Interaction of γ-Polyglutamic Acid/Polyethyleneimine/Plasmid DNA Ternary Complexes with Serum Components Plays a Crucial Role in Transfection in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Plasmid DNA
2.4. Preparation of Binary Complexes
2.5. Preparation of Ternary Complexes
2.6. Preparation of Serum Components
2.7. In Vivo Gene Transfer
2.8. Physicochemical Characteristics Measurement
2.9. Microscopic Image Analysis
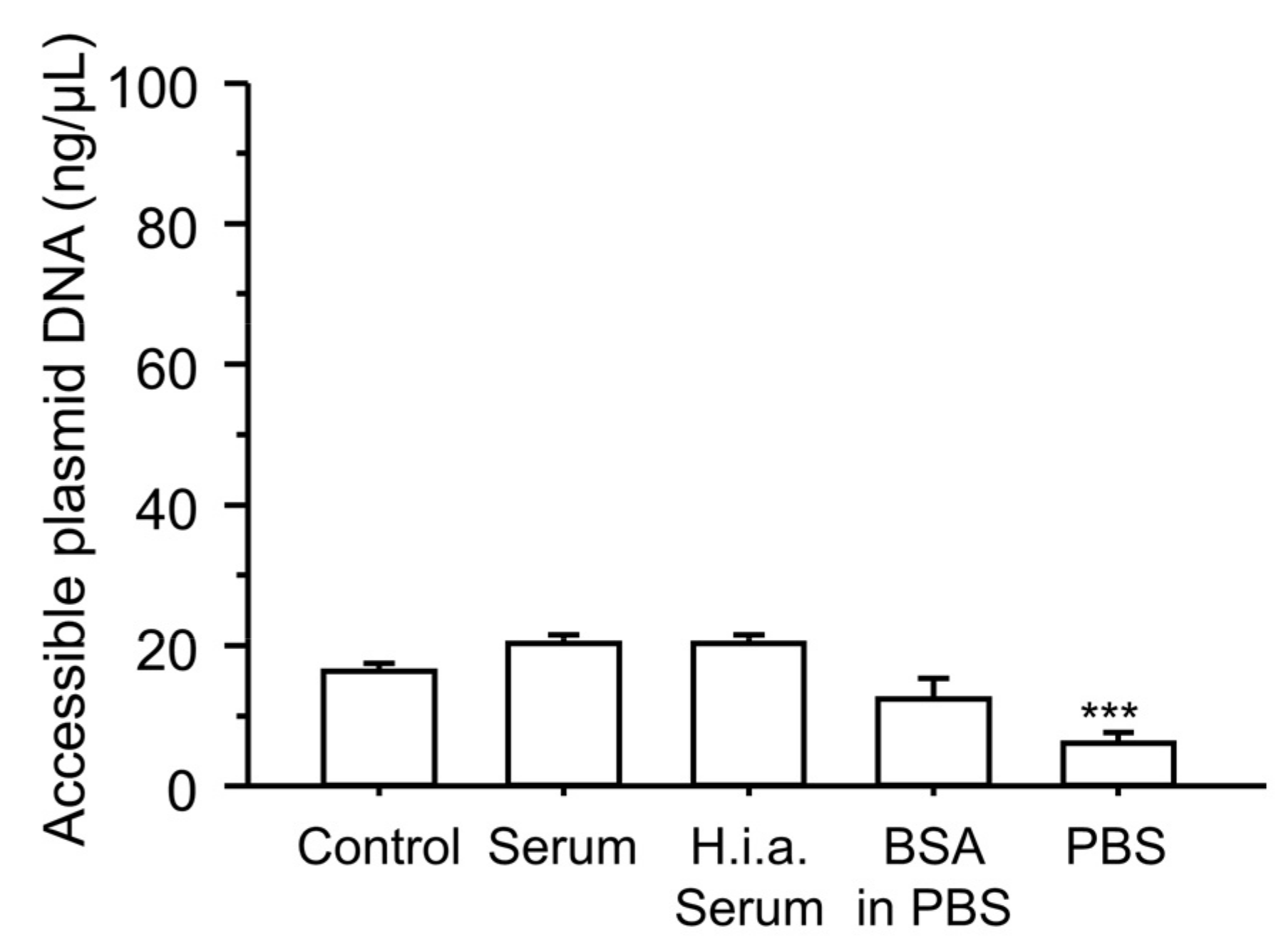
2.10. Plasmid DNA Accessibility
2.11. Statistical Analysis
3. Results
3.1. Effect of Interaction with Serum on In Vivo Transfection Using Binary Complexes
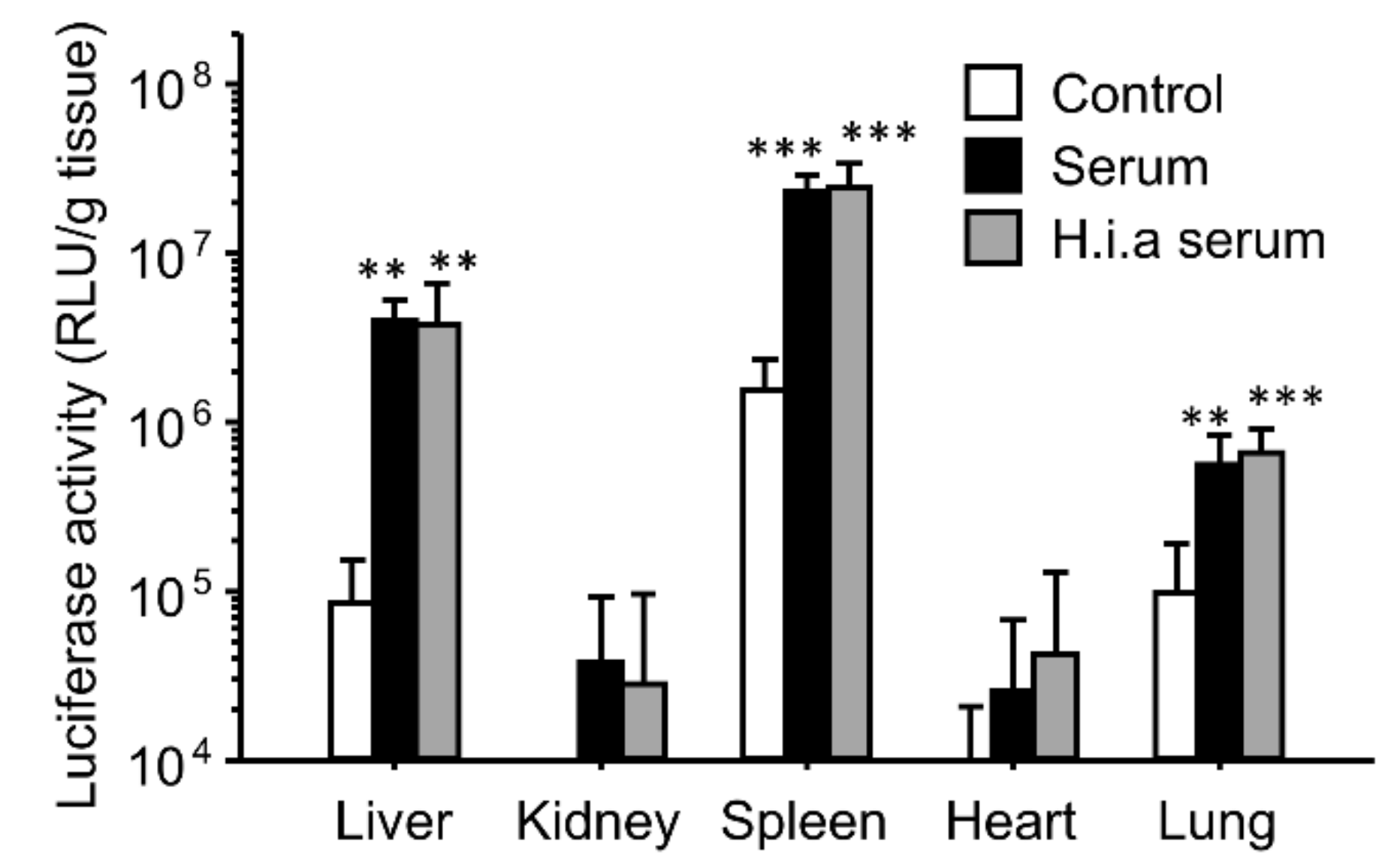
3.2. Effect of Interaction with Serum on In Vivo Transfection Using Ternary Complexes
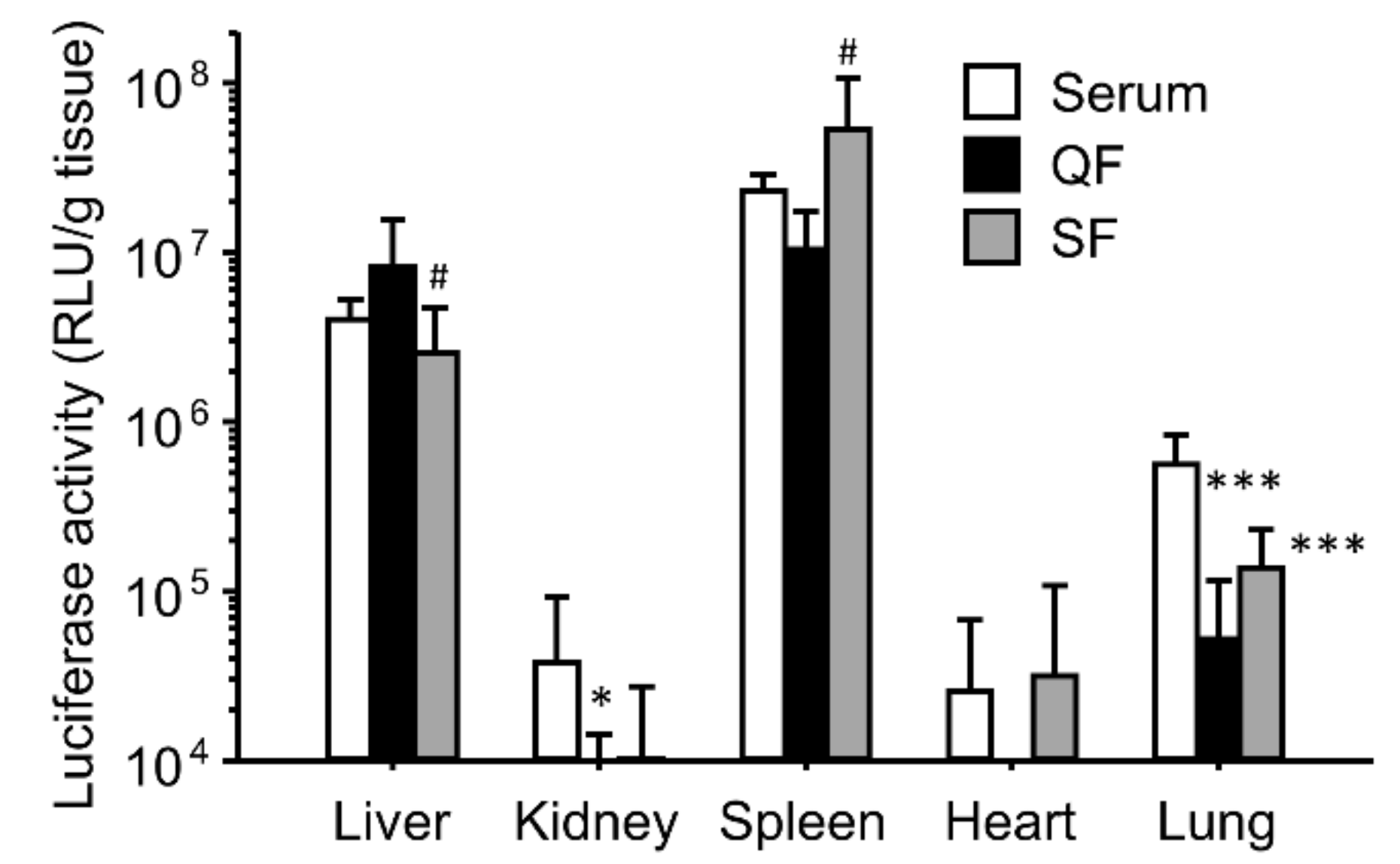
3.3. Effect of Separation of Serum Components on the Enhancing Effects of the Gene Expression for Ternary Complexes
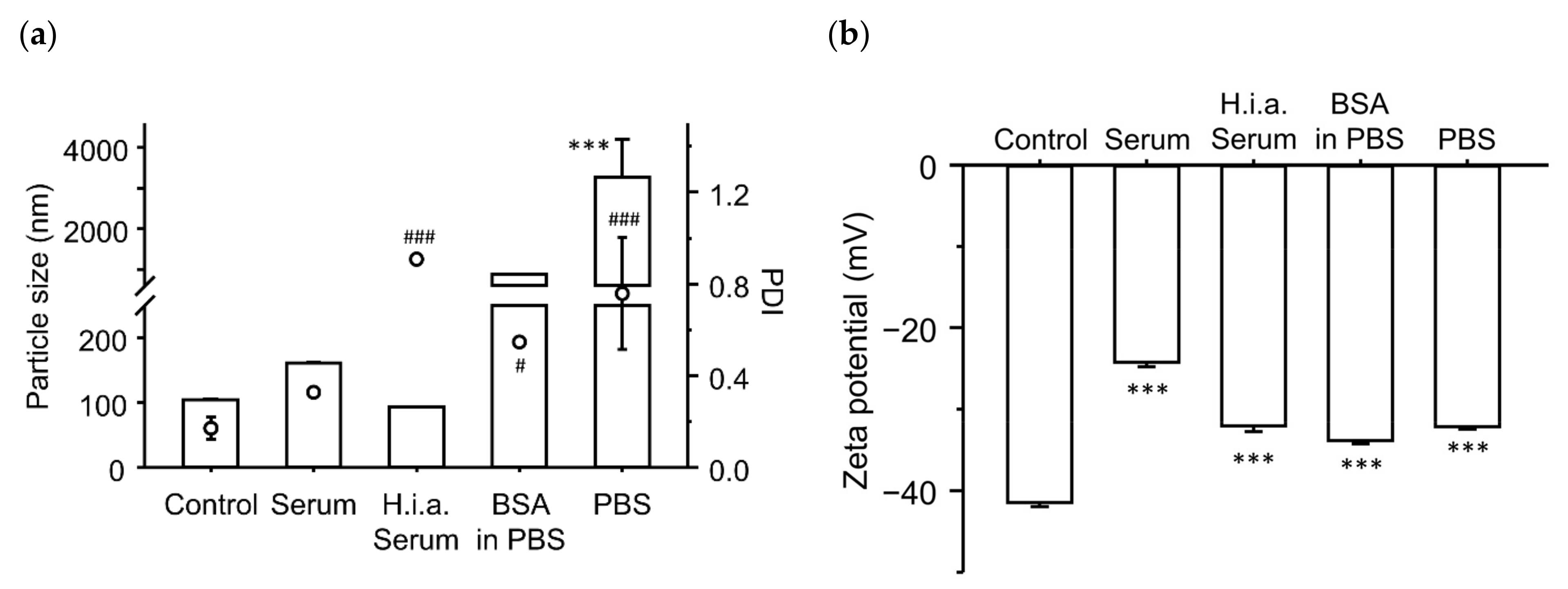
3.4. Effect of BSA and PBS on the Enhancing Effects of the Gene Expression for Ternary Complexes
3.5. Effect of Incubation with Serum Components on the Physicochemical Characteristics of the Ternary Complexes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-Puente, D.H.; Pérez-Trujillo, J.J.; Zavala-Flores, L.M.; García-García, A.; Villanueva-Olivo, A.; Rodríguez-Rocha, H.; Valdés, J.; Saucedo-Cárdenas, O.; Montes de Oca-Luna, R.; Loera-Arias, M.J. Plasmid DNA for Therapeutic Applications in Cancer. Pharmaceutics 2022, 14, 1861. [Google Scholar] [CrossRef] [PubMed]
- Grisch-Chan, H.M.; Schwank, G.; Harding, C.O.; Thöny, B. State-of-the-Art 2019 on Gene Therapy for Phenylketonuria. Hum. Gene Ther. 2019, 30, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J Gene Med. 2018, 20, e3015. [Google Scholar] [CrossRef] [PubMed]
- Schnell, M.A.; Zhang, Y.; Tazelaar, J.; Gao, G.P.; Yu, Q.C.; Qian, R.; Chen, S.J.; Varnavski, A.N.; LeClair, C.; Raper, S.E.; et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 2001, 3, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Khatri, A.; Shelke, R.; Guan, S.; Somanathan, S. Higher Seroprevalence of Anti-Adeno-Associated Viral Vector Neutralizing Antibodies Among Racial Minorities in the United States. Hum. Gene Ther. 2022, 33, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Kim, K.S.; Liu, D. Nonviral gene delivery: What we know and what is next. AAPS J. 2007, 9, E92–E104. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Zanta, M.A.; Boussif, O.; Adib, A.; Behr, J.P. In vitro gene delivery to hepatocytes with galactosylated polyethylenimine. Bioconjug Chem. 1997, 8, 839–844. [Google Scholar] [CrossRef]
- Godbey, W.T.; Wu, K.K.; Hirasaki, G.J.; Mikos, A.G. Improved packing of poly(ethylenimine)/DNA complexes increases transfection effi-ciency. Gene Ther. 1999, 6, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Kunath, K.; von Harpe, A.; Fischer, D.; Petersen, H.; Bickel, U.; Voigt, K.; Kissel, T. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: Comparison of physicochemical properties, transfection efficiency and in vivo distribution withhigh-molecular-weight polyethylenimine. J. Control Release 2003, 89, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.K.; Suh, D.; Kim, J.M.; Choi, H.G.; Shin, K.; Ko, J.J. Polyethylenimine-mediated cellular uptake, nucleus trafficking and expression of cytokine plasmid DNA. Gene Ther. 2002, 9, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Lu, J.J.; Ge, Q.; Zhang, C.; Chen, J.; Klibanov, A.M. Full deacylation of polyethylenimine dramatically boosts its gene delivery ef-ficiency and specificity to mouse lung. Proc. Natl. Acad. Sci. USA 2005, 102, 5679–5684. [Google Scholar] [CrossRef]
- Gomez, J.P.; Pichon, C.; Midoux, P. Ability of plasmid DNA complexed with histidinylated lPEI and lPEI to cross in vitro lung and muscle vascular endothelial barriers. Gene 2013, 525, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Dabbaghi, M.; Hashemi, K.; Oskuee, R.K.; Afkhami-Goli, A. Reverse relation between cytotoxicity and Polyethylenimine/DNA ratio, the effect of using HEPES-buffered saline (HBS) medium in gene delivery. Toxicol. In Vitro 2022, 83, 105414. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Kaczmarek, J.C.; Bose, S.; Kauffman, K.J.; Mir, F.; Heartlein, M.W.; DeRosa, F.; Langer, R.; Anderson, D.G. Inhaled Nanoformulated mRNA Polyplexes for Protein Production in Lung Epithelium. Adv. Mater. 2019, 31, e1805116. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Zhou, D.; Alshehri, F.; Lara-Sáez, I.; Lyu, Y.; Creagh-Flynn, J.; Xu, Q.; Sigen, A.; Zhang, J.; Wang, W. Manipulation of Transgene Expression in Fibroblast Cells by a Multifunctional Linear-Branched Hybrid Poly(β-Amino Ester) Synthesized through an Oligomer Combination Approach. Nano Lett. 2019, 19, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Cutlar, L.; Gao, Y.; Wang, W.; O’Keeffe-Ahern, J.; McMahon, S.; Duarte, B.; Larcher, F.; Rodriguez, B.J.; Greiser, U.; et al. The transition from linear to highly branched poly(β-amino ester)s: Branching matters for gene delivery. Sci. Adv. 2016, 2, e1600102. [Google Scholar] [CrossRef]
- Perni, S.; Prokopovich, P. Poly-beta-amino-esters nano-vehicles based drug delivery system for cartilage. Nanomedicine 2017, 13, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Petkova, A.I.; Kubajewska, I.; Vaideanu, A.; Schätzlein, A.G.; Uchegbu, I.F. Gene Targeting to the Cerebral Cortex Following Intranasal Administration of Polyplexes. Pharmaceutics 2022, 14, 1136. [Google Scholar] [CrossRef] [PubMed]
- Rodolfo, C.; Eusébio, D.; Ventura, C.; Nunes, R.; Florindo, H.F.; Costa, D.; Sousa, Â. Design of Experiments to Achieve an Efficient Chitosan-Based DNA Vaccine Delivery System. Pharmaceutics 2021, 13, 1369. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lu, Y.; Cao, W.; Aleem, M.T.; Liu, J.; Luo, J.; Yan, R.; Xu, L.; Song, X.; Li, X. Nano DNA Vaccine Encoding Toxoplasma gondii Histone Deacetylase SIR2 Enhanced Protective Immunity in Mice. Pharmaceutics 2021, 13, 1582. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, G.; Guido, C.; Russo, A.; Giglio, A.; Rizzello, L.; Testini, M.; Cortese, B.; D’Amone, S.; Gigli, G.; Palamà, I.E. Hybrid Polyelectrolyte Nanocomplexes for Non-Viral Gene Delivery with Favorable Efficacy and Safety Profile. Pharmaceutics 2022, 14, 1310. [Google Scholar] [CrossRef] [PubMed]
- Zenze, M.; Daniels, A.; Singh, M. Dendrimers as Modifiers of Inorganic Nanoparticles for Therapeutic Delivery in Cancer. Pharmaceutics 2023, 15, 398. [Google Scholar] [CrossRef] [PubMed]
