The Processing Space of the Spray-Dried Mannitol-Leucine System for Pulmonary Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Spray Draying
2.2.2. Droplet Size Distribution
2.2.3. Solubility
2.2.4. Theoretical Formulation Model
2.2.5. Powder X-ray Diffraction (PXRD)
2.2.6. Aerodynamic Particle Size Distribution
2.2.7. Optical Particle Size Distribution
2.2.8. Scanning Electron Microscopy (SEM)
2.2.9. Raman Spectroscopy
2.2.10. X-ray Photoelectron Spectroscopy (XPS)
2.2.11. Karl Fischer (KF) Titration
2.2.12. Gas Chromatography (GC)
2.2.13. Stability Analysis
2.2.14. Data Visualization
3. Results and Discussion
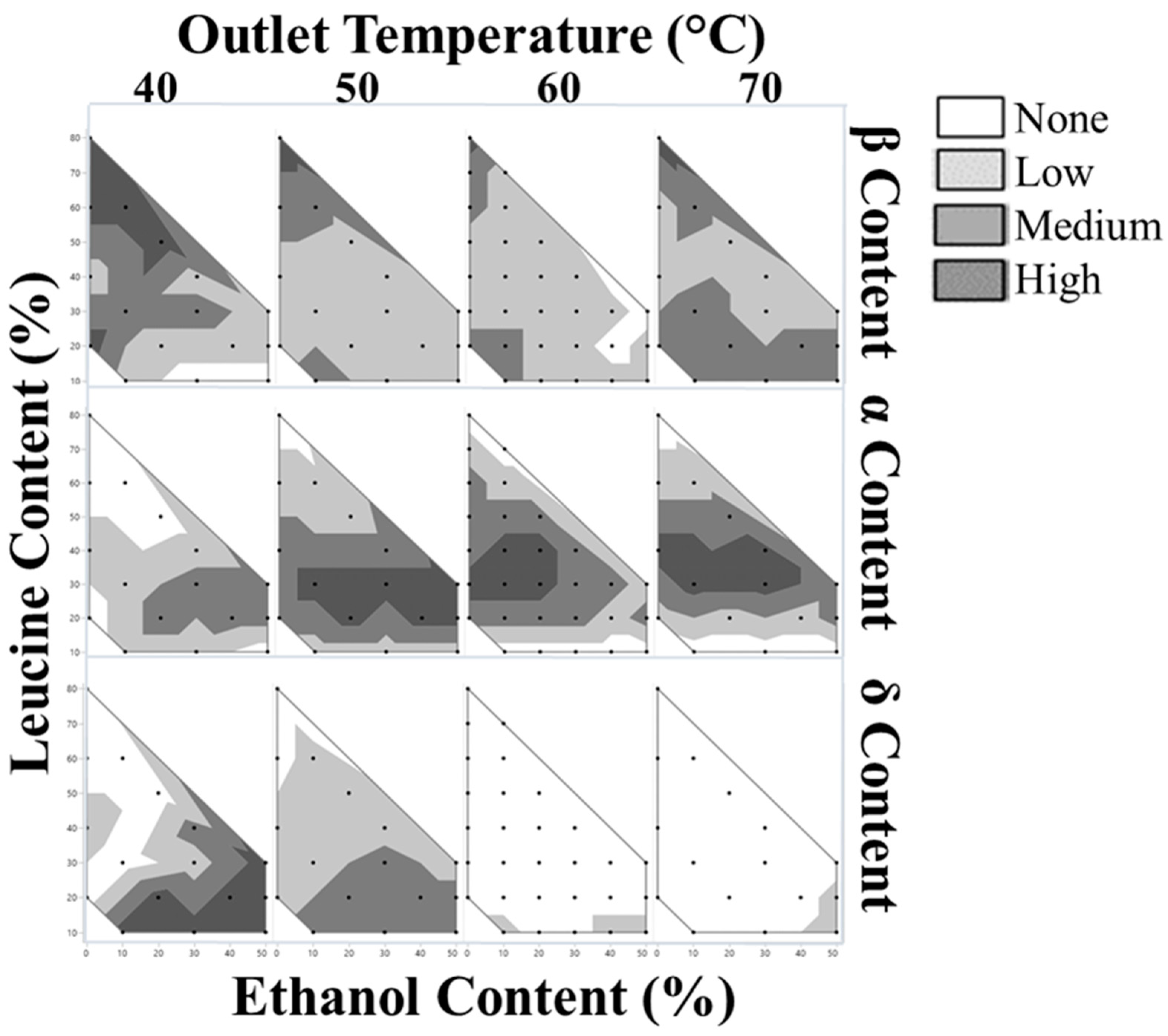
3.1. Prediction of Crystallization Windows
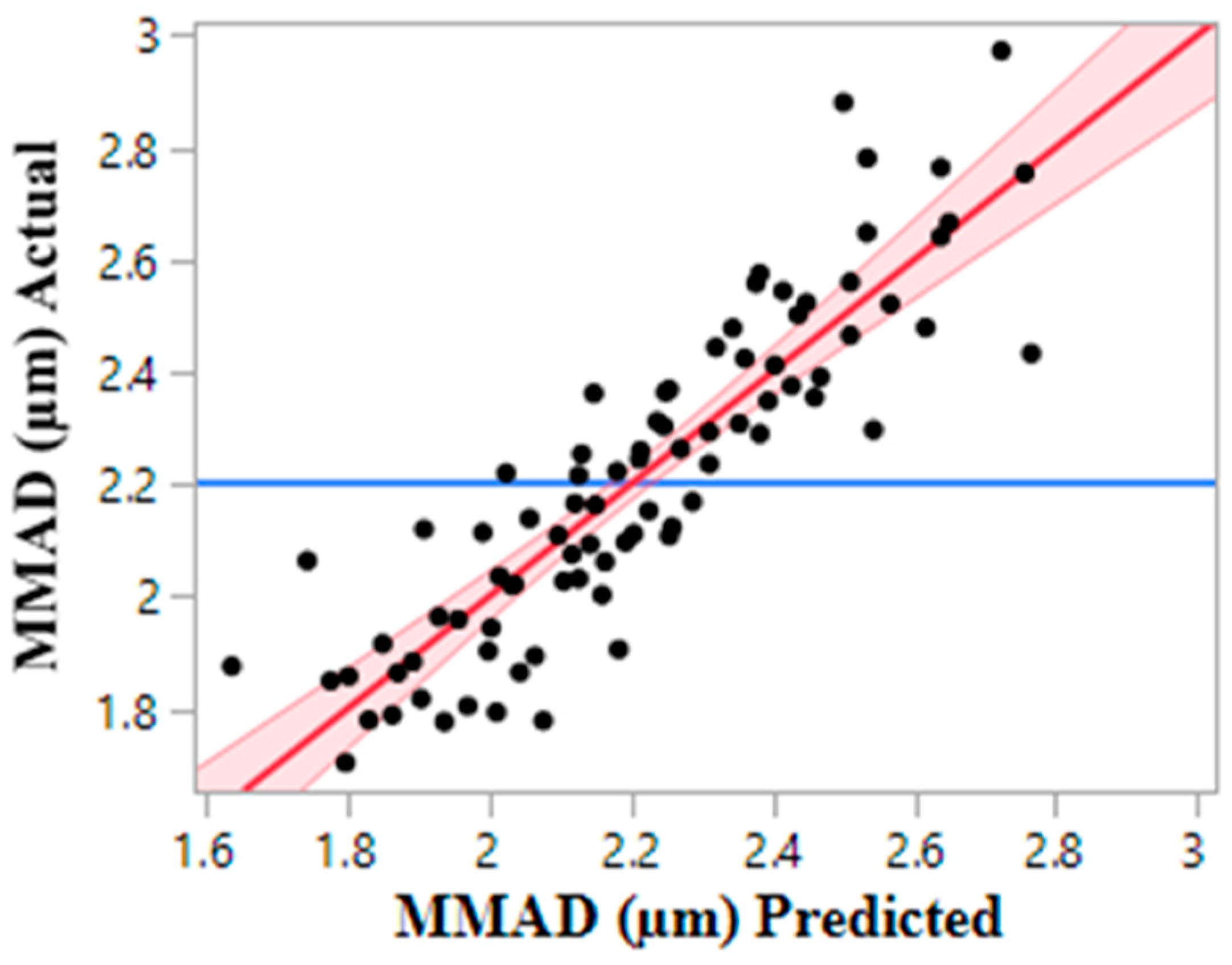
3.2. Particle Size
3.2.1. Aerodynamic Size Distribution
3.2.2. Optical Particle Size Distribution
3.3. Solid State and Chemical Composition
3.3.1. Powder X-ray Diffraction (PXRD)
3.3.2. Raman Spectroscopy
3.3.3. X-ray Photoelectric Spectroscopy (XPS)
3.4. Particle Morphology
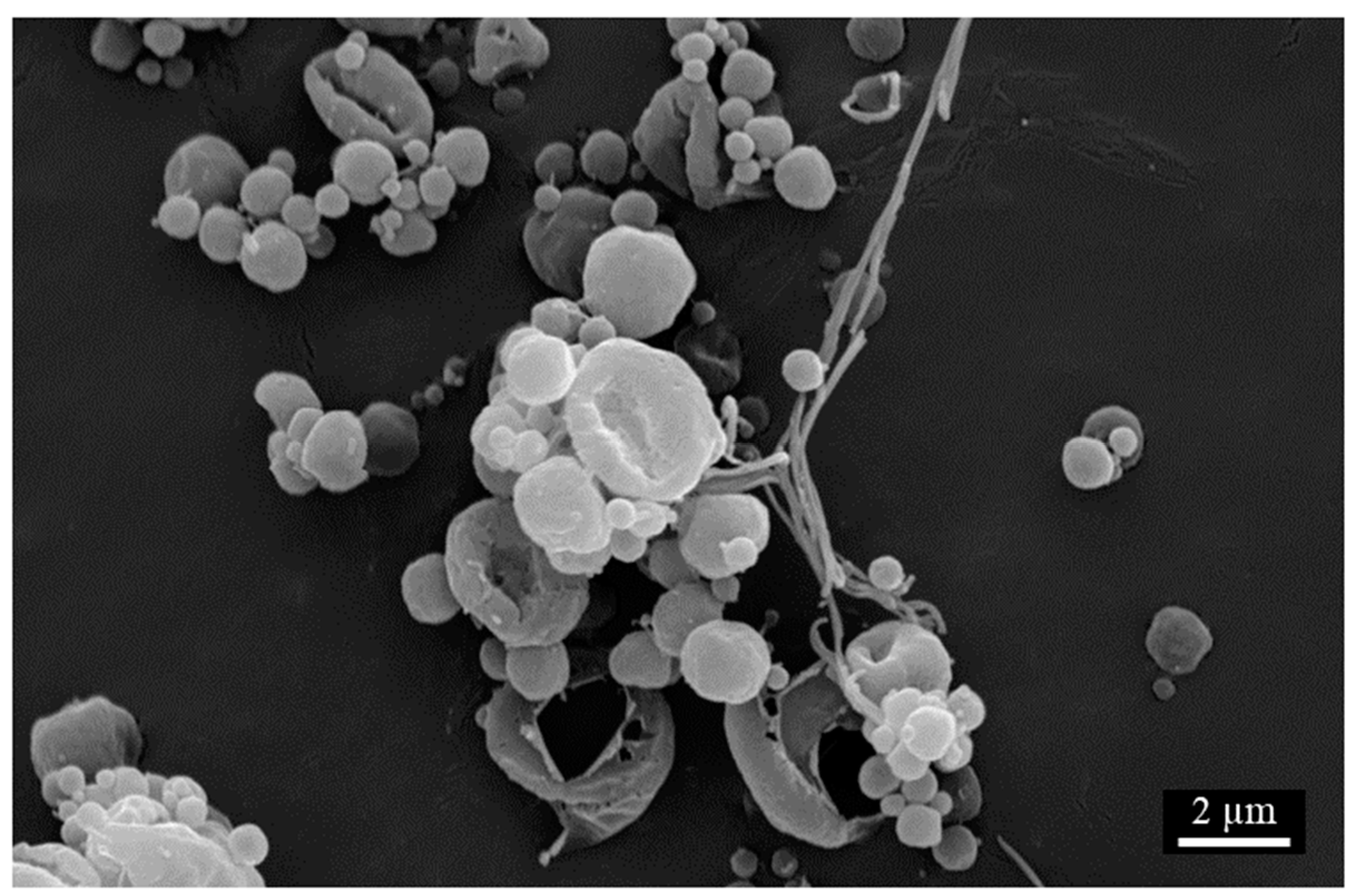
Scanning Electron Microscopy (SEM)
3.5. Residual Solvent
3.6. Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef] [PubMed]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Designing enhanced spray dried particles for inhalation: A review of the impact of excipients and processing parameters on particle properties. Powder Technol. 2021, 384, 313–331. [Google Scholar] [CrossRef]
