Development and Evaluation of Docetaxel-Loaded Nanostructured Lipid Carriers for Skin Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
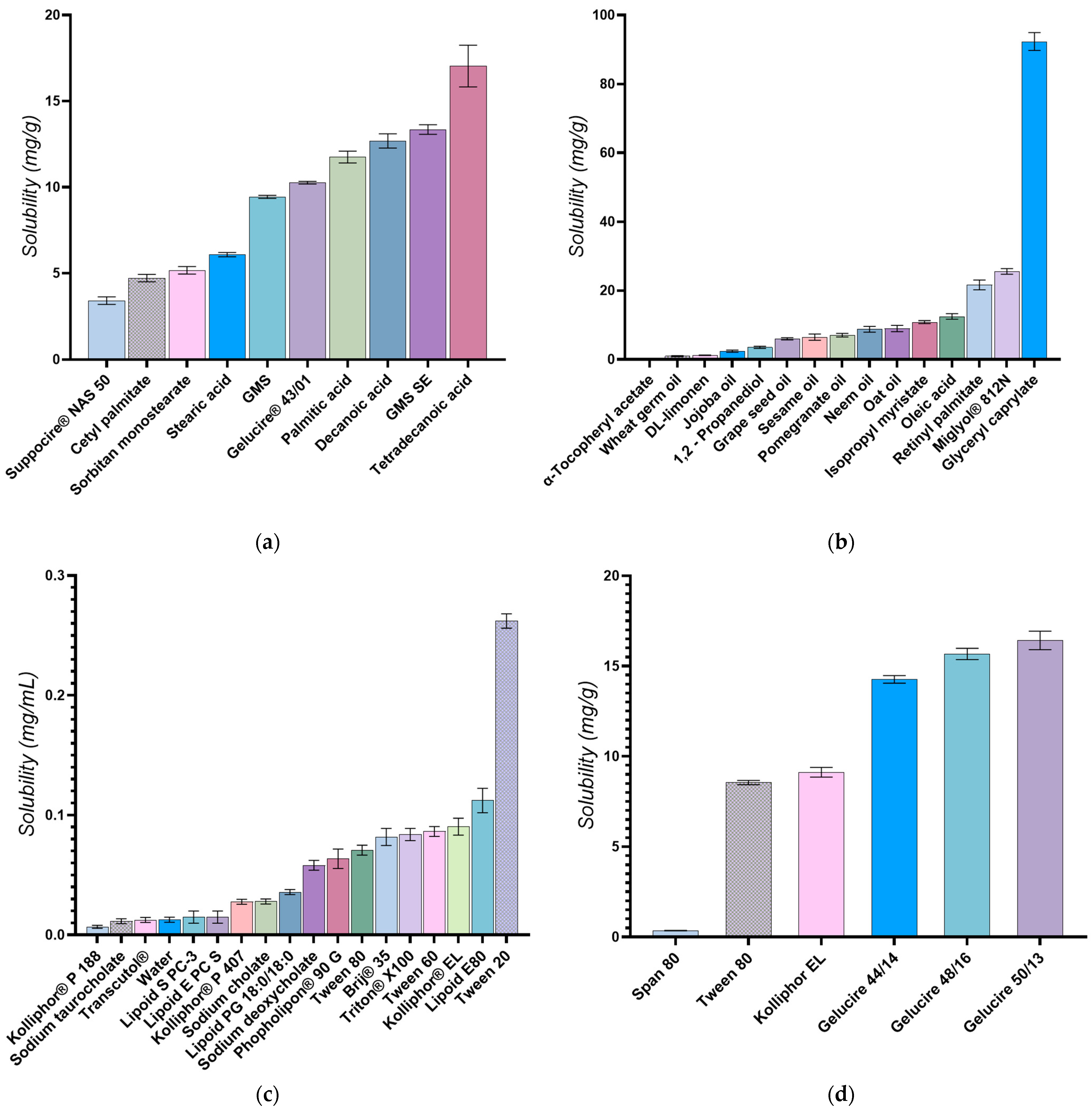
2.2. Selection of Lipids and Surfactants
2.2.1. Selection of the Liquid Lipids
2.2.2. Selection of the Solid Lipids
2.2.3. Selection of Surfactants
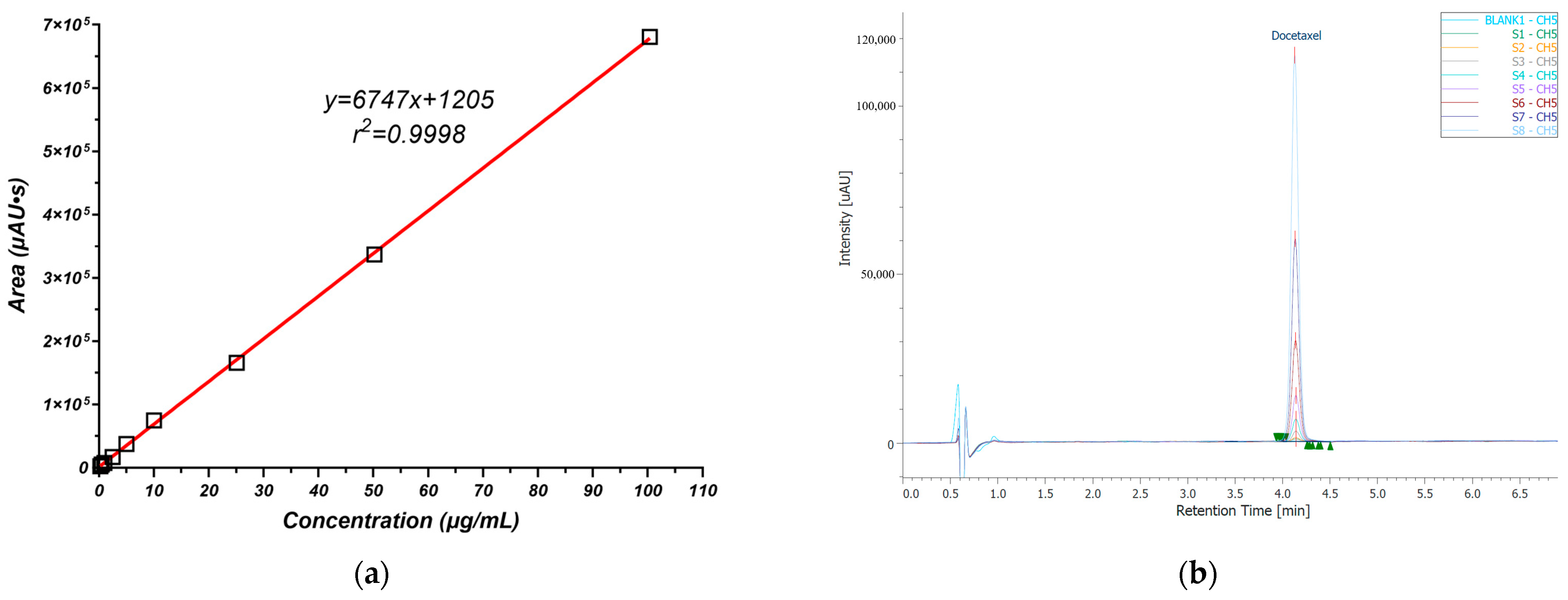
2.2.4. Docetaxel Quantitative Analysis
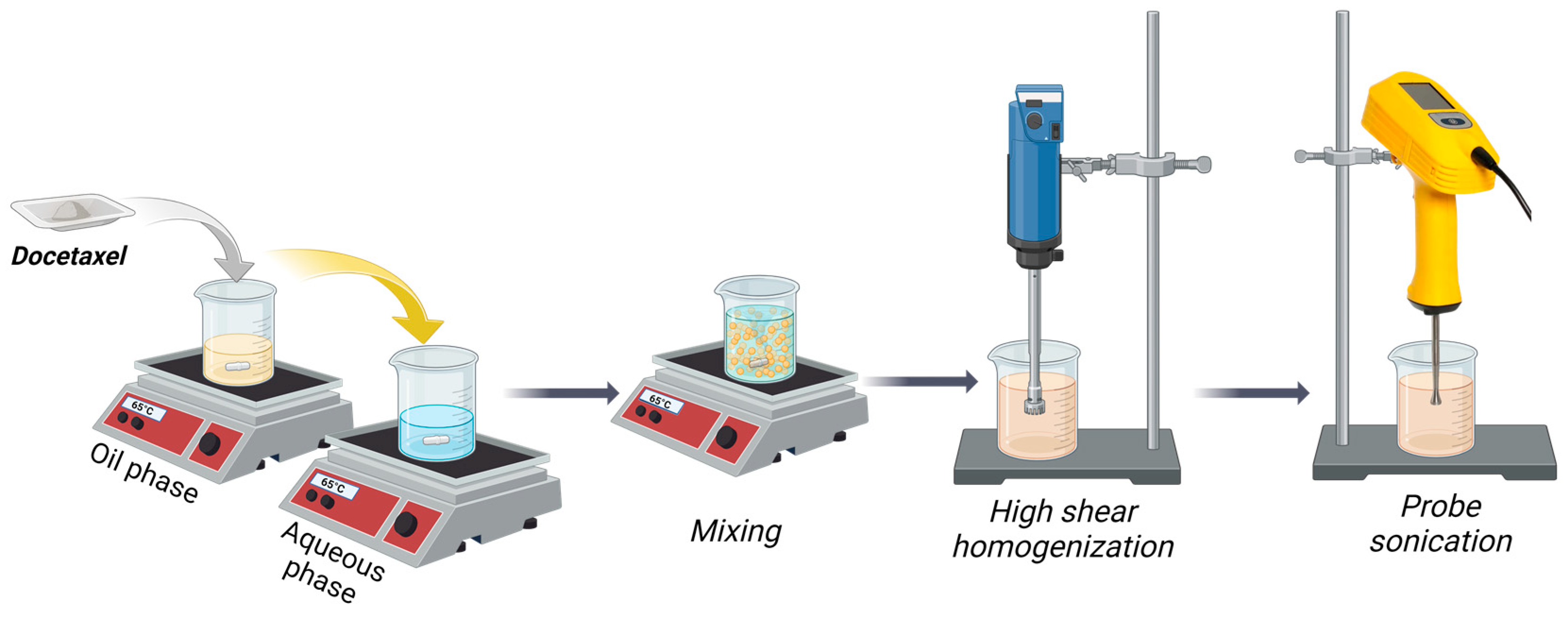
2.3. Preparation of Docetaxel-Loaded NLCs
2.4. Experimental Design
2.5. NLC Characterization
2.5.1. Nanoparticle Size and Polydispersity Index (PDI) by Dynamic Light Scattering (DLS)
2.5.2. Zeta Potential Determination
2.5.3. Entrapment Efficiency
2.5.4. In Vitro Release of NLC-DTX
2.5.5. Scanning Electron Microscopy (SEM)
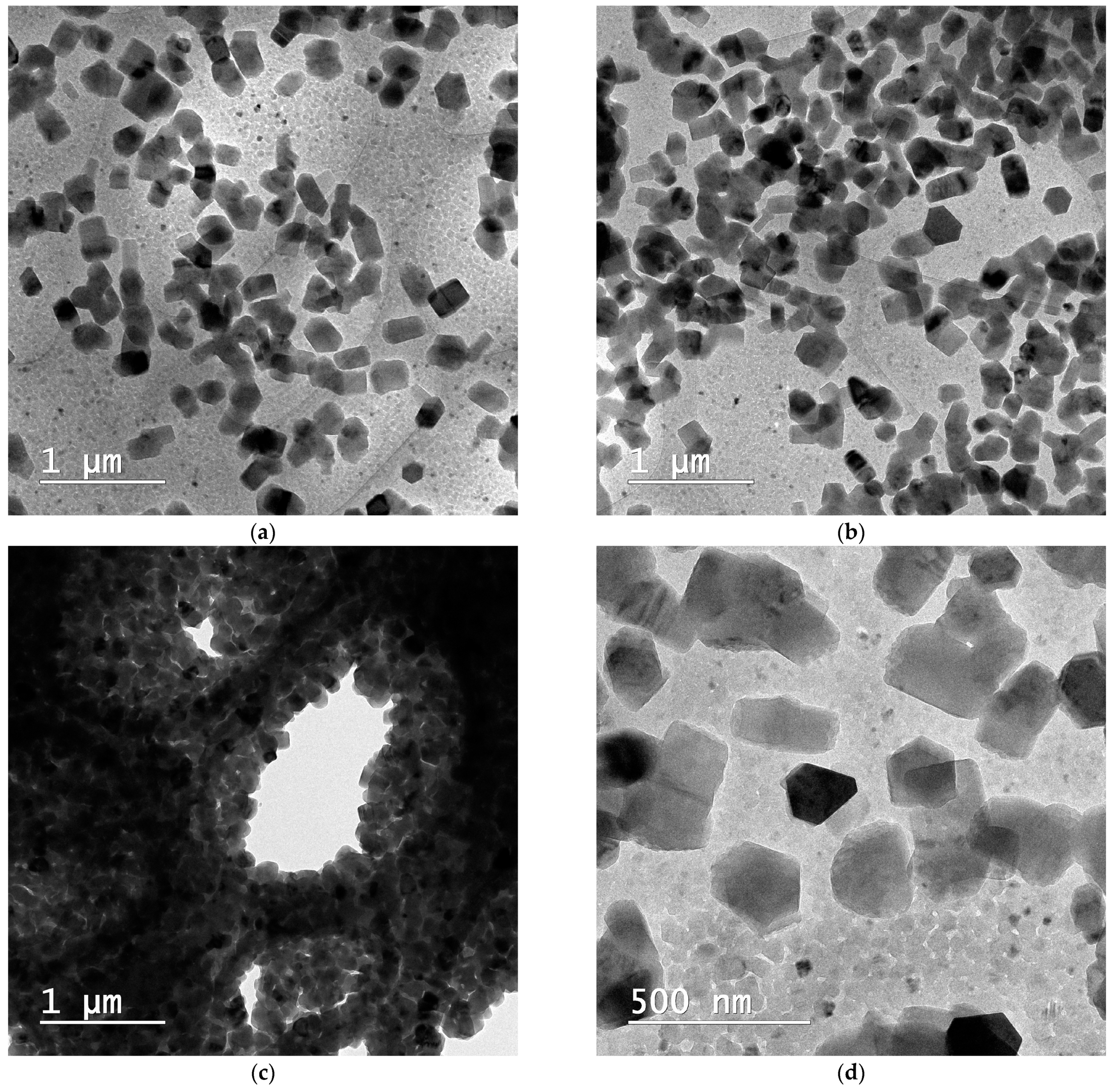
2.5.6. Cryo-Transmission Electron Microscopy (Cryo-TEM)
2.5.7. X-ray Diffraction
2.5.8. Thermal Analysis
2.6. In Vitro Biological Testing
2.6.1. Cell Culture and Treatments
2.6.2. Cytotoxicity Assay
2.6.3. Apoptosis Analysis
2.6.4. Cell Cycle Analysis
3. Results and Discussion
3.1. Docetaxel Quantitative Analysis
3.2. Selection of Lipids and Surfactants