- Srinageshwar, B.; Florendo, M.; Clark, B.; Johnson, K.; Munro, N.; Peruzzaro, S.; Antcliff, A.; Andrews, M.; Figacz, A.; Swanson, D.; et al. A Mixed-Surface Polyamidoamine Dendrimer for In Vitro and In Vivo Delivery of Large Plasmids. Pharmaceutics 2020, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Eliyahu, H.; Servel, N.; Domb, A.J.; Barenholz, Y. Lipoplex-induced hemagglutination: Potential involvement in intravenous gene delivery. Gene Ther. 2002, 9, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Dekie, L.; Toncheva, V.; Dubruel, P.; Schacht, E.H.; Barrett, L.; Seymour, L.W. Poly-L-glutamic acid derivatives as vectors for gene therapy. J. Control Release 2000, 65, 187–202. [Google Scholar] [CrossRef]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Poly(ethylenimine) and its role in gene delivery. J. Control Release 1999, 60, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Goula, D.; Remy, J.S.; Erbacher, P.; Wasowicz, M.; Levi, G.; Abdallah, B.; Demeneix, B.A. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. Gene Ther. 1998, 5, 712–717. [Google Scholar] [CrossRef]
- Fischer, D.; Bieber, T.; Li, Y.; Elsässer, H.P.; Kissel, T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: Effect of molecular weight on transfection efficiency and cytotoxicity. Pharm. Res. 1999, 16, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.K.; Kim, J.P.; Yoon, H.; Kim, J.M.; Yang, J.S.; Kim, C.K. Prolonged organ retention and safety of plasmid DNA administered in polyethylenimine complexes. Gene Ther. 2001, 8, 1587–1592. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trubetskoy, V.S.; Wong, S.C.; Subbotin, V.; Budker, V.G.; Loomis, A.; Hagstrom, J.E.; Wolff, J.A. Recharging cationic DNA complexes with highly charged polyanions for in vitro and in vivo gene delivery. Gene Ther. 2003, 10, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Iida-Tanaka, N.; Koyama, Y. Efficient in vivo gene transfection by stable DNA/PEI complexes coated by hyaluronic acid. J. Drug Target. 2008, 16, 276–281. [Google Scholar] [CrossRef]