- Finlay, W.H. The Mechanics of Inhaled Pharmaceutical Aerosols: An Introduction; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Patton, J.S.; Byron, P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, B.; Zhao, Y.-Y. Dry Powder for Pulmonary Delivery: A Comprehensive Review. Pharmaceutics 2021, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Hastedt, J.E.; Bäckman, P.; Clark, A.R.; Doub, W.; Hickey, A.; Hochhaus, G.; Kuehl, P.J.; Lehr, C.-M.; Mauser, P.; McConville, J.; et al. Scope and Relevance of a Pulmonary Biopharmaceutical Classification System AAPS/FDA/USP Workshop 16–17 March 2015 in Baltimore, MD; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Dabbagh, A.; Abu Kasim, N.H.; Yeong, C.H.; Wong, T.W.; Rahman, N.A. Critical Parameters for Particle-Based Pulmonary Delivery of Chemotherapeutics. J. Aerosol Med. Pulm. Drug Deliv. 2017, 31, 139–154. [Google Scholar] [CrossRef]
- Molina, C.; Kaialy, W.; Nokhodchi, A. The crucial role of leucine concentration on spray dried mannitol-leucine as a single carrier to enhance the aerosolization performance of Albuterol sulfate. J. Drug Deliv. Sci. Technol. 2019, 49, 97–106. [Google Scholar] [CrossRef]
- Vehring, R.; Foss, W.R.; Lechuga-Ballesteros, D. Particle formation in spray drying. J. Aerosol Sci. 2007, 38, 728–746. [Google Scholar] [CrossRef]
- Ordoubadi, M.; Shepard, K.B.; Wang, H.; Wang, Z.; Pluntze, A.M.; Churchman, J.P.; Vehring, R. On the Physical Stability of Leucine-Containing Spray-Dried Powders for Respiratory Drug Delivery. Pharmaceutics 2023, 15, 435. [Google Scholar] [CrossRef]
- Boraey, M.A.; Hoe, S.; Sharif, H.; Miller, D.P.; Lechuga-Ballesteros, D.; Vehring, R. Improvement of the dispersibility of spray-dried budesonide powders using leucine in an ethanol–water cosolvent system. Powder Technol. 2013, 236, 171–178. [Google Scholar] [CrossRef]