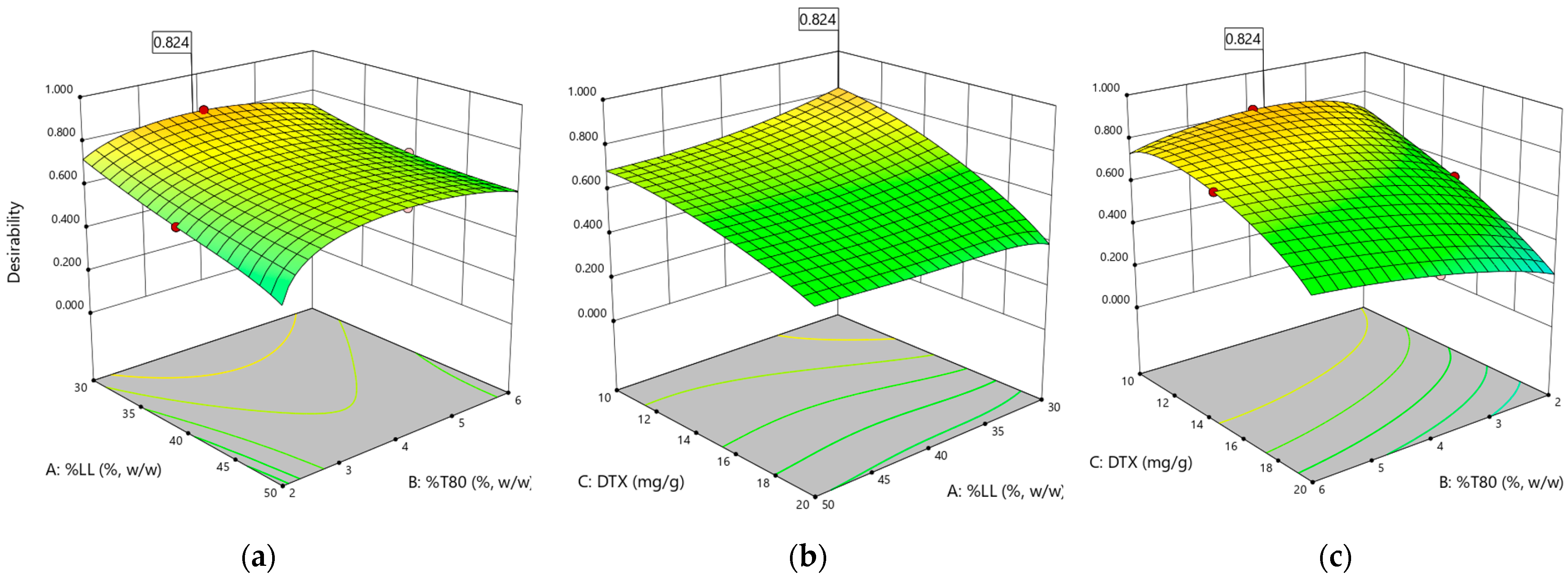
3.3. Experimental Design
| Size (nm) = 193.3 + 21.81*A − 67.36*B + 48.74*B2 |
| PDI = 0.2146 + 0.0221*B + 0.0033*C + −0.0509*BC + 0.0726*C2 |
| ZP (mV) = −24.15 + 2.64*A + 2.74*B + 2.95*C + 2.80*C2 |
| Size SS (nm) =283.09 − 45.61*A + 38.71*C − 44.23*AC |
| Released 24 h (%) =36.82 − 0.56*A + 4.43*B − 5.14*C + 5.57*BC + 5.06*A2 |
3.4. Characterization of the Optimized NLC Formulation
3.4.1. Size, PDI, Zeta Potential, and Entrapment Efficiency
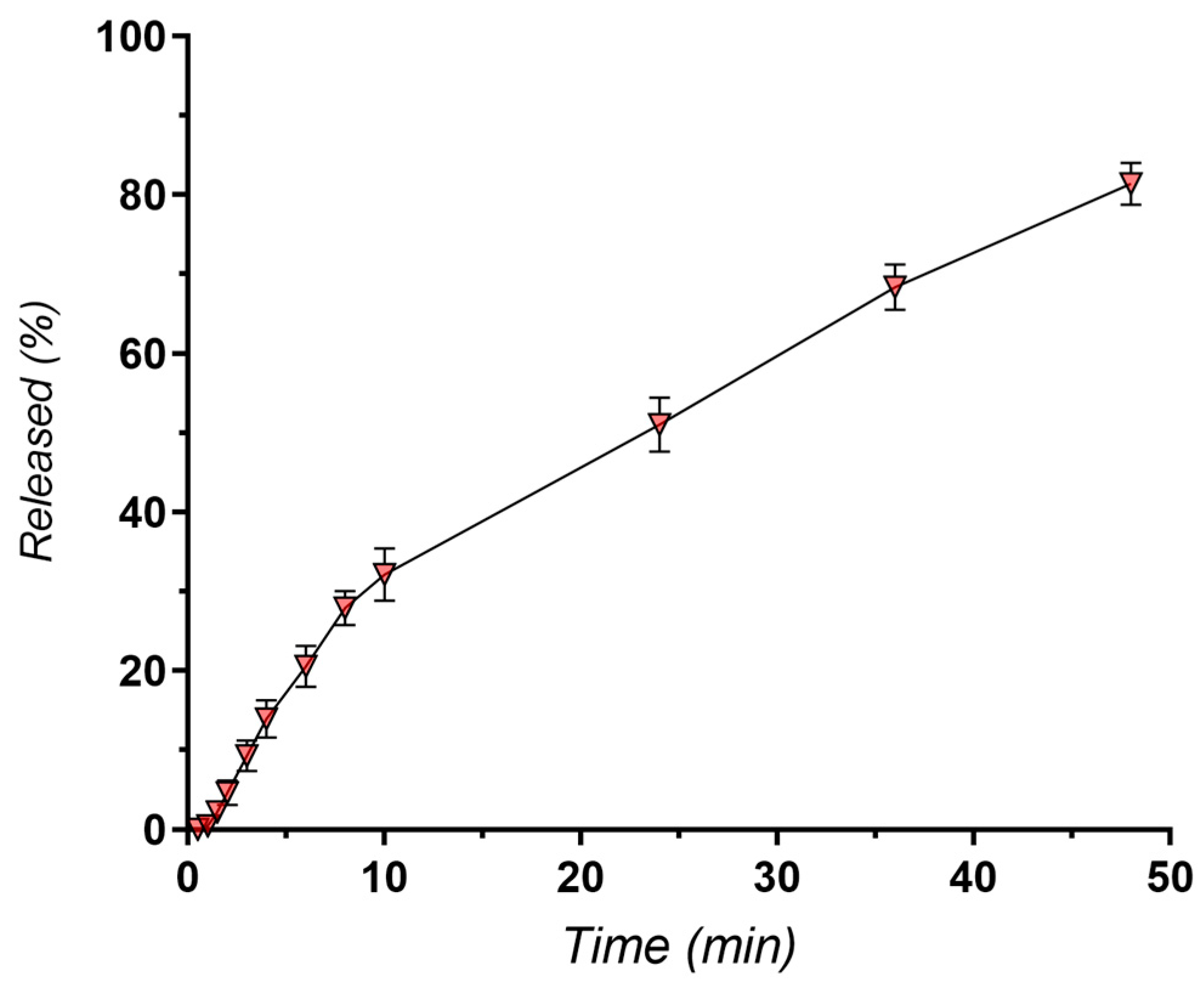
3.4.2. In Vitro Release Kinetics
3.4.3. Scanning Electron Microscopy (SEM)
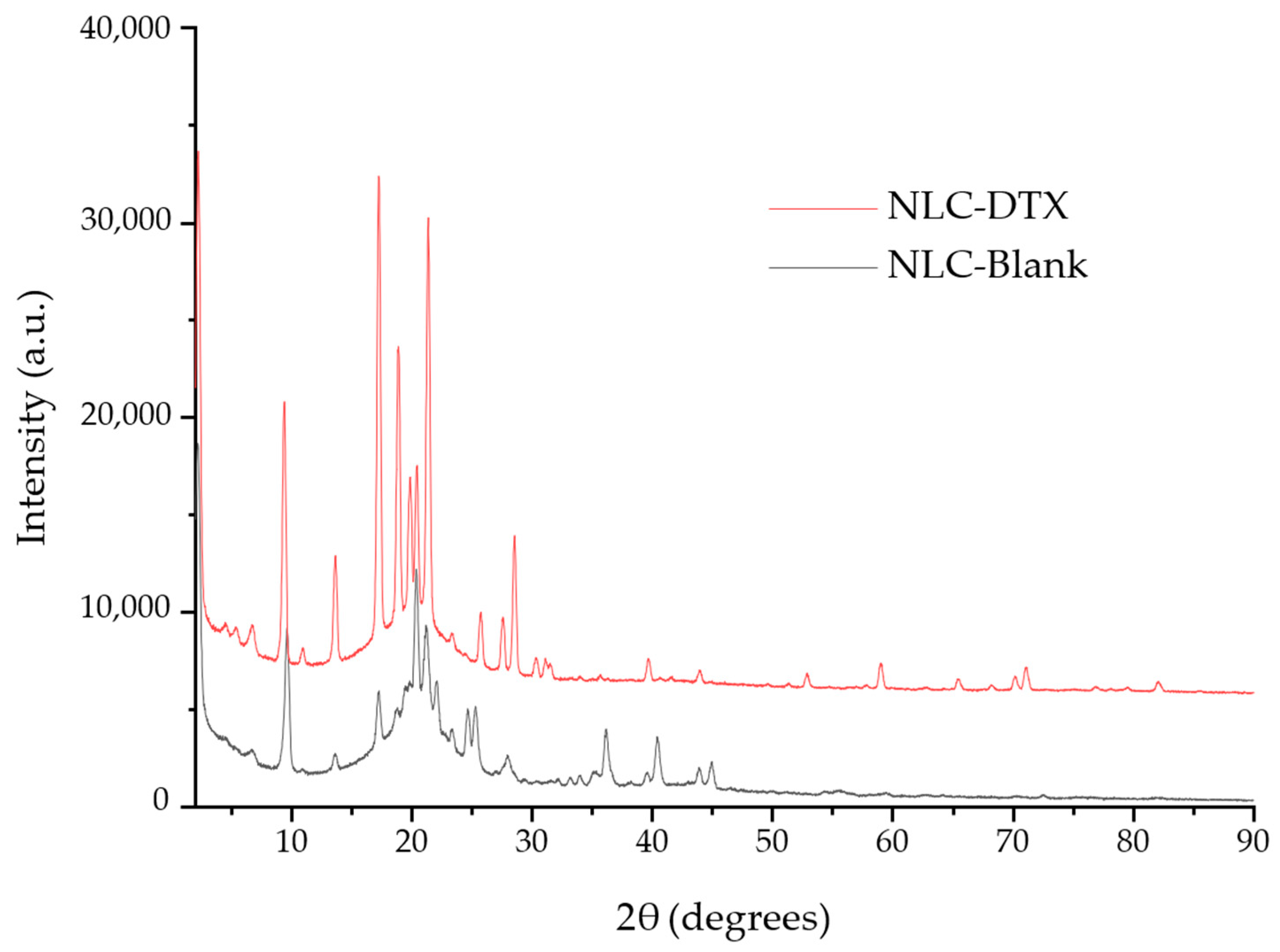
3.4.4. NLC Nanostructure Evaluation through Combined XRD and Cryo-TEM Analysis
3.4.5. Thermal Analysis
3.5. In Vitro Biological Testing
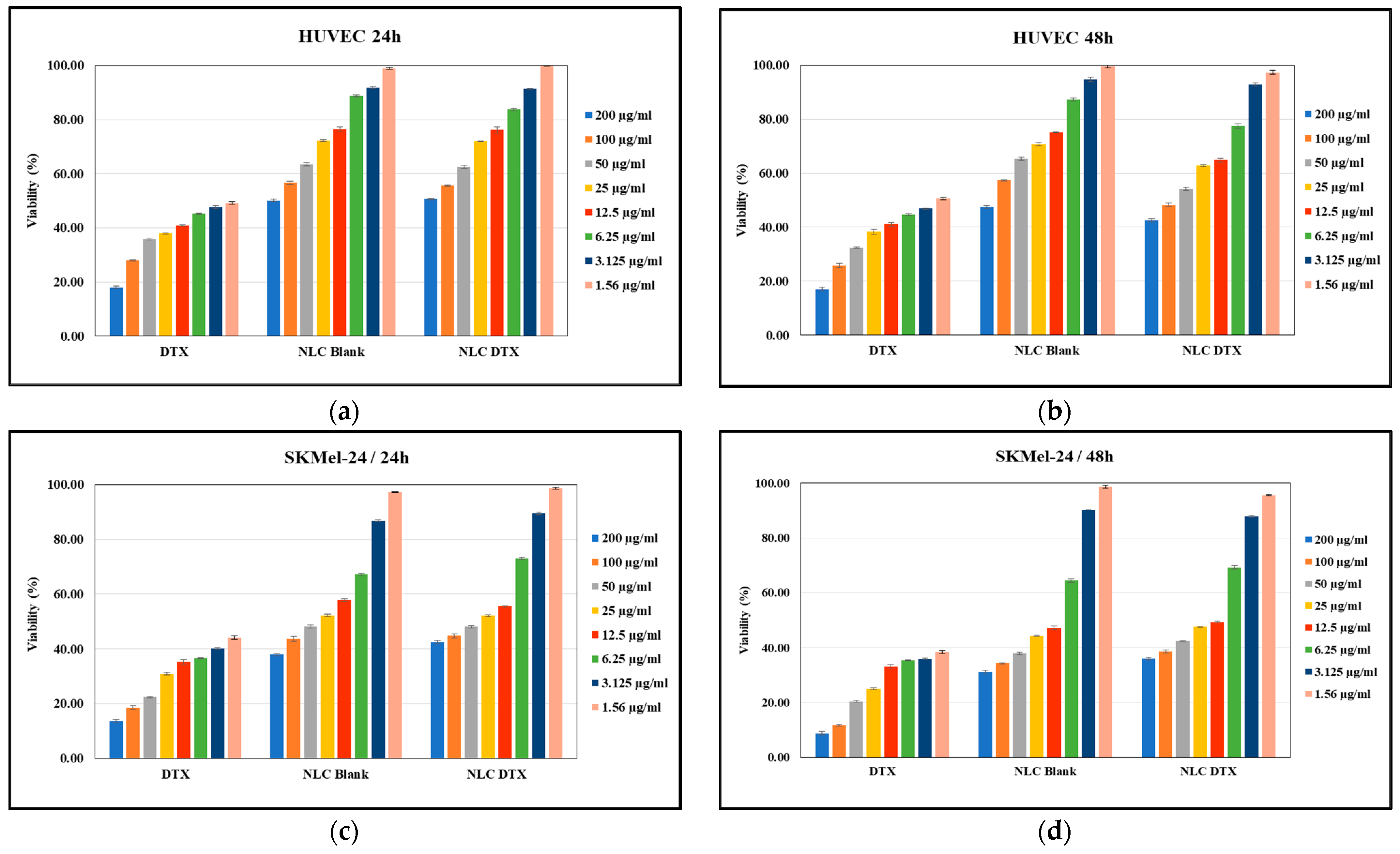
3.5.1. Cell Viability Assay
3.5.2. Apoptotic Process Induced by the Analyzed Formulations
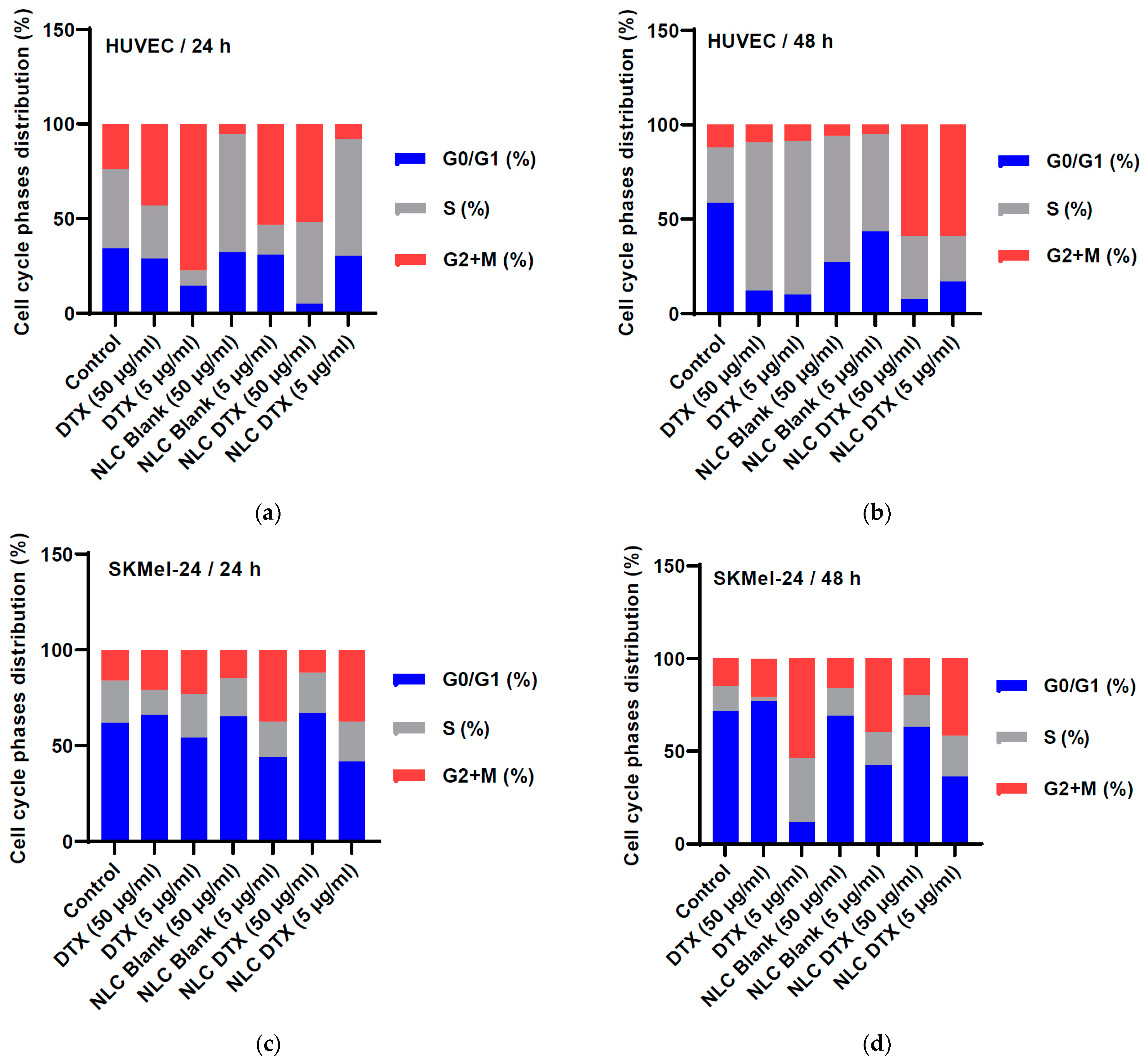
3.5.3. Cell Cycle Phases
3.6. Clinical and Industrial Translation Aspects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.; Sharma, A.; Jain, V. An Overview of Nanostructured Lipid Carriers and Its