The Integration of Advanced Drug Delivery Systems into Conventional Adjuvant Therapies for Peri-Implantitis Treatment
Abstract
:1. Introduction
1.1. Clinical Characteristics of Peri-Implantitis
1.2. Peri-Implantitis Conventional Non-Surgical Therapies
| Treatment Type | Efficacy | Ref. | |
|---|---|---|---|
| Systemic Antibiotics | Chemical Agents | ||
| Sr(OH)2 | Proven bacterial inhibition (p < 0.001) | [33] | |
| Chlorhexidine (C22H30Cl2N10) H2O2 | Proven reduction in anaerobic bacteria | [34] | |
| Chlorhexidine (C22H30Cl2N10) H2O2 PO4H3 Cetylpyridinium chloride (C21H38ClN) | Proven, it allows osseointegration within a short time period | [35] | |
| PO4H3 | Proven efficacy (3 months) | [36] | |
| 0.12% Chlorhexidine (C22H30Cl2N10) Timolol (C13H24N4O3S) Eucalyptol (C10H18O) Menthol (C10H20O) Methyl salicylate (C8H8O3) | Proven, better than combined systemic treatment | [32] | |
|
Clindamycin Amoxicillin Doxycycline Metronidazole | Proven, but bacterial resistance appears | [30] | |
| Chlorhexidine (C22H30Cl2N10) H2O2 | Proven reduction in anaerobic bacteria | [37] | |
| Chloramine gel (NH2Cl) Chlorhexidine chips (C22H30Cl2N10) | Proven only short-term clinical efficacy (3 months) (p < 0.001) | [38] | |
|
Tetracycline Doxycycline Ciprofloxacin Sulfonamides | Proven, reduction in inflammatory pockets in the area | [29] | |
| 0.12% Chlorhexidine (C22H30Cl2N10) | Proven (3-month period) | [17] | |
| Chlorhexidine (C22H30Cl2N10) Cetylpyridinium chloride (C21H38ClN) | Proven, bacterial reduction but no clinical benefit | [39] | |
2. Drug Delivery Systems for Peri-Implantitis Management
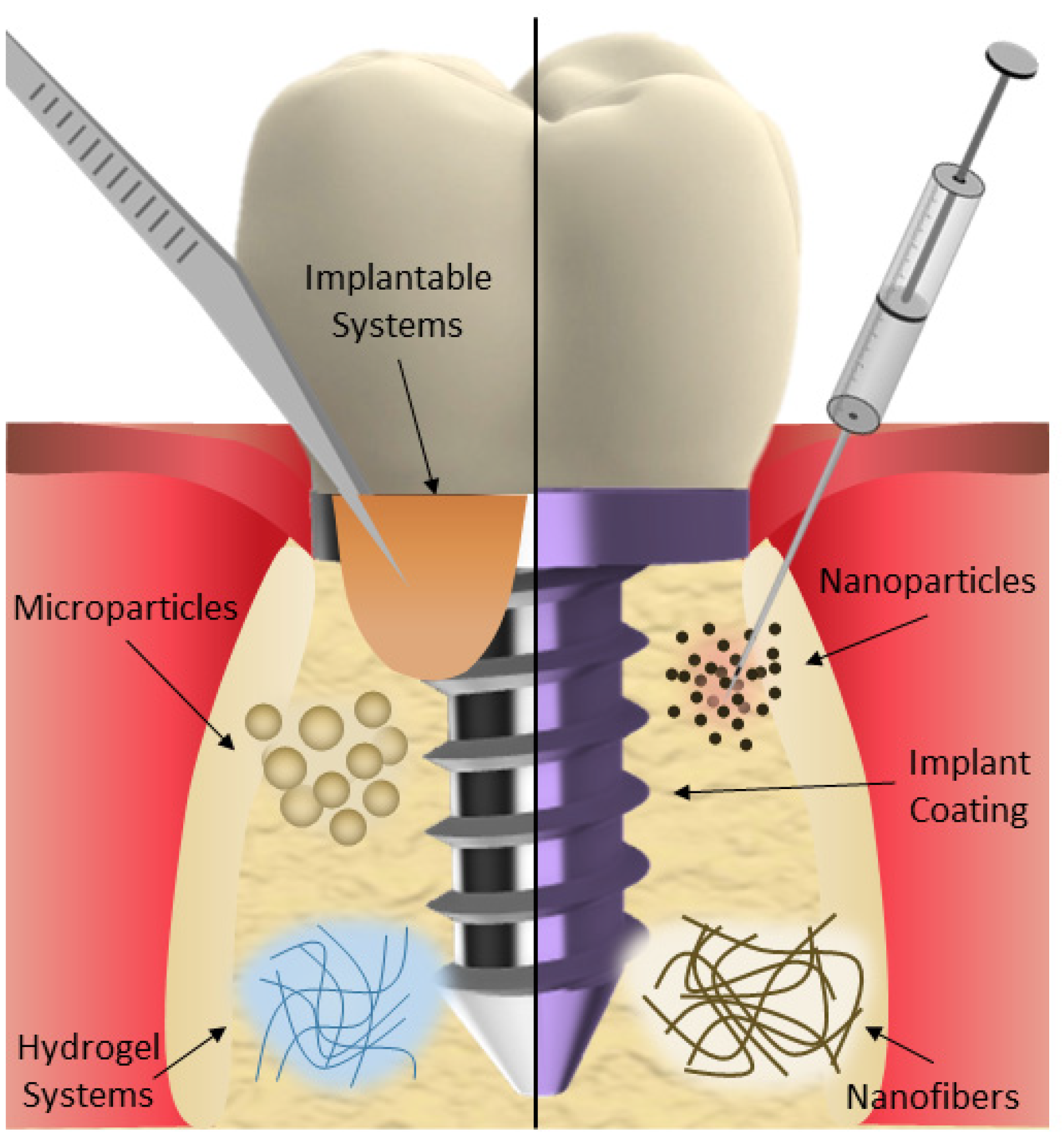
2.1. Micro- and Nanoparticles
| Type of Drug Carrier | Drug | Efficacy | Ref. |
|---|---|---|---|
| Micro- and nanoparticles | |||
| Gelatine microspheres | Minocycline | Antibacterial activity against P. gingivalis and F. nucleatum and promoted osteogenic differentiation in vitro. | [53] |
| Chitosan-coated alginate microspheres | Minocycline | Controlled release of the drug and bacteriostatic effects. | [54] |
| Fe3O4/CaCO3 microspheres | Minocycline | Great drug delivery and magnetic targeting properties and osteoinductive potential. | [55] |
| Gelatine microspheres | Icariin | Promotes bone formation and alleviates inflammation. | [56] |
| PLGA microspheres | Insulin | Stimulation of the osteogenic differentiation of the stem cells and peri-implant bone regeneration. | [58] |
| PLGA microspheres | Insulin | Higher peri-implant bone formation and improved osseointegration. | [59] |
| PLGA microspheres (Arestin®) | Minocycline | Reduction in total bacteria loading, with a higher impact on A. actinomycetemcomitans. | [60] |
| PLGA nanoparticles | Methylene blue | Antibacterial activity against P. gingivalis without deteriorating the surfaces and compromising the mechanical properties of dental implants. | [61] |
| PCL nanoparticles | Curcumin | Antimicrobial and antibiofilm properties, with better effects when associated with blue light. | [62] |
| Nanostructured lipid carriers (NLC) | Docosahexaenoic acid (DHA) | Enhances the anti-inflammatory bioavailability of DHA, preventing the activation of certain inflammatory pathways. | [63] |
| Silver nanoparticles | Silver nanoparticles | Antibacterial efficacy against P. gingivalis. | [64] |
| Nanofibers | |||
| Nanocrystals in nanofibers | Curcumin | Increased bioavailability of the drug and enhanced release properties. | [67] |
| PLA nanofibers | Metronidazole | Improved sustained drug release and in vitro antibacterial effect. | [68] |
| PCL nanofibers | Oxytetracycline HCl | Improved sustained drug release and in vitro antibacterial effect. | [69] |
| PLA nanofibers | Quercetin | In vitro antibacterial activity and pH-dependent drug release. | [70] |
| Electrospun membranes | Resveratrol | In vitro antibacterial activity and pH-dependent drug release. | [71] |
| Implantable systems | |||
| PerioChip® | Clorhexidine digluconate | Better treatment outcomes than using adjuvant therapies alone | [72,73,74,75] |
| Titania nanotube arrays implant | Silver nanoparticles | Biocompatible to osteoblasts, osteoinductive properties, and strong antimicrobial properties in vitro. | [76] |