- Orson, F.M.; Song, L.; Gautam, A.; Densmore, C.L.; Bhogal, B.S.; Kinsey, B.M. Gene delivery to the lung using pro-tein/polyethylenimine/plasmid complexes. Gene Ther. 2002, 9, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Kitahara, T.; Fumoto, S.; Nishida, K.; Nakamura, J.; Niidome, T.; Kodama, Y.; Nakagawa, H.; To, H.; Sasaki, H. Ternary complexes of pDNA, polyethylenimine, and gamma-polyglutamic acid for gene delivery systems. Biomaterials 2009, 30, 2846–2853. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Kitahara, T.; Kawakami, S.; Higuchi, Y.; Yamaguchi, A.; Nakagawa, H.; Kodama, Y.; Hamamoto, T.; Hashida, M.; Sasaki, H. Gamma-polyglutamic acid-coated vectors for effective and safe gene therapy. J. Control Release 2010, 142, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, F.; Nishioka, T.; Saito, H.; Baba, T.; Okuda, A.; Matsumoto, O.; Taga, T.; Yamashita, F.; Takakura, Y.; Hashida, M. Interaction between DNA-cationic liposome complexes and erythrocytes is an important factor in systemic gene transfer via the intravenous route in mice: The role of the neutral helper lipid. Gene Ther. 2001, 8, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.P.; Huang, L. Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA. Gene Ther. 1997, 4, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Yan, H.; Zhou, Z.; Tang, J.; Liu, X.; Hartmann, R.; Parak, W.J.; Feliu, N.; Shen, Y. Detailed investigation on how the protein corona modulates the physicochemical properties and gene delivery of polyethylenimine (PEI) polyplexes. Biomater. Sci. 2018, 6, 1800–1817. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Fumoto, S.; Nakashima, M.; Shimokawa, K.; Miyamoto, H.; Nishida, K. The role of fibronectin in pulmonary gene transfer following intravenous administration of lipoplex in mice. Biol. Pharm. Bull. 2013, 36, 1807–1813. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lausted, C.; Hu, Z.; Hood, L. Quantitative serum proteomics from surface plasmon resonance imaging. Mol. Cell. Proteom. 2008, 7, 2464–2474. [Google Scholar] [CrossRef]
- Fumoto, S.; Yamamoto, T.; Okami, K.; Maemura, Y.; Terada, C.; Yamayoshi, A.; Nishida, K. Understanding In Vivo Fate of Nucleic Acid and Gene Medicines for the Rational Design of Drugs. Pharmaceutics 2021, 13, 159. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Sakamoto, K.; Mizuno, S.; Sakaguchi, J.; Miyamoto, H.; Mine, T.; Sasaki, H.; Fumoto, S.; Nishida, K. Multiple components in serum contribute to hepatic transgene expression by lipoplex in mice. J. Gene Med. 2011, 13, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Almofti, M.R.; Harashima, H.; Shinohara, Y.; Almofti, A.; Li, W.; Kiwada, H. Lipoplex size determines lipofection efficiency with or without serum. Mol. Membr. Biol. 2003, 20, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, G.; Callipo, L.; De Sanctis, S.C.; Cavaliere, C.; Pozzi, D.; Laganà, A. Surface adsorption of protein corona controls the cell internali-zation mechanism of DC-Chol-DOPE/DNA lipoplexes in serum. Biochim. Biophys. Acta 2010, 1798, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Prabha, S.; Arya, G.; Chandra, R.; Ahmed, B.; Nimesh, S. Effect of size on biological properties of nanoparticles employed in gene delivery. Artif. Cells Nanomed Biotechnol. 2016, 44, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ogris, M.; Steinlein, P.; Kursa, M.; Mechtler, K.; Kircheis, R.; Wagner, E. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Ther. 1998, 5, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Fumoto, S.; Kawakami, S.; Ito, Y.; Shigeta, K.; Yamashita, F.; Hashida, M. Enhanced hepatocyte-selective in vivo gene expression by stabilized galactosylated liposome/plasmid DNA complex using sodium chloride for complex formation. Mol. Ther. 2004, 10, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Fumoto, S.; Yoshikawa, N.; Peng, J.; Miyamoto, H.; Tanaka, M.; Nishida, K. Diffusion coefficient of cationic liposomes during lipoplex formation determines transfection efficiency in HepG2 cells. Int. J. Pharm. 2023, 637, 122881. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Fumoto, S.; Yoshikawa, K.; Hu, D.; Okami, K.; Kato, R.; Nakashima, M.; Miyamoto, H.; Nishida, K. Interaction of Lipoplex with Albumin Enhances Gene Expression in Hepatitis Mice. Pharmaceutics 2020, 12, 341. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Zhao, Z.; Guo, Y.; Chen, H.; Liu, P.; Zhao, M.; Guo, J. Biomolecules meet organic frameworks: From synthesis strategies to diverse applications. Nanoscale 2024, 16, 4529–4541. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.F.; Hsu, H.K.; Lin, C.C.; Cheng, Y.M.; Hsu, K.H. Novel PEI/Poly-γ-Gutamic Acid Nanoparticles for High Efficient siRNA and Plasmid DNA Co-Delivery. Molecules 2017, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, X.; Sun, Q.; Dong, X.; Zhu, Z.; Yang, C. Incorporation of poly(γ-glutamic acid) in lipid nanoparticles for enhanced mRNA delivery efficiency in vitro and in vivo. Acta Biomater. 2024, 177, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Dasgerdi, N.K.; Gumus, N.; Bayraktutan, H.; Jackson, D.; Polra, K.; McKay, P.F.; Atyabi, F.; Dinarvand, R.; Shattock, R.J.; Martinez-Pomares, L.; et al. Charge neutralized poly(β-amino ester) polyplex nanoparticles for delivery of self-amplifying RNA. Nanoscale Adv. 2024, 6, 1409–1422. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, H.M.; Dhaliwal, H.K.; Aljaeid, B.M.; Alhakamy, N.A.; Banjar, Z.M.; Amiji, M.M. Optimization of the Conditions for Plasmid DNA Delivery and Transfection with Self-Assembled Hyaluronic Acid-Based Nanoparticles. Mol. Pharm. 2019, 16, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, M.; Yu, Q.; Fei, W.; Li, T.; Zhu, L.; Yao, Y.; Zheng, C.; Zhang, X. Hyaluronic Acid-Modified Nanoplatforms as a Vector for Targeted Delivery of Autophagy-Related Gene to the Endometriotic Lesions in Mice. Front. Bioeng. Biotechnol. 2022, 10, 918368. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Pascual, M.; Gómez-Aguado, I.; Rodríguez-Castejón, J.; Rodríguez-Gascón, A.; Muntoni, E.; Battaglia, L.; Del Pozo-Rodríguez, A.; Solinís Aspiazu, M.Á. Topical Administration of SLN-Based Gene Therapy for the Treatment of Corneal Inflammation by De Novo IL-10 Production. Pharmaceutics 2020, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, M.; Hashemi, M.; Farzadnia, M.; Zarei-Ghanavati, S.; Malaekeh-Nikouei, B. Development of targeted gene delivery system based on liposome and PAMAM dendrimer functionalized with hyaluronic acid and TAT peptide: In vitro and in vivo studies. Biotechnol. Prog. 2022, 38, e3278. [Google Scholar] [CrossRef] [PubMed]