Application in Drug Delivery through Different Routes. Adv. Pharm. Bull. 2023, 13, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Lancelot, A.; Sierra, T.; Serrano, J.L. Nanostructured Liquid-Crystalline Particles for Drug Delivery. Expert Opin. Drug. Deliv. 2014, 11, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.C.; Cai, Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Z.; Wang, L.; Zhang, C.; Zhang, N. Nanostructured Lipid Carriers as Novel Carrier for Parenteral Delivery of Docetaxel. Colloids Surf. B Biointerfaces 2011, 85, 262–269. [Google Scholar] [CrossRef] [PubMed]
- de Moura, L.D.; Ribeiro, L.N.M.; de Carvalho, F.V.; Rodrigues da Silva, G.H.; Lima Fernandes, P.C.; Brunetto, S.Q.; Ramos, C.D.; Velloso, L.A.; de Araújo, D.R.; de Paula, E. Docetaxel and Lidocaine Co-Loaded (Nlc-in-Hydrogel) Hybrid System Designed for the Treatment of Melanoma. Pharmaceutics 2021, 13, 1552. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations Based on Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for Cutaneous Use: A Review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid Nanoparticles (SLN, NLC) in Cosmetic and Pharmaceutical Dermal Products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Zhao, L.; Almásy, L.; Garamus, V.M.; Willumeit, R.; Zou, A. Preparation and Characterization of a Nanostructured Lipid Carrier for a Poorly Soluble Drug. Colloids Surf. A Physicochem. Eng. Asp. 2014, 455, 36–43. [Google Scholar] [CrossRef]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Müller, R.H. Development of a Controlled Release Formulation Based on SLN and NLC for Topical Clotrimazole Delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Sharma, S.; Rawal, S.; Patel, B.; Patel, M.M. Fabrication, Optimization, and in Vitro Evaluation of Docetaxel-Loaded Nanostructured Lipid Carriers for Improved Anticancer Activity. J. Liposome Res. 2020, 30, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Li, S.; Yin, R.; Liu, H.; Xu, L. Effect of Surfactants on the Formation and Characterization of a New Type of Colloidal Drug Delivery System: Nanostructured Lipid Carriers. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 210–216. [Google Scholar] [CrossRef]
