Fine-Tuning the Amphiphilic Properties of Carbosilane Dendritic Networks towards High-Swelling Thermogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Synthesis of Linear Polymers P(SH)2
2.2. General Methods
2.3. Synthesis of Dendritic Hydrogels
2.3.1. Synthesis of Dendritic Hydrogels
2.3.2. Post-Functionalization of Dendritic Hydrogels
2.4. Characterization of Hydrogels
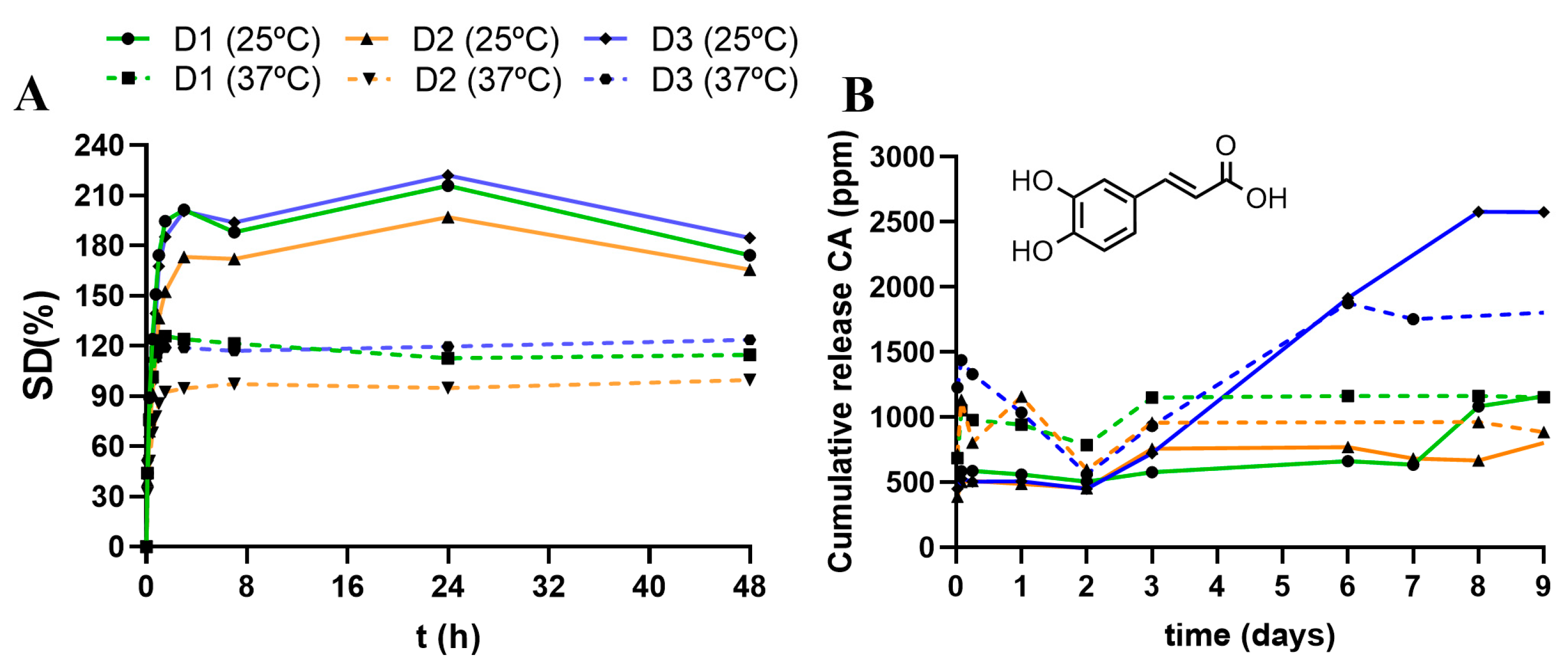
2.4.1. Swelling Degree (SD%)
2.4.2. Crosslinking Degree (CD%)
2.4.3. Caffeic Acid Loading and Release Assay
3. Results
3.1. Synthesis of Linear Polymers
3.2. Synthesis and Characterization of Dendritic Hydrogels
3.2.1. Impact of the Linear Polymer P(SH)2
3.2.2. Impact of the Dendritic Crosslinker
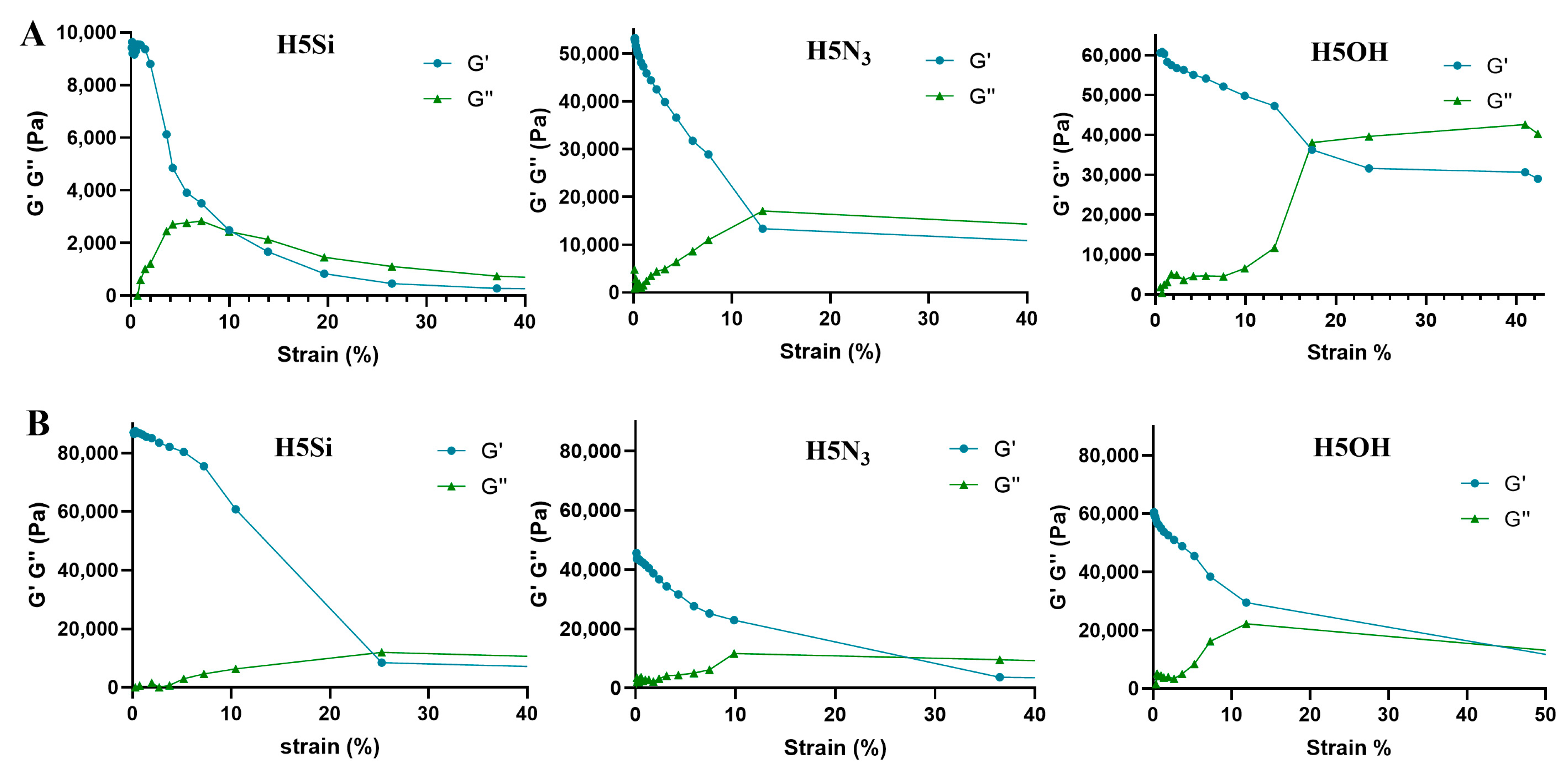
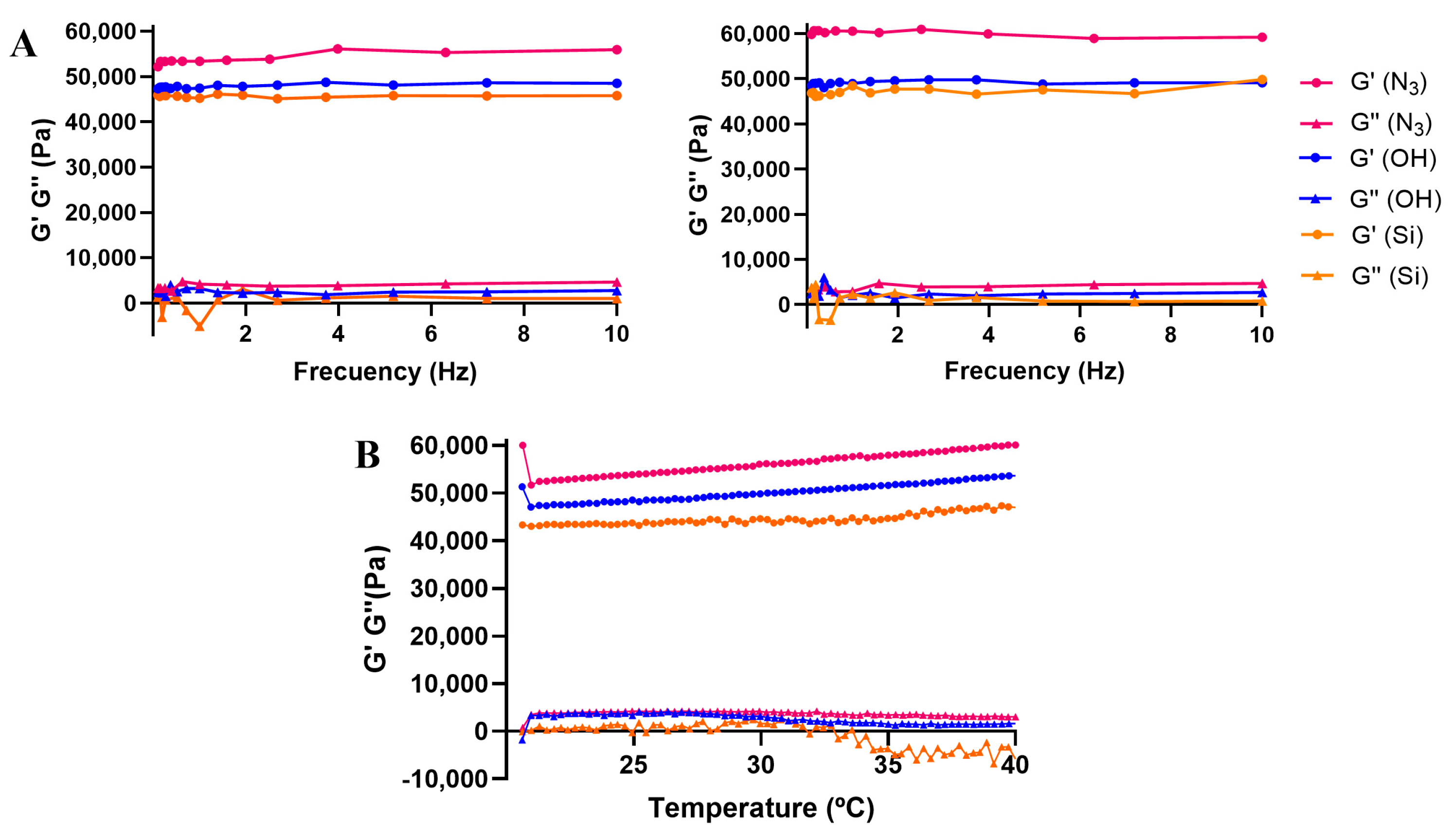
3.3. Mechanical Characterization of Dendritic Hydrogels
3.4. Post-Functionalization of Dendritic Networks
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, Y.; Zhao, J.; Johnson, J.A. Polymer networks: From plastics and gels to porous frameworks. Angew. Chem. Int. Ed. 2019, 59, 5022–5049. [Google Scholar] [CrossRef]
- Danielsen, S.P.O.; Beech, H.K.; Wang, S.; El-Zaatari, B.M.; Wang, X.; Sapir, L.; Ouchi, T.; Wang, Z.; Johnson, P.N.; Hu, Y.; et al. Molecular characterization of polymer networks. Chem. Rev. 2021, 121, 5042–5092. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-based hydrogels applied in drug delivery: An overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-responsive hydrogels: From recent progress to biomedical applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-l.; Kan, C.-w.; Wang, W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 11658. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Y.; Hu, X.; Gong, H.; Li, R.; Cox, H.; Zhang, J.; Waigh, T.A.; Xu, H.; Lu, J.R. Reversible thermoresponsive peptide–PNIPAM hydrogels for controlled drug delivery. Biomacromolecules 2019, 20, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.-Y.; Zhao, J.-Y.; Hsieh, Y.-R.; Lin, J.-H.; Chen, F.-Y.; Chakravarthy, R.D.; Chung, P.-C.; Lin, H.-C.; Hung, S.-C. Reverse thermo-responsive hydrogels prepared from Pluronic F127 and gelatin composite materials. RSC Adv. 2017, 7, 21252–21257. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Georgiou, T.K. Pre-clinical and clinical applications of thermoreversible hydrogels in biomedical engineering: A review. Polym. Int. 2021, 70, 1433–1448. [Google Scholar] [CrossRef]
- Olofsson, K.; Andrén, O.C.J.; Malkoch, M. Recent advances on crosslinked dendritic networks. J. Appl. Polym. Sci. 2013, 131, 39876. [Google Scholar] [CrossRef]
- Gitsov, I.; Zhu, C. Amphiphilic hydrogels constructed by Poly(ethylene glycol) and shape-persistent dendritic fragments. Macromolecules 2002, 35, 8418–8427. [Google Scholar] [CrossRef]
