Comparative Cytotoxicity of Menthol and Eucalyptol: An In Vitro Study on Human Gingival Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Culture
2.2. Sample Preparation
2.3. IC50 Assay
2.4. Cell Apoptosis/Necrosis Assay: Annexin-V-FITC and 7-AAD Staining
2.5. MTT Assay
2.6. Horizontal Scratch Wound Assay
2.7. Intracellular ROS Measurement
2.8. Statistical Analysis
3. Results
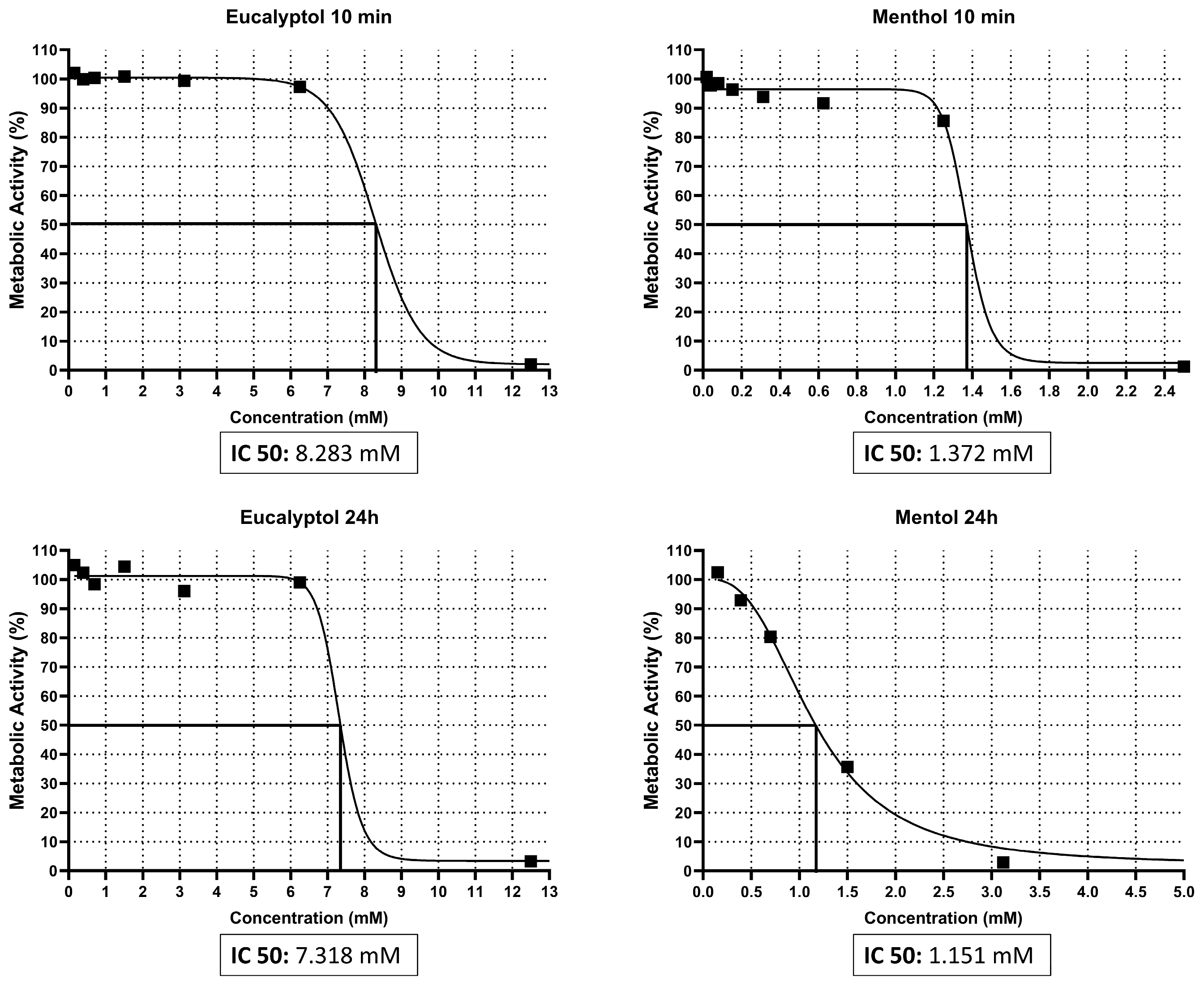
3.1. IC50 Assay
3.2. Cell Apoptosis/Necrosis Assay: Annexin-V-FITC and 7-AAD Staining
3.3. MTT Assay
3.4. Horizontal Scratch Wound Assay
3.5. Intracellular ROS Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical Composition and Anti-Inflammatory, Cytotoxic and Antioxidant Activities of Essential Oil from Leaves of Mentha piperita Grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.M.F.; Bona, N.P.; Pedra, N.S.; Da Cunha, K.F.; Fiorentini, A.M.; Stefanello, F.M.; Zavareze, E.R.; Dias, A.R.G. Risk assessment of in vitro cytotoxicity, antioxidant and antimicrobial activities of Mentha piperita L. essential oil. J. Toxicol. Environ. Health Part A 2022, 85, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Dolghi, A.; Coricovac, D.; Dinu, S.; Pinzaru, I.; Dehelean, C.A.; Grosu, C.; Chioran, D.; Merghes, P.E.; Sarau, C.A. Chemical and Antimicrobial Characterization of Mentha piperita L. and Rosmarinus officinalis L. Essential Oils and In Vitro Potential Cytotoxic Effect in Human Colorectal Carcinoma Cells. Molecules 2022, 27, 6106. [Google Scholar] [CrossRef] [PubMed]
- Farco, J.A.; Grundmann, O. Menthol—Pharmacology of an important naturally medicinal “cool”. Mini Rev. Med. Chem. 2013, 13, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, H.; Mazumder, A.; Salahuddin; Yadav, R.K.; Chauhan, B.; Abdulah, M.M. Camphor and Menthol as Anticancer Agents: Synthesis, Structure-Activity Relationship and Interaction with Cancer Cell Lines. Anticancer Agents Med. Chem. 2023, 23, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, H.; Gautam, L.K.; Capalash, N. Unraveling the molecular mechanism of l-menthol against cervical cancer based on network pharmacology, molecular docking and in vitro analysis. Mol. Divers 2023, 27, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Ghavam, M. In vitro biological potential of the essential oil of some aromatic species used in Iranian traditional medicine. Inflammopharmacology 2022, 30, 855–874. [Google Scholar] [CrossRef]
- Lu, H.-F.; Liu, J.-Y.; Hsueh, S.-C.; Yang, Y.-Y.; Yang, J.-S.; Tan, T.-W.; Kok, L.-F.; Lu, C.-C.; Lan, S.-H.; Wu, S.-Y.; et al. (-)-Menthol inhibits WEHI-3 leukemia cells in vitro and in vivo. In Vivo 2007, 21, 285–289. [Google Scholar]