| Chip | Silver nanoparticles | Significant antimicrobial activity against P. aeruginosa. | [77] |
| PLGA extrudates | Minocycline and doxycyclin | In vitro controlled release of drugs over 42 days. | [78] |
| Hydrogels | |||
| Atridox® gel system | Doxycycline hyclate | Favorable clinical outcomes when used in combination with conventional therapies. | [79,80] |
| Ozonized gel | Ozone | Better outcome than chlorhexidine on specific clinical periodontal parameters. | [81] |
| Alginate hydrogel | Gingival and human bone marrow mesenchymal stem cells | Promising outcomes for bone tissue engineering with in vitro antimicrobial properties. | [82] |
| Gelatine hydrogel | Antimicrobial peptide | Antimicrobial activity against P. gingivalis. Ability to support the growth of autologous bone in mice. | [83] |
| Chitosan hydrogel | Tannic acid | Antibacterial efficacy against P. gingivalis and F. nucleatum. | [84] |
| Hyaluronic acid-chitosan hydrogel | Dexamethasone | Sustained release of the drug. In vitro inhibition of S. aureus and E. coli and downregulation of the expression levels of several inflammation factors. | [85] |
| Thermosensitive micellar hydrogel | Ibuprofen and basic fibroblast growth factor | In vitro proliferation and adhesion of human gingival fibroblasts while inhibiting inflammation. | [86] |
| Thermo-reversible hydrogel | Doxycycline and/or lipoxin A4 | Decreases the subgingival bacterial load and specific pro-inflammatory markers. | [87] |
| Redox gel | Nitroxide radicals | Reduction in oxidative damage in a rat peri-implantitis model, providing protection against bone resorption and loss of bone density. | [88] |
| Coatings | |||
| Alcoholic solution | Chlorhexidine gluconate | Effective control of bacterial loading in the peri-implant tissue, with the ability to influence the quality of the microbiota. | [89] |
| Suspension | Totarol | Efficient contact killing and inhibition effects on S. gordonii. | [90] |
| Multilayer coating | Tetracycline | Burst release under neutral and acidic conditions, showing robust antibacterial efficacy against P. gingivalis. | [91] |
| Polymeric solution | Ciprofloxacine | Short-term antibacterial effect. | [92] |
| Abutment coating | Tannic acid, cerium, and minocycline | Effective isolation of the immune microenvironment from pathogen invasion. | [93] |
| PLGA coating | Doxycyline | Drug release faster at acidic pHs. | [94] |
| Niosomes thin films | Minocycline | Controlled drug release for up to 7 days. | [95] |
| Porous polymeric coatings | N-halamine | Antibacterial effectiveness persisted for an extended period in vitro, in animal models, and in the human oral cavity. | [96] |
| Bioactive glass | Strontium | Stimulation of osteoblasts and inhibition of osteoclast activities in vitro. | [97] |
2.2. Nanofibers
2.3. Implantable Systems
2.4. Hydrogel Systems
2.5. Coatings
3. Discussion and Future Perspectives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Müller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 67–76. [Google Scholar] [CrossRef]
- Vignoletti, F.; Di Domenico, G.L.; Di Martino, M.; Montero, E.; de Sanctis, M. Prevalence and risk indicators of peri-implantitis in a sample of university-based dental patients in Italy: A cross-sectional study. J. Clin. Periodontol. 2019, 46, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Juodzbalys, G. Diagnostic Principles of Peri-Implantitis: A Systematic Review and Guidelines for Peri-Implantitis Diagnosis Proposal. J. Oral Maxillofac. Res. 2016, 7, e8. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Ström, C. Peri-implantitis in partially edentulous patients: Association with inadequate plaque control. Clin. Oral Implant. Res. 2009, 20, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gárces, M.A.; Gay-Escoda, C. Periimplantitis. Med. Oral Patol. Oral Y Cir. Bucal 2004, 9, 69–74, 63–69. [Google Scholar]
- Roccuzzo, M.; Mirra, D.; Pittoni, D.; Ramieri, G.; Roccuzzo, A. Reconstructive treatment of peri-implantitis infrabony defects of various configurations: 5-year survival and success. Clin. Oral Implant. Res. 2021, 32, 1209–1217. [Google Scholar] [CrossRef]