- Mongkhontreerat, S.; Andrén, O.C.J.; Boujemaoui, A.; Malkoch, M. Dendritic hydrogels: From exploring various crosslinking chemistries to introducing functions and naturally abundant resources. J. Polym. Sci. A 2015, 53, 2431–2439. [Google Scholar] [CrossRef]
- Apartsin, E.; Caminade, A.-M. Single-component physical hydrogels of dendritic molecules. J. Compos. Sci. 2023, 7, 26. [Google Scholar] [CrossRef]
- Kabanov, V.A.; Zezin, A.B.; Rogacheva, V.B.; Panova, T.V.; Bykova, E.V.; Joosten, J.G.H.; Brackman, J. Self-organization of cationic dendrimers in polyanionic hydrogels. J. Faraday Discuss. 2005, 128, 341. [Google Scholar] [CrossRef] [PubMed]
- Recio-Ruiz, J.; Carloni, R.; Ranganathan, S.; Muñoz-Moreno, L.; Carmena, M.J.; Ottaviani, M.F.; de la Mata, F.J.; García-Gallego, S. Amphiphilic dendritic hydrogels with carbosilane nanodomains: Preparation and characterization as drug delivery systems. Chem. Mater. 2023, 35, 2797–2807. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Sánchez, S.; Heredero-Bermejo, I.; de la Mata, F.J.; García-Gallego, S. Bifunctional carbosilane dendrimers for the design of multipurpose hydrogels with antibacterial action. Chem. Mater. 2024, 36, 266–274. [Google Scholar] [CrossRef]