- Kijpornyongpan, T.; Sereemaspun, A.; Chanchao, C. Dose-Dependent Cytotoxic Effects of Menthol on Human Malignant Melanoma A-375 Cells: Correlation with TRPM8 Transcript Expression. Asian Pac. J. Cancer Prev. 2014, 15, 1551–1556. [Google Scholar] [CrossRef]
- Nagai, K.; Fukuno, S.; Omachi, A.; Omotani, S.; Hatsuda, Y.; Myotoku, M.; Konishi, H. Enhanced anti-cancer activity by menthol in HepG2 cells exposed to paclitaxel and vincristine: Possible involvement of CYP3A4 downregulation. Drug Metab. Pers. Ther. 2019, 34, 20180029. [Google Scholar] [CrossRef]
- Nagai, K.; Tamura, M.; Murayama, R.; Fukuno, S.; Ito, T.; Konishi, H. Development of multi-drug resistance to anticancer drugs in HepG2 cells due to MRP2 upregulation on exposure to menthol. PLoS ONE 2023, 18, e0291822. [Google Scholar] [CrossRef]
- Yang, B.; Du, S.; Lu, Y.; Jia, S.; Zhao, M.; Bai, J.; Li, P.; Wu, H. Influence of paeoniflorin and menthol on puerarin transport across MDCK and MDCK-MDR1 cells as blood–brain barrier in vitro model. J. Pharm. Pharmacol. 2018, 70, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, C.A.; Armstrong, M.J.; Bradt, C.I.; Hill, R.B.; Greenwood, S.K.; Galloway, S.M. Chromosome aberrations in vitro related to cytotoxicity of nonmutagenic chemicals and metabolic poisons. Environ. Mol. Mutagen. 1998, 31, 316–326. [Google Scholar] [CrossRef]
- Dörsam, B.; Wu, C.-F.; Efferth, T.; Kaina, B.; Fahrer, J. The eucalyptus oil ingredient 1,8-cineol induces oxidative DNA damage. Arch. Toxicol. 2015, 89, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.-M.; Lin, J.-Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.F.R.; Sanglard, D.; Soković, M. Camphor and Eucalyptol—Anticandidal Spectrum, Antivirulence Effect, Efflux Pumps Interference and Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 483. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Gupta, P.; Srivastava, A.K.; Poluri, K.M.; Prasad, R. Eucalyptol/β-cyclodextrin inclusion complex loaded gellan/PVA nanofibers as antifungal drug delivery system. Int. J. Pharm. 2021, 609, 121163. [Google Scholar] [CrossRef]
- Tulbah, A.S.; Bader, A.; Ong, H.X.; Traini, D. In vitro evaluation of nebulized eucalyptol nano-emulsion formulation as a potential COVID-19 treatment. Saudi Pharm. J. 2022, 30, 1691–1699. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Marques, M.E.A.; Salvador, D.M.F. In vitro cytotoxic and non-genotoxic effects of gutta-percha solvents on mouse lymphoma cells by single cell gel (comet) assay. Braz. Dent. J. 2006, 17, 228–232. [Google Scholar] [CrossRef]