- Ordoubadi, M.; Gregson, F.K.; Wang, H.; Nicholas, M.; Gracin, S.; Lechuga-Ballesteros, D.; Reid, J.P.; Finlay, W.H.; Vehring, R. On the particle formation of leucine in spray drying of inhalable microparticles. Int. J. Pharm. 2020, 592, 120102. [Google Scholar] [CrossRef]
- Kawakami, K. Crystallization Tendency of Pharmaceutical Glasses: Relevance to Compound Properties, Impact of Formulation Process, and Implications for Design of Amorphous Solid Dispersions. Pharmaceutics 2019, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Karthika, S.; Radhakrishnan, T.K.; Kalaichelvi, P. A Review of Classical and Nonclassical Nucleation Theories. Cryst. Growth Des. 2016, 16, 6663–6681. [Google Scholar] [CrossRef]
- Iyer, R.; Jovanovska, V.P.; Berginc, K.; Jaklič, M.; Fabiani, F.; Harlacher, C.; Huzjak, T.; Sanchez-Felix, M.V. Amorphous Solid Dispersions (ASDs): The Influence of Material Properties, Manufacturing Processes and Analytical Technologies in Drug Product Development. Pharmaceutics 2021, 13, 1682. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Vehring, R. Leucine enhances the dispersibility of trehalose-containing spray-dried powders on exposure to a high-humidity environment. Int. J. Pharm. 2021, 601, 120561. [Google Scholar] [CrossRef] [PubMed]
- Ordoubadi, M.; Gregson, F.K.; Melhem, O.; Barona, D.; Miles, R.E.; D’Sa, D.; Gracin, S.; Lechuga-Ballesteros, D.; Reid, J.P.; Finlay, W.H.; et al. Multi-solvent microdroplet evaporation: Modeling and measurement of spray-drying kinetics with inhalable pharmaceutics. Pharmaceutical 2019, 36, 1–17. [Google Scholar]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Leucine as an excipient in spray dried powder for inhalation. Drug Discov. Today 2021, 26, 2384–2396. [Google Scholar] [CrossRef]