- Naharros-Molinero, A.; Caballo-González, M.A.; de la Mata, F.J.; García-Gallego, S. Direct and reverse Pluronic micelles: Design and characterization of promising drug delivery nanosystems. Pharmaceutics 2022, 14, 2628. [Google Scholar] [CrossRef] [PubMed]
- Elluru, M.; Ma, H.; Hadjiargyrou, M.; Hsiao, B.S.; Chu, B. Synthesis and characterization of biocompatible hydrogel using Pluronics-based block copolymers. Polymer 2013, 54, 2088–2095. [Google Scholar] [CrossRef]
- Strašák, T.; Čermák, J.; Sýkora, J.; Horský, J.; Walterová, Z.; Jaroschik, F.; Harakat, D. Carbosilane metallodendrimers with titanocene dichloride end groups. Organometallics 2012, 31, 6779–6786. [Google Scholar] [CrossRef]
- Mencia, G.; Lozano-Cruz, T.; Valiente, M.; de la Mata, F.J.; Cano, J.; Gómez, R. New ionic carbosilane dendrons possessing fluorinated tails at different locations on the skeleton. Molecules 2020, 25, 807. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, L.J.; Pérez-Madrigal, M.M.; Arno, M.C.; Dove, A.P. Nonswelling Thiol–Yne Cross-Linked Hydrogel Materials as Cytocompatible Soft Tissue Scaffolds. Biomacromolecules 2018, 19, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, C.; Pan, Y.; Zhang, Y.; Wang, J.; Huang, M.; Wang, Y.; Wang, K.; Gou, M.; Tu, M.; et al. Synthesis and characterization of a thermosensitive hydrogel based on biodegradable amphiphilic PCL-Pluronic (L35)-PCL block copolymers. Colloid Surf. A 2007, 302, 430–438. [Google Scholar] [CrossRef]