- Izham, M.N.M.; Hussin, Y.; Rahim, N.F.C.; Aziz, M.N.M.; Yeap, S.K.; Rahman, H.S.; Masarudin, M.J.; Mohamad, N.E.; Abdullah, R.; Alitheen, N.B. Physicochemical characterization, cytotoxic effect and toxicity evaluation of nanostructured lipid carrier loaded with eucalyptol. BMC Complement. Med. Ther. 2021, 21, 254. [Google Scholar] [CrossRef]
- Nikolić, B.; Vasilijević, B.; Mitić-Ćulafić, D.; Vuković-Gačić, B.; Knežević-Vukćević, J. Comparative study of genotoxic, antigenotoxic and cytotoxic activities of monoterpenes camphor, eucalyptol and thujone in bacteria and mammalian cells. Chem. Biol. Interact. 2015, 242, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.A.; Matsumoto, M.A.; Marques, M.E.; Salvadori, D.M. Biocompatibility of gutta-percha solvents using in vitro mammalian test-system. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, e106–e109. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.L.; López-García, S.; Forner, L.; Rodríguez-Lozano, F.J.; García-Bernal, D.; Sánchez-Bautista, S.; Puig-Herreros, C.; Rosell-Clari, V.; Oñate-Sánchez, R.E. Are Endodontic Solvents Cytotoxic? An In Vitro Study on Human Periodontal Ligament Stem Cells. Pharmaceutics 2022, 14, 2415. [Google Scholar] [CrossRef] [PubMed]
- Omaiye, E.E.; Luo, W.; McWhirter, K.J.; Pankow, J.F.; Talbot, P. Flavour chemicals, synthetic coolants and pulegone in popular mint-flavoured and menthol-flavoured e-cigarettes. Tob. Control 2022, 31, e3–e9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lucas, J.H.; Pang, C.; Zhao, R.; Rahman, I. Tobacco and menthol flavored nicotine-free electronic cigarettes induced inflammation and dysregulated repair in lung fibroblast and epithelium. Respir. Res. 2024, 25, 23. [Google Scholar] [CrossRef] [PubMed]
- Omaiye, E.E.; McWhirter, K.J.; Luo, W.; Tierney, P.A.; Pankow, J.F.; Talbot, P. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci. Rep. 2019, 9, 2468. [Google Scholar] [CrossRef] [PubMed]
- Noriyasu, A.; Konishi, T.; Mochizuki, S.; Sakurai, K.; Tanaike, Y.; Matsuyama, K.; Uezu, K.; Kawano, T. Menthol-enhanced cytotoxicity of cigarette smoke demonstrated in two bioassay models. Tob. Induc. Dis. 2013, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Nethery, R.C.; Herring, A.H.; Tarran, R. Flavored little cigar smoke induces cytotoxicity and apoptosis in airway epithelia. Cell Death Discov. 2017, 3, 17019. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.; Kelty, J.S.; Laskin, J.D.; Laskin, D.L.; Gow, A.J. Menthol flavoring in e-cigarette condensate causes pulmonary dysfunction and cytotoxicity in precision cut lung slices. Am. J. Physiol. Cell Mol. Physiol. 2023, 324, L345–L357. [Google Scholar] [CrossRef]
