Aptamers for the Delivery of Plant-Based Compounds: A Review
Abstract
:1. Introduction
2. Sources of Natural Products
2.1. Natural Compounds from Plants
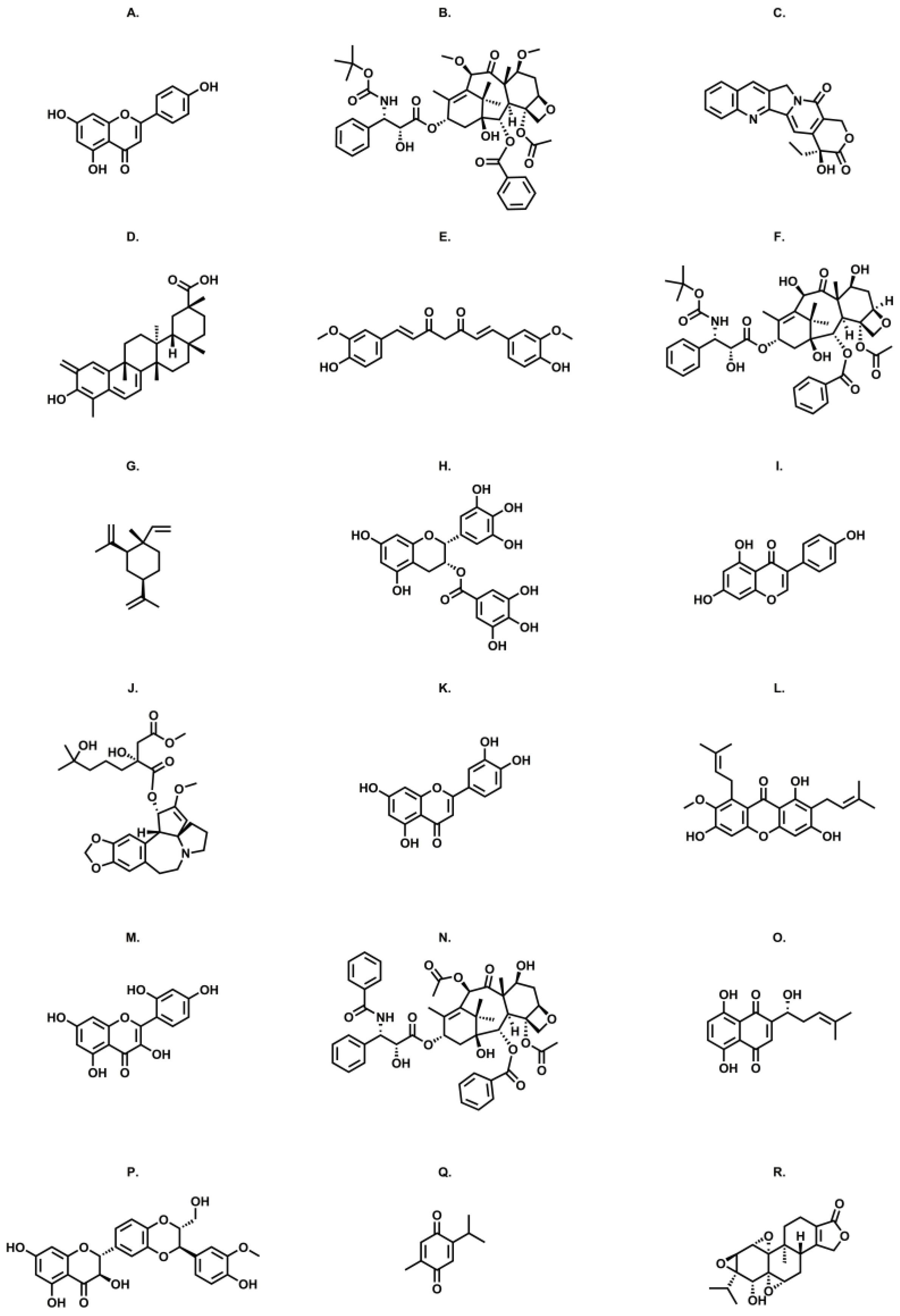
2.1.1. Alkaloids
2.1.2. Phenolic Compounds
2.1.3. Terpenes and Terpenoids
Top of Form
3. Enhancing Therapeutic Potential: Overcoming Challenges in Natural Product-Based Drug Development
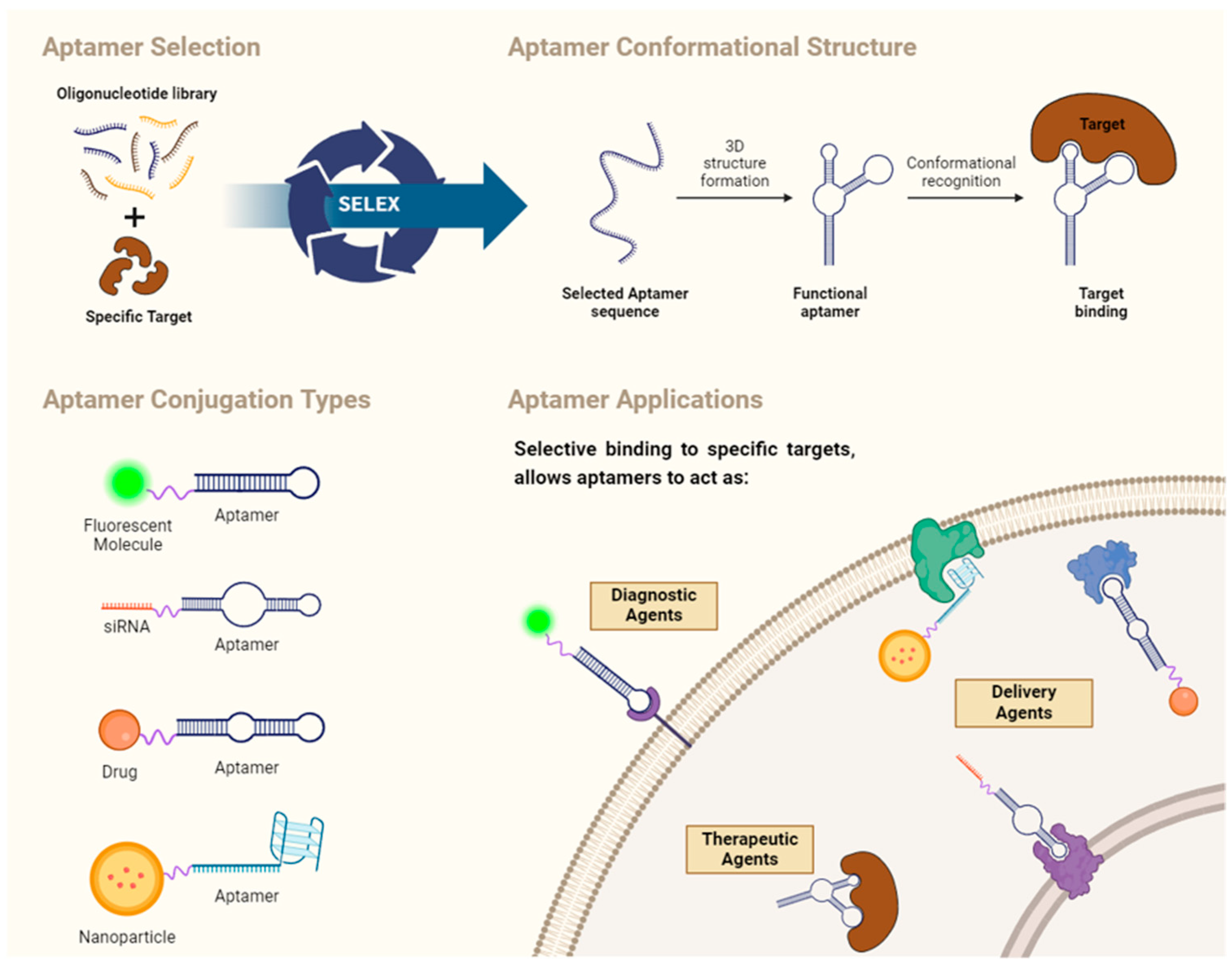
4. Aptamers: General Concepts
4.1. SELEX
4.2. Aptamer Structure
4.3. Structure and Affinity of Aptamers
4.4. Aptamer Applications
Aptamer-Based Drug Carriers for Delivery
5. Current Plant-Based Natural Products, Aptamers and Delivery Systems
5.1. Alkaloids Based Aptamer-Carriers
Homoharringtonine
5.2. Phenolic Compound-Based Aptamer Carriers
5.2.1. Apigenin
5.2.2. Curcumin
5.2.3. Epigallocatechin Gallate
5.2.4. Genistein
5.2.5. Mangosteen
5.2.6. Morin
5.3. Terpenes and Terpenoids Based Aptamer Carriers
5.3.1. Celastrol
5.3.2. Thymoquinone
5.3.3. Triptolide
5.4. Synergistic Therapy
5.4.1. Curcumin
5.4.2. Elemene
5.4.3. Luteolin
5.4.4. Shikonin
5.4.5. Silibinin
6. Aptamers and Semi-Synthetic Products Derived from Plants
6.1. Camptothecin—Aptamer Carriers
6.2. Taxanes—Aptamer Carriers
6.2.1. Cabazitaxel
6.2.2. Docetaxel
6.2.3. Paclitaxel
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Mushtaq, S.; Abbasi, B.H.; Uzair, B.; Abbasi, R. Natural products as reservoirs of novel therapeutic agents. EXCLI J. 2018, 17, 420–451. [Google Scholar] [CrossRef] [PubMed]
- Chopra, B.; Dhingra, A.K. Natural products: A lead for drug discovery and development. Phytother. Res. 2021, 35, 4660–4702. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Baldassari, S.; Balboni, A.; Drava, G.; Donghia, D.; Canepa, P.; Ailuno, G.; Caviglioli, G. Phytochemicals and Cancer Treatment: Cell-Derived and Biomimetic Vesicles as Promising Carriers. Pharmaceutics 2023, 15, 1445. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055–6074. [Google Scholar] [CrossRef]
- Simoben, C.V.; Babiaka, S.B.; Aurélien, A.; Aurélien, A.; Moumbock, F.A.; Namba-Nzanguim, C.T.; Eni, D.B.; Medina-Franco, J.L.; Günther, S.; Ntie-Kang, F.; et al. Challenges in natural product-based drug discovery assisted with in silico-based methods. RSC Adv. 2023, 13, 31578–31594. [Google Scholar] [CrossRef] [PubMed]
- Jain, H.; Chella, N. Methods to improve the solubility of therapeutical natural products: A review. Env. Chem. Lett. 2021, 19, 111–121. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef]
- Aiswarya, P.U.; Raj, G.; John, J.; Mohan, K.M.; John, F.; George, J. Aptamers: Features, Synthesis and Applications. Chem. Biodivers. 2023, 20, e202301008. [Google Scholar] [CrossRef]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Ismail, M.A.; Esawi, E.; Qaqish, B.; Al Bawab, A.; Ismail, S.I. Aptamers Chemistry: Chemical Modifications and Conjugation Strategies. Molecules 2020, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Zhuo, Z.; Pan, Y.; Yu, Y.; Li, F.; Liu, J.; Wang, L.; Wu, X.; Li, D.; Wan, Y.; et al. Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces 2021, 13, 9500–9519. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.A.; Joo, B.J.; Lee, J.S.; Ryu, G.; Han, M.; Kim, W.Y.; Park, H.H.; Lee, J.H.; Lee, C.S. Phytochemicals as Anti-Inflammatory Agents in Animal Models of Prevalent Inflammatory Diseases. Molecules 2020, 25, 5932. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Alam Khan, S.; et al. Antidiabetic Phytochemicals From Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef] [PubMed]
- Olofinsan, K.; Abrahamse, H.; George, B.P. Therapeutic Role of Alkaloids and Alkaloid Derivatives in Cancer Management. Molecules 2023, 28, 5578. [Google Scholar] [CrossRef]
- Rana, P.; Shrama, A.; Mandal, C.C. Molecular insights into phytochemicals-driven break function in tumor microenvironment. J. Food Biochem. 2021, 45, e13824. [Google Scholar] [CrossRef]
- Kongkham, B.; Prabakaran, D.; Puttaswamy, H. Opportunities and challenges in managing antibiotic resistance in bacteria using plant secondary metabolites. Fitoterapia 2020, 147, 104762. [Google Scholar] [CrossRef] [PubMed]
- Stincone, P.; Brandelli, A. Marine bacteria as source of antimicrobial compounds. Crit. Rev. Biotechnol. 2020, 40, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Farinacci, P.; Mevissen, M.; Ayrle, H.; Maurer, V.; Sorensen Dalgaard, T.; Melzig, M.F.; Walkenhorst, M. Medicinal Plants for Prophylaxis and Therapy of Common Infectious Diseases in Poultry—A Systematic Review of in Vivo Studies. Planta Med. 2022, 88, 200–217. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, P.; Rajput, V.D.; Mishra, S.K.; Tiwari, K.N.; Singh, A.K.; Minkina, T.; Pandey, A.K. Phytochemicals, Antioxidant, Anti-inflammatory Studies, and Identification of Bioactive Compounds Using GC–MS of Ethanolic Novel Polyherbal Extract. Appl. Biochem. Biotechnol. 2023, 195, 4447–4468. [Google Scholar] [CrossRef]
- Mopuri, R.; Islam, M.S. Medicinal plants and phytochemicals with anti-obesogenic potentials: A review. Biomed. Pharmacother. 2017, 89, 1442–1452. [Google Scholar] [CrossRef]
- Biesinger, S.; Michaels, H.A.; Quadros, A.S.; Qian, Y.; Rabovsky, A.B.; Badger, R.S.; Jalili, T. A combination of isolated phytochemicals and botanical extracts lowers diastolic blood pressure in a randomized controlled trial of hypertensive subjects. Eur. J. Clin. Nutr. 2016, 70, 10–16. [Google Scholar] [CrossRef]
- Afsheen, N.; Khalil-Ur-Rehman Jahan, N.; Ijaz, M.; Manzoor, A.; Khan, K.M.; Hina, S. Cardioprotective and Metabolomic Profiling of Selected Medicinal Plants against Oxidative Stress. Oxid. Med. Cell Longev. 2018, 2018, 9819360. [Google Scholar] [CrossRef]
- Kumar, A.; Nirmal, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Guan, R.; Van Le, Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Jianguang, Z.; et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef]
- Liu, L.Y.; Yang, Y.K.; Wang, J.N.; Ren, J.G. Steroidal alkaloids from Solanum nigrum and their cytotoxic activities. Phytochemistry 2022, 202, 113317. [Google Scholar] [CrossRef]
- Silva, L.C.; Correia, A.F.; Gomes, J.V.D.; Romão, W.; Motta, L.C.; Fagg, C.W.; Magalhães, P.O.; Silveira, D.; Fonseca-Bazzo, Y.M. Lycorine Alkaloid and Crinum americanum L. (Amaryllidaceae) Extracts Display Antifungal Activity on Clinically Relevant Candida Species. Molecules 2022, 27, 2976. [Google Scholar] [CrossRef]
- Li, Q.; Yang, K.X.; Zhao, Y.L.; Qin, X.J.; Yang, X.W.; Liu, L.; Liu, Y.-P.; Luo, X.-D. Potent anti-inflammatory and analgesic steroidal alkaloids from Veratrum taliense. J. Ethnopharmacol. 2016, 179, 274–279. [Google Scholar] [CrossRef]