- Elmowafy, M.; Ibrahim, H.M.; Ahmed, M.A.; Shalaby, K.; Salama, A.; Hefesha, H. Atorvastatin-Loaded Nanostructured Lipid Carriers (Nlcs): Strategy to Overcome Oral Delivery Drawbacks. Drug Deliv. 2017, 24, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, E.; Demir, E.S.; Ekinci, M.; Ozgenc, E.; Ilem-Ozdemir, D.; Senyigit, Z.; Gonzalez-Alvarez, I.; Bermejo, M. An Innovative Formulation Based on Nanostructured Lipid Carriers for Imatinib Delivery: Pre-Formulation, Cellular Uptake and Cytotoxicity Studies. Nanomaterials 2022, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Kharkar, P.B.; Talkar, S.S.; Patravale, V.B. An Industrially Viable Technique for Fabrication of Docetaxel NLCs for Oncotherapy. Int. J. Pharm. 2020, 577, 119028. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Shao, J.; Tan, B.; Guan, S.; Liu, Z.; Zhao, Z.; He, F.; Zhao, J. Targeted Lung Cancer Therapy: Preparation and Optimization of Transferrin-Decorated Nanostructured Lipid Carriers as Novel Nanomedicine for Co-Delivery of Anticancer Drugs and DNA. Int. J. Nanomed. 2015, 10, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Emami, J.; Rezazadeh, M.; Varshosaz, J.; Tabbakhian, M.; Aslani, A. Formulation of LDL Targeted Nanostructured Lipid Carriers Loaded with Paclitaxel: A Detailed Study of Preparation, Freeze Drying Condition, and in Vitro Cytotoxicity. J. Nanomater. 2012, 2012, 358782. [Google Scholar] [CrossRef]