- O’Farrell, H.E.; Brown, R.; Brown, Z.; Milijevic, B.; Ristovski, Z.D.; Bowman, R.V.; Fong, K.M.; Vaughan, A.; Yang, I.A. E-cigarettes induce toxicity comparable to tobacco cigarettes in airway epithelium from patients with COPD. Toxicol. Vitr. 2021, 75, 105204. [Google Scholar] [CrossRef]
- Go, Y.Y.; Mun, J.Y.; Chae, S.-W.; Chang, J.; Song, J.-J. Comparison between in vitro toxicities of tobacco- and menthol-flavored electronic cigarette liquids on human middle ear epithelial cells. Sci. Rep. 2020, 10, 2544. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Effects of E-Cigarette Refill Liquid Flavorings with and without Nicotine on Human Retinal Pigment Epithelial Cells: A Preliminary Study. Int. J. Environ. Res. Public Health 2021, 18, 11655. [Google Scholar] [CrossRef] [PubMed]
- Putzhammer, R.; Doppler, C.; Jakschitz, T.; Heinz, K.; Förste, J.; Danzl, K.; Messner, B.; Bernhard, D. Vapours of US and EU Market Leader Electronic Cigarette Brands and Liquids Are Cytotoxic for Human Vascular Endothelial Cells. PLoS ONE 2016, 11, e0157337. [Google Scholar] [CrossRef] [PubMed]
- Faggion, C.M. Guidelines for Reporting Pre-clinical In Vitro Studies on Dental Materials. J. Evid. Based Dent. Pract. 2012, 12, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Gironés, J.; López-García, S.; Pecci-Lloret, M.R.; Pecci-Lloret, M.P.; Lozano, F.J.R.; García-Bernal, D. In vitro biocompatibility testing of 3D printing and conventional resins for occlusal devices. J. Dent. 2022, 123, 104163. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Gironés, J.; López-García, S.; Pecci-Lloret, M.R.; Pecci-Lloret, M.P.; García-Bernal, D. Influence of dual-cure and self-cure abutment cements for crown implants on human gingival fibroblasts biological properties. Ann. Anat.-Anat. Anzeiger 2022, 239, 151829. [Google Scholar] [CrossRef] [PubMed]
- Scelza, M.F.Z.; Oliveira, L.R.L.; Carvalho, F.B.; Faria, S.C.-R. In vitro evaluation of macrophage viability after incubation in orange oil, eucalyptol, and chloroform. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, e24–e27. [Google Scholar] [CrossRef] [PubMed]
- Naksawat, M.; Norkaew, C.; Charoensedtasin, K.; Roytrakul, S.; Tanyong, D. Anti-leukemic effect of menthol, a peppermint compound, on induction of apoptosis and autophagy. PeerJ 2023, 11, e15049. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, C.; Mu, L.; Hu, H.; Qin, X. Menthol induces apoptosis and inhibits proliferation and migration of nonsmall cell lung carcinoma in vitro and in vivo through Akt pathway. Clin. Respir. J. 2023, 17, 1265–1275. [Google Scholar] [CrossRef]
- da Costa, A.O.; de Assis, M.C.; Ede Marques, A.; Plotkowski, M.C. Comparative analysis of three methods to assess viability of mammalian cells in culture. Biocell 1999, 23, 65–72. [Google Scholar]
