms and assessment of substrate specificity. Pharmacogenet. Genom. 2011, 21, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Hirvensalo, P.; Tornio, A.; Launiainen, T.; Paile-Hyvärinen, M.; Tapaninen, T.; Neuvonen, M.; Backman, J.T.; Niemi, M. UGT1A3 and Sex Are Major Determinants of Telmisartan Pharmacokinetics-A Comprehensive Pharmacogenomic Study. Clin. Pharmacol. Ther. 2020, 108, 885–895. [Google Scholar] [CrossRef]
- Campbell, S.D.; de Morais, S.M.; Xu, J.J. Inhibition of human organic anion transporting polypeptide OATP 1B1 as a mechanism of drug-induced hyperbilirubinemia. Chem. Biol. Interact. 2004, 150, 179–187. [Google Scholar] [CrossRef]
- Takehara, I.; Yoshikado, T.; Ishigame, K.; Mori, D.; Furihata, K.-I.; Watanabe, N.; Ando, O.; Maeda, K.; Sugiyama, Y.; Kusuhara, H. Comparative Study of the Dose-Dependence of OATP1B Inhibition by Rifampicin Using Probe Drugs and Endogenous Substrates in Healthy Volunteers. Pharm. Res. 2019, 35, 138, Erratum in Pharm Res. 2019, 36, 55. [Google Scholar] [CrossRef]
- Zhang, W.; He, Y.; Gan, Z.; Fan, L.; Li, Q.; Wang, A.; Liu, Z.; Deng, S.; Huang, Y.; Xu, L.; et al. OATP1B1 polymorphism is a major determinant of serum bilirubin level but not associated with rifampicin-mediated bilirubin elevation. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1240–1244. [Google Scholar] [CrossRef]
- Jabir, R.S.; Naidu, R.; Annuar, M.A.; Ho, G.F.; Munisamy, M.; Stanslas, J. Pharmacogenetics of taxanes: Impact of gene polymorphisms of drug transporters on pharmacokinetics and toxicity. Pharmacogenomics 2012, 13, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Mbatchi, L.C.; Schmitt, A.; Thomas, F.; Cazaubon, Y.; Robert, J.; Lumbroso, S.; Brouillet, J.-P.; Pourquier, P.; Chatelut, E.; Boyer, J.-C.; et al. Polymorphisms in SLCO1B3 and NR1I2 as genetic determinants of hematotoxicity of carboplatin and paclitaxel combination. Pharmacogenomics 2015, 16, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Cascorbi, I. P-glycoprotein: Tissue distribution, substrates, and functional consequences of genetic variations. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 261–283. [Google Scholar] [CrossRef]
- Hu, R.; Barratt, D.T.; Coller, J.K.; Sallustio, B.C.; Somogyi, A.A. CYP3A5*3 and ABCB1 61A>G Significantly Influence Dose-adjusted Trough Blood Tacrolimus Concentrations in the First Three Months Post-Kidney Transplantation. Basic Clin. Pharmacol. Toxicol. 2018, 123, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.D.; Haas, D.W.; Motsinger, A.A.; Donahue, J.P.; Erdem, H.; Raffanti, S.; Rebeiro, P.; George, A.L.; Kim, R.B.; Haines, J.L.; et al. Drug transporter and metabolizing enzyme gene variants and nonnucleoside reverse-transcriptase inhibitor hepatotoxicity. Clin. Infect. Dis. 2006, 43, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.P.; Lohse, A.W.; Burchard, G.D.; Fischer, L.; Nashan, B.; Zimmermann, M.; Marx, A.; Kluge, S. Low N-acetyltransferase 2 activity in isoniazid-associated acute hepatitis requiring liver transplantation. Transpl. Int. 2010, 23, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Chua, A.P.G.; Aminkeng, F.; Chee, C.B.E.; Jin, S.; Loh, M.; Gan, S.H.; Wang, Y.T.; Brunham, L.R. Association and clinical utility of NAT2 in the prediction of isoniazid-induced liver injury in Singaporean patients. PLoS ONE 2017, 12, e0186200. [Google Scholar] [CrossRef]
- Ma, X.; Idle, J.R.; Gonzalez, F.J. The pregnane X receptor: From bench to bedside. Expert Opin. Drug Metab. Toxicol. 2008, 4, 895–908. [Google Scholar] [CrossRef]
- Smythe, W.; Khandelwal, A.; Merle, C.; Rustomjee, R.; Gninafon, M.; Lo, M.B.; Sow, O.B.; Olliaro, P.L.; Lienhardt, C.; Horton, J.; et al. A semimechanistic pharmacokinetic-enzyme turnover model for rifampin autoinduction in adult tuberculosis patients. Antimicrob. Agents Chemother. 2012, 56, 2091–2098. [Google Scholar] [CrossRef]
- He, P.; Court, M.H.; Greenblatt, D.J.; von Moltke, L.L. Human pregnane X receptor: Genetic polymorphisms, alternative mRNA splice variants, and cytochrome P450 3A metabolic activity. J. Clin. Pharmacol. 2006, 46, 1356–1369. [Google Scholar] [CrossRef]
- Oleson, L.; von Moltke, L.L.; Greenblatt, D.J.; Court, M.H. Identification of polymorphisms in the 3′-untranslated region of the human pregnane X receptor (PXR) gene associated with variability in cytochrome P450 3A (CYP3A) metabolism. Xenobiotica 2010, 40, 146–162. [Google Scholar] [CrossRef]
- Rana, M.; Coshic, P.; Goswami, R.; Tyagi, R.K. Influence of a critical single nucleotide polymorphism on nuclear receptor PXR-promoter function. Cell Biol. Int. 2017, 41, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Goodwin, B.; Willson, T.M. The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocr. Rev. 2002, 23, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, K.A.; Isbel, N.M.; Lee, K.J.; Bergmann, T.K.; Johnson, D.W.; McWhinney, B.C.; Ungerer, J.P.J.; Campbell, S.B.; Leary, D.R.; Bialasiewicz, S.; et al. NR1I2 polymorphisms are related to tacrolimus dose-adjusted exposure and BK viremia in adult kidney transplantation. Transplantation 2012, 94, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, A.; Cusato, J.; Sekaggya-Wiltshire, C.; von Braun, A.; Motta, I.; Turyasingura, G.; Castelnuovo, B.; Fehr, J.; Di Perri, G.; Lamorde, M. The Influence of Pharmacogenetic Variants in HIV/Tuberculosis Coinfected Patients in Uganda in the SOUTH Study. Clin. Pharmacol. Ther. 2019, 106, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Hustert, E.; Zibat, A.; Presecan-Siedel, E.; Eiselt, R.; Mueller, R.; Fuss, C.; Brehm, I.; Brinkmann, U.; Eichelbaum, M.; Wojnowski, L.; et al. Natural protein variants of pregnane X receptor with altered transactivation activity toward CYP3A4. Drug Metab. Dispos. 2001, 29, 1454–1459. [Google Scholar]
- Zhuang, X.; Li, L.; Liu, T.; Zhang, R.; Yang, P.; Wang, X.; Dai, L. Mechanisms of isoniazid and rifampicin-induced liver injury and the effects of natural medicinal ingredients: A review. Front. Pharmacol. 2022, 13, 1037814. [Google Scholar] [CrossRef]
- Sookoian, S.; Castaño, G.O.; Burgueño, A.L.; Gianotti, T.F.; Rosselli, M.S.; Pirola, C.J. The nuclear receptor PXR gene variants are associated with liver injury in nonalcoholic fatty liver disease. Pharmacogenet. Genom. 2010, 20, 1–8. [Google Scholar] [CrossRef]
| No./Total (%) | Median (IQR) | ||
|---|---|---|---|
| Demographic and anthropometric characteristics | |||
| Biological sex | Male | 35/46 (76.1) | |
| Female | 11/46 (23.9) | ||
| Age, years | 46 (38–55) | ||
| <60 years | 41/46 (89.1) | ||
| ≥60 years | 5/46 (10.9) | ||
| Body weight, kg | 64 (55–73) | ||
| BMI a | Underweight | 11/46 (23.9) | |
| Normal weight | 27/46 (58.7) | ||
| Overweight | 8/46 (17.4) | ||
| Smoking status | Smoker | 35/46 (76.1) | |
| Non-smoker | 11/46 (23.9) | ||
| Increased alcohol consumption | Yes | 15/46 (32.6) | |
| No | 31/46 (67.4) | ||
| Baseline blood biochemical parameters | |||
| ALAT, U/L | 17 (13–25) | ||
| ASAT, U/L b | 18 (15–27) | ||
| Total bilirubin, µmol/L c | 6.3 (5.2–8.7) | ||
| Conjugated bilirubin, µmol/L b | 3.2 (2.4–4.0) | ||
| Rifampicin-related data | |||
| RIF dose, mg/kg | 9.4 (8.3–10.9) | ||
| RIF Cmax, µg/mL | 2.09 (0.39–5.63) | ||
| ≥8 µg/mL | 4/46 (8.7) | ||
| <8 µg/mL | 42/46 (91.3) | ||
| RIF AUC0–6 h, µg × h/mL | 12.75 (6.29–22.34) | ||
| Gene | SNP ID a (Nucleotide Change) | Genomic Coordinates b | Region c | Type of Substitution | Allele Frequency | HWE p-Value d |
|---|---|---|---|---|---|---|
| SLCO1B1 | rs2306283 (A > G) | chr12:21176804 | Exon 5 | nonsyn. | A (0.65) G (0.35) | 0.713 |
| rs4149056 (T > C) | chr12:21178615 | Exon 6 | nonsyn. | T (0.84) C (0.16) | 0.810 | |
| rs11045819 (C > A) | chr12:21176879 | Exon 5 | nonsyn. | C (0.92) A (0.08) | 0.577 | |
| SLCO1B3 | rs60140950 (G > C) | chr12:20875274 | Exon 9 | nonsyn. | G (0.92) C (0.08) | 0.577 |
| ABCB1 | rs1045642 (A > G) | chr7:87509329 | Exon 28 | syn. | A (0.57) G (0.43) | 0.676 |
| rs9282564 (T > C) | chr7:87600124 | Exon 4 | nonsyn. | T (0.88) C (0.12) | 0.357 | |
| NR1I2 | rs3814055 (C > T) | chr3:119781188 | UTR5 | N/A | C (0.62) T (0.38) | 0.401 |
| rs2276707 (C > T) | chr3:119815306 | Intron 6 | N/A | C (0.78) T (0.22) | 0.474 | |
| rs3732357 (G > A) | chr3:119812011 | Intron 4 | N/A | G (0.35) A (0.65) | 0.351 | |
| rs3732359 (G > A) | chr3:119817582 | UTR3 | N/A | G (0.33) A (0.67) | 0.457 |
| Gene | SNP ID a | Genotype b | Genotype Frequency, n (%) | Pharmacokinetic Parameters | |||
|---|---|---|---|---|---|---|---|
| Cmax, µg/mL c | p-Value d | AUC0–6 h, µg × h/mL c | p-Value d | ||||
| SLCO1B1 | rs2306283 | AA | 19 (41.3) | 1.13 (0.37–6.13) | 0.145 | 10.39 (5.21–22.25) | 0.148 |
| AG + GG | 27 (58.7) | 3.06 (0.39–5.47) | 15.98 (6.95–22.62) | ||||
| rs4149056 | TT | 32 (69.6) | 1.15 (0.40–6.26) | 0.689 | 10.72 (5.43–23.07) | 0.635 | |
| TC + CC | 14 (30.4) | 3.20 (0.19–4.13) | 14.95 (9.36–19.97) | ||||
| rs11045819 | CC | 39 (84.8) | 2.18 (0.37–6.13) | 0.850 | 12.88 (6.55–22.62) | 0.999 | |
| CA+ AA | 7 (15.2) | 0.89 (0.39–5.37) | 10.32 (5.49–21.07) | ||||
| SLCO1B3 | rs60140950 | GG | 39 (84.8) | 2.18 (0.37–6.13) | 0.850 | 12.88 (6.55–22.62) | 0.999 |
| GC + CC | 7 (15.2) | 0.89 (0.39–5.37) | 10.32 (5.49–21.07) | ||||
| ABCB1 | rs1045642 | AA | 14 (30.4) | 3.30 (0.47–6.40) | 0.302 | 16.29 (9.87–23.86) | 0.346 |
| AG + GG | 32 (69.6) | 1.44 (0.35–5.02) | 11.29 (5.43–22.05) | ||||
| rs9282564 | TT | 35 (76.1) | 1.17 (0.35–5.47) | 0.466 | 11.04 (5.49–22.62) | 0.435 | |
| CT + CC | 11 (23.9) | 3.01 (0.48–6.13) | 15.98 (8.56–21.45) | ||||
| NR1I2 | rs3814055 | CC | 19 (41.3) | 2.18 (0.89–6.53) | 0.746 | 15.98 (5.41–23.39) | 0.917 |
| CT + TT | 27 (58.7) | 1.13 (0.19–5.47) | 11.53 (6.55–22.25) | ||||
| rs2276707 | CC | 29 (63.0) | 3.01 (0.31–6.42) | 0.354 | 15.98 (5.88–23.39) | 0.510 | |
| CT + TT | 17 (37.0) | 1.71 (0.43–3.33) | 11.04 (6.15–18.04) | ||||
| rs3732357 | GG | 7 (15.2) | 3.16 (0.48–8.08) | 0.077 | 22.25 (8.56–31.04) | 0.026 | |
| GA + AA | 39 (84.8) | 1.71 (0.35–5.37) | 11.53 (5.41–21.07) | ||||
| rs3732359 | GG | 6 (13.0) | 3.02 (0.46–9.70) | 0.211 | 19.12 (7.79–38.33) | 0.058 | |
| GA + AA | 40 (87.0) | 1.85 (0.35–5.44) | 12.13 (5.70–21.36) | ||||
| Gene | SNP ID a | Genotype b | Genotype Frequency, n (%) | p-Value d | OR (95% CI) | |
|---|---|---|---|---|---|---|
| DILI Group (n = 6) c | Non-DILI Group (n = 40) | |||||
| SLCO1B1 | rs2306283 | AA | 2 (33.3) | 17 (42.5) | 0.963 | 1.07 (0.05–22.80 |
| AG + GG | 4 (66.6) | 23 (57.5) | ||||
| rs4149056 | TT | 3 (50.0) | 29 (72.5) | 0.571 | 2.49 (0.11–58.54) | |
| TC + CC | 3 (50.0) | 11 (27.5) | ||||
| rs11045819 | CC | 5 (83.3) | 34 (85.0) | 0.230 | 8.27 (0.26–260.17) | |
| CA+ AA | 1 (16.6) | 6 (15.0) | ||||
| SLCO1B3 | rs60140950 | GG | 5 (83.3) | 34 (85.0) | 0.230 | 8.27 (0.26–260.17) |
| GC + CC | 1 (16.6) | 6 (15.0) | ||||
| ABCB1 | rs1045642 | AA | 2 (33.3) | 12 (30.0) | 0.706 | 0.55 (0.02–12.68) |
| AG + GG | 4 (66.6) | 28 (70.0) | ||||
| rs9282564 | TT | 5 (83.3) | 30 (75.0) | 0.299 | 0.11 (0.002–7.00) | |
| CT + CC | 1 (16.6) | 10 (25.0) | ||||
| NR1I2 | rs3814055 | CC | 3 (50.0) | 16 (40.0) | 0.447 | 0.28 (0.01–7.46) |
| CT + TT | 3 (50.0) | 24 (60.0) | ||||
| rs2276707 | CC | 4 (66.6) | 25 (62.5) | 0.681 | 0.55 (0.03–9.59) | |
| CT + TT | 2 (33.3) | 15 (37.5) | ||||
| rs3732357 | GG | 0 (0.0) | 7 (17.5) | 0.997 | N/A | |
| GA + AA | 6 (100.0) | 33 (82.5) | ||||
| rs3732359 | GG | 0 (0.0) | 6 (15.0) | 0.998 | N/A | |
| GA + AA | 6 (100.0) | 34 (85.0) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kivrane, A.; Ulanova, V.; Grinberga, S.; Sevostjanovs, E.; Viksna, A.; Ozere, I.; Bogdanova, I.; Zolovs, M.; Ranka, R. Exploring Variability in Rifampicin Plasma Exposure and Development of Anti-Tuberculosis Drug-Induced Liver Injury among Patients with Pulmonary Tuberculosis from the Pharmacogenetic Perspective. Pharmaceutics 2024, 16, 388. https://doi.org/10.3390/pharmaceutics16030388
Kivrane A, Ulanova V, Grinberga S, Sevostjanovs E, Viksna A, Ozere I, Bogdanova I, Zolovs M, Ranka R. Exploring Variability in Rifampicin Plasma Exposure and Development of Anti-Tuberculosis Drug-Induced Liver Injury among Patients with Pulmonary Tuberculosis from the Pharmacogenetic Perspective. Pharmaceutics. 2024; 16(3):388. https://doi.org/10.3390/pharmaceutics16030388
Chicago/Turabian StyleKivrane, Agnija, Viktorija Ulanova, Solveiga Grinberga, Eduards Sevostjanovs, Anda Viksna, Iveta Ozere, Ineta Bogdanova, Maksims Zolovs, and Renate Ranka. 2024. "Exploring Variability in Rifampicin Plasma Exposure and Development of Anti-Tuberculosis Drug-Induced Liver Injury among Patients with Pulmonary Tuberculosis from the Pharmacogenetic Perspective" Pharmaceutics 16, no. 3: 388. https://doi.org/10.3390/pharmaceutics16030388
APA StyleKivrane, A., Ulanova, V., Grinberga, S., Sevostjanovs, E., Viksna, A., Ozere, I., Bogdanova, I., Zolovs, M., & Ranka, R. (2024). Exploring Variability in Rifampicin Plasma Exposure and Development of Anti-Tuberculosis Drug-Induced Liver Injury among Patients with Pulmonary Tuberculosis from the Pharmacogenetic Perspective. Pharmaceutics, 16(3), 388. https://doi.org/10.3390/pharmaceutics16030388







