Advances in 2,3-Dimethylmaleic Anhydride (DMMA)-Modified Nanocarriers in Drug Delivery Systems
Abstract
:1. Introduction
2. Properties of Maleic Anhydride and Its Derivatives
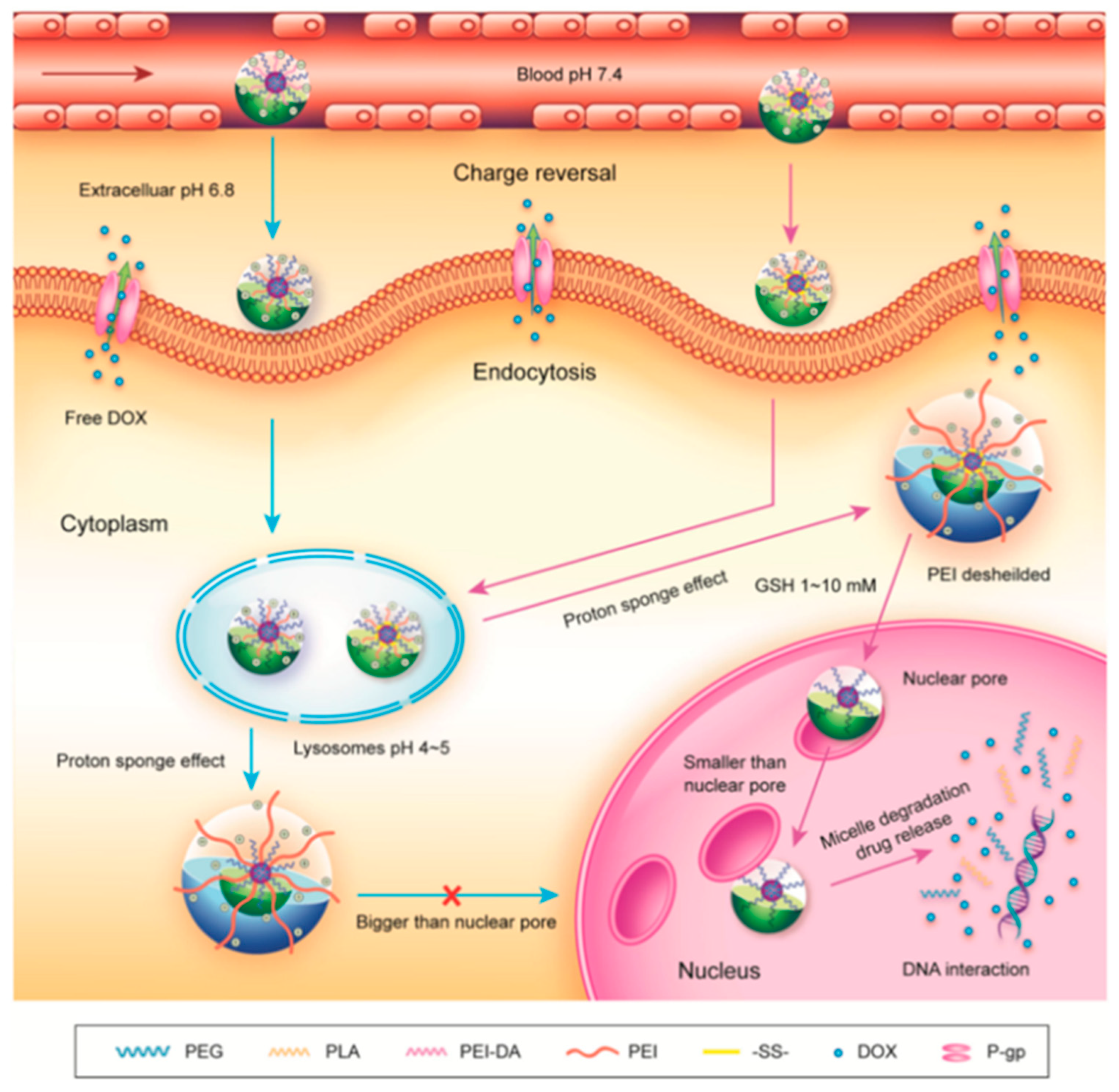
3. Advances in DMMA-Modified Smart Nanomedicine Delivery Systems
4. Conclusions
5. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Poon, W.; Kingston, B.R.; Ouyang, B.; Ngo, W.; Chan, W.C.W. A framework for designing delivery systems. Nat. Nanotechnol. 2020, 15, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zheng, R.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2022. J. Natl. Cancer Cent. 2024, 4, 43–57. [Google Scholar] [CrossRef]
- Swetha, K.L.; Roy, A. Tumor heterogeneity and nanoparticle-mediated tumor targeting: The importance of delivery system personalization. Drug Deliv. Transl. Res 2018, 8, 1508–1526. [Google Scholar] [CrossRef]
- Du, J.-Z.; Mao, C.-Q.; Yuan, Y.-Y.; Yang, X.-Z.; Wang, J. Tumor extracellular acidity-activated nanoparticles as drug delivery systems for enhanced cancer therapy. Biotechnol. Adv. 2014, 32, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tan, S.; Li, S.; Shen, Q.; Wang, K. Cancer drug delivery in the nano era: An overview and perspectives (Review). Oncol Rep 2017, 38, 611–624. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Credit, K.; Henderson, K.; Deverkadra, R.; He, Z.; Wiig, H.; Vanpelt, H.; Flessner, M.F. Intraperitoneal Immunotherapy for Metastatic Ovarian Carcinoma: Resistance of Intratumoral Collagen to Antibody Penetration. Clin. Cancer Res. 2006, 12, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.-H.; Rubin, K.; Pietras, K.; Östman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Newby, J.M.; Lin, C.M.; Zhang, L.; Xu, F.; Kim, W.Y.; Forest, M.G.; Lai, S.K.; Milowsky, M.I.; Wobker, S.E.; et al. The Binding Site Barrier Elicited by Tumor-Associated Fibroblasts Interferes Disposition of Nanoparticles in Stroma-Vessel Type Tumors. ACS Nano 2016, 10, 9243–9258. [Google Scholar] [CrossRef] [PubMed]
- Michael, M.; Doherty, M.M. Tumoral Drug Metabolism: Overview and Its Implications for Cancer Therapy. J. Clin. Oncol. 2005, 23, 205–229. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Sui, M.; Liu, W.; Shen, Y. Nuclear drug delivery for cancer chemotherapy. J. Control. Release 2011, 155, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Panté, N.; Kann, M. Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Mol. Biol. Cell 2002, 13, 425–434. [Google Scholar] [CrossRef]