- Shaikh, S.B.; Tung, W.C.; Pang, C.; Lucas, J.; Li, D.; Rahman, I. Flavor Classification/Categorization and Differential Toxicity of Oral Nicotine Pouches (ONPs) in Oral Gingival Epithelial Cells and Bronchial Epithelial Cells. Toxics 2022, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Fatima, T.; Abrar, H.; Jahan, N.; Shamim, S.; Ahmed, N.; Ali, A.B.; Begum, I.; Ahmed, W. Molecular marker identification, antioxidant, antinociceptive, and anti-inflammatory responsiveness of malonic acid capped silver nanoparticle. Front. Pharmacol. 2024, 14, 1319613. [Google Scholar] [CrossRef] [PubMed]
- Gomathi, R.; Umamaheswari, T.N.; Prethipa, R. Evaluation of Antioxidant, Anti-inflammatory, and Antimicrobial Activities of Raspberry Fruit Extract: An In Vitro Study. Cureus 2024, 16, e54045. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Gundogan, G.I.; Durmus, S.; Ozturk, G.C.; Kucukyesil, N.; Acar, Y.T.; Ba, R.B.; Kig, C. A comparative study of the effects of gutta-percha solvents on human osteoblasts and murine fibroblasts. Aust. Endod. J. 2021, 47, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pan, H.; Liu, W.; Liu, E.; Pang, Y.; Gao, H.; He, Q.; Liao, W.; Yao, Y.; Zeng, J.; et al. Menthol: An underestimated anticancer agent. Front. Pharmacol. 2023, 14, 1148790. [Google Scholar] [CrossRef]
- Lim, J.; Lee, J.E.; Park, C.-J.; Park, J.-B. Evaluation of the Effects of Mouthwash on the Morphology and Cell Viability of Osteoblast-Like Cells. Biomed. Res. Int. 2022, 2022, 5884974. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puig-Herreros, C.; Sanz, J.L.; García-Bernal, D.; Rodríguez-Lozano, F.J.; Murcia, L.; Forner, L.; Ghilotti, J.; Oñate-Sánchez, R.E.; López-García, S. Comparative Cytotoxicity of Menthol and Eucalyptol: An In Vitro Study on Human Gingival Fibroblasts. Pharmaceutics 2024, 16, 521. https://doi.org/10.3390/pharmaceutics16040521
Puig-Herreros C, Sanz JL, García-Bernal D, Rodríguez-Lozano FJ, Murcia L, Forner L, Ghilotti J, Oñate-Sánchez RE, López-García S. Comparative Cytotoxicity of Menthol and Eucalyptol: An In Vitro Study on Human Gingival Fibroblasts. Pharmaceutics. 2024; 16(4):521. https://doi.org/10.3390/pharmaceutics16040521
Chicago/Turabian StylePuig-Herreros, Clara, José Luis Sanz, David García-Bernal, Francisco Javier Rodríguez-Lozano, Laura Murcia, Leopoldo Forner, James Ghilotti, Ricardo E. Oñate-Sánchez, and Sergio López-García. 2024. "Comparative Cytotoxicity of Menthol and Eucalyptol: An In Vitro Study on Human Gingival Fibroblasts" Pharmaceutics 16, no. 4: 521. https://doi.org/10.3390/pharmaceutics16040521