- Mangal, S.; Meiser, F.; Tan, G.; Gengenbach, T.; Denman, J.; Rowles, M.R.; Larson, I.; Morton, D.A. Relationship between surface concentration of l-leucine and bulk powder properties in spray dried formulations. Eur. J. Pharm. Biopharm. 2015, 94, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Ordoubadi, M.; Wang, H.; Vehring, R. Mechanistic Formulation Design of Spray-Dried Powders. KONA Powder Part. J. 2023, 40, 149–171. [Google Scholar] [CrossRef]
- Sibum, I.; Hagedoorn, P.; de Boer, A.H.; Frijlink, H.W.; Grasmeijer, F. Challenges for pulmonary delivery of high powder doses. Int. J. Pharm. 2018, 548, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Penha, F.M.; Gopalan, A.; Meijlink, J.C.; Ibis, F.; Eral, H.B. Selective crystallization of d-mannitol polymorphs using surfactant self-assembly. Cryst. Growth Des. 2021, 21, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Jia, N.; Li, H.; Hao, H.; Li, C. Polymorphism of D-mannitol: Crystal structure and the crystal growth mechanism. Chin. J. Chem. Eng. 2017, 25, 358–362. [Google Scholar] [CrossRef]
- Lyu, F.; Liu, J.J.; Zhang, Y.; Wang, X.Z. Combined control of morphology and polymorph in spray drying of mannitol for dry powder inhalation. J. Cryst. Growth 2017, 467, 155–161. [Google Scholar] [CrossRef]
- Hancock, B.C.; Shamblin, S.L. Water vapour sorption by pharmaceutical sugars. Pharm. Sci. Technol. Today 1998, 1, 345–351. [Google Scholar] [CrossRef]