- Zhao, C.; Fan, T.; Yang, Y.; Wu, M.; Li, L.; Zhou, Z.; Jian, Y.; Zhang, Q.; Huang, Y. Preparation, Macrophages Targeting Delivery and Anti-Inflammatory Study of Pentapeptide Grafted Nanostructured Lipid Carriers. Int. J. Pharm. 2013, 450, 11–20. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Qiao, H.; Ni, J.M.; Shi, Y.B.; Qiang, Y. Preparation of Isoliquiritigenin-Loaded Nanostructured Lipid Carrier and the in Vivo Evaluation in Tumor-Bearing Mice. Eur. J. Pharm. Sci. 2013, 49, 411–422. [Google Scholar] [CrossRef]
- Trucillo, P.; Cardea, S.; Baldino, L.; Reverchon, E. Production of Liposomes Loaded Alginate Aerogels Using Two Supercritical CO2 Assisted Techniques. J. CO2 Util. 2020, 39, 101161. [Google Scholar] [CrossRef]
- Arjunan, N.; Thiruvengadam, V.; Sushil, S.N. Nanoparticle-Mediated DsRNA Delivery for Precision Insect Pest Control: A Comprehensive Review. Mol. Biol. Rep. 2024, 51, 355. [Google Scholar] [CrossRef]