- Mongkhontreerat, S.; Öberg, K.; Erixon, L.; Löwenhielm, P.; Hult, A.; Malkoch, M. UV initiated thiol–ene chemistry: A facile and modular synthetic methodology for the construction of functional 3D networks with tunable properties. J. Mater. Chem. A 2013, 1, 13732–13737. [Google Scholar] [CrossRef]
- Granskog, V.; García-Gallego, S.; von Kieseritzky, J.; Rosendahl, J.; Stenlund, P.; Zhang, Y.; Petronis, S.; Lyvén, B.; Arner, M.; Håkansson, J.; et al. High-performance Thiol–Ene composites unveil a new era of adhesives suited for bone repair. Adv. Funct. Mater. 2018, 28, 1800372. [Google Scholar] [CrossRef]
- Chung, H.J.; Park, T.G. Self-assembled and nanostructured hydrogels for drug delivery and tissue engineering. NanoToday 2009, 4, 429–437. [Google Scholar] [CrossRef]
- Chun, K.W.; Lee, J.B.; Kim, S.H.; Park, T.G. Controlled release of plasmid DNA from photo-cross-linked pluronic hydrogels. Biomaterials 2005, 26, 3319–3326. [Google Scholar] [CrossRef]
- Kim, M.R.; Park, T.G. Temperature-responsive and degradable hyaluronic acid/Pluronic composite hydrogels for controlled release of human growth hormone. J. Control Release 2002, 80, 69–77. [Google Scholar] [CrossRef]
- Cabrera-Munguía, D.A.; Caldera-Villalobos, M.; Flores-Guía, T.E.; Cano-Salazar, L.F.; Claudio-Rizo, J.A. Composites in hydrogel state with nanostructured components for biomedical applications. In Nanotechnology for Biomedical Applications; Materials horizons: From nature to nanomaterials; Gopi, S., Balakrishnan, P., Mubarak, N.M., Eds.; Springer: Singapore, 2022. [Google Scholar]