- García Díaz, J.; Tuenter, E.; Escalona Arranz, J.C.; Llauradó Maury, G.; Cos, P.; Pieters, L. Antiplasmodial activity of alkaloids from Croton linearis leaves. Exp. Parasitol. 2022, 236, 108254. [Google Scholar] [CrossRef]
- Song, M.; Ying, Z.; Ying, X.; Jia, L.; Yang, G. Three novel alkaloids from Portulaca oleracea L. and their anti-inflammatory bioactivities. Fitoterapia 2022, 156, 105087. [Google Scholar] [CrossRef]
- Polbuppha, I.; Teerapongpisan, P.; Phukhatmuen, P.; Suthiphasilp, V.; Maneerat, T.; Charoensup, R.; Andersen, R.J.; Laphookhieo, S. Alkaloids and Styryl lactones from Goniothalamus ridleyi King and Their α-Glucosidase Inhibitory Activity. Molecules 2023, 28, 1158. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, M.; Jin-Peng, L.; Li, J.F.; Zhang, K.Y.; Zhi, H.; Zhang, H.; Sun, J. Antioxidant, Anti-inflammatory and Cytotoxic Activities of Polyphenols Extracted from Chroogomphus rutilus. Chem. Biodivers. 2020, 17, e1900479. [Google Scholar] [CrossRef]
- Bouslamti, M.; El Barnossi, A.; Kara, M.; Alotaibi, B.S.; Al Kamaly, O.; Assouguem, A.; Lyoussi, B.; Benjelloun, A.S. Total Polyphenols Content, Antioxidant and Antimicrobial Activities of Leaves of Solanum elaeagnifolium Cav. from Morocco. Molecules 2022, 27, 4322. [Google Scholar] [CrossRef]
- Lyu, J.l.; Ryu, J.; Jin, C.H.; Kim, D.G.; Kim, J.M.; Seo, K.S.; Kim, J.-B.; Kim, S.H.; Ahn, J.-W.; Kang, S.-Y.; et al. Phenolic compounds in extracts of Hibiscus acetosella (Cranberry Hibiscus) and their antioxidant and antibacterial properties. Molecules 2020, 25, 4190. [Google Scholar] [CrossRef]
- Balea, S.S.; Pârvu, A.E.; Pop, N.; Marín, F.Z.; Pârvu, M. Polyphenolic compounds, antioxidant, and cardioprotective effects of pomace extracts from Fetească neagră cultivar. Oxid. Med. Cell Longev. 2018, 2018, 8194721. [Google Scholar] [CrossRef]
- Chen, W.; Shen, X.; Ma, L.; Chen, R.; Yuan, Q.; Zheng, Y.; Li, C.; Peng, G. Phenolic Compounds from Polygonum chinense Induce Growth Inhibition and Apoptosis of Cervical Cancer SiHa Cells. Biomed. Res. Int. 2020, 2020, 8868508. [Google Scholar] [CrossRef]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxid. Med. Cell Longev. 2022, 2022, 6037303. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, C.; SiTu, Y.; Shen, Z.; Chen, Y.; Zhang, Z.; Tang, C.; Jiang, T. Anoectochilus roxburghii flavonoids extract ameliorated the memory decline and reduced neuron apoptosis via modulating SIRT1 signaling pathway in senescent mice. J. Ethnopharmacol. 2022, 296, 115361. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Malik, A.; Adhami, V.M.; Asim, M.; Hafeez, B.B.; Sarfaraz, S.; Mukhtar, H. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene 2008, 27, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; De Mejia, E.G. Flavonoid apigenin modified gene expression associated with inflammation and cancer and induced apoptosis in human pancreatic cancer cells through inhibition of GSK-3β/NF-κB signaling cascade. Mol. Nutr. Food Res. 2013, 57, 2112–2127. [Google Scholar] [CrossRef] [PubMed]
- Margină, D.; Ilie, M.; Grădinaru, D.; Androutsopoulos, V.P.; Kouretas, D.; Tsatsakis, A.M. Natural products-friends or foes? Toxicol. Lett. 2015, 236, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.P.; Sabadin, G.A.; Sousa, I.C.; Gomes, M.N.; Soares, A.M.S.; Monteiro, C.M.O.; Vaz, I.S.; Costa-Junior, L.M. Effects of carvacrol and thymol on the antioxidant and detoxifying enzymes of Rhipicephalus microplus (Acari: Ixodidae). Ticks Tick. Borne Dis. 2022, 13, 101929. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Ads, E.N.; Hassan, S.I.; Rajendrasozhan, S.; Hetta, M.H.; Aly, S.H.; Ali, M.A. Isolation, Structure Elucidation and Antimicrobial Evaluation of Natural Pentacyclic Triterpenoids and Phytochemical Investigation of Different Fractions of Ziziphus spina-christi (L.) Stem Bark Using LCHRMS Analysis. Molecules 2022, 27, 1805. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Zhu, Y.; Ouyang, Z.; Du, H.; Wang, M.; Wang, J.; Sun, H.; Kong, L.; Xu, Q.; Ma, H.; Sun, Y. New opportunities and challenges of natural products research: When target identification meets single-cell multiomics. Acta Pharm. Sin. B 2022, 12, 4011–4039. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Xing, H. Chemistries and applications of DNA-natural product conjugate. Front. Chem. 2022, 10, 984916. [Google Scholar] [CrossRef]
- Sflakidou, E.; Leonidis, G.; Foroglou, E.; Siokatas, C.; Sarli, V. Recent Advances in Natural Product-Based Hybrids as Anti-Cancer Agents. Molecules 2022, 27, 6632. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Adachi, T.; Nakamura, Y. Aptamers: A review of their chemical properties and modifications for therapeutic application. Molecules 2019, 24, 4229. [Google Scholar] [CrossRef]
- Srivastava, S.; Abraham, P.R.; Mukhopadhyay, S. Aptamers: An Emerging Tool for Diagnosis and Therapeutics in Tuberculosis. Front. Cell Infect. Microbiol. 2021, 11, 656421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef]
- Zhu, C.; Feng, Z.; Qin, H.; Chen, L.; Yan, M.; Li, L.; Qu, F. Recent progress of SELEX methods for screening nucleic acid aptamers. Talanta 2024, 266, 124998. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.F.; Ling, M.; Kacherovsky, N.; Pun, S.H. Aptamers 101: Aptamer discovery and in vitro applications in biosensors and separations. Chem. Sci. 2023, 14, 4961–4978. [Google Scholar] [CrossRef]
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol. Adv. 2019, 37, 28–50. [Google Scholar] [CrossRef]
- McCloskey, C.M.; Li, Q.; Yik, E.J.; Chim, N.; Ngor, A.K.; Medina, E.; Grubisic, I.; Keh, L.C.T.; Poplin, R.; Chaput, J.C. Evolution of Functionally Enhanced α-l-Threofuranosyl Nucleic Acid Aptamers. ACS Synth. Biol. 2021, 10, 3190–3199. [Google Scholar] [CrossRef]
- New, R.R.C.; Bui, T.T.T.; Bogus, M. Binding Interactions of Peptide Aptamers. Molecules 2020, 25, 6055. [Google Scholar] [CrossRef]
- Fan, R.; Tao, X.; Zhai, X.; Zhu, Y.; Li, Y.; Chen, Y.; Dong, D.; Yang, S.; Lv, L. Application of aptamer-drug delivery system in the therapy of breast cancer. Biomed. Pharmacother. 2023, 161, 114444. [Google Scholar] [CrossRef]
- Fu, Z.; Xiang, J. Aptamer-functionalized nanoparticles in targeted delivery and cancer therapy. Int. J. Mol. Sci. 2020, 21, 9123. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Y.; Yuan, W.F.; Ai, W.B.; Ai, Y.W.; Wang, J.J.; Chu, L.Y.; Zhang, Y.-Q.; Wu, J.-F. An exploration of aptamer internalization mechanisms and their applications in drug delivery. Expert. Opin. Drug Deliv. 2019, 16, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Vorobyeva, M.A.; Davydova, A.S.; Vorobjev, P.E.; Pyshnyi, D.V.; Venyaminova, A.G. Key aspects of nucleic acid library design for in vitro selection. Int. J. Mol. Sci. 2018, 19, 470. [Google Scholar] [CrossRef]
- Khan, S.; Hussain, A.; Fahimi, H.; Aliakbari, F.; Haj Bloukh, S.; Edis, Z.; Babadaei, M.M.N.; Izadi, Z.; Varnamkhasti, B.S.; Jahanshahi, F.; et al. A review on the therapeutic applications of aptamers and aptamer-conjugated nanoparticles in cancer, inflammatory and viral diseases. Arab. J. Chem. 2022, 15, 103626. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, Y.; Li, X.; Wang, S.; Dong, Y. Development of a chimeric aptamer and an AuNPs aptasensor for highly sensitive and specific identification of Aflatoxin B1. Sens. Actuators B Chem. 2020, 319, 128250. [Google Scholar] [CrossRef]
- Santos, T.; Salgado, G.F.; Cabrita, E.J.; Cruz, C. G-Quadruplexes and Their Ligands: Biophysical Methods to Unravel G-Quadruplex/Ligand Interactions. Pharmaceuticals 2021, 14, 769. [Google Scholar] [CrossRef]
- Moreira, D.; Leitão, D.; Lopes-Nunes, J.; Santos, T.; Figueiredo, J.; Miranda, A.; Alexandre, D.; Tomaz, C.; Mergny, J.-L.; Cruz, C. G-Quadruplex Aptamer-Ligand Characterization. Molecules 2022, 27, 6781. [Google Scholar] [CrossRef]
- Lee, M.; Shin, S.; Kim, S.; Park, N. Recent Advances in Biological Applications of Aptamer-Based Fluorescent Biosensors. Molecules 2023, 28, 7327. [Google Scholar] [CrossRef]
- Sequeira-Antunes, B.; Ferreira, H.A. Nucleic Acid Aptamer-Based Biosensors: A Review. Biomedicines 2023, 11, 3201. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Santos, T.; Carvalho, J.; Alexandre, D.; Jardim, A.; Caneira, C.F.; Vaz, V.; Pereira, B.; Godinho, R.; Brito, D.; et al. Aptamer-based approaches to detect nucleolin in prostate cancer. Talanta 2021, 226, 122037. [Google Scholar] [CrossRef]
- Aljohani, M.M.; Cialla-May, D.; Popp, J.; Chinnappan, R.; Al-Kattan, K.; Zourob, M. Aptamers: Potential Diagnostic and Therapeutic Agents for Blood Diseases. Molecules 2022, 27, 383. [Google Scholar] [CrossRef] [PubMed]
- Van den Avont, A.; Sharma-Walia, N. Anti-nucleolin aptamer AS1411: An advancing therapeutic. Front. Mol. Biosci. 2023, 10, 1217769. [Google Scholar] [CrossRef]
- Dailey, M.M.; Clarke Miller, M.; Bates, P.J.; Lane, A.N.; Trent, J.O. Resolution and characterization of the structural polymorphism of a single quadruplex-forming sequence. Nucleic Acids Res. 2010, 38, 4877–4888. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Ga, L.; Ai, J.; Wang, Y. Progress in cancer drug delivery based on AS1411 oriented nanomaterials. J. Nanobiotechnol. 2022, 20, 57. [Google Scholar] [CrossRef]
- Carvalho, J.; Paiva, A.; Cabral Campello, M.P.; Paulo, A.; Mergny, J.L.; Salgado, G.F.; Queiroz, J.A.; Cruz, C. Aptamer-based Targeted Delivery of a G-quadruplex Ligand in Cervical Cancer Cells. Sci. Rep. 2019, 9, 7945. [Google Scholar] [CrossRef]
- Figueiredo, J.; Lopes-Nunes, J.; Carvalho, J.; Antunes, F.; Ribeiro, M.; Campello, M.P.C.; Paulo, A.; Paiva, A.; Salgado, G.F.; Queiroz, J.A.; et al. AS1411 derivatives as carriers of G-quadruplex ligands for cervical cancer cells. Int. J. Pharm. 2019, 568, 118511. [Google Scholar] [CrossRef]
- Gao, F.; Yin, J.; Chen, Y.; Guo, C.; Hu, H.; Su, J. Recent advances in aptamer-based targeted drug delivery systems for cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 1–9. [Google Scholar] [CrossRef]
- Kim, M.; Kim, D.M.; Kim, K.S.; Jung, W.; Kim, D.E. Applications of cancer cell-specific aptamers in targeted delivery of anticancer therapeutic agents. Molecules 2018, 23, 830. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S.; Chanda, K.; Balamurali, M.M. Recent Advancements of Aptamers in Cancer Therapy. ACS Omega 2023, 8, 32231–32243. [Google Scholar] [CrossRef]
- Li, S.L.; Jiang, P.; Jiang, F.L.; Liu, Y. Recent Advances in Nanomaterial-Based Nanoplatforms for Chemodynamic Cancer Therapy. Adv. Funct. Mater. 2021, 31, 635. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucl. 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Wu, J. Personalized Medicine the Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Park, D.; Lee, S.J.; Park, J. Aptamer-Based Smart Targeting and Spatial Trigger–Response Drug-Delivery Systems for Anticancer Therapy. Biomedicines 2024, 12, 187. [Google Scholar] [CrossRef]
- Rabiee, N.; Chen, S.; Ahmadi, S.; Veedu, R.N. Aptamer-engineered (nano)materials for theranostic applications. Theranostics 2023, 13, 5183–5206. [Google Scholar] [CrossRef]
- Tang, J.-F.; Li, G.-L.; Zhang, T.; Du, Y.-M.; Huang, S.-Y.; Ran, J.-H.; Li, J.; Chen, D.-L. Homoharringtonine inhibits melanoma cells proliferation in vitro and vivo by inducing DNA damage, apoptosis, and G2/M cell cycle arrest. Arch. Biochem. Biophys. 2021, 700, 108774. [Google Scholar] [CrossRef]
- Cao, W.; Liu, Y.; Zhang, R.; Zhang, B.; Wang, T.; Zhu, X.; Mei, L.; Chen, H.; Zhang, H.; Ming, P.; et al. Homoharringtonine induces apoptosis and inhibits STAT3 via IL-6/JAK1/STAT3 signal pathway in Gefitinib-resistant lung cancer cells. Sci. Rep. 2015, 5, 8477. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, W.; Pan, Y.; Jia, L. An anticancer agent-loaded PLGA nanomedicine with glutathione-response and targeted delivery for the treatment of lung cancer. J. Mater. Chem. B 2020, 8, 655–665. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Dhara, M.; Al Hoque, A.; Sen, R.; Dutta, D.; Mukherjee, B.; Paul, B.; Laha, S. Phosphorothioated amino-AS1411 aptamer functionalized stealth nanoliposome accelerates bio-therapeutic threshold of apigenin in neoplastic rat liver: A mechanistic approach. J. Nanobiotechnol. 2023, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Shekhar Dey, N.; Mukherjee, B.; Maji, R.; Sankar Satapathy, B. Development of Linker-Conjugated Nanosize Lipid Vesicles: A Strategy for Cell Selective Treatment in Breast Cancer. Curr. Cancer Drug Targets 2015, 16, 357–372. [Google Scholar] [CrossRef]
- Mashreghi, M.; Zamani, P.; Moosavian, S.A.; Jaafari, M.R. Anti-Epcam Aptamer (Syl3c)-Functionalized Liposome for Targeted Delivery of Doxorubicin: In Vitro And In Vivo Antitumor Studies in Mice Bearing C26 Colon Carcinoma. Nanoscale Res. Lett. 2020, 15, 101. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Wang, L.; Chen, L.; Goh, B.C. Curcumin in cancer therapy: Exploring molecular mechanisms and overcoming clinical challenges. Cancer Lett. 2023, 570, 216332. [Google Scholar] [CrossRef]
- Li, L.; Xiang, D.; Shigdar, S.; Yang, W.; Li, Q.; Lin, J.; Liu, K.; Li, L. Epithelial cell adhesion molecule aptamer functionalized PLGA-lecithin-curcumin-PEG nanoparticles for targeted drug delivery to human colorectal adenocarcinoma cells. Int. J. Nanomed. 2014, 9, 1083–1096. [Google Scholar] [CrossRef]