- Jain, A.; Mehra, N.K.; Nahar, M.; Jain, N.K. Topical Delivery of Enoxaparin Using Nanostructured Lipid Carrier. J. Microencapsul. 2013, 30, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Wu, C.T. Optimization of Nanostructured Lipid Carriers for Lutein Delivery. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 149–156. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, D.; Dong, X.; Sun, H.; Song, C.; Wang, C.; Kong, D. Folate-Modified Lipid–Polymer Hybrid Nanoparticles for Targeted Paclitaxel Delivery. Int. J. Nanomed. 2015, 10, 2101–2114. [Google Scholar] [CrossRef]
- Colombo, S.; Cun, D.; Remaut, K.; Bunker, M.; Zhang, J.; Martin-Bertelsen, B.; Yaghmur, A.; Braeckmans, K.; Nielsen, H.M.; Foged, C. Mechanistic Profiling of the SiRNA Delivery Dynamics of Lipid-Polymer Hybrid Nanoparticles. J. Control. Release 2015, 201, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Gu, X.; Bai, H.; Yang, R.H.; Dong, C.D.; Liu, J.P. Nanostructured Lipid Carriers Constituted from High-Density Lipoprotein Components for Delivery of a Lipophilic Cardiovascular Drug. Int. J. Pharm. 2010, 391, 313–321. [Google Scholar] [CrossRef]