- Salvi, G.E.; Stähli, A.; Imber, J.-C.; Sculean, A.; Roccuzzo, A. Physiopathology of peri-implant diseases. Clin. Implant Dent. Relat. Res. 2023, 25, 629–639. [Google Scholar] [CrossRef]
- Alves, C.H.; Russi, K.L.; Rocha, N.C.; Bastos, F.; Darrieux, M.; Parisotto, T.M.; Girardello, R. Host-microbiome interactions regarding peri-implantitis and dental implant loss. J. Transl. Med. 2022, 20, 425. [Google Scholar] [CrossRef]
- Schmid, E.; Eick, S.; Sculean, A.; Salvi, G.E. Peri-Implant Diseases: Characteristics of the Microbiota and of the Host Response in Humans—A Narrative Review. Monogr. Oral Sci. 2021, 29, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Venugopal, A.; Marya, A.; Scribante, A. Evaluation of Adjuvant Systems in Non-Surgical Peri-Implant Treatment: A Literature Review. Healthcare 2022, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Øen, M.; Leknes, K.N.; Lund, B.; Bunæs, D.F. The efficacy of systemic antibiotics as an adjunct to surgical treatment of peri-implantitis: A systematic review. BMC Oral Health 2021, 21, 666. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Osorio, M.T.; Vallecillo-Rivas, M.; Toledano-Osorio, M.; Rodríguez-Archilla, A.; Toledano, R.; Osorio, R. Efficacy of local antibiotic therapy in the treatment of peri-implantitis: A systematic review and meta-analysis. J. Dent. 2021, 113, 103790. [Google Scholar] [CrossRef] [PubMed]
- Parlar, A.; Bosshardt, D.D.; Cetiner, D.; Schafroth, D.; Unsal, B.; Haytaç, C.; Lang, N.P. Effects of decontamination and implant surface characteristics on re-osseointegration following treatment of peri-implantitis. Clin. Oral Implant. Res. 2009, 20, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Jepsen, S.; Obreja, K.; Galarraga-Vinueza, M.E.; Ramanauskaite, A. Surgical therapy of peri-implantitis. Periodontol. 2000 2022, 88, 145–181. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, P.; Wilson, T.G., Jr. Detoxification of implant surfaces affected by peri-implant disease: An overview of surgical methods. Int. J. Dent. 2013, 2013, 740680. [Google Scholar] [CrossRef] [PubMed]
- Chala, M.; Anagnostaki, E.; Mylona, V.; Chalas, A.; Parker, S.; Lynch, E. Adjunctive Use of Lasers in Peri-Implant Mucositis and Peri-Implantitis Treatment: A Systematic Review. Dent. J. 2020, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Hentenaar, D.F.M.; De Waal, Y.C.M.; Stewart, R.E.; Van Winkelhoff, A.J.; Meijer, H.J.A.; Raghoebar, G.M. Erythritol airpolishing in the non-surgical treatment of peri-implantitis: A randomized controlled trial. Clin. Oral Implant. Res. 2021, 32, 840–852. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Schwarz, F.; Cafferata, E.A.; Sahrmann, P. Photo/mechanical and physical implant surface decontamination approaches in conjunction with surgical peri-implantitis treatment: A systematic review. J. Clin. Periodontol. 2023, 50, 317–335. [Google Scholar] [CrossRef]
- Meyle, J. Mechanical, chemical and laser treatments of the implant surface in the presence of marginal bone loss around implants. Eur. J. Oral Implantol. 2012, 5, S71–S81. [Google Scholar]
- Roccuzzo, A.; De Ry, S.P.; Sculean, A.; Roccuzzo, M.; Salvi, G.E. Current Approaches for the Non-surgical Management of Peri-implant Diseases. Curr. Oral Health Rep. 2020, 7, 274–282. [Google Scholar] [CrossRef]
- Passarelli, P.C.; Netti, A.; Lopez, M.A.; Giaquinto, E.F.; De Rosa, G.; Aureli, G.; Bodnarenko, A.; Papi, P.; Starzyńska, A.; Pompa, G.; et al. Local/Topical Antibiotics for Peri-Implantitis Treatment: A Systematic Review. Antibiotics 2021, 10, 1298. [Google Scholar] [CrossRef]
- Figuero, E.; Graziani, F.; Sanz, I.; Herrera, D.; Sanz, M. Management of peri-implant mucositis and peri-implantitis. Periodontol. 2000 2014, 66, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Şahiner, A.; Halat, E.; Alğın Yapar, E. Comparison of bactericidal and fungicidal efficacy of antiseptic formulations according to EN 13727 and EN 13624 standards. Turk. J. Med. Sci. 2019, 49, 1564–1567. [Google Scholar] [CrossRef]