- Wang, Z.; Ordoubadi, M.; Wang, H.; Vehring, R. Morphology and formation of crystalline leucine microparticles from a co-solvent system using multi-orifice monodisperse spray drying. Aerosol Sci. Technol. 2021, 55, 901–919. [Google Scholar] [CrossRef]
- He, G.; Bhamidi, V.; Tan, R.B.H.; Kenis, P.J.A.; Zukoski, C.F. Determination of critical supersaturation from microdroplet evaporation experiments. Cryst. Growth Des. 2006, 6, 1175–1180. [Google Scholar] [CrossRef]
- Wang, H.; Boraey, M.A.; Williams, L.; Lechuga-Ballesteros, D.; Vehring, R. Low-frequency shift dispersive Raman spectroscopy for the analysis of respirable dosage forms. Int. J. Pharm. 2014, 469, 197–205. [Google Scholar] [CrossRef]
- Carrigy, N.; Vehring, R. Vehring, R. Vehring, Engineering stable spray-dried biologic powder for inhalation. In Pharmaceutical Inhalation Aerosol Technology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 291–326. [Google Scholar]
- Littringer, E.M.; Noisternig, M.F.; Mescher, A.; Schroettner, H.; Walzel, P.; Griesser, U.J.; Urbanetz, N.A. The morphology and various densities of spray dried mannitol. Powder Technol. 2013, 246, 193–200. [Google Scholar] [CrossRef]
- Boraey, M.A.; Vehring, R. Diffusion controlled formation of microparticles. J. Aerosol Sci. 2014, 67, 131–143. [Google Scholar] [CrossRef]
- Baldelli, A.; Vehring, R. Analysis of cohesion forces between monodisperse microparticles with rough surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 179–189. [Google Scholar] [CrossRef]










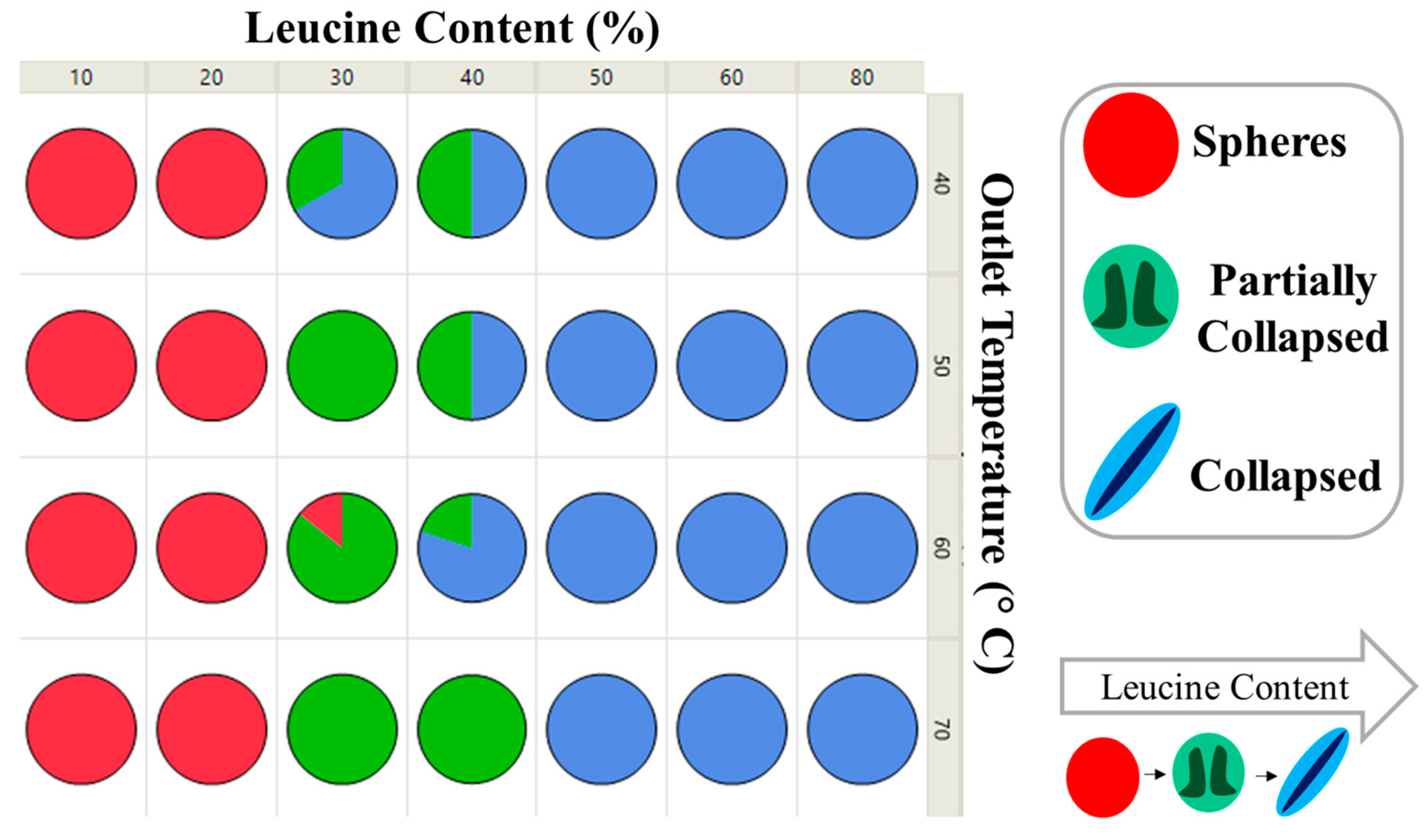