- Beck, M.; Hurt, E. The nuclear pore complex: Understanding its function through structural insight. Nat. Rev. Mol. Cell Biol. 2016, 18, 73–89. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Kang, S.; Kim, Y.; Song, Y.; Choi, J.U.; Park, E.; Choi, W.; Park, J.; Lee, Y. Comparison of pH-sensitive degradability of maleic acid amide derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 2364–2367. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Liu, J.Y.; Li, S.Q.; Wang, J.; Li, N.N.; Zhou, J.N.; Chen, H.X. Application of Nano Drug Delivery System (NDDS) in Cancer Therapy: A Perspective. Recent Pat. Anti-Canc. Drug Discov. 2023, 18, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Yan, Z.Q.; Zhong, J.; He, D.N.; Lu, W.Y. Peptide-Mediated Nano Drug Delivery System for Tumor Targeting. Prog. Chem. 2013, 25, 1052–1060. [Google Scholar]
- Zhang, J.; Liu, F.; Huang, L. Implications of pharmacokinetic behavior of lipoplex for its inflammatory toxicity. Adv. Drug Deliv. Rev. 2005, 57, 689–698. [Google Scholar] [CrossRef]
- Levchenko, T.S.; Rammohan, R.; Lukyanov, A.N.; Whiteman, K.R.; Torchilin, V.P. Liposome clearance in mice: The effect of a separate and combined presence of surface charge and polymer coating. Int. J. Pharm. 2002, 240, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Ankone, M.; Pieters, E.; Schiffelers, R.M.; Hennink, W.E.; Feijen, J. Circulation kinetics and biodistribution of dual-labeled polymersomes with modulated surface charge in tumor-bearing mice: Comparison with stealth liposomes. J. Control. Release 2011, 155, 282–288. [Google Scholar] [CrossRef]
- Wan, D.; Zhu, Q.; Zhang, J.; Chen, X.; Li, F.; Liu, Y.; Pan, J. Intracellular and extracellular enzymatic responsive micelle for intelligent therapy of cancer. Nano Res. 2022, 16, 2851–2858. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Wang, Y.; Cheng, J.; Ji, S.; Zhuang, X.; Chen, X. Sequentially Responsive Shell-Stacked Nanoparticles for Deep Penetration into Solid Tumors. Adv. Mater. 2017, 29, 1701170. [Google Scholar] [CrossRef]
- Zhong, J.; Li, L.; Zhu, X.; Guan, S.; Yang, Q.; Zhou, Z.; Zhang, Z.; Huang, Y. A smart polymeric platform for multistage nucleus-targeted anticancer drug delivery. Biomaterials 2015, 65, 43–55. [Google Scholar] [CrossRef]
- Shi, M.H.; Zhang, J.L.; Wang, Y.; Han, Y.Y.; Zhao, X.L.; Hu, H.Y.; Qiao, M.X.; Chen, D.W. Blockage of the IDO1 pathway by charge-switchable nanoparticles amplifies immunogenic cell death for enhanced cancer immunotherapy. Acta Biomater. 2022, 150, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sun, Z.; Lu, L. Targeted Engineering of Medicinal Chemistry for Cancer Therapy: Recent Advances and Perspectives. Angew. Chem. Int. Ed. 2020, 60, 5626–5643. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, L.; Morin, E.E.; Liu, M.; Schwendeman, A. Survey of Clinical Translation of Cancer Nanomedicines—Lessons Learned from Successes and Failures. Acc. Chem. Res. 2019, 52, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Veider, F.; Sanchez Armengol, E.; Bernkop-Schnürch, A. Charge-Reversible Nanoparticles: Advanced Delivery Systems for Therapy and Diagnosis. Small 2023, 20, 2304713. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Deng, Y.; Chen, X.; Ji, J. Rational Design of Cancer Nanomedicine for Simultaneous Stealth Surface and Enhanced Cellular Uptake. ACS Nano 2019, 13, 954–977. [Google Scholar] [CrossRef] [PubMed]
- Man, S.L.; Gao, W.Y.; Wei, C.L.; Liu, C.X. Anticancer Drugs from Traditional Toxic Chinese Medicines. Phytother. Res. 2012, 26, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, Y.; Zhang, C.Y.; Fang, T. Co-Delivery of Paclitaxel and Doxorubicin by pH-Responsive Prodrug Micelles for Cancer Therapy. Int. J. Nanomed. 2020, 15, 3319–3331. [Google Scholar] [CrossRef] [PubMed]
- Suma, T.; Miyata, K.; Anraku, Y.; Watanabe, S.; Christie, R.J.; Takemoto, H.; Shioyama, M.; Gouda, N.; Ishii, T.; Nishiyama, N.; et al. Smart multilayered assembly for biocompatible siRNA delivery featuring dissolvable silica, endosome-disrupting polycation, and detachable PEG. ACS Nano 2012, 6, 6693–6705. [Google Scholar] [CrossRef]
- Gao, W.W.; Langer, R.; Farokhzad, O.C. Poly(ethylene glycol) with Observable Shedding. Angew. Chem. Int. Ed. 2010, 49, 6567–6571. [Google Scholar] [CrossRef]
- Cho, E.C.; Zhang, Q.; Xia, Y. The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nat. Nanotechnol. 2011, 6, 385–391. [Google Scholar] [CrossRef]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Dong, C.; Fan, W.; Jiang, H.; Xiang, J.; Qiu, N.; Piao, Y.; Xie, T.; Luo, Y.; Li, Z.; et al. Tumor extravasation and infiltration as barriers of nanomedicine for high efficacy: The current status and transcytosis strategy. Biomaterials 2020, 240, 119902. [Google Scholar] [CrossRef]
- Lee, Y.; Miyata, K.; Oba, M.; Ishii, T.; Fukushima, S.; Han, M.; Koyama, H.; Nishiyama, N.; Kataoka, K. Charge-Conversion Ternary Polyplex with Endosome Disruption Moiety: A Technique for Efficient and Safe Gene Delivery. Angew. Chem. Int. Ed. 2008, 47, 5163–5166. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. Biomed. Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Cho, E.C.; Xie, J.; Wurm, P.A.; Xia, Y. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano Lett. 2009, 9, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Petros, R.A.; Ropp, P.A.; DeSimone, J.M. Reductively labile PRINT particles for the delivery of doxorubicin to HeLa cells. J. Am. Chem. Soc. 2008, 130, 5008–5009. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Zhu, L.; Liu, Z.; Cheng, R.; Meng, F.; Cui, J.H.; Ji, S.J.; Zhong, Z. Reversibly stabilized multifunctional dextran nanoparticles efficiently deliver doxorubicin into the nuclei of cancer cells. Angew. Chem. Int. Ed. 2009, 48, 9914–9918. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur. J. Pharm. Biopharm. 2009, 71, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Tang, D.; Liu, C.; Zhang, Q.; Tang, L.; Lu, Y.; Xiao, H. Biodegradable Polymer with Effective Near-Infrared-II Absorption as Photothermal Agent for Deep Tumor Therapy. Adv. Mater. 2021, 34, 2105976. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor pH targeting nanotechnology. J. Control. Release 2008, 132, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.D.; Mart, R.J.; Webb, S.J.; Ulijn, R.V. Enzyme-responsive hydrogel particles for the controlled release of proteins: Designing peptide actuators to match payload. Soft Matter 2008, 4, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Gondi, C.S.; Rao, J.S. Cathepsin B as a cancer target. Expert Opin. Ther. Targets 2013, 17, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Mijanović, O.; Branković, A.; Panin, A.N.; Savchuk, S.; Timashev, P.; Ulasov, I.; Lesniak, M.S. Cathepsin B: A sellsword of cancer progression. Cancer Lett. 2019, 449, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Cai, H.; Wei, Q.; Tang, X.; Zhang, Q.; Kopytynski, M.; Yang, J.; Yi, Y.; Zhang, H.; Gong, Q.; et al. Enhanced chemo-photodynamic therapy of an enzyme-responsive prodrug in bladder cancer patient-derived xenograft models. Biomaterials 2021, 277, 121061. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shim, M.K.; Yang, S.; Moon, Y.; Song, S.; Choi, J.; Kim, J.; Kim, K. Combination of cancer-specific prodrug nanoparticle with Bcl-2 inhibitor to overcome acquired drug resistance. J. Control. Release 2021, 330, 920–932. [Google Scholar] [CrossRef]
- Knapp, J.P.; Kakish, J.E.; Bridle, B.W.; Speicher, D.J. Tumor Temperature: Friend or Foe of Virus-Based Cancer Immunotherapy. Biomedicines 2022, 10, 2024. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, D.Y.; Li, L.; Sun, K.X. Charge reversal nano-systems for tumor therapy. J. Nanobiotechnol. 2022, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Cardone, R.A.; Casavola, V.; Reshkin, S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 2005, 5, 786–795. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Z.; Wu, W.; Ding, C.; Li, J. Dual pH-responsive micelles with both charge-conversional property and hydrophobic–hydrophilic transition for effective cellular uptake and intracellular drug release. Polym. Chem. 2016, 7, 2202–2208. [Google Scholar] [CrossRef]