- Menezes, K.M.; Fernandes-Costa, A.N.; Silva-Neto, R.D.; Calderon, P.S.; Gurgel, B.C. Efficacy of 0.12% Chlorhexidine Gluconate for Non-Surgical Treatment of Peri-Implant Mucositis. J. Periodontol. 2016, 87, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Boccia, G.; Di Spirito, F.; D’Ambrosio, F.; Di Palo, M.P.; Giordano, F.; Amato, M. Local and Systemic Antibiotics in Peri-Implantitis Management: An Umbrella Review. Antibiotics 2023, 12, 114. [Google Scholar] [CrossRef]
- Baus-Domínguez, M.; Bakkali, S.; Hermida-Cabrera, P.; Serrera-Figallo, M.A.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D. A Systematic Review and Meta-Analysis of Systemic and Local Antibiotic Therapy in the Surgical Treatment of Peri-Implantitis. Antibiotics 2023, 12, 1223. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic resistance in human chronic periodontitis microbiota. J. Periodontol. 2014, 85, 160–169. [Google Scholar] [CrossRef]
- Verdugo, F.; Laksmana, T.; Uribarri, A. Systemic antibiotics and the risk of superinfection in peri-implantitis. Arch. Oral Biol. 2016, 64, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzi, V.; Escobar, E.C.; Cortelli, J.R.; Haas, A.N.; Andrade, A.K.; Pannuti, C.M.; Almeida, E.R.; Costa, F.O.; Cortelli, S.C.; Rode Sde, M. Antimicrobial mouthrinse use as an adjunct method in peri-implant biofilm control. Braz. Oral Res. 2014, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, H.; Neilands, J.; Svensäter, G.; Stavropoulos, A. Antimicrobial Potential of Strontium Hydroxide on Bacteria Associated with Peri-Implantitis. Antibiotics 2021, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Dostie, S.; Alkadi, L.T.; Owen, G.; Bi, J.; Shen, Y.; Haapasalo, M.; Larjava, H.S. Chemotherapeutic decontamination of dental implants colonized by mature multispecies oral biofilm. J. Clin. Periodontol. 2017, 44, 403–409. [Google Scholar] [CrossRef]
- Garaicoa-Pazmino, C.; Sinjab, K.; Wang, H.-L. Current Protocols for the Treatment of Peri-implantitis. Curr. Oral Health Rep. 2019, 6, 209–217. [Google Scholar] [CrossRef]
- Hentenaar, D.F.M.; De Waal, Y.C.M.; Strooker, H.; Meijer, H.J.A.; Van Winkelhoff, A.J.; Raghoebar, G.M. Implant decontamination with phosphoric acid during surgical peri-implantitis treatment: A RCT. Int. J. Implant Dent. 2017, 3, 33. [Google Scholar] [CrossRef]
- Rismanchian, M.; Nosouhian, S.; Shahabouee, M.; Davoudi, A.; Nourbakhshian, F. Effect of conventional and contemporary disinfectant techniques on three peri-implantitis associated microbiotas. Am. J. Dent. 2017, 30, 23–26. [Google Scholar]
- Roos-Jansåker, A.M.; Almhöjd, U.S.; Jansson, H. Treatment of peri-implantitis: Clinical outcome of chloramine as an adjunctive to non-surgical therapy, a randomized clinical trial. Clin. Oral Implant. Res. 2017, 28, 43–48. [Google Scholar] [CrossRef] [PubMed]
- de Waal, Y.C.; Raghoebar, G.M.; Huddleston Slater, J.J.; Meijer, H.J.; Winkel, E.G.; van Winkelhoff, A.J. Implant decontamination during surgical peri-implantitis treatment: A randomized, double-blind, placebo-controlled trial. J. Clin. Periodontol. 2013, 40, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Alghamdi, A.S.; Ahmed, A.; Mikami, T.; Ahmed, H.B.; Tenenbaum, H.C. Clinical efficacy of antibiotics in the treatment of peri-implantitis. Int. Dent. J. 2013, 63, 169–176. [Google Scholar] [CrossRef]
- Mouratidou, A.; Karbach, J.; d’Hoedt, B.; Al-Nawas, B. Antibiotic susceptibility of cocultures in polymicrobial infections such as peri-implantitis or periodontitis: An in vitro model. J. Periodontol. 2011, 82, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Almazin, S.M.; Dziak, R.; Andreana, S.; Ciancio, S.G. The effect of doxycycline hyclate, chlorhexidine gluconate, and minocycline hydrochloride on osteoblastic proliferation and differentiation in vitro. J. Periodontol. 2009, 80, 999–1005. [Google Scholar] [CrossRef]