- Nabar, N.; Dacoba, T.G.; Covarrubias, G.; Romero-Cruz, D.; Hammond, P.T. Electrostatic adsorption of polyanions onto lipid nanoparticles controls uptake, trafficking, and transfection of RNA and DNA therapies. Proc. Natl. Acad. Sci. USA 2024, 121, e2307809121. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Ohkubo, C.; Kurosaki, T.; Egashira, K.; Sato, K.; Fumoto, S.; Nishida, K.; Higuchi, N.; Kitahara, T.; Nakamura, T.; et al. Secure and effective gene delivery system of plasmid DNA coated by polynucleotide. J. Drug Target. 2015, 23, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sugiura, K.; Hasegawa, A.; Ouchi, W.; Yoshimoto, T.; Mizoguchi, I.; Inaba, T.; Hamada, K.; Eriguchi, M.; Koyama, Y. Microbial An-tigen-Presenting Extracellular Vesicles Derived from Genetically Modified Tumor Cells Promote Antitumor Activity of Den-dritic Cells. Pharmaceutics 2021, 13, 57. [Google Scholar] [CrossRef]
- Lin, W.J.; Lee, W.C. Polysaccharide-modified nanoparticles with intelligent CD44 receptor targeting ability for gene delivery. Int. J. Nanomed. 2018, 13, 3989–4002. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, T.; Fumoto, S.; Kurosaki, T.; Nakashima, M.; Miyamoto, H.; Sasaki, H.; Nishida, K. Interaction of γ-Polyglutamic Acid/Polyethyleneimine/Plasmid DNA Ternary Complexes with Serum Components Plays a Crucial Role in Transfection in Mice. Pharmaceutics 2024, 16, 522. https://doi.org/10.3390/pharmaceutics16040522
Ko T, Fumoto S, Kurosaki T, Nakashima M, Miyamoto H, Sasaki H, Nishida K. Interaction of γ-Polyglutamic Acid/Polyethyleneimine/Plasmid DNA Ternary Complexes with Serum Components Plays a Crucial Role in Transfection in Mice. Pharmaceutics. 2024; 16(4):522. https://doi.org/10.3390/pharmaceutics16040522
Chicago/Turabian StyleKo, Tomotaka, Shintaro Fumoto, Tomoaki Kurosaki, Moe Nakashima, Hirotaka Miyamoto, Hitoshi Sasaki, and Koyo Nishida. 2024. "Interaction of γ-Polyglutamic Acid/Polyethyleneimine/Plasmid DNA Ternary Complexes with Serum Components Plays a Crucial Role in Transfection in Mice" Pharmaceutics 16, no. 4: 522. https://doi.org/10.3390/pharmaceutics16040522
APA StyleKo, T., Fumoto, S., Kurosaki, T., Nakashima, M., Miyamoto, H., Sasaki, H., & Nishida, K. (2024). Interaction of γ-Polyglutamic Acid/Polyethyleneimine/Plasmid DNA Ternary Complexes with Serum Components Plays a Crucial Role in Transfection in Mice. Pharmaceutics, 16(4), 522. https://doi.org/10.3390/pharmaceutics16040522