| Formulation Name | Feed Concentration (%) | Outlet Temperature (Txx) | |||||
|---|---|---|---|---|---|---|---|
| LxxEtxx | Ethanol: Water | Leucine | Mannitol | 40 °C | 50 °C | 60 °C | 70 °C |
| L10Et0 | 0:100 | 0.2 | 1.8 | m,x | |||
| L20Et0 | 0:100 | 0.4 | 1.6 | m,x | s | m,x | s |
| L20Et0(c) | 0:100 | 0 | 1.6 | m | m | ||
| L30Et0 | 0:100 | 0.6 | 1.4 | m,x | |||
| L40Et0 | 0:100 | 0.8 | 1.2 | s | s | m,x | s |
| L50Et0 | 0:100 | 1.0 | 1.0 | m,x | |||
| L60Et0 | 0:100 | 1.2 | 0.8 | m | s | m | s |
| L70Et0 | 0:100 | 1.4 | 0.6 | s | |||
| L80Et0 | 0:100 | 1.6 | 0.4 | s | s | m | s |
| L80Et0(c) | 0:100 | 0 | 0.4 | m | |||
| L10Et10 | 10:90 | 0.2 | 1.8 | s | s | m | s |
| L20Et10 | 10:90 | 0.4 | 1.6 | s | |||
| L30Et10 | 10:90 | 0.6 | 1.4 | s | s | s | s |
| L40Et10 | 10:90 | 0.8 | 1.2 | s | |||
| L50Et10 | 10:90 | 1.0 | 1.0 | s | |||
| L60Et10 | 10:90 | 1.2 | 0.8 | s | s | s | s |
| L70Et10 | 10:90 | 1.4 | 0.6 | s | |||
| L10Et20 | 20:80 | 0.2 | 1.8 | m | |||
| L10Et20(c) | 20:80 | 0 | 1.8 | m | |||
| L20Et20 | 20:80 | 0.4 | 1.6 | s | s | s | s |
| L30Et20 | 20:80 | 0.6 | 1.4 | m,x * | m,x | ||
| L40Et20 | 20:80 | 0.8 | 1.2 | s | |||
| L50Et20 | 20:80 | 1.0 | 1.0 | s | s | m | s |
| L50Et20(c) | 20:80 | 0 | 1.0 | m | |||
| L10Et30 | 30:70 | 0.2 | 1.8 | s | s | s | s |
| L20Et30 | 30:70 | 0.4 | 1.6 | m,x | |||
| L20Et30(c) | 30:70 | 0 | 1.6 | m | |||
| L30Et30 | 30:70 | 0.6 | 1.4 | s | s | s | s |
| L40Et30 | 30:70 | 0.8 | 1.2 | s | s | m | s |
| L10Et40 | 40:60 | 0.2 | 1.8 | s | |||
| L20Et40 | 40:60 | 0.4 | 1.6 | s | s | s | s |
| L30Et40 | 40:60 | 0.6 | 1.4 | m,x * | m,x | ||