- Mandal, B.; Mittal, N.K.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Development and in Vitro Evaluation of Core-Shell Type Lipid-Polymer Hybrid Nanoparticles for the Delivery of Erlotinib in Non-Small Cell Lung Cancer. Eur. J. Pharm. Sci. 2016, 81, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Al-Sanea, M.M. Nanostructured Lipid Carriers (NLCs) as Drug Delivery Platform: Advances in Formulation and Delivery Strategies. Saudi Pharm. J. 2021, 29, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Salvi, V.R.; Pawar, P. Nanostructured Lipid Carriers (NLC) System: A Novel Drug Targeting Carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- Gorle, A.; Pawar, T.; Mahhirao, J. Design, Development and Characterization of Nanostructure Lipid Carriers (NLCs) by HPH Method Loaded with Anticancer Drug. J. Drug Deliv. Ther. 2023, 13, 58–69. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Badran, M.M.; Ali, H.M.; Abdel-Bakky, M.S.; Ibrahim, H.M. Multifunctional Carbamazepine Loaded Nanostructured Lipid Carrier (NLC) Formulation. Int. J. Pharm. 2018, 550, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Urban, K.; Mehrmal, S.; Uppal, P.; Giesey, R.L.; Delost, G.R. The Global Burden of Skin Cancer: A Longitudinal Analysis from the Global Burