Liposome Encapsulation Enhances the Antidiabetic Efficacy of Silibinin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Liposomal Preparation
2.3. Determination of the Encapsulation Efficiency
2.4. Liposomal Characterization
2.5. Animals
2.6. Experimental Design
2.7. Determination of Biochemical Parameters of Diabetes
2.8. Histological and Immunohistochemical Examination of the Pancreas
2.9. Statistical Analysis
3. Results
3.1. Encapsulation Efficiency, Vesicle Size, PDI, Zeta Potential, and Mobility of Silibinin-Loaded Liposomes
3.2. Effects of Silibinin and Silibinin-Loaded Liposomes on Glucose, Glycated Hemoglobin, and Insulin Levels in Non-Diabetic and Diabetic Rats
3.3. Impact of Silibinin and Silibinin-Loaded Liposomes on Biochemical Parameters in Non-Diabetic and Diabetic Rats
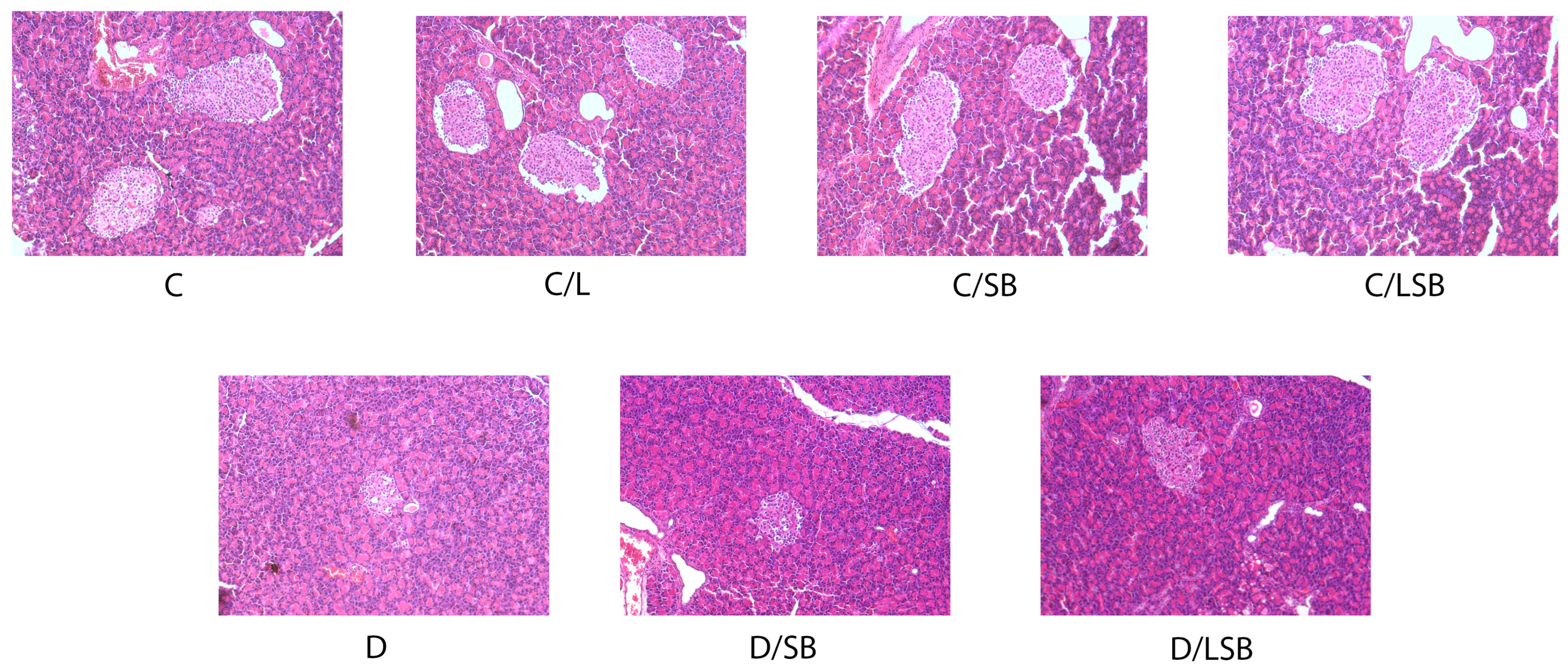
3.4. Histological Examination of the Pancreas following Treatment with Silibinin and Silibinin-Loaded Liposomes in Non-Diabetic and Diabetic Rats