- Lee, J.B.; Yoon, J.J.; Lee, D.S.; Park, T.G. Photo-crosslinkable, thermo-sensitive and biodegradable pluronic hydrogels for sustained release of protein. J. Biomater. Sci. Polym. Ed. 2004, 15, 1571–1583. [Google Scholar] [CrossRef]
- Yoo, H.S. Photo-cross-linkable and thermo-responsive hydrogels containing chitosan and Pluronic for sustained release of human growth hormone (hGH). J. Biomater. Sci. Polym. Ed. 2007, 18, 1429–1441. [Google Scholar] [CrossRef]
- Lupu, A.; Gradinaru, L.M.; Rusu, D.; Bercea, M. Self-healing of Pluronic® F127 hydrogels in the presence of various polysaccharides. Gels 2023, 9, 719. [Google Scholar] [CrossRef] [PubMed]
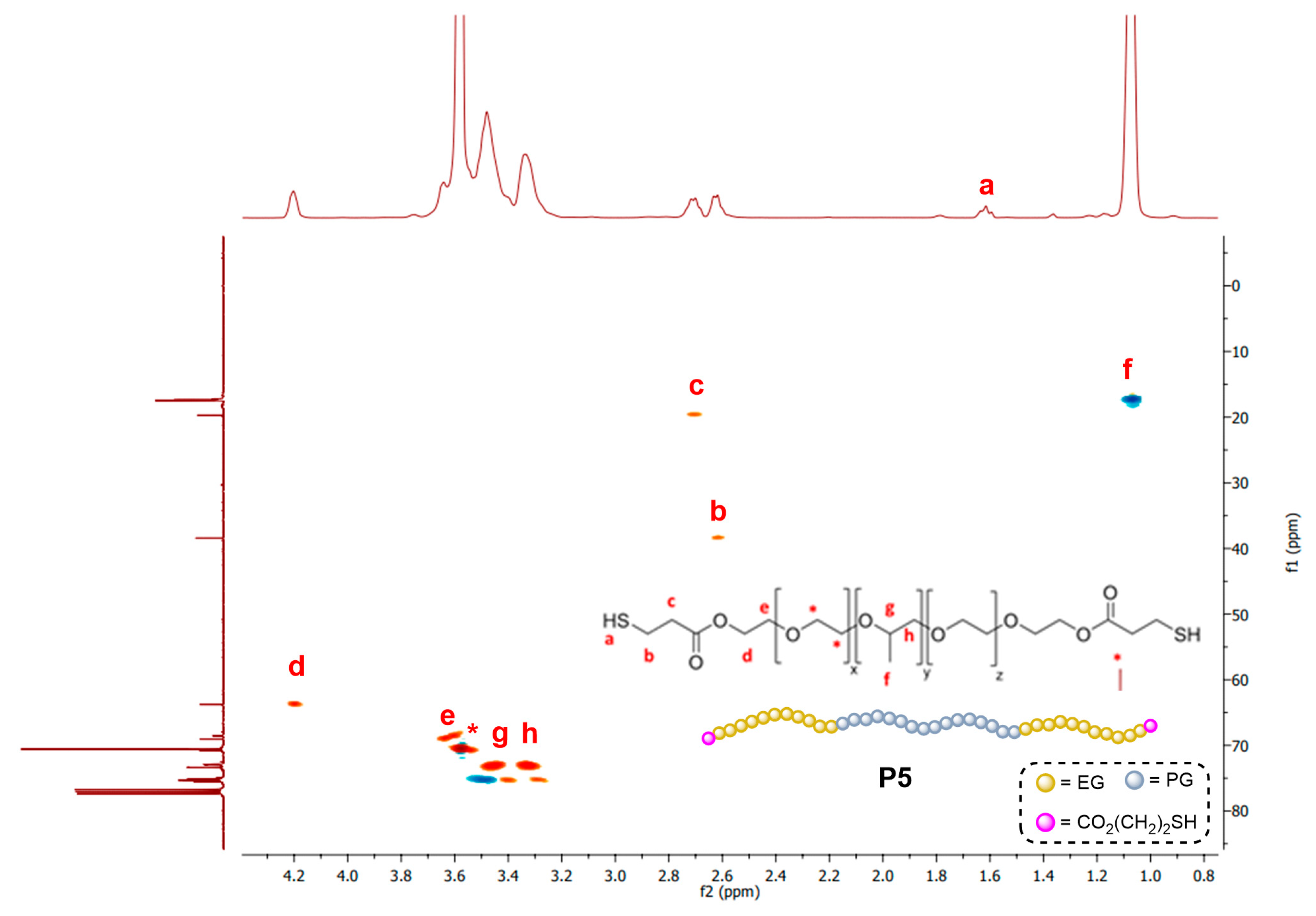



| Polymer | Mn a (g/mol) | %PEG | HLB b | Dendritic Hydrogel | CD (%) | SD (%) c 25/37 °C |
|---|---|---|---|---|---|---|
| PEG1k(SH)2 (P1) | 1000 | 100 | 18 | Hy[(Si-G1V8)x(P1)] (H1Si) | 91 (3 h) | 276/271 |
| PPG1k(SH)2 (P2) | 1000 | 0 | 10.78 | Hy[(Si-G1V8)x(P2)] (H2Si) | 94 (3 h) | 2/3 |
| PLUL31(SH)2 (P3) | 1100 | 10 | 11.80 | Hy[(Si-G1V8)x(P3)] (H3Si) | 94 (3 h) | 6/5 |
| PLUL61(SH)2 (P4) | 2000 | 10 | 10.05 | Hy[(Si-G1V8)x(P4)] (H4Si) | 71 (3 h) | 11/9 |
| Hy[(N3-G3V8)x(P4)] (H4N3) | 92 (3 h) | d | ||||
| Hy[(HO-G3V8)x(P4)] (H4OH) | 92 (3 h) | d | ||||
| PLUL35(SH)2 (P5) | 1900 | 50 | 18.10 | Hy[(Si-G1V8)x(P5)] (H5Si) | 90 (4 h) | 216/112 |
| Hy[(N3-G3V8)x(P5)] (H5N3) | 76 (4 h) | 198/95 | ||||
| Hy[(HO-G3V8)x(P5)] (H5OH) | 81 (4 h) | 222/120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Sánchez, S.; Barrios-Gumiel, A.; de la Mata, F.J.; García-Gallego, S. Fine-Tuning the Amphiphilic Properties of Carbosilane Dendritic Networks towards High-Swelling Thermogels. Pharmaceutics 2024, 16, 495. https://doi.org/10.3390/pharmaceutics16040495
Muñoz-Sánchez S, Barrios-Gumiel A, de la Mata FJ, García-Gallego S. Fine-Tuning the Amphiphilic Properties of Carbosilane Dendritic Networks towards High-Swelling Thermogels. Pharmaceutics. 2024; 16(4):495. https://doi.org/10.3390/pharmaceutics16040495
Chicago/Turabian StyleMuñoz-Sánchez, Silvia, Andrea Barrios-Gumiel, Francisco Javier de la Mata, and Sandra García-Gallego. 2024. "Fine-Tuning the Amphiphilic Properties of Carbosilane Dendritic Networks towards High-Swelling Thermogels" Pharmaceutics 16, no. 4: 495. https://doi.org/10.3390/pharmaceutics16040495
APA StyleMuñoz-Sánchez, S., Barrios-Gumiel, A., de la Mata, F. J., & García-Gallego, S. (2024). Fine-Tuning the Amphiphilic Properties of Carbosilane Dendritic Networks towards High-Swelling Thermogels. Pharmaceutics, 16(4), 495. https://doi.org/10.3390/pharmaceutics16040495