| L10Et50 | 50:50 | 0.2 | 1.8 | s | s | s | s |
| L20Et50 | 50:50 | 0.4 | 1.6 | m,x | s | m,x | s |
| L20Et50(c) | 50:50 | 0 | 1.6 | m | m | ||
| L30Et50 | 50:50 | 0.6 | 1.4 | s | s | s | s |
| Formulation | (ms) | (ms) | (μm) |
|---|---|---|---|
| L10Et0T60 | 0.52 | 1.07 | 3.07 |
| L20Et0T60 | 1.08 | 0.96 | 3.08 |
| L30Et0T60 | 1.53 | 0.83 | 3.52 |
| L40Et0T60 | 1.94 | 0.71 | 3.88 |
| L50Et0T60 | 2.32 | 0.58 | 4.18 |
| L60Et0T60 | 2.66 | 0.42 | 4.44 |
| L80Et0T60 | 3.32 | 0.01 | 4.89 |
| L10Et10T60 | 0.53 | 1.08 | 3.07 |
| L10Et20T60 | 0.54 | 1.11 | 3.07 |
| L30Et20T60 | 1.58 | 0.86 | 3.52 |
| L50Et20T60 | 2.4 | 0.59 | 4.19 |
| L20Et30T60 | 1.14 | 1.02 | 3.07 |
| L40Et30T60 | 2.08 | 0.75 | 3.89 |
| L30Et40T60 | 1.68 | 0.91 | 3.54 |
| L20Et50T60 | 1.21 | 1.07 | 3.10 |
| L20Et0T40 | 1.6 | 1.44 | 3.04 |
| L60Et0T40 | 3.94 | 0.66 | 4.38 |
| L30Et20T40 | 2.32 | 1.28 | 3.48 |
| L30Et40T40 | 2.12 | 1.16 | 3.51 |
| L20Et50T40 | 1.69 | 1.52 | 3.05 |
| Formulation Name | Normalized δ Content | Mannitol Control | Normalized δ Content |
|---|---|---|---|
| LxxEtxxTxx | (%) | LxxEtxxTxx(c) | (%) |
| L20Et0T60 | 0 | L20Et0(c) | 0 |
| L60Et0T60 | 0 | - | - |
| L80Et0T60 | 0 | L80Et0(c) | 0 |
| L10Et10T60 | 0 | - | - |
| L10Et20T60 | 1 ± 5 | L10Et20(c) | 0 |
| L30Et20T60 | 0 | - | - |
| L50Et20T60 | 0 | L50Et20(c) | 0 |
| L20Et30T60 | 0 | L20Et30(c) | 0 |
| L40Et30T60 | 0 | - | - |
| L30Et40T60 | 0 | - | - |
| L20Et50T60 | 0 | L20Et50(c) | 0 |
| L20Et0T40 | 1 ± 5 | L20Et0(c) | 0 |
| L60Et0T40 | 10 ± 8 | - | - |
| L30Et20T40 | 0 | - | - |
| L30Et40T40 | 20 ± 6 | - | - |
| L20Et50T40 | 40 ± 5 | L20Et50(c) | 45 ± 5 |
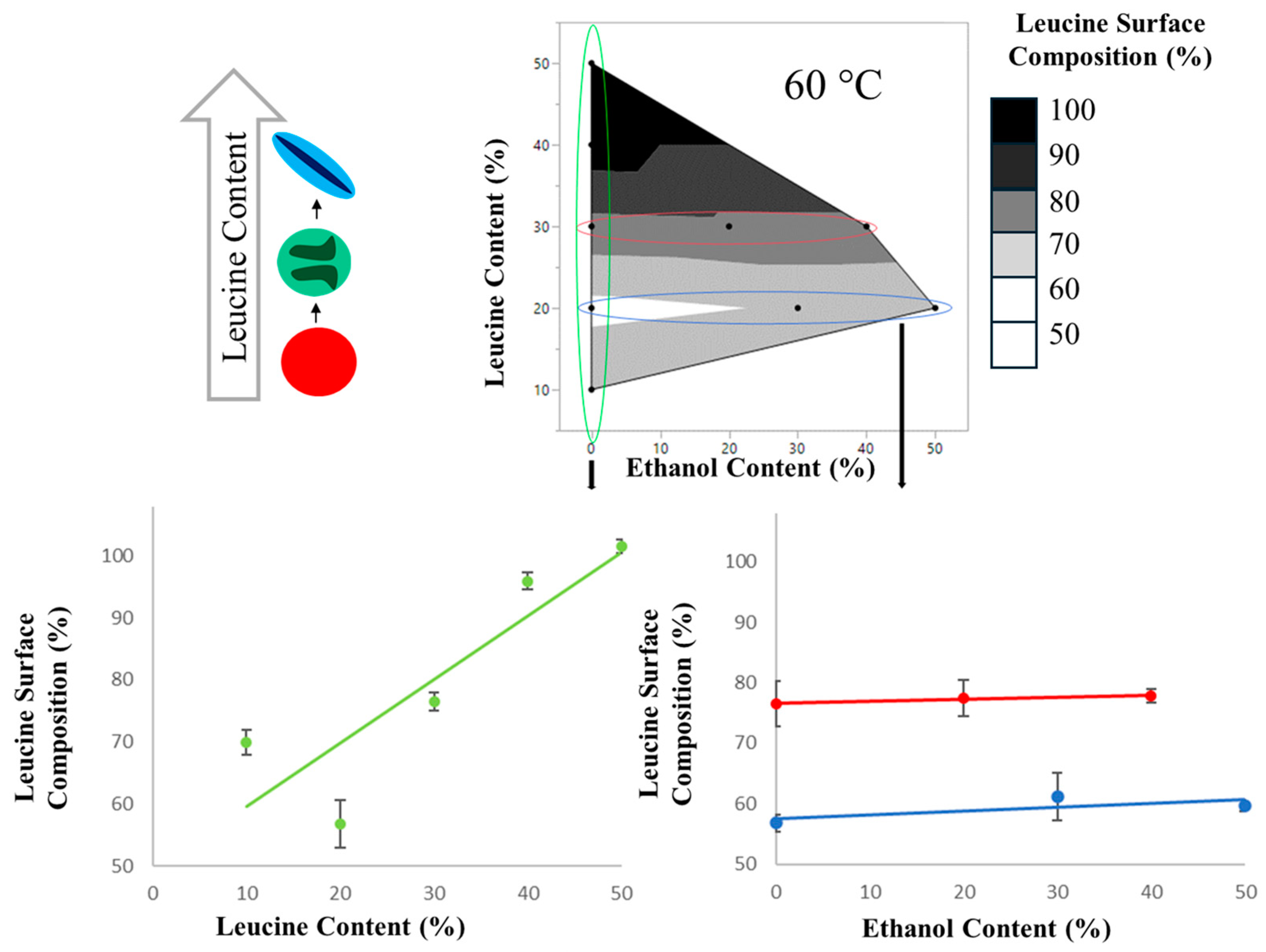
| Binary Formulation | Leucine Surface Composition | Binary Formulation | Leucine Surface Composition |
|---|---|---|---|
| LxxEtxxT60 | (%) | LxxEtxxT40 | (%) |
| L10Et0T60 | 70 ± 2 | - | - |
| L20Et0T60 | 57 ± 4 | L20Et0T40 | 70 ± 2 |
| L30Et0T60 | 77 ± 2 | - | - |
| L40Et0T60 | 96 ± 2 | - | - |
| L50Et0T60 | 102 ± 2 | - | - |
| L30Et20T60 | 78 ± 1 | L30Et20T40 | 93 ± 3 |
| L20Et30T60 | 61 ± 3 | - | - |
| L30Et40T60 | 78 ± 1 | L30Et40T40 | 84 ± 2 |
| L20Et50T60 | 60 ± 1 | L20Et50T40 | 63 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweizer, R.T.; Ordoubadi, M.; Prather, C.A.; Vehring, R.; Shepard, K.B. The Processing Space of the Spray-Dried Mannitol-Leucine System for Pulmonary Drug Delivery. Pharmaceutics 2024, 16, 398. https://doi.org/10.3390/pharmaceutics16030398
Schweizer RT, Ordoubadi M, Prather CA, Vehring R, Shepard KB. The Processing Space of the Spray-Dried Mannitol-Leucine System for Pulmonary Drug Delivery. Pharmaceutics. 2024; 16(3):398. https://doi.org/10.3390/pharmaceutics16030398
Chicago/Turabian StyleSchweizer, Riley T., Mani Ordoubadi, Cody A. Prather, Reinhard Vehring, and Kimberly B. Shepard. 2024. "The Processing Space of the Spray-Dried Mannitol-Leucine System for Pulmonary Drug Delivery" Pharmaceutics 16, no. 3: 398. https://doi.org/10.3390/pharmaceutics16030398
APA StyleSchweizer, R. T., Ordoubadi, M., Prather, C. A., Vehring, R., & Shepard, K. B. (2024). The Processing Space of the Spray-Dried Mannitol-Leucine System for Pulmonary Drug Delivery. Pharmaceutics, 16(3), 398. https://doi.org/10.3390/pharmaceutics16030398