- Ma, Q.; Qian, W.; Tao, W.; Zhou, Y.; Xue, B. Delivery of curcumin nanoliposomes using surface modified with CD133 aptamers for prostate cancer. Drug Des. Devel Ther. 2019, 13, 4021–4033. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Ding, X.; He, L.; Wu, S.; Wei, Y.; Wang, Z. Selection, identification and application of a DNA aptamer against Listeria monocytogenes. Food Control 2013, 33, 239–243. [Google Scholar] [CrossRef]
- Kalantari, E.; Asgari, M.; Nikpanah, S.; Salarieh, N.; Asadi Lari, M.H.; Madjd, Z. Co-Expression of Putative Cancer Stem Cell Markers CD44 and CD133 in Prostate Carcinomas. Pathol. Oncol. Res. 2017, 23, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Sun, J.; Li, H.; Liu, W.; Zhang, Y.; Li, B.; Qian, W.; Wang, H.; Chen, J.; Guo, Y. Lyophilized HER2-specific PEGylated immunoliposomes for active siRNA gene silencing. Biomaterials 2010, 31, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Alibolandi, M.; Hoseini, F.; Mohammadi, M.; Ramezani, P.; Einafshar, E.; Taghdisi, S.M.; Ramezani, M.; Abnous, K. Curcumin-entrapped MUC-1 aptamer targeted dendrimer-gold hybrid nanostructure as a theranostic system for colon adenocarcinoma. Int. J. Pharm. 2018, 549, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.M.; Matthews, C.S.; Missailidis, S. DNA aptamers that bind to MUC1 tumour marker: Design and characterization of MUC1-binding single-stranded DNA aptamers. Tumor Biol. 2006, 27, 289–301. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Aptamer functionalized curcumin-loaded human serum albumin (HSA) nanoparticles for targeted delivery to HER-2 positive breast cancer cells. Int. J. Biol. Macromol. 2019, 130, 109–116. [Google Scholar] [CrossRef]
- Kim, T.H.; Jiang, H.H.; Youn, Y.S.; Park, C.W.; Tak, K.K.; Lee, S.; Kim, H.; Jon, S.; Chen, X.; Lee, K.C. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int. J. Pharm. 2011, 403, 285–291. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Jon, S.; Khademhosseini, A.; Tran, T.N.T.; LaVan, D.A.; Langer, R. Nanoparticle-Aptamer BioconjugatesA New Approach for Targeting Prostate Cancer Cells. Cancer Res. 2004, 64, 7668–7672. [Google Scholar] [CrossRef] [PubMed]
- Marchì, C.; Annaratone, L.; Marques, A.; Casorzo, L.; Berrino, E.; Sapino, A. NC-ND license Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin. Cancer Biol. 2021, 72, 123–135. [Google Scholar] [CrossRef]
- Sanjanwala, D.; Patravale, V. Aptamers and nanobodies as alternatives to antibodies for ligand-targeted drug delivery in cancer. Drug Discov. Today 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Alshaer, W.; Hillaireau, H.; Fattal, E. Aptamer-guided nanomedicines for anticancer drug delivery. Adv. Drug Deliv. Rev. 2018, 134, 122–137. [Google Scholar] [CrossRef]
- Fu, Z.; Xiang, J. Aptamers, the nucleic acid antibodies, in cancer therapy. Int. J. Mol. Sci. 2020, 21, 2793. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, D.; Zeng, Z.; Huang, L.; Lin, X.; Hong, S. Aptamer-Based Probes for Cancer Diagnostics and Treatment. Life 2022, 12, 1937. [Google Scholar] [CrossRef]
- Sheikh, A.; Md, S.; Alhakamy, N.A.; Kesharwani, P. Recent development of aptamer conjugated chitosan nanoparticles as cancer therapeutics. Int. J. Pharm. 2022, 620, 121751. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, L.; Huang, X.; Zhu, S.; Ma, C.; Wang, H. Identification and antioxidant abilities of enzymatic-transesterification (−)-epigallocatechin-3-o-gallate stearyl derivatives in non-aqueous systems. Antioxidants 2021, 10, 1282. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, L.; Yang, L.; Wang, X. Epigallocatechin gallate is the most effective catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Med. Sci. Monit. 2018, 24, 8198–8206. [Google Scholar] [CrossRef]
- Chang, X.; Rong, C.; Chen, Y.; Yang, C.; Hu, Q.; Mo, Y.; Zhang, C.; Gu, X.; Zhang, L.; He, W.; et al. (-)-Epigallocatechin-3-gallate attenuates cognitive deterioration in Alzheimer[U+05F3]s disease model mice by upregulating neprilysin expression. Exp. Cell Res. 2015, 334, 136–145. [Google Scholar] [CrossRef]
- Chen, B.H.; Hsieh, C.H.; Tsai, S.Y.; Wang, C.Y.; Wang, C.C. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef] [PubMed]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Rotoli, D.; Santana-Viera, L.; Ibba, M.L.; Esposito, C.L.; Catuogno, S. Advances in oligonucleotide aptamers for NSCLC targeting. Int. J. Mol. Sci. 2020, 21, 6075. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.Y.L.; Xu, M.L.; Jin, Y.; Wu, Q.; Dong, T.T.X.; Tsim, K.W.K. Genistein, a phytoestrogen in soybean, induces the expression of acetylcholinesterase via G protein-coupled receptor 30 in PC12 cells. Front. Mol. Neurosci. 2018, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Cheng, D.; Wei, S.; Wang, X.; Niu, Y.; Qi, W.; Wang, C. Preventive effect of genistein on AOM/DSS-induced colonic neoplasm by modulating the PI3K/AKT/FOXO3 signaling pathway in mice fed a high-fat diet. J. Funct. Foods 2018, 46, 237–242. [Google Scholar] [CrossRef]
- Chan, K.K.L.; Siu, M.K.Y.; Jiang, Y.; Wang, J.; Leung, T.H.Y.; Ngan, H.Y.S. Estrogen receptor modulators genistein, daidzein and ERB-041 inhibit cell migration, invasion, proliferation and sphere formation via modulation of FAK and PI3K/AKT signaling in ovarian cancer. Cancer Cell Int. 2018, 18, 65. [Google Scholar] [CrossRef]
- Nauman, M.C.; Johnson, J.J. The purple mangosteen (Garcinia mangostana): Defining the anticancer potential of selected xanthones. Pharmacol. Res. 2022, 175, 106032. [Google Scholar] [CrossRef] [PubMed]
- Kalick, L.S.; Khan, H.A.; Maung, E.; Baez, Y.; Atkinson, A.N.; Wallace, C.E.; Day, F.; Delgadillo, B.E.; Mondal, A.; Watanapokasin, R.; et al. Mangosteen for malignancy prevention and intervention: Current evidence, molecular mechanisms, and future perspectives. Pharmacol. Res. 2023, 188, 106630. [Google Scholar] [CrossRef] [PubMed]
- Bonafè, F.; Pazzini, C.; Marchionni, S.; Guarnieri, C.; Muscari, C. Complete disaggregation of MCF-7-derived breast tumour spheroids with very low concentrations of α-mangostin loaded in CD44 thioaptamer-tagged nanoparticles. Int. J. Med. Sci. 2019, 16, 33–42. [Google Scholar] [CrossRef]
- Aravind, A.; Jeyamohan, P.; Nair, R.; Veeranarayanan, S.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. AS1411 aptamer tagged PLGA-lecithin-PEG nanoparticles for tumor cell targeting and drug delivery. Biotechnol. Bioeng. 2012, 109, 2920–2931. [Google Scholar] [CrossRef]
- Jin, H.; Lee, W.S.; Eun, S.Y.; Jung, J.H.; Park, H.S.; Kim, G.; Choi, Y.H.; Ryu, C.H.; Jung, J.M.; Hong, S.C.; et al. Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB-231 partly through suppression of the Akt pathway. Int. J. Oncol. 2014, 45, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.G.; Lee, S.; Kim, J.; Yang, S.; Lee, M.; Ahn, J.; Lee, H.; Chang, S.-C.; Ha, N.-C.; Lee, J. Anti-Inflammatory and Neuroprotective Effects of Morin in an MPTP-Induced Parkinson’s Disease Model. Int. J. Mol. Sci. 2022, 23, 10578. [Google Scholar] [CrossRef] [PubMed]
- Yazdanshenas, R.; Gharib, F. Spectrophotometric determination of preferential solvation and solvation shell composition of morin hydrate in some water-aliphatic alcohol mixed solvents. J. Mol. Liq. 2017, 243, 414–419. [Google Scholar] [CrossRef]
- Ding, X.; Yin, C.; Zhang, W.; Sun, Y.; Zhang, Z.; Yang, E.; Sun, D.; Wang, W. Designing Aptamer-Gold Nanoparticle-Loaded pH-Sensitive Liposomes Encapsulate Morin for Treating Cancer. Nanoscale Res. Lett. 2020, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Wu, Y.; Zhou, M.; Lin, R.; Ge, P.; Chen, X.; Zhou, H.; Zhang, X.; Xie, J. Precise delivery of celastrol by PEGylated aptamer dendrimer nanoconjugates for enormous therapeutic effect via superior intratumor penetration over antibody counterparts. Cancer Lett. 2023, 579, 216461. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Singh Tuli, H.; Sak, K.; Mukherjee, T.; Bishayee, A. Molecular targets of celastrol in cancer: Recent trends and advancements. Crit. Rev. Oncol. Hematol. 2018, 128, 70–81. [Google Scholar] [CrossRef]
- Wang, C.; Dai, S.; Zhao, X.; Zhang, Y.; Gong, L.; Fu, K.; Ma, C.; Peng, C.; Li, Y. Celastrol as an emerging anticancer agent: Current status, challenges and therapeutic strategies. Biomed. Pharmacother. 2023, 163, 114882. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Niu, B.; Wu, Y.; Xu, W.; Li, M.; Sun, H.; Zhou, H.; Zhang, X.; Xie, J. Enhanced cancer therapy of celastrol in vitro and in vivo by smart dendrimers delivery with specificity and biosafety. Chem. Eng. J. 2020, 383, 123228. [Google Scholar] [CrossRef]
- Dong, H.; Han, L.; Wu, Z.-S.; Zhang, T.; Xie, J.; Ma, J.; Wang, J.; Li, T.; Gao, Y.; Shao, J.; et al. Supporting Information Biostable aptamer rings conjugated for targeting two biomarkers on circulating tumor cells in vivo with great precision. Chem. Mater. 2017, 29, 10312–10325. [Google Scholar] [CrossRef]
- Malik, S.; Singh, A.; Negi, P.; Kapoor, V.K. Thymoquinone: A small molecule from nature with high therapeutic potential. Drug Discov. Today 2021, 26, 2716–2725. [Google Scholar] [CrossRef]
- Mahmoud, Y.K.; Abdelrazek, H.M.A. Cancer: Thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomed. Pharmacother. 2019, 115, 108783. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.M.; Centner, C.S.; Bates, P.J.; Malik, M.T.; Kopechek, J.A. Delivery of thymoquinone to cancer cells with as1411-conjugated nanodroplets. PLoS ONE 2020, 15, e0233466. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Peng, T.; Peng, Y.; Ai, L.; Deng, Z.; Wang, X.Q.; Tan, W. Molecularly Engineering Triptolide with Aptamers for High Specificity and Cytotoxicity for Triple-Negative Breast Cancer. J. Am. Chem. Soc. 2020, 142, 2699–2703. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; He, Z.; Ge, W.; Zhao, Z. Application of aptamer functionalized nanomaterials in targeting therapeutics of typical tumors. Front. Bioeng. Biotechnol. 2023, 11, 1092901. [Google Scholar] [CrossRef]
- Li, Q.; Maier, S.H.; Li, P.; Peterhansl, J.; Belka, C.; Mayerle, J.; Mahajan, U.M. Aptamers: A novel targeted theranostic platform for pancreatic ductal adenocarcinoma. Radiat. Oncol. 2020, 15, 189. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B.; Xu, X.; Zhuang, B.; Li, H.; Yin, J.; Cong, M.; Xu, W.; Lu, A. Toward targeted therapy in chemotherapy-resistant pancreatic cancer with a smart triptolide nanomedicine. Oncotarget 2016, 7, 8360–8372. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.; Zhu, C.; Xiang, C. Anti prostate cancer therapy: Aptamer-functionalized, curcumin and cabazitaxel co-delivered, tumor targeted lipid-polymer hybrid nanoparticles. Biomed. Pharmacother. 2020, 127, 110181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cao, C.; Li, N.; Yuan, S. SYL3C aptamer-anchored microemulsion co-loading β-elemene and PTX enhances the treatment of colorectal cancer. Drug Deliv. 2019, 26, 886–897. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, W.; Chen, J. Binary Nanodrug-Delivery System Designed for Leukemia Therapy: Aptamer-and Transferrin-Codecorated Daunorubicin-and Luteolin-Coloaded Nanoparticles. Drug Des. Devel Ther. 2023, 17, 1–13. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Z.; Zhang, G.; Lin, F.; Liu, Y.; Zhang, Y.; Feng, J.; Chen, W.; Meng, Q.; Chen, L. AS1411 Aptamer/Hyaluronic Acid-Bifunctionalized Microemulsion Co-Loading Shikonin and Docetaxel for Enhanced Antiglioma Therapy. J. Pharm. Sci. 2019, 108, 3684–3694. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Wu, G.; Kong, J.; Yuan, S.; Chen, L. A Magnetic T7 Peptide&AS1411 Aptamer-Modified Microemulsion for Triple Glioma-Targeted Delivery of Shikonin and Docetaxel. J. Pharm. Sci. 2021, 110, 2946–2954. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, M.; Haghiralsadat, B.F.; Abazari, O.; Hemati, M.; Dayati, P.; Jaliani, H.Z.; Motlagh, N.S.H.; Naghib, S.M.; Moradi, A. HB5 aptamer-tagged graphene oxide for co-delivery of doxorubicin and silibinin, and highly effective combination therapy in breast cancer. Cancer Nanotechnol. 2023, 14, 59. [Google Scholar] [CrossRef]
- Kim, D.H.; Seo, J.M.; Shin, K.J.; Yang, S.G. Design and clinical developments of aptamer-drug conjugates for targeted cancer therapy. Biomater. Res. 2021, 25, 42. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, Y.; Wang, Y.; Zhang, M.; Luo, Y.; Tang, J.; Wang, Z.; Wang, D.; Hao, L.; Wang, Z. Paclitaxel-loaded and A10-3.2 aptamer-targeted poly(lactide-co-glycolic acid) nanobubbles for ultrasound imaging and therapy of prostate cancer. Int. J. Nanomed. 2017, 12, 5313–5330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid-polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhou, Y.; Wang, R.; Wang, J.; Chen, Z. Aptamers as Smart Ligands for Targeted Drug Delivery in Cancer Therapy. Pharmaceutics 2022, 14, 2561. [Google Scholar] [CrossRef] [PubMed]
- Song, G.-Q.; Wu, P.; Dong, X.-M.; Cheng, L.-H.; Lu, H.-Q.; Lin, Y.-Y.; Tang, W.-Y.; Xie, T.; Zhou, J.-L. Elemene induces cell apoptosis via inhibiting glutathione synthesis in lung adenocarcinoma. J. Ethnopharmacol. 2023, 311, 116409. [Google Scholar] [CrossRef]
- Jiang, Z.; Jacob, J.A.; Loganathachetti, D.S.; Nainangu, P.; Chen, B. β-Elemene: Mechanistic Studies on Cancer Cell Interaction and Its Chemosensitization Effect. Front. Pharmacol. 2017, 8, 105. [Google Scholar] [CrossRef]
- Xie, Q.; Li, F.; Fang, L.; Liu, W.; Gu, C. The Antitumor Efficacy of β -Elemene by Changing Tumor Inflammatory Environment and Tumor Microenvironment. Biomed. Res. Int. 2020, 2020, 6892961. [Google Scholar] [CrossRef]
- Shang, X.; Guan, Z.; Zhang, S.; Shi, L.; You, H. Predicting the aptamer SYL3C-EpCAM complex’s structure with the Martini-based simulation protocol. Phys. Chem. Chem. Phys. 2021, 23, 7066–7079. [Google Scholar] [CrossRef]
- Gao, H.L.; Yu, X.J.; Feng, Y.Q.; Yang, Y.; Hu, H.B.; Zhao, Y.Y.; Zhang, J.-H.; Liu, K.-L.; Zhang, Y.; Fu, L.-Y.; et al. Luteolin Attenuates Hypertension via Inhibiting NF-κB-Mediated Inflammation and PI3K/Akt Signaling Pathway in the Hypothalamic Paraventricular Nucleus. Nutrients 2023, 15, 502. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nazim, U.M.D.; Park, S.Y. Luteolin sensitizes human liver cancer cells to TRAIL-induced apoptosis via autophagy and JNK-mediated death receptor 5 upregulation. Int. J. Oncol. 2019, 54, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Chen, L.; Li, H. The dietary compound luteolin inhibits pancreatic cancer growth by targeting BCL-2. Food Funct 2018, 9, 3018–3027. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Chen, Y.R.; Chow, J.M.; Chien, M.H.; Yang, S.F.; Wen, Y.C.; Lee, W.; Tseng, T. Stimulation of Fas/FasL-mediated apoptosis by luteolin through enhancement of histone H3 acetylation and c-Jun activation in HL-60 leukemia cells. Mol. Carcinog. 2018, 57, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Pei, S.N.; Qi, J.; Zeng, Z.; Iyer, S.P.; Lin, P.; Tung, C.-H.; Zu, Y. Oligonucleotide aptamer-drug conjugates for targeted therapy of acute myeloid leukemia. Biomaterials 2015, 67, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Mayca Pozo, F.; Tian, D.; Geng, X.; Yao, X.; Zhang, Y.; Tang, J. Shikonin Inhibits Cancer Through P21 Upregulation and Apoptosis Induction. Front. Pharmacol. 2020, 11, 861. [Google Scholar] [CrossRef]
- Yan, C.; Li, Q.; Sun, Q.; Yang, L.; Liu, X.; Luo, K.; Zhao, Y.; Shi, M.; Li, X.; Luo, K. Promising Nanomedicines of Shikonin for Cancer Therapy. Int. J. Nanomed. 2023, 18, 1195–1218. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Li, Z.L.; Wu, J.Y.; Lu, F.J.; Chen, C.H. An oxidative stress mechanism of shikonin in human glioma cells. PLoS ONE 2014, 9, e94180. [Google Scholar] [CrossRef]
- Rehfeld, M.; Matschke, J.; Hagel, C.; Willenborg, K.; Glatzel, M.; Bernreuther, C. Differential expression of stem cell markers in proliferating cells in glioma. J. Cancer Res. Clin. Oncol. 2021, 147, 2969–2982. [Google Scholar] [CrossRef]
- Jahanafrooz, Z.; Motamed, N.; Rinner, B.; Mokhtarzadeh, A.; Baradaran, B. Silibinin to improve cancer therapeutic, as an apoptotic inducer, autophagy modulator, cell cycle inhibitor, and microRNAs regulator. Life Sci. 2018, 213, 236–247. [Google Scholar] [CrossRef]
- Liu, Z.; Duan, J.H.; Song, Y.M.; Ma, J.; Wang, F.D.; Lu, X.; Yang, X.-D. Novel HER2 Aptamer Selectively Delivers Cytotoxic Drug to HER2-positive Breast Cancer Cells in Vitro. J. Transl. Med. 2012, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.; Wani, M.; Cook, C.; Palmer, K.; McPhail, A.; Sim, G. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inibitor from Camptotheca acuminata 1,2. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Zeng, C.W.; Zhang, X.J.; Lin, K.Y.; Ye, H.; Feng, S.Y.; Zhang, H.; Chen, Y.-Q. Camptothecin induces apoptosis in cancer cells via microRNA-125b-mediated mitochondrial pathways. Mol. Pharmacol. 2012, 81, 578–586. [Google Scholar] [CrossRef]
- Li, L.H.; Frã¤ser, T.J.; Olin, E.J.; Bhuyan, B.K. Action of Camptothecin on Mammalian Cells in Culture1. Cancer Res. 1972, 32, 2643–2650. [Google Scholar] [PubMed]
- Ferraro, C.; Quemeneur, L.; Fournel, S.; Prigent, A.-F.; Revillard, J.-P.; Bonnefoy-Berard, N. The topoisomerase inhibitors camptothecin and etoposide induce a CD95-independent apoptosis of activated peripheral lymphocytes. Cell Death Differ. 2000, 7, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Ding, J.; Yu, L.; Ma, D.; Ding, J. Improved Solubility and Bioactivity of Camptothecin Family Antitumor Drugs with Supramolecular Encapsulation by Water-Soluble Pillar[6]arene. ACS Omega 2017, 2, 5283–5288. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, A.; Kundu, B.; Sarkar, D.; Goon, S.; Mondal, M.A. Topoisomerase I inhibitors: Challenges, progress and the road ahead. Eur. J. Med. Chem. 2022, 236, 114304. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, K.H.; Kim, D.W.; Kim, S.W.; Kim, H.R.; Kim, J.H.; Choi, J.-H.; An, H.J.; Kim, J.-S.; Jang, J.-S.; et al. A randomised phase 2b study comparing the efficacy and safety of belotecan vs. topotecan as monotherapy for sensitive-relapsed small-cell lung cancer. Br. J. Cancer 2021, 124, 713–720. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, J.; Sun, Y.; Yang, C.; Zhang, S.; Wang, R.; Tan, W. Programmable Repurposing of Existing Drugs as Pharmaceutical Elements for the Construction of Aptamer-Drug Conjugates. ACS Appl. Mater. Interfaces 2021, 13, 9457–9463. [Google Scholar] [CrossRef]
- Onstenk, W.; Sieuwerts, A.M.; Kraan, J.; Van, M.; Nieuweboer, A.J.M.; Mathijssen, R.H.J.; De Laere, B.; Dirix, L.Y. Efficacy of Cabazitaxel in Castration-resistant Prostate Cancer Is Independent of the Presence of AR-V7 in Circulating Tumor Cells. Eur. Urol. 2015, 68, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Brian Bundy, M.; Wenzel, L.; Huang, H. Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Pezaro, C.J.; Omlin, A.G.; Altavilla, A.; Lorente, D.; Ferraldeschi, R.; Bianchini, D.; Dearnaley, D.; Parker, C.; de Bono, J.S.; Attard, G. Activity of cabazitaxel in castration-resistant prostate cancer progressing after docetaxel and next-generation endocrine agents. Eur. Urol. 2014, 66, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Miles, D.; Kim, S.B.; Im, Y.H.; Im, S.A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Walsh, V.; Goodman, J. From taxol to Taxol®: The changing identities and ownership of an anti-cancer drug. Med. Anthropol. Cross Cult. Stud. Health Illn. 2002, 21, 307–336. [Google Scholar] [CrossRef] [PubMed]
- Khing, T.M.; Choi, W.S.; Kim, D.M.; Po, W.W.; Thein, W.; Shin, C.Y.; Sohn, U.D. The effect of paclitaxel on apoptosis, autophagy and mitotic catastrophe in AGS cells. Sci. Rep. 2021, 11, 23490. [Google Scholar] [CrossRef] [PubMed]
- Fumoleau, P.; Chevallier, B.; Kerbrat, P.; Krakowski, Y.; Misset, J.-L.; Maugard-Louboutin, C.; Dieras, V.; Azli, N.; Bougon, N.; Riva, A.; et al. A Multicentre Phase II Study of the Efficacy and Safety of Docetaxel as First-Line Treatment of Advanced Breast Cancer: Report of the Clinical Screening Group of the EORTC; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1996. [Google Scholar]
- Zhang, G.; Fang, W. A new synthesis route of cabazitaxel. J. Chin. Pharm. Sci. 2012, 21, 472–476. [Google Scholar] [CrossRef]
- Mathew, A.E.; Mejillano, M.R.; Nath, J.P.; Himes, R.H.; Stella, V.J. Synthesis and Evaluation of Some Water-Soluble Prodrugs and Derivatives of Taxol with Antitumor Activity. J. Med. Chem. 1992, 35, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Guastalla, J.P.; Diéras, V. The taxanes: Toxicity and quality of life considerations in advanced ovarian cancer. Br. J. Cancer 2003, 89, S16–S22. [Google Scholar] [CrossRef]
- Fellner, S.; Bauer, B.; Miller, D.S.; Schaffrik, M.; Fankhänel, M.; Spruß, T.; Bernhardt, G.; Graeff, C.; Färber, L.; Gschaidmeier, H.; et al. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J. Clin. Investig. 2002, 110, 1309–1318. [Google Scholar] [CrossRef]
- Engels, F.K.; Mathot, R.A.A.; Verweij, J. Alternative drug formulations of docetaxel: A review. Anticancer. Drugs 2007, 18, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, N. How nanotechnology can enhance docetaxel therapy. Int. J. Nanomed. 2013, 8, 2927–2941. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lu, J.; Liu, J.; Liang, C.; Wang, M.; Wang, L.; Li, D.; Yao, H.; Zhang, Q.; Wen, J.; et al. A water-soluble nucleolin aptamer-paclitaxel conjugate for tumor-specific targeting in ovarian cancer. Nat. Commun. 2017, 8, 1390. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Arruda, B.; Mendes, M.G.A.; Freitas PGC de Reis, A.V.F.; Soares Lima, T.; Crisóstomo, L.C.C.F.; Nogueira, K.A.B.; Pessoa, C.; Petrilli, R.; Eloy, J.O. Nanocarriers for delivery of taxanes: A review on physicochemical and biological aspects. J. Drug Deliv. Sci. Technol. 2023, 80, 104070. [Google Scholar] [CrossRef]
- Cheng, Y.; Ou, Z.; Li, Q.; Yang, J.; Hu, M.; Zhou, Y.; Zhuang, X.; Zhang, Z.J.; Guan, S. Cabazitaxel liposomes with aptamer modification enhance tumor-targeting efficacy in nude mice. Mol. Med. Rep. 2019, 19, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Lin, S.; Yang, F.; Situ, J.; Lin, S.; Luo, Y. Aptamer-Conjugated Multifunctional Polymeric Nanoparticles as Cancer-Targeted, MRI-Ultrasensitive Drug Delivery Systems for Treatment of Castration-Resistant Prostate Cancer. Biomed. Res. Int. 2020, 2020, 9186583. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, X.; Duan, J.; Yang, X.D. Targeted treatment of colon cancer with aptamer-guided albumin nanoparticles loaded with docetaxel. Int. J. Nanomed. 2020, 15, 6737–6748. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhang, Z.; Wang, B.; Chen, G.; Zhang, Y.; Deng, H.; Tang, Z.; Mao, J.; Wang, L. Combination chemotherapy of lung cancer–co-delivery of docetaxel prodrug and cisplatin using aptamer-decorated lipid–polymer hybrid nanoparticles. Drug Des. Devel Ther. 2020, 14, 2249–2261. [Google Scholar] [CrossRef]
- Li, B.; Feng, Z.; He, L.; Li, W.; Wang, Q.; Liu, J.; Huang, J.; Zheng, Y.; Ma, Y.; Yang, X.; et al. Self-Assembled Supramolecular Nanoparticles for Targeted Delivery and Combination Chemotherapy. ChemMedChem 2018, 13, 2037–2044. [Google Scholar] [CrossRef]
- Zhang, R.; Zhong, L.; Liu, X.; Liu, X.; Chen, Q.; Wu, P.; He, J.; Li, Y.; Zhao, Y.; Liu, Z.; et al. Microfluidic PLGA microcapsules with PD-L1 aptamers and docetaxel encapsulation for enhancing tumor immunity. Appl. Mater. Today 2022, 27, 101484. [Google Scholar] [CrossRef]
- Ghassami, E.; Varshosaz, J.; Jahanian-Najafabadi, A.; Minaiyan, M.; Rajabi, P.; Hayati, E. Pharmacokinetics and in vitro/in vivo antitumor efficacy of aptamer-targeted ecoflex® nanoparticles for docetaxel delivery in ovarian cancer. Int. J. Nanomed. 2018, 13, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Zolbanin, N.M.; Jafari, R.; Majidi, J.; Atyabi, F.; Yousefi, M.; Jadidi-Niaragh, F.; Aghebati-Maleki, L.; Shanehbandi, D.; Zangbar, M.-S.S.; Nayebi, A.M. Targeted co-delivery of docetaxel and cMET siRNA for treatment of mucin1 overexpressing breast cancer cells. Adv. Pharm. Bull. 2018, 8, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Allan, A.L. Anti-proliferative and anti-migratory effects of EGFR and c-Met tyrosine kinase inhibitors in triple negative breast cancer cells. Precis. Cancer Med. 2021, 4, 2. [Google Scholar] [CrossRef]
- Zolbanin, N.M.; Jafari, R.; Majidi, J.; Atyabi, F.; Yousefi, M.; Jadidi-Niaragh, F.; Zangbar, M.-S.S.; Asadi, M.; Nayebi, A.M. Apoptotic effects of Mucin1 aptamer-conjugated nanoparticles containing docetaxel and c-Met siRNA on SKBR3 human metastatic breast cancer cells. Jundishapur J. Nat. Pharm. Prod. 2019, 14, e67023. [Google Scholar] [CrossRef]
- Kong, N.; Deng, M.; Sun, X.N.; Chen, Y.D.; Sui, X.B. Polydopamine-functionalized CA-(PCL-ran-PLA) nanoparticles for target delivery of docetaxel and chemo-photothermal therapy of breast cancer. Front. Pharmacol. 2018, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, J.; Ding, F.; Pan, G.; Li, J.; Feng, J.; Zhu, X.; Zhang, C. Stressing the Role of DNA as a Drug Carrier: Synthesis of DNA–Drug Conjugates through Grafting Chemotherapeutics onto Phosphorothioate Oligonucleotides. Adv. Mater. 2019, 31, e1807533. [Google Scholar] [CrossRef] [PubMed]
- Mie, M.; Matsumoto, R.; Mashimo, Y.; Cass, A.E.G.; Kobatake, E. Development of drug-loaded protein nanoparticles displaying enzymatically-conjugated DNA aptamers for cancer cell targeting. Mol. Biol. Rep. 2019, 46, 261–269. [Google Scholar] [CrossRef]
- Duan, T.; Xu, Z.; Sun, F.; Wang, Y.; Zhang, J.; Luo, C.; Wang, M. HPA aptamer functionalized paclitaxel-loaded PLGA nanoparticles for enhanced anticancer therapy through targeted effects and microenvironment modulation. Biomed. Pharmacother. 2019, 117, 109121. [Google Scholar] [CrossRef]
- Wu, X.; Li, F.; Li, Y.; Yu, Y.; Liang, C.; Zhang, B.; Zhao, C.; Lu, A.; Zhang, G. A PD-L1 aptamer selected by loss-gain cell-SELEX conjugated with paclitaxel for treating triple-negative breast cancer. Med. Sci. Monit. 2020, 26, e925583. [Google Scholar] [CrossRef]
- Guo, S.; Vieweger, M.; Zhang, K.; Yin, H.; Wang, H.; Li, X.; Li, S.; Hu, S.; Sparreboom, A.; Evers, B.M.; et al. Ultra-thermostable RNA nanoparticles for solubilizing and high-yield loading of paclitaxel for breast cancer therapy. Nat. Commun. 2020, 11, 972. [Google Scholar] [CrossRef]
- Kang, S.J.; Jeong, H.Y.; Kim, M.W.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Im, C.S.; Song, I.H.; Lee, T.S.; Park, Y.S. Anti-EGFR lipid micellar nanoparticles co-encapsulating quantum dots and paclitaxel for tumor-targeted theranosis. Nanoscale 2018, 10, 19338–19350. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Huang, Y.; Huang, S.; Zhuang, J.; Chen, P.; Wang, Y.; Zhang, L. Targeted delivery of cancer drug paclitaxel to chordomas tumor cells via an RNA nanoparticle harboring an EGFR aptamer. Colloids Surf. B Biointerfaces 2022, 212, 112366. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Fu, W.; Lin, S.; Tian, T.; Li, S.; Shao, X.; Zhang, Y.; Zhang, T.; Tang, Z.; Zhou, Y.; et al. Targeted and effective glioblastoma therapy via aptamer-modified tetrahedral framework nucleic acid-paclitaxel nanoconjugates that can pass the blood brain barrier. Nanomedicine 2019, 21, 102061. [Google Scholar] [CrossRef] [PubMed]
- Engelberg, S.; Netzer, E.; Assaraf, Y.G.; Livney, Y.D. Selective eradication of human non-small cell lung cancer cells using aptamer-decorated nanoparticles harboring a cytotoxic drug cargo. Cell Death Dis. 2019, 10, 702. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dlie, Z.Y.; Chakraborty, S.; Roy, S.; Mukherjee, B.; Besra, S.E.; Dewanjee, S.; Mukherjee, A.; Ojha, P.K.; Kumar, V.; et al. Aptamer-Functionalized Drug Nanocarrier Improves Hepatocellular Carcinoma toward Normal by Targeting Neoplastic Hepatocytes. Mol. Ther. Nucleic Acids 2020, 20, 34–49. [Google Scholar] [CrossRef]
| Compound | Nanocarrier | Formulations | Aptamer | Oligonucleotide | Main structure | Target | Clinical Application | Main Results Using Aptamer-Based Drug Carriers | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Apigenin | PEGylated liposomes | Free apigenin Apigenin-LP Apigenin-PEG-LP Apt-Apigenin-PEG-LP | AS1411 | 5′-GGT GGT GGT GGT TGT GGT GGT GGT GG-3′ | G-quadruplex | Nucleolin | Hepatocellular carcinoma | Higher cytotoxic potential Higher percentage of apoptosis Improved bioavailability Higher accumulation in the tumor site | [93] | |
| Celastrol | PEGylated PAMAM dendrimers G5 | Free Ce Ab-Ce-PAMAM Apt-Ce-PAMAM | SYL3C | 5′-CAC TAC AGA GGT TGC GTC TGT CCC ACG TTG TCA TGG GGG GTT GGC CTG-3′ | Hairpin | EpCAM | Colorectal Adenocarcinoma | Higher accumulation Enhanced intratumoral penetration Higher efficacy | [135] | |
| Curcumin | PEGylated PAMAM G5 dendrimers loaded with AuNPs | Free CUR PEG-AuPAMAM-CUR Apt-PEG-AuPAMAM-CUR | MUC-1 | 5′-GCAGTTG ATCCTTTGGATACCCTGGTTTTTTTTTT-3′ | Hairpin | MUC-1 | Colorectal Adenocarcinoma | High reduction in tumor volume Improved survival rate of mice Higher cellular uptake and internalization Higher cytotoxicity | [106] | |
| Curcumin | Human serum albumin nanoparticle | Free CUR HSANP-CUR Apt-HSANP-CUR | HB5 | 5′-AACCGCCCAAATCCCTAAGAGTCT GCACTTGTCATTTTGTATATGTATTTGGTTTTTGGCTCTCACAGACACACTA CACACGCACA-3′ | Hairpin | HER2 | Breast cancer | Increased curcumin cell uptake Higher cytotoxicity for cancer cells Less cytotoxicity for other cells | [109] | |
| Liposomes | Free CUR CUR-LP Apt-CUR-LP | A15 | 5ʹ-CCCUCCUACAUAGGG-3ʹ | Hairpin | CD133 | Prostate cancer | Less hemolytic effect Higher cancer cell internalization Higher accumulation on cancer tissues Higher reduction in tumor size | [102] | ||
| Homoharringtonine | Polymeric nanoparticles | Free HHT Apt-HHT-POL | EGFR | 5′-CGGCUUUGCCGCUAUAAUGCA CGGAUUUAAUCGCCGUAGAAAAGCAUGUCAAAGCCG-3′ | Hairpin | EGFR | Lung cancer | Higher apoptotic levels Fewer toxic effects on liver function | [91] | |
| α-Mangosteen | Lipid–polymer combinational nanoparticles | MG-POL Apt-MG-POL | CD44 | 5′-GAGATTCATCACGCGCATAGTCTTGGGACGGTGTTAAACGAAAGGGGACGACCGACTATGCGATGATGTCTTC-3′ | Hairpin | CD44 | Breast cancer | Higher reduction and disaggregation of tumor spheroids Higher global efficacy Reduced clearance | [129] | |
| Morin | Liposomes | Free Morin Morin-LP Apt-AU@morin-LP | AS1411 | 5′-GGT GGT GGT GGT TGT GGT GGT GGT GG-3′ | G-quadruplex | Nucleolin | Gastric cancer | Higher cytotoxicity Lower toxicity for non-cancerous cells Increased apoptotic potential Decrease in cancer cell density Reduction of tumor weight and size in mice Prolonged survival of mice | [134] | |
| Thymoquinone | Nanodroplet | Free TQ TQ-ND Apt-TQ-ND | AS1411 | 5′-GGTGGTGGTGGTTGTGGTGGT GGTGG-3′ | G-quadruplex | Nucleolin | Breast cancer | Cytotoxic potential was relatively the same for aptamer-modified and untargeted nanoemulsions | [142] | |
| Triptolide | - | Free TP Apt-TP | AS1411 | 5′-GGTGGTGGTGGTTGTGGTGGT GGTGG-3′ | G-quadruplex | Nucleolin | TNBC | Higher efficiency in inhibiting tumor growth and inducing apoptosis Less physical toxicity | [143] | |
| Synergistic therapy | Curcumin and cabazitaxel | Lipid-polymer hybrid nanoparticles | Free CUR/CTX Apt-CTX-POL Apt-CUR/CTX-POL | A10-3.2 | 5′-GGGAGGACGA UGCGGAUCA GCCAUGUUUACG UCACUCCU-3′ | Hairpin | PSMA | Prostate cancer | Superior cell inhibition (compared with individual cabazitaxel or curcumin) Increased accumulation in the tumor | [147] |
| β-elemene and paclitaxel | Microemulsion | Free β-elemene/PTX Apt-ME-β-elemene/PTX | SYL3C | 5′-CAC TAC AGA GGT TGC GTC TGT CCC ACG TTG TCA TGG GGG GTT GGC CTG-3′ | Hairpin | EpCAM | Colorectal cancer | Superior tumor growth suppression Extended mice survival Higher apoptotic levels | [148] | |
| Luteolin and daunorubicin | Lipid-polymer hybrid nanoparticles * | Free Dn Free Lut Free Dn/Lut Apt-Dn Tf-Lut Apt-Dn/Lut Tf-Dn/Lut Apt/Tf-Dn/Lut | CD117 | 5′-GGGGCCGGGGC AAGGGGGGG GTACCGTGGTAGGAC-3′ | G-quadruplex | CD117 | Acute myeloid leukemia | Enhanced tumor distribution Higher cytotoxicity Superior tumor growth suppression with Apt/Tf-Dn/LUT NP | [149] | |
| Shikonin and docetaxel | Hyaluronic acid-based microemulsion | SKN/DTX Apt-SKN/DTX-ME | AS1411 | 5′-GGTGGTGGTGGT TGTGGTGGT GGTGG-3′ | G-quadruplex | Nucleolin | Glioma | Increased cell uptake Higher apoptotic levels Increased cytotoxicity Enhanced permeability Enhanced brain-specific accumulation Superior tumor growth suppression Extended mice survival | [150] | |
| Microemulsion * | SKN/DTX T7-SKN/DTX AS1411-SKN/DTX T7/AS1411-SKN/DTX Fe3O4@T7/AS1411-SKN/DTX | AS1411 | 5′-GGTGGTGGTGGT TGTGGTGGT GGTGG-3′ | G-quadruplex | Nucleolin | Glioma | Higher cellular uptake Stronger apoptosis Mice treated with Fe3O4@T7/AS1411/SKN&DTX-M exhibited the highest drug distribution and increased survival rates, with no notable toxicity observed Inhibition of CD133+ and CD44+ cells within glioma segments | [151] | ||
| Silibinin and doxorubicin | Carboxylated graphene Oxide | Free FOX Free Sili cGO-DOX-Sili Apt-cGO-DOX/Sili | HB5 | 5′-(AACCGCCCAAATC (dNP)60CTACACACCCACA)-3′ | Hairpin | HER2 | Breast cancer | Higher cytotoxicity Higher internalization induced higher apoptotic levels | [152] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamboa, J.; Lourenço, P.; Cruz, C.; Gallardo, E. Aptamers for the Delivery of Plant-Based Compounds: A Review. Pharmaceutics 2024, 16, 541. https://doi.org/10.3390/pharmaceutics16040541
Gamboa J, Lourenço P, Cruz C, Gallardo E. Aptamers for the Delivery of Plant-Based Compounds: A Review. Pharmaceutics. 2024; 16(4):541. https://doi.org/10.3390/pharmaceutics16040541
Chicago/Turabian StyleGamboa, Joana, Pedro Lourenço, Carla Cruz, and Eugenia Gallardo. 2024. "Aptamers for the Delivery of Plant-Based Compounds: A Review" Pharmaceutics 16, no. 4: 541. https://doi.org/10.3390/pharmaceutics16040541