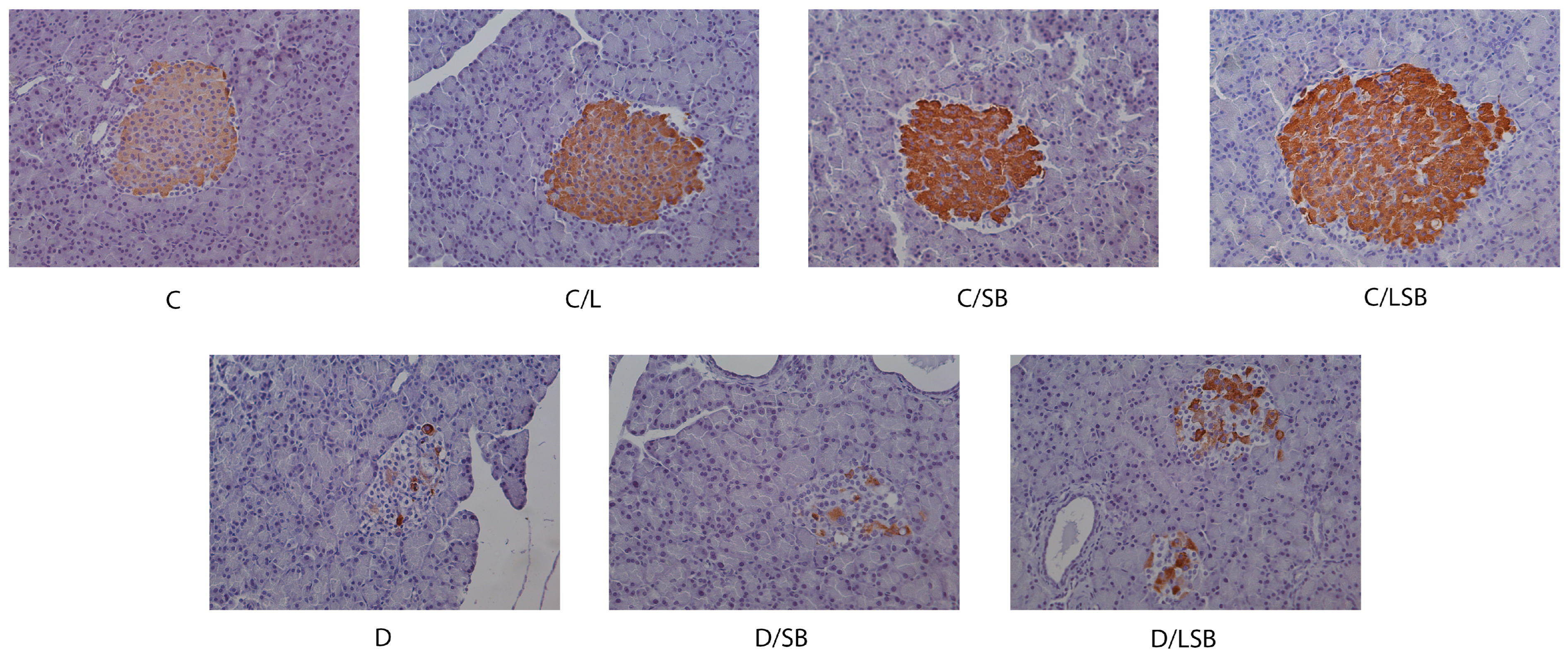
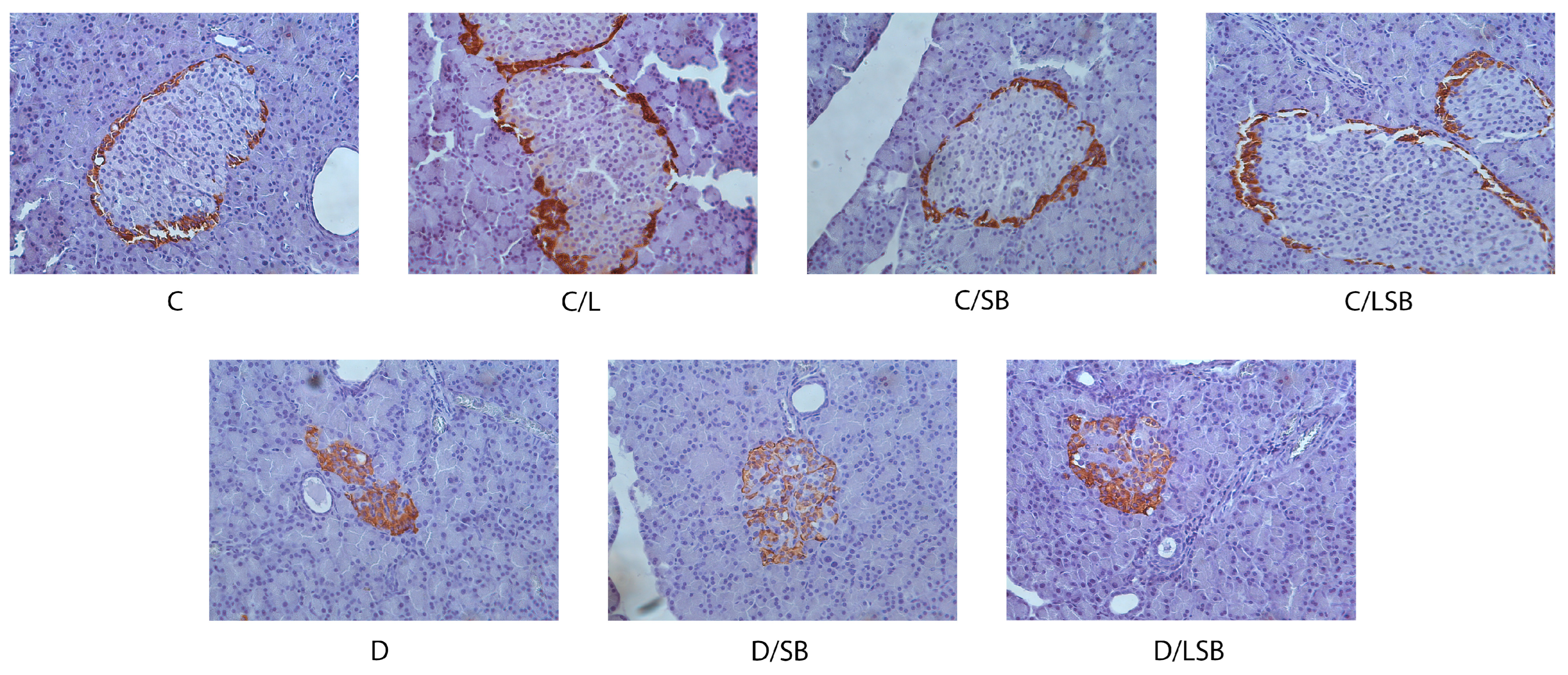
3.5. Effects of Silibinin and Silibinin-Loaded Liposomes on Insulin, Glucagon, and Glut2 in the Pancreatic Islets of Non-Diabetic and Diabetic Rats
3.6. Silibinin and Silibinin-Loaded Liposomes Reduce Collagen Deposition in the Pancreas of Diabetic Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- IDF. Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 1 March 2024).
- Dinić, S.; Arambašić Jovanović, J.; Uskoković, A.; Mihailović, M.; Grdović, N.; Tolić, A.; Rajić, J.; Đorđević, M.; Vidaković, M. Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Front. Endocrinol. 2022, 13, 1006376. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Masters, S.L.; Dunne, A.; Subramanian, S.L.; Hull, R.L.; Tannahill, G.M.; Sharp, F.A.; Becker, C.; Franchi, L.; Yoshihara, E.; Chen, Z.; et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 2010, 11, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Spranger, J.; Kroke, A.; Möhlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Brunner, E.J.; Rathmann, W.; Strassburger, K.; Tabák, A.G.; Schloot, N.C.; Witte, D.R. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: The Whitehall II study. Diabetes Care 2009, 32, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Edelman, S.V.; Polonsky, W.H. Type 2 Diabetes in the Real World: The Elusive Nature of Glycemic Control. Diabetes Care 2017, 40, 1425. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, M.M.; Tolić, A.; Rajić, J.; Mihailović, M.; Arambašić Jovanović, J.; Uskoković, A.; Grdović, N.; Đorđević, M.B.; Mišić, D.; Šiler, B.; et al. Centaurium erythraea methanol extract improves the functionality of diabetic liver and kidney by mitigating hyperglycemia-induced oxidative stress. J. Funct. Foods 2022, 90, 104975. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. A review of nanostructured delivery systems for the encapsulation, protection, and delivery of silymarin: An emerging nutraceutical. Food Res. Int. 2022, 156, 111314. [Google Scholar] [CrossRef] [PubMed]
- Taleb, A.; Ahmad, K.A.; Ihsan, A.U.; Qu, J.; Lin, N.; Hezam, K.; Koju, N.; Hui, L.; Qilong, D. Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases. Biomed. Pharmacother. 2018, 102, 689–698. [Google Scholar] [CrossRef]
- Liu, C.H.; Jassey, A.; Hsu, H.Y.; Lin, L.T. Antiviral activities of silymarin and derivatives. Molecules 2019, 24, 1552. [Google Scholar] [CrossRef]
- Zhao, F.; Li, X. Evaluation of immunomodulatory activity of silymarin extract from Silybum marianum in mice of health food. Adv. J. Food Sci. Technol. 2015, 8, 278–282. [Google Scholar] [CrossRef]
- Ahmad, U.; Akhtar, J.; Singh, S.P.; Ahmad, F.J.; Siddiqui, S. Silymarin nanoemulsion against human hepatocellular carcinoma: Development and optimization. Artif. Cells Nanomed. Biotechnol. 2018, 46, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Li, D.; Zhang, S.; Ikejima, T.; Jia, Y.; Wang, D.; Xu, F. Role of silibinin in the management of diabetes mellitus and its complications. Arch. Pharm. Res. 2018, 41, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yang, J.; Negishi, H.; Sun, Y.; Li, D.; Zhang, X.; Hayashi, T.; Gao, M.; Ikeda, K.; Ikejima, T. Silibinin decreases hepatic glucose production through the activation of gut-brain-liver axis in diabetic rats. Food Funct. 2018, 9, 4926. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.J.; Somani, R.; Gilhotra, R. Silibinin ameliorates hyperglycaemia, hyperlipidemia and prevent oxidative stress in streptozotocin induced diabetes in Sprague Dawley rats. Int. J. Pharm. Res. Allied. Sci. 2016, 5, 136–144. [Google Scholar]
- Voroneanu, L.; Nistor, I.; Dumea, R.; Apetrii, M.; Covic, A. Silymarin in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Diabetes Res. 2016, 2016, 5147468. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Angelico, R. Formulation Strategies for Enhancing the Bioavailability of Silymarin: The State of the Art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.; Balanč, B.; Volić, M.; Pećinar, I.; Živković, J.; Šavikin, K.P. Rosehip Extract-Loaded Liposomes for Potential Skin Application: Physicochemical Properties of Non- and UV-Irradiated Liposomes. Plants 2023, 12, 3063. [Google Scholar] [CrossRef]
- Jash, A.; Ubeyitogullari, A.; Rizvi, S. Liposomes for oral delivery of protein and peptide-based therapeutics: Challenges, formulation strategies, and advances. J. Mater. Chem. B 2021, 9, 4773–4792. [Google Scholar] [CrossRef]
- Lee, M.K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef]
- Čutović, N.; Marković, T.; Carević, T.; Stojković, D.; Bugarski, B.; Jovanović, A. Liposomal and Liposomes-Film Systems as Carriers for Bioactives from Paeonia tenuifolia L. Petals: Physicochemical Characterization and Biological Potential. Pharmaceutics 2023, 15, 2742. [Google Scholar] [CrossRef]
- Ricci, M.; Oliva, R.; Del Vecchio, P.; Paolantoni, M.; Morresi, A.; Sassi, P. DMSO-induced perturbation of thermotropic properties of cholesterol-containing DPPC liposomes. Biochim. Biophys. Acta 2016, 1858, 3024–3031. [Google Scholar] [CrossRef]
- Jovanović, A.; Balanč, B.; Djordjević, V.; Ota, A.; Skrt, M.; Šavikin, K.; Bugarski, B.; Nedović, V.; Poklar-Ulrih, N. Effect of gentisic acid on the structural-functional properties of liposomes incorporating β-sitosterol. Colloids Surf. B Biointerfaces 2019, 183, 110422. [Google Scholar] [CrossRef]
- Jovanović, A.; Dinić, S.; Uskoković, A.; Arambašić Jovanović, J.; Grdović, N.; Melita, V.; Mirjana, M. The characterisation of silymarin and silibinin liposomes. J. Eng. Process. Manag. 2022, 14, 41–46. [Google Scholar] [CrossRef]
- Isailović, B.; Kostić, I.; Zvonar, A.; Đorđević, V.; Gašperlin, M.; Nedović, V.; Bugarski, B. Resveratrol loaded liposomes produced by different techniques. Innov. Food Sci. Emerg. Technol. 2013, 19, 181–189. [Google Scholar] [CrossRef]
- Masiello, P.; Broca, C.; Gross, R.; Roye, M.; Manteghetti, M.; Hillaire-Buys, D.; Novelli, M.; Ribes, G. Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 1998, 47, 224–229. [Google Scholar] [CrossRef]
- Pai, M.H.; Huang, K.H.; Wu, C.H.; Yeh, S.L. Effects of dietary arginine on inflammatory mediator and receptor of advanced glycation endproducts (RAGE) expression in rats with streptozotocin-induced type 2 diabetes. Br. J. Nutr. 2010, 104, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Masiello, P.; Novelli, M.; Fierabracci, V.; Bergamini, E. Protection by 3-aminobenzamide and nicotinamide against streptozotocin-induced beta-cell toxicity in vivo and in vitro. Res. Commun. Chem. Pathol. Pharmacol. 1990, 69, 17–32. [Google Scholar] [PubMed]
- Drabkin, A.; Austin, H. Spectrophotometric studies preparations from washed blood cells. J. Biol. Chem. 1935, 112, 51–55. [Google Scholar] [CrossRef]
- Parker, K.M.; England, J.D.; DaCosta, J.; Hess, E.L.; Goldstein, D.E. Improved colorimetric assay for glycosylated hemoglobin. Clin. Chem. 1981, 27, 669–672. [Google Scholar] [CrossRef]
- Dupuy, A.M.; Badiou, S.; Descomps, B.; Cristol, J.P. Immunoturbidimetric determination of C-reactive protein (CRP) and high-sensitivity CRP on heparin plasma. Comparison with serum determination. Clin. Chem. Lab. Med. 2003, 41, 948–949. [Google Scholar] [CrossRef]
- Di Pierro, F.; Callegari, A.; Carotenuto, D.; Tapia, M.M. Clinical efficacy, safety and tolerability of BIO-C (micronized Silymarin) as a galactagogue. Acta Biomed. 2008, 79, 205–210. [Google Scholar] [PubMed]
- Shriram, R.G.; Moin, A.; Alotaibi, H.F.; Khafagy, E.S.; Al Saqr, A.; Abu Lila, A.S.; Charyulu, R.N. Phytosomes as a Plausible Nano-Delivery System for Enhanced Oral Bioavailability and Improved Hepatoprotective Activity of Silymarin. Pharmaceuticals 2022, 15, 790. [Google Scholar] [CrossRef]
- Das, S.; Roy, P.; Pal, R.; Auddy, R.G.; Chakraborti, A.S.; Mukherjee, A. Engineered silybin nanoparticles educe efficient control in experimental diabetes. PLoS ONE 2014, 9, e101818. [Google Scholar] [CrossRef] [PubMed]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Ardani, H.K.; Imawan, C.; Handayani, W.; Djuhana, D.; Harmoko, A.; Fauzia, V. Enhancement of the stability of silver nanoparticles synthesized using aqueous extract of Diospyros discolor Willd. leaves using polyvinyl alcohol. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012056. [Google Scholar] [CrossRef]
- Arias-Alpizar, G.; Kong, L.; Vlieg, R.C.; Rabe, A.; Papadopoulou, P.; Meijer, M.S.; Bonnet, S.; Vogel, S.; van Noort, J.; Kros, A.; et al. Light-triggered switching of liposome surface charge directs delivery of membrane impermeable payloads in vivo. Nat. Commun. 2020, 11, 3638. [Google Scholar] [CrossRef] [PubMed]
- Kechai, N.; Geiger, S.; Fallacara, A.; Canero Infante, I.; Nicolas, V.; Ferrary, E.; Huang, N.; Bochot, A.; Agnely, F. Mixtures of hyaluronic acid and liposomes for drug delivery: Phase behavior, microstructure and mobility of liposomes. Int. J. Pharm. 2017, 523, 246–259. [Google Scholar] [CrossRef]
- Wadhwa, K.; Pahwa, R.; Kumar, M.; Kumar, S.; Sharma, P.C.; Singh, G.; Verma, R.; Mittal, V.; Singh, I.; Kaushik, D.; et al. Mechanistic Insights into the Pharmacological Significance of Silymarin. Molecules 2022, 27, 5327. [Google Scholar] [CrossRef]
- Chen, K.; Xu, J.; He, H.; Zhao, L.; Xiong, J.; Mo, Z. Protective effect of silibinin on islet beta cells in C57BL/6 J mice fed a high fat diet. J. Cent. South Univ. Med. Sci. 2015, 40, 165–170. [Google Scholar]
- Alabdan, M.A. Silymarin Ameliorates Metabolic Risk Factors and Protects against Cardiac Apoptosis in Streptozotocin-Induced Diabetic Rats. Biomed. Biotechnol. 2015, 3, 20–27. [Google Scholar]
- Guigas, B.; Naboulsi, R.; Villanueva, G.R.; Taleux, N.; Lopez-Novoa, J.M.; Leverve, X.M.; El-Mir, M.Y. The flavonoid silibinin decreases glucose-6-phosphate hydrolysis in perfused rat hepatocytes by an inhibitory effect on glucose-6-phosphatase. Cell Physiol. Biochem. 2007, 20, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Digel, M.; Küch, E.-M.; Stremmel, W.; Füllekrug, J. Silybin and dehydrosilybin decrease glucose uptake by inhibiting GLUT proteins. J. Cell. Biochem. 2011, 112, 849–859. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, M.; Liu, W.W.; Hao, W.B.; Tashiro, S.; Onodera, S.; Ikejima, T. In vivo recovery effect of silibinin treatment on streptozotocin-induced diabetic mice is associated with the modulations of Sirt-1 expression and autophagy in pancreatic beta-cell. J. Asian Nat. Prod. Res. 2012, 14, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Raya, L.; Juárez, J.; Pérez, J.; González, I. Effect of Silymarin in Pdx-1 expression and the proliferation of pancreatic β-cells in a pancreatectomy model. Phytomedicine 2014, 21, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Raya, L.; Pérez, J.; González, I.; Pérez, S. Silymarin Induces Expression of Pancreatic Nkx6.1 Transcription Factor and β-Cells Neogenesis in a Pancreatectomy Model. Molecules 2014, 19, 4654–4668. [Google Scholar] [CrossRef] [PubMed]
- Tysseling, V.M.; Kessler, J.A. Biomaterials for Central Nervous System Regeneration. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 321–333. [Google Scholar]
- Parhofer, K.G. Interaction between Glucose and Lipid Metabolism: More than Diabetic Dyslipidemia. Diabetes Metab. J. 2015, 39, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.J.; Cho, S.Y.; Kim, E.Y.; Choi, H.S. Blockade of lipid accumulation by silibinin in adipocytes and zebrafish. Chem. Biol. Interact. 2015, 227, 53–62. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, D.; Pan, Y.; Qu, W.; Hao, H.; Wang, X.; Liu, Z.; Xie, S. Nanoparticles for antiparasitic drug delivery. Drug Deliv. 2019, 26, 1206–1221. [Google Scholar] [CrossRef]
- Barros, B.S.V.; Santos, D.C.; Pizarro, M.H.; del Melo, L.G.N.; Gomes, M.B. Type 1 diabetes and non-alcoholic fatty liver disease: When should we be concerned? A nationwide study in Brazil. Nutrients 2017, 9, E878. [Google Scholar] [CrossRef] [PubMed]
- Calzadilla Bertot, L.; Adams, L.A. The natural course of non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2016, 17, E774. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xie, T.; Chang, Z.; Wang, L.; Xie, X.; Kou, Y.; Xu, H.; Gao, X. A New Type of Liquid Silymarin Proliposome Containing Bile Salts: Its Preparation and Improved Hepatoprotective Effects. PLoS ONE 2015, 10, e0143625. [Google Scholar] [CrossRef] [PubMed]
- Vessal, G.; Akmali, M.; Najafi, P.; Moein, M.R.; Sagheb, M.M. Silymarin and Milk Thistle Extract May Prevent the Progression of Diabetic Nephropathy in Streptozotocin-Induced Diabetic Rats. Ren. Fail. 2010, 32, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Sheela, N.; Jose, M.A.; Sathyamurthy, D.; Kumar, B.N. Effect of Silymarin on Streptozotocin-Nicotinamide-Induced Type 2 Diabetic Nephropathy in Rats. Iran. J. Kidney Dis. 2013, 7, 117–123. [Google Scholar] [PubMed]
- Fallahzadeh, M.K.; Dormanesh, B.; Sagheb, M.M.; Roozbeh, J.; Vessal, G.; Pakfetrat, M.; Daneshbod, Y.; Kamali-Sarvestani, E.; Lankarani, K.B. Effect of Addition of Silymarin to Renin-Angiotensin System Inhibitors on Proteinuria in Type 2 Diabetic Patients with Overt Nephropathy: A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Kidney Dis. 2012, 60, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.M.O.; Agostini, L.D.C.; Lima, W.G.; Camini, F.C.; Costa, D.C. Silymarin Attenuates Hepatic and Pancreatic Redox Imbalance Independent of Glycemic Regulation in the Alloxan-induced Diabetic Rat Model. Biomed. Environ. Sci. 2020, 33, 690–700. [Google Scholar] [PubMed]
- Bulboacă, A.E.; Porfire, A.; Bolboacă, S.D.; Nicula, C.A.; Feștilă, D.G.; Roman, A.; Râjnoveanu, R.M.; Râjnoveanu, A.; Dogaru, G.; Boarescu, P.M.; et al. Protective Effects of Liposomal Curcumin on Oxidative Stress/Antioxidant Imbalance, Metalloproteinases 2 and -9, Histological Changes and Renal Function in Experimental Nephrotoxicity Induced by Gentamicin. Antioxidants 2021, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Dorcely, B.; Katz, K.; Jagannathan, R.; Chiang, S.S.; Oluwadare, B.; Goldberg, I.J.; Bergman, M. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes Metab. Syndr. Obes. 2017, 10, 345–361. [Google Scholar] [CrossRef]