- Kubasiewicz-Ross, P.; Fleischer, M.; Pitułaj, A.; Hadzik, J.; Nawrot-Hadzik, I.; Bortkiewicz, O.; Dominiak, M.; Jurczyszyn, K. Evaluation of the three methods of bacterial decontamination on implants with three different surfaces. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2020, 29, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, A.; Bertl, K.; Winning, L.; Polyzois, I. What is the influence of implant surface characteristics and/or implant material on the incidence and progression of peri-implantitis? A systematic literature review. Clin. Oral Implant. Res. 2021, 32, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Hu, H.; Wang, Y.; Lai, W.; Jian, F. Efficacy of Probiotics as Adjunctive Therapy to Nonsurgical Treatment of Peri-Implant Mucositis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 541752. [Google Scholar] [CrossRef] [PubMed]
- Máximo, M.B.; de Mendonça, A.C.; Renata Santos, V.; Figueiredo, L.C.; Feres, M.; Duarte, P.M. Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin. Oral Implant. Res. 2009, 20, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Shibli, J.A.; Ferrari, D.S.; Siroma, R.S.; Figueiredo, L.C.; Faveri, M.; Feres, M. Microbiological and clinical effects of adjunctive systemic metronidazole and amoxicillin in the non-surgical treatment of peri-implantitis: 1 year follow-up. Braz. Oral Res. 2019, 33, e080. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Schmucker, A.; Becker, J. Efficacy of alternative or adjunctive measures to conventional treatment of peri-implant mucositis and peri-implantitis: A systematic review and meta-analysis. Int. J. Implant Dent. 2015, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Persson, G.R.; Heitz-Mayfield, L.J.; Frei, M.; Lang, N.P. Adjunctive local antibiotic therapy in the treatment of peri-implantitis II: Clinical and radiographic outcomes. Clin. Oral Implant. Res. 2007, 18, 281–285. [Google Scholar] [CrossRef]
- Renvert, S.; Lessem, J.; Dahlén, G.; Renvert, H.; Lindahl, C. Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri-implantitis: A randomized clinical trial. J. Periodontol. 2008, 79, 836–844. [Google Scholar] [CrossRef]
- Muthukuru, M.; Zainvi, A.; Esplugues, E.O.; Flemmig, T.F. Non-surgical therapy for the management of peri-implantitis: A systematic review. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 77–83. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Peng, X.; Zhou, X.; Zou, J.; Cheng, L. Emerging Applications of Drug Delivery Systems in Oral Infectious Diseases Prevention and Treatment. Molecules 2020, 25, 516. [Google Scholar] [CrossRef]
- Liu, N.; Huang, S.; Guo, F.; Wang, D.; Zuo, Y.; Li, F.; Liu, C. Calcium phosphate cement with minocycline hydrochloride-loaded gelatine microspheres for peri-implantitis treatment. J. Dent. 2023, 136, 104624. [Google Scholar] [CrossRef]
- Yoon, S.W.; Kim, M.J.; Paeng, K.W.; Yu, K.A.; Lee, C.K.; Song, Y.W.; Cha, J.K.; Sanz, M.; Jung, U.W. Locally Applied Slow-Release of Minocycline Microspheres in the Treatment of Peri-Implant Mucositis: An Experimental In Vivo Study. Pharmaceutics 2020, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Li, X.; Li, Q.; Lyu, J.; Wang, W.; Zhuang, L.; Xu, Y. Magnetic drug-loaded osteoinductive Fe3O4/CaCO3 hybrid microspheres system: Efficient for sustained release of antibiotics. J. Phys. D Appl. Phys. 2020, 53, 245401. [Google Scholar] [CrossRef]
- Liu, N.; Huang, S.; Guo, F.; Zhai, S.; Wang, D.; Li, F.; Liu, C. Calcium phosphate cement with icariin-loaded gelatin microspheres as a local drug delivery system for bone regeneration. Biomed. Eng. Online 2022, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308. [Google Scholar] [CrossRef]
- Wang, X.; Qi, F.; Xing, H.; Zhang, X.; Lu, C.; Zheng, J.; Ren, X. Uniform-sized insulin-loaded PLGA microspheres for improved early-stage peri-implant bone regeneration. Drug Deliv. 2019, 26, 1178–1190. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Qi, F.; Zhao, J. The effect of a single injection of uniform-sized insulin-loaded PLGA microspheres on peri-implant bone formation. RSC Adv. 2018, 8, 40417–40425. [Google Scholar] [CrossRef]
- Persson, G.R.; Salvi, G.E.; Heitz-Mayfield, L.J.A.; Lang, N.P. Antimicrobial therapy using a local drug delivery system (Arestin®) in the treatment of peri-implantitis. I: Microbiological outcomes. Clin. Oral Implant. Res. 2006, 17, 386–393. [Google Scholar] [CrossRef]
- Aldegheishem, A.; Alharthi, R.; Al-Qahtani, Y.M.; Soliman, M.; Mostafa, M.S.; Mohsin, S.F.; Eldwakhly, E. Mechanical and antibacterial efficacy of photo-sonodynamic therapy via methylene blue-loaded nanoparticles over dental implants for treating peri-implantitis. Photodiagnosis Photodyn. Ther. 2022, 40, 103188. [Google Scholar] [CrossRef] [PubMed]
- Tonon, C.C.; Panariello, B.; Chorilli, M.; Spolidorio, D.M.P.; Duarte, S. Effect of curcumin-loaded photoactivatable polymeric nanoparticle on peri-implantitis-related biofilm. Photodiagnosis Photodyn. Ther. 2022, 40, 103150. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, Z.; Li, B.; He, J.; Liu, Y.; Zhang, N.; Li, X.; Cai, Q.; Meng, W. Docosahexaenoic Acid-Loaded Nanostructured Lipid Carriers for the Treatment of Peri-Implantitis in Rats. Int. J. Mol. Sci. 2023, 24, 1872. [Google Scholar] [CrossRef] [PubMed]
- Halkai, K.R.; Mudda, J.A.; Shivanna, V.; Rathod, V.; Halkai, R.S. Biosynthesis, Characterization and Antibacterial Efficacy of Silver Nanoparticles Derived from Endophytic Fungi against P. gingivalis. J. Clin. Diagn. Res. JCDR 2017, 11, zc92–zc96. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Poortman, J.; Peters, R.J.; Wijma, E.; Kramer, E.; Makama, S.; Puspitaninganindita, K.; Marvin, H.J.; Peijnenburg, A.A.; Hendriksen, P.J. Characterization of translocation of silver nanoparticles and effects on whole-genome gene expression using an in vitro intestinal epithelium coculture model. ACS Nano 2011, 5, 4091–4103. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jahan, S.A.; Zhang, J.; Bianchi, M.B.; Volpe-Zanutto, F.; Baviskar, S.M.; Rodriguez-Abetxuko, A.; Mishra, D.; Magee, E.; Gilmore, B.F.; et al. Curcumin nanocrystals-in-nanofibres as a promising platform for the management of periodontal disease. Int. J. Pharm. 2023, 648, 123585. [Google Scholar] [CrossRef]
- Reise, M.; Kranz, S.; Guellmar, A.; Wyrwa, R.; Rosenbaum, T.; Weisser, J.; Jurke, A.; Schnabelrauch, M.; Heyder, M.; Watts, D.C.; et al. Coaxial electrospun nanofibers as drug delivery system for local treatment of periodontitis. Dent. Mater. 2023, 39, 132–139. [Google Scholar] [CrossRef]
- Dias, A.M.; da Silva, F.G.; Monteiro, A.P.F.; Pinzón-García, A.D.; Sinisterra, R.D.; Cortés, M.E. Polycaprolactone nanofibers loaded oxytetracycline hydrochloride and zinc oxide for treatment of periodontal disease. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109798. [Google Scholar] [CrossRef] [PubMed]
- Di Cristo, F.; Valentino, A.; De Luca, I.; Peluso, G.; Bonadies, I.; Calarco, A.; Di Salle, A. PLA Nanofibers for Microenvironmental-Responsive Quercetin Release in Local Periodontal Treatment. Molecules 2022, 27, 2205. [Google Scholar] [CrossRef]
- Bonadies, I.; Di Cristo, F.; Valentino, A.; Peluso, G.; Calarco, A.; Di Salle, A. pH-Responsive Resveratrol-Loaded Electrospun Membranes for the Prevention of Implant-Associated Infections. Nanomaterials 2020, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Frankenthal, S.; Levi, G.; Elimelech, R.; Shoshani, E.; Rosenfeld, O.; Tagger-Green, N.; Shlomi, B. Treatment of peri-implantitis using multiple applications of chlorhexidine chips: A double-blind, randomized multi-centre clinical trial. J. Clin. Periodontol. 2012, 39, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Singh, P.; Dev, Y.; Sardana, S.; Rathee, K.; Sethi, M. Effectiveness of Controlled Release Chlorhexidine Chip as an Adjunctive to Scaling and Root Planning for the Treatment of Chronic Periodontitis. J. Contemp. Dent. Pract. 2019, 20, 1402–1405. [Google Scholar] [PubMed]
- Sahrmann, P.; Bettschart, C.; Wiedemeier, D.B.; Al-Majid, A.; Attin, T.; Schmidlin, P.R. Treatment of Peri-Implant Mucositis with Repeated Application of Chlorhexidine Chips or Gel during Supportive Therapy—A Randomized Clinical Trial. Dent. J. 2019, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Romanos, G.; Kang, P.; Travan, S.; Schmidt, S.; Papathanasiou, E.; Tatarakis, N.; Tandlich, M.; Liberman, L.H.; Horwitz, J.; et al. Repeated delivery of chlorhexidine chips for the treatment of peri-implantitis: A multicenter, randomized, comparative clinical trial. J. Periodontol. 2021, 92, 11–20. [Google Scholar] [CrossRef]
- Dong, Y.; Ye, H.; Liu, Y.; Xu, L.; Wu, Z.; Hu, X.; Ma, J.; Pathak, J.L.; Liu, J.; Wu, G. pH dependent silver nanoparticles releasing titanium implant: A novel therapeutic approach to control peri-implant infection. Colloids Surf. B Biointerfaces 2017, 158, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, K.; Dinda, A.K.; Kottarath, S.K.; Chaudhari, P.K.; Verma, F. Mucoadhesive silver nanoparticle-based local drug delivery system for peri-implantitis management in COVID-19 era. Part 1: Antimicrobial and safety in-vitro analysis. J. Oral Biol. Craniofacial Res. 2022, 12, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Kirchberg, M.; Eick, S.; Buchholz, M.; Kiesow, A.; Sarembe, S.; Mäder, K. Extrudates of lipophilic tetracycline complexes: A new option for periodontitis therapy. Int. J. Pharm. 2019, 572, 118794. [Google Scholar] [CrossRef] [PubMed]
- Javali, M.A.; Vandana, K.L. A comparative evaluation of atrigel delivery system (10% doxycycline hyclate) Atridox with scaling and root planing and combination therapy in treatment of periodontitis: A clinical study. J. Indian Soc. Periodontol. 2012, 16, 43–48. [Google Scholar] [CrossRef]
- Büchter, A.; Kleinheinz, J.; Meyer, U.; Joos, U. Treatment of severe peri-implant bone loss using autogenous bone and a bioabsorbable polymer that delivered doxycycline (Atridox). Br. J. Oral Maxillofac. Surg. 2004, 42, 454–456. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Gallo, S.; Pérez-Albacete Martínez, C.; Maté Sánchez de Val, J.E.; Parisi, L.; Gariboldi, A.; Scribante, A. Ozonized Hydrogels vs. 1% Chlorhexidine Gel for the Clinical and Domiciliary Management of Peri-Implant Mucositis: A Randomized Clinical Trial. J. Clin. Med. 2023, 12, 1464. [Google Scholar] [CrossRef] [PubMed]
- Diniz, I.M.A.; Chen, C.; Ansari, S.; Zadeh, H.H.; Moshaverinia, M.; Chee, D.; Marques, M.M.; Shi, S.; Moshaverinia, A. Gingival Mesenchymal Stem Cell (GMSC) Delivery System Based on RGD-Coupled Alginate Hydrogel with Antimicrobial Properties: A Novel Treatment Modality for Peri-Implantitis. J. Prosthodont. 2016, 25, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Sani, E.S.; Lara, R.P.; Aldawood, Z.; Bassir, S.H.; Nguyen, D.; Kantarci, A.; Intini, G.; Annabi, N. An Antimicrobial Dental Light Curable Bioadhesive Hydrogel for Treatment of Peri-Implant Diseases. Matter 2019, 1, 926–944. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Hu, J.; Yuan, Y.; Wang, X.; Jiang, Q. Photothermal-promoted multi-functional gallic acid grafted chitosan hydrogel containing tannic acid miniaturized particles for peri-implantitis. Int. J. Biol. Macromol. 2023, 253, 127366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, Q.; Wang, Y. Preparation and characterization of antibacterial and anti-inflammatory hyaluronic acid-chitosan-dexamethasone hydrogels for peri-implantitis repair. J. Biomater. Appl. 2022, 36, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhi, M.; Feng, Z.; Gao, P.; Yuan, Y.; Zhang, C.; Wang, Y.; Dong, A. Sustained co-delivery of ibuprofen and basic fibroblast growth factor by thermosensitive nanoparticle hydrogel as early local treatment of peri-implantitis. Int. J. Nanomed. 2019, 14, 1347–1358. [Google Scholar] [CrossRef]
- Wang, B.; Booij-Vrieling, H.E.; Bronkhorst, E.M.; Shao, J.; Kouwer, P.H.J.; Jansen, J.A.; Walboomers, X.F.; Yang, F. Antimicrobial and anti-inflammatory thermo-reversible hydrogel for periodontal delivery. Acta Biomater. 2020, 116, 259–267. [Google Scholar] [CrossRef]
- Ozawa, R.; Saita, M.; Sakaue, S.; Okada, R.; Sato, T.; Kawamata, R.; Sakurai, T.; Hamada, N.; Kimoto, K.; Nagasaki, Y. Redox injectable gel protects osteoblastic function against oxidative stress and suppresses alveolar bone loss in a rat peri-implantitis model. Acta Biomater. 2020, 110, 82–94. [Google Scholar] [CrossRef]
- Carinci, F.; Lauritano, D.; Bignozzi, C.A.; Pazzi, D.; Candotto, V.; Santos de Oliveira, P.; Scarano, A. A New Strategy Against Peri-Implantitis: Antibacterial Internal Coating. Int. J. Mol. Sci. 2019, 20, 3897. [Google Scholar] [CrossRef]
- Xu, Z.; Krajewski, S.; Weindl, T.; Loeffler, R.; Li, P.; Han, X.; Geis-Gerstorfer, J.; Wendel, H.-P.; Scheideler, L.; Rupp, F. Application of totarol as natural antibacterial coating on dental implants for prevention of peri-implantitis. Mater. Sci. Eng. C 2020, 110, 110701. [Google Scholar] [CrossRef]
- de Avila, E.D.; Castro, A.G.B.; Tagit, O.; Krom, B.P.; Löwik, D.; van Well, A.A.; Bannenberg, L.J.; Vergani, C.E.; van den Beucken, J.J.J.P. Anti-bacterial efficacy via drug-delivery system from layer-by-layer coating for percutaneous dental implant components. Appl. Surf. Sci. 2019, 488, 194–204. [Google Scholar] [CrossRef]
- Jaworska, J.; Jelonek, K.; Jaworska-Kik, M.; Musiał-Kulik, M.; Marcinkowski, A.; Szewczenko, J.; Kajzer, W.; Pastusiak, M.; Kasperczyk, J. Development of antibacterial, ciprofloxacin-eluting biodegradable coatings on Ti6Al7Nb implants to prevent peri-implant infections. J. Biomed. Mater. Res. Part A 2020, 108, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tan, X.; Zheng, L.; Tang, H.; Hu, S.; Zhai, Q.; Jing, X.; Liang, P.; Zhang, Y.; He, Q.; et al. A Dual-Antioxidative Coating on Transmucosal Component of Implant to Repair Connective Tissue Barrier for Treatment of Peri-Implantitis. Adv. Healthc. Mater. 2023, 12, 2301733. [Google Scholar] [CrossRef] [PubMed]
- Alécio, A.B.W.; Ferreira, C.F.; Babu, J.; Shokuhfar, T.; Jo, S.; Magini, R.; Garcia-Godoy, F. Doxycycline Release of Dental Implants with Nanotube Surface, Coated with Poly Lactic-Co-Glycolic Acid for Extended pH-controlled Drug Delivery. J. Oral Implantol. 2019, 45, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Wongsuwan, N.; Dwivedi, A.; Tancharoen, S.; Nasongkla, N. Development of dental implant coating with minocycline-loaded niosome for antibacterial application. J. Drug Deliv. Sci. Tec. 2020, 56, 101555. [Google Scholar] [CrossRef]
- Wu, S.; Xu, J.; Zou, L.; Luo, S.; Yao, R.; Zheng, B.; Liang, G.; Wu, D.; Li, Y. Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection. Nat. Commun. 2021, 12, 3303. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rawlinson, S.C.; Hill, R.G.; Fortune, F. Strontium-substituted bioactive glasses in vitro osteogenic and antibacterial effects. Dent. Mater. 2016, 32, 412–422. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Zhao, L. Antibacterial, angiogenic, and osteogenic activities of Ca, P, Co, F, and Sr compound doped titania coatings with different Sr content. Sci. Rep. 2019, 9, 14203. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Wu, X.; Wang, L. Management of peri-implantitis: A systematic review, 2010–2015. SpringerPlus 2016, 5, 105. [Google Scholar] [CrossRef]

| Material | Advantages | Disadvantages |
|---|---|---|
| Chlorhexidine | None | No co-adjuvant effects |
| Chemical agents (H2O2, H3PO4, EDTA, etc.) | Controversy | Corrosion with pH < 3 |
| Systemic antibiotics | Limited evidence | |
| Local antibiotics | Limited evidence | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seoane-Viaño, I.; Seoane-Gigirey, M.; Bendicho-Lavilla, C.; Gigirey, L.M.; Otero-Espinar, F.J.; Seoane-Trigo, S. The Integration of Advanced Drug Delivery Systems into Conventional Adjuvant Therapies for Peri-Implantitis Treatment. Pharmaceutics 2024, 16, 769. https://doi.org/10.3390/pharmaceutics16060769
Seoane-Viaño I, Seoane-Gigirey M, Bendicho-Lavilla C, Gigirey LM, Otero-Espinar FJ, Seoane-Trigo S. The Integration of Advanced Drug Delivery Systems into Conventional Adjuvant Therapies for Peri-Implantitis Treatment. Pharmaceutics. 2024; 16(6):769. https://doi.org/10.3390/pharmaceutics16060769
Chicago/Turabian StyleSeoane-Viaño, Iria, Mariola Seoane-Gigirey, Carlos Bendicho-Lavilla, Luz M. Gigirey, Francisco J. Otero-Espinar, and Santiago Seoane-Trigo. 2024. "The Integration of Advanced Drug Delivery Systems into Conventional Adjuvant Therapies for Peri-Implantitis Treatment" Pharmaceutics 16, no. 6: 769. https://doi.org/10.3390/pharmaceutics16060769
APA StyleSeoane-Viaño, I., Seoane-Gigirey, M., Bendicho-Lavilla, C., Gigirey, L. M., Otero-Espinar, F. J., & Seoane-Trigo, S. (2024). The Integration of Advanced Drug Delivery Systems into Conventional Adjuvant Therapies for Peri-Implantitis Treatment. Pharmaceutics, 16(6), 769. https://doi.org/10.3390/pharmaceutics16060769