- Li, L.; Sun, W.; Zhong, J.; Yang, Q.; Zhu, X.; Zhou, Z.; Zhang, Z.; Huang, Y. Multistage Nanovehicle Delivery System Based on Stepwise Size Reduction and Charge Reversal for Programmed Nuclear Targeting of Systemically Administered Anticancer Drugs. Adv. Funct. Mater. 2015, 25, 4101–4113. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Bai, H.; Shen, J.; Chen, X.; Ping, Y.; Tang, G. A Cooperative Dimensional Strategy for Enhanced Nucleus-Targeted Delivery of Anticancer Drugs. Adv. Funct. Mater. 2017, 27, 1700339. [Google Scholar] [CrossRef]
- Xu, P.; Van Kirk, E.A.; Zhan, Y.; Murdoch, W.J.; Radosz, M.; Shen, Y. Targeted Charge-Reversal Nanoparticles for Nuclear Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 4999–5002. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, C.; Li, F.; Chen, D. pH-activated size reduction of large compound nanoparticles for in vivo nucleus-targeted drug delivery. Biomaterials 2016, 85, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Zhang, Y.; Xiao, C.; Zhuang, X.; Chen, X. Polyion complex micelles with gradient pH-sensitivity for adjustable intracellular drug delivery. Polym. Chem. 2015, 6, 397–405. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.; Radosz, M.; Van Kirk, E.; Murdoch, W.J. pH-responsive nanoparticles for cancer drug delivery. Drug Deliv. Syst. 2008, 437, 183–216. [Google Scholar]
- Wang, C.; Cheng, L.; Liu, Y.; Wang, X.; Ma, X.; Deng, Z.; Li, Y.; Liu, Z. Imaging-Guided pH-Sensitive Photodynamic Therapy Using Charge Reversible Upconversion Nanoparticles under Near-Infrared Light. Adv. Funct. Mater. 2013, 23, 3077–3086. [Google Scholar] [CrossRef]
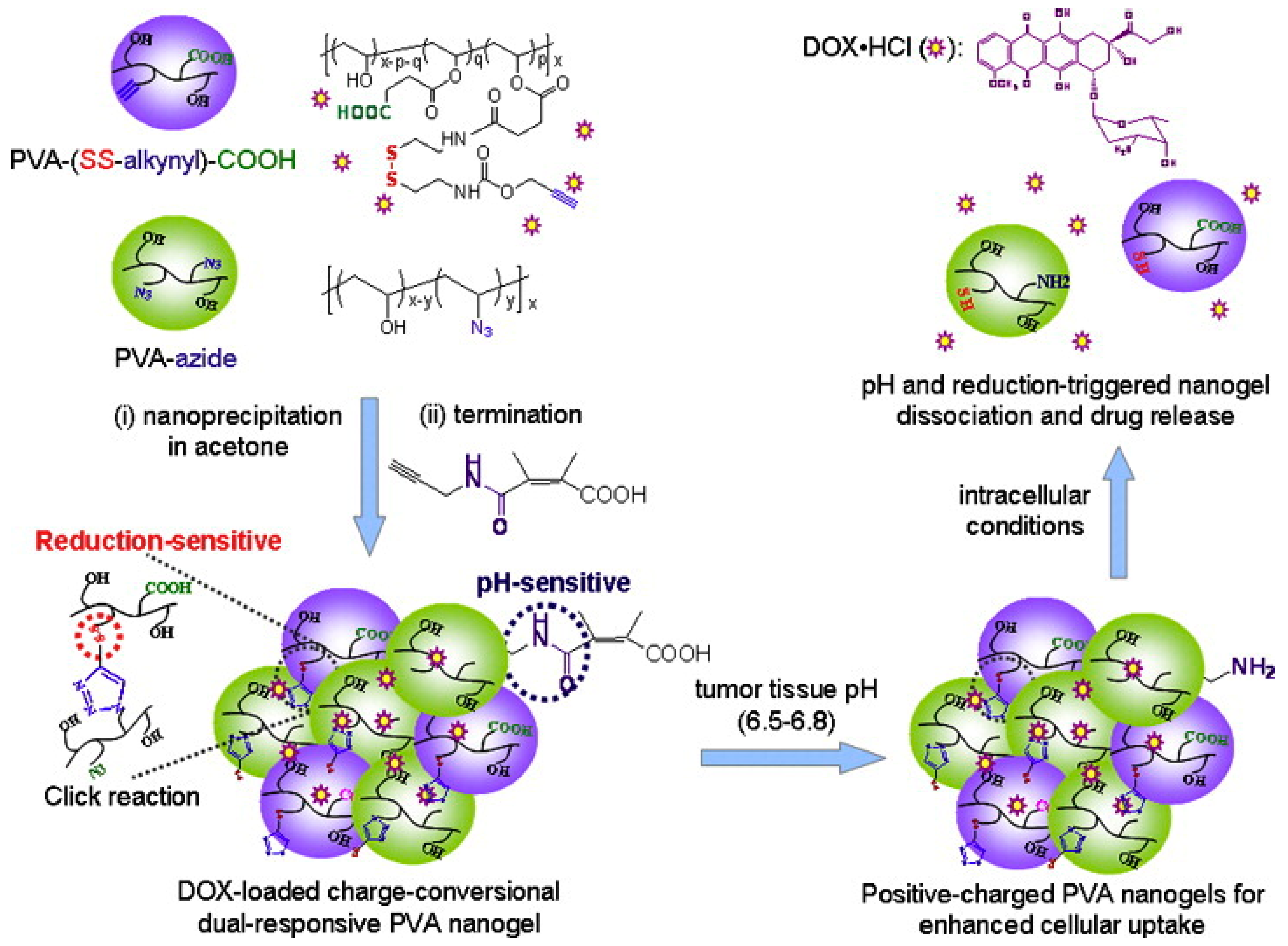
- Chen, W.; Achazi, K.; Schade, B.; Haag, R. Charge-conversional and reduction-sensitive poly(vinyl alcohol) nanogels for enhanced cell uptake and efficient intracellular doxorubicin release. J. Control. Release 2015, 205, 15–24. [Google Scholar] [CrossRef]
- Sun, T.-M.; Du, J.-Z.; Yan, L.-F.; Mao, H.-Q.; Wang, J. Self-assembled biodegradable micellar nanoparticles of amphiphilic and cationic block copolymer for siRNA delivery. Biomaterials 2008, 29, 4348–4355. [Google Scholar] [CrossRef]
- Jing-Jing, Z.; Xiao-Jie, C.; Wen-Dong, Y.; Ying-Hui, W.; Hang-Sheng, Z.; Hong-Yue, Z.; Zhi-Hong, Z.; Bin-Hui, W.; Fan-Zhu, L. Fabrication of A Folic Acid-Modified Arsenic Trioxide Prodrug Liposome and Assessment of its Anti-Hepatocellular Carcinoma Activity. Digit. Chin. Med. 2020, 3, 260–274. [Google Scholar] [CrossRef]
- Bugno, J.; Hsu, H.-J.; Pearson, R.M.; Noh, H.; Hong, S. Size and Surface Charge of Engineered Poly(amidoamine) Dendrimers Modulate Tumor Accumulation and Penetration: A Model Study Using Multicellular Tumor Spheroids. Mol. Pharm. 2016, 13, 2155–2163. [Google Scholar] [CrossRef]
- Wang, G.H.; Chen, H.; Cai, Y.Y.; Li, L.; Yang, H.K.; Li, Q.; He, Z.J.; Lin, J.T. Efficient gene vector with size changeable and nucleus targeting in cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 568–575. [Google Scholar] [CrossRef]
- Dutta, K.; Das, R.; Medeiros, J.; Kanjilal, P.; Thayumanavan, S. Charge-Conversion Strategies for Nucleic Acid Delivery. Adv. Funct. Mater. 2021, 31, 2011103. [Google Scholar] [CrossRef]
- Tang, S.; Meng, Q.S.; Sun, H.P.; Su, J.H.; Yin, Q.; Zhang, Z.W.; Yu, H.J.; Chen, L.L.; Gu, W.W.; Li, Y.P. Dual pH-sensitive micelles with charge-switch for controlling cellular uptake and drug release to treat metastatic breast cancer. Biomaterials 2017, 114, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Pan, Q.; Gao, W.; Pu, Y.; Luo, K.; He, B. Correction to “Reversing Chemotherapy Resistance by a Synergy between Lysosomal pH-Activated Mitochondrial Drug Delivery and Erlotinib-Mediated Drug Efflux Inhibition”. ACS Appl. Mater. Interfaces 2022, 14, 19077. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.C.; Lin, H.C.; Zhang, W.C.; Tang, S.J.; Liu, D.J.; Cai, J.F. Poly(L-ornithine)-Grafted Zinc Phthalocyanines as Dual-Functional Antimicrobial Agents with Intrinsic Membrane Damage and Photothermal Ablation Capacity. ACS Infect. Dis. 2021, 7, 2917–2929. [Google Scholar] [CrossRef] [PubMed]
- Tangsangasaksri, M.; Takemoto, H.; Naito, M.; Maeda, Y.; Sueyoshi, D.; Kim, H.J.; Miura, Y.; Ahn, J.; Azuma, R.; Nishiyama, N.; et al. siRNA-Loaded Polyion Complex Micelle Decorated with Charge-Conversional Polymer Tuned to Undergo Stepwise Response to Intra-Tumoral and Intra-Endosomal pHs for Exerting Enhanced RNAi Efficacy. Biomacromolecules 2016, 17, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Du, J.-Z.; Li, H.-J.; Wang, J. Tumor-Acidity-Cleavable Maleic Acid Amide (TACMAA): A Powerful Tool for Designing Smart Nanoparticles to Overcome Delivery Barriers in Cancer Nanomedicine. Acc. Chem. Res. 2018, 51, 2848–2856. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Li, C.; Shen, X. Charge-reversal nanocarriers: An emerging paradigm for smart cancer nanomedicine. J. Control. Release 2020, 319, 46–62. [Google Scholar] [CrossRef]
- Kunzmann, A.; Andersson, B.; Thurnherr, T.; Krug, H.; Scheynius, A.; Fadeel, B. Toxicology of engineered nanomaterials: Focus on biocompatibility, biodistribution and biodegradation. Biochim. Biophys. Acta 2011, 1810, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.J.; Lloyd, G.J. Structure and efficiency in intramolecular and enzymic catalysis: Intramolecular general base catalysis. Hydrolysis of monoaryl malonates. J. Chem. Soc. Perkin Trans. 1976, 8, 1753–1761. [Google Scholar] [CrossRef]
- Han, K.; Zhang, W.-Y.; Zhang, J.; Lei, Q.; Wang, S.-B.; Liu, J.-W.; Zhang, X.-Z.; Han, H.-Y. Acidity-Triggered Tumor-Targeted Chimeric Peptide for Enhanced Intra-Nuclear Photodynamic Therapy. Adv. Funct. Mater. 2016, 26, 4351–4361. [Google Scholar] [CrossRef]
- Zhou, Q.; Hou, Y.; Zhang, L.; Wang, J.; Qiao, Y.; Guo, S.; Fan, L.; Yang, T.; Zhu, L.; Wu, H. Dual-pH Sensitive Charge-reversal Nanocomplex for Tumor-targeted Drug Delivery with Enhanced Anticancer Activity. Theranostics 2017, 7, 1806–1819. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, R.; Li, Y.; Luo, X.; Hua, Z.; Wang, X.; Xue, Z.; Zhang, Z.; Lu, C.; Lu, A.; et al. MTX-PEG-modified CG/DMMA polymeric micelles for targeted delivery of doxorubicin to induce synergistic autophagic death against triple-negative breast cancer. Breast Cancer Res. BCR 2023, 25, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, D.; Liu, J.; Feng, B.; Zhou, F.; Zhang, H.; Zhou, L.; Yin, Q.; Zhang, Z.; Cao, Z.; et al. Acidity-Triggered Ligand-Presenting Nanoparticles To Overcome Sequential Drug Delivery Barriers to Tumors. Nano Lett. 2017, 17, 5429–5436. [Google Scholar] [CrossRef]
- El-Sawy, H.S.; Al-Abd, A.M.; Ahmed, T.A.; El-Say, K.M.; Torchilin, V.P. Stimuli-Responsive Nano-Architecture Drug-Delivery Systems to Solid Tumor Micromilieu: Past, Present, and Future Perspectives. ACS Nano 2018, 12, 10636–10664. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, L.; Lu, J.; Deng, X.; Wu, Y. Tumor Acidity-Induced Sheddable Polyethylenimine-Poly(trimethylene carbonate)/DNA/Polyethylene Glycol-2,3-Dimethylmaleicanhydride Ternary Complex for Efficient and Safe Gene Delivery. ACS Appl. Mater 2016, 8, 6400–6410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Shen, Y.; Tang, J.; Fan, M.; Van Kirk, E.A.; Murdoch, W.J.; Radosz, M. Charge-Reversal Drug Conjugate for Targeted Cancer Cell Nuclear Drug Delivery. Adv. Funct. Mater. 2009, 19, 3580–3589. [Google Scholar] [CrossRef]
- Zhou, Q.; Shao, S.; Wang, J.; Xu, C.; Xiang, J.; Piao, Y.; Zhou, Z.; Yu, Q.; Tang, J.; Liu, X.; et al. Enzyme-activatable polymer–drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 2019, 14, 799–809. [Google Scholar] [CrossRef]
- Mikhail, A.S.; Allen, C. Block copolymer micelles for delivery of cancer therapy: Transport at the whole body, tissue and cellular levels. J. Control. Release 2009, 138, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Z.; Nie, X.; Zeng, W.; Zhang, Y.; Deng, Y.; Chen, H.; Zeng, X.; Ma, H.; Zheng, Y.; et al. pH-Sensitive and Charge-Reversal Polymeric Nanoplatform Enhanced Photothermal/Photodynamic Synergistic Therapy for Breast Cancer. Front. Biomed. Biotechnol. 2022, 10, 836468. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; He, C.; Chu, X.; Chu, Y.; Ding, Y. Charge-reversal ZnO-based nanospheres for stimuli-responsive release of multiple agents towards synergistic cancer therapy. Chem. Eng. J. 2020, 395, 125177. [Google Scholar] [CrossRef]
- Feng, T.; Ai, X.; An, G.; Yang, P.; Zhao, Y. Charge-Convertible Carbon Dots for Imaging-Guided Drug Delivery with Enhanced in Vivo Cancer Therapeutic Efficiency. ACS Nano 2016, 10, 4410–4420. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zeng, Y.; Ruan, H.; Zhang, Z.; Gong, T.; Sun, X. Ternary Nanoparticles with a Sheddable Shell Efficiently Deliver MicroRNA-34a against CD44-Positive Melanoma. Mol. Pharm. 2017, 14, 3152–3163. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-L.; Li, F.; Tang, Y.; Yang, S.-D.; Li, J.-Z.; Yuan, Z.-Q.; Liu, Y.; Zhou, X.-F.; Liu, C.; Zhang, X.-N. Stepwise pH-responsive nanoparticles for enhanced cellular uptake and on-demand intracellular release of doxorubicin. Int. J. Nanomed. 2017, 12, 4241–4256. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, P.; Li, C.; Guo, Y.; Sun, K. In vitro/vivo antitumor study of modified-chitosan/carboxymethyl chitosan “boosted” charge-reversal nanoformulation. Carbohydr. Polym. 2021, 269, 118268. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wei, X.; Jing, Y.; Zhou, S. Size Changeable Nanocarriers with Nuclear Targeting for Effectively Overcoming Multidrug Resistance in Cancer Therapy. Adv. Mater. 2015, 27, 6450–6456. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Peng, F.; Shi, X.; Leong, D.T. Targeting Endothelial Cell Junctions with Negatively Charged Gold Nanoparticles. Chem. Mater. 2018, 30, 3759–3767. [Google Scholar] [CrossRef]
- Kandula, S.; Singh, P.K.; Kaur, G.A.; Tiwari, A. Trends in smart drug delivery systems for targeting cancer cells. Mater. Sci. Eng. B 2023, 297, 116816. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, D.; Wu, Y.; Liu, Y.; Liu, Y.; Pan, J. Advances in 2,3-Dimethylmaleic Anhydride (DMMA)-Modified Nanocarriers in Drug Delivery Systems. Pharmaceutics 2024, 16, 809. https://doi.org/10.3390/pharmaceutics16060809
Wan D, Wu Y, Liu Y, Liu Y, Pan J. Advances in 2,3-Dimethylmaleic Anhydride (DMMA)-Modified Nanocarriers in Drug Delivery Systems. Pharmaceutics. 2024; 16(6):809. https://doi.org/10.3390/pharmaceutics16060809
Chicago/Turabian StyleWan, Dong, Yanan Wu, Yujun Liu, Yonghui Liu, and Jie Pan. 2024. "Advances in 2,3-Dimethylmaleic Anhydride (DMMA)-Modified Nanocarriers in Drug Delivery Systems" Pharmaceutics 16, no. 6: 809. https://doi.org/10.3390/pharmaceutics16060809