- Du Clos, T.W. Function of C-reactive protein. Ann. Med. 2000, 32, 274–278. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, J.; Lee, M.Y.; Sudhanva, M.S.; Devakumar, S.; Jeon, Y.J. Silymarin Inhibits Cytokine-Stimulated Pancreatic Beta Cells by Blocking the ERK1/2 Pathway. Biomol. Ther. 2014, 22, 282–287. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Wang, Y.; Zhu, T. Silymarin Improved Diet-Induced Liver Damage and Insulin Resistance by Decreasing Inflammation in Mice. Pharm. Biol. 2016, 54, 2995–3000. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Huang, C.; Iovanna, J.; Santofimia-Castaño, P. Targeting Fibrosis: The Bridge That Connects Pancreatitis and Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 4970. [Google Scholar] [CrossRef]
- Jia, J.-D.; Bauer, M.; Cho, J.J.; Ruehl MMilani SBoigk GRiecken, E.O.; Schuppan, D. Antifibrotic effect of silymarin in rat secondary biliary fibrosis is mediated by downregulation of procollagen α1(I) and TIMP-1. J. Hepatol. 2001, 35, 392–398. [Google Scholar] [CrossRef]
- Clichici, S.; Olteanu, D.; Nagy, A.L.; Oros, A.; Filip, A.; Mircea, P.A. Silymarin Inhibits the Progression of Fibrosis in the Early Stages of Liver Injury in CCl4-Treated Rats. J. Med. Food 2015, 18, 290–298. [Google Scholar] [CrossRef]
- Ou, Q.; Weng, Y.; Wang, S.; Zhao, Y.; Zhang, F.; Zhou, J.; Wu, X. Silybin Alleviates Hepatic Steatosis and Fibrosis in NASH Mice by Inhibiting Oxidative Stress and Involvement with the NF-B Pathway. Dig. Dis. Sci. 2018, 63, 3398–3408. [Google Scholar] [CrossRef]
- Yin, W.; Kimbrough, C.W.; Gomez-Gutierrez, J.G.; Burns, C.T.; Chuong, P.; Grizzle, W.E.; McNally, L.R. Tumor specific liposomes improve detection of pancreatic adenocarcinoma in vivo using optoacoustic tomography. J. Nanobiotechnol. 2015, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.; Ćujić, D.; Gnjatović, M.; Stepanović, S.; Dinić, S.; Mihailović, M.; Bugarski, B. Storage and UV stability of small unilamellar liposomes with encapsulated silymarin and silibinin. In Proceeding Book, Proceedings of the International Conference on Advanced Technologies (ICAT’22), Van, Turkey, 25–27 November 2022; Bay, O.F., Ed.; Plusbase Akademi Publishing: Çumra, Turkey, 2022; pp. 448–452. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinić, S.; Arambašić Jovanović, J.; Uskoković, A.; Jovanović, A.; Grdović, N.; Rajić, J.; Đorđević, M.; Sarić, A.; Bugarski, B.; Vidaković, M.; et al. Liposome Encapsulation Enhances the Antidiabetic Efficacy of Silibinin. Pharmaceutics 2024, 16, 801. https://doi.org/10.3390/pharmaceutics16060801
Dinić S, Arambašić Jovanović J, Uskoković A, Jovanović A, Grdović N, Rajić J, Đorđević M, Sarić A, Bugarski B, Vidaković M, et al. Liposome Encapsulation Enhances the Antidiabetic Efficacy of Silibinin. Pharmaceutics. 2024; 16(6):801. https://doi.org/10.3390/pharmaceutics16060801
Chicago/Turabian StyleDinić, Svetlana, Jelena Arambašić Jovanović, Aleksandra Uskoković, Aleksandra Jovanović, Nevena Grdović, Jovana Rajić, Marija Đorđević, Ana Sarić, Branko Bugarski, Melita Vidaković, and et al. 2024. "Liposome Encapsulation Enhances the Antidiabetic Efficacy of Silibinin" Pharmaceutics 16, no. 6: 801. https://doi.org/10.3390/pharmaceutics16060801





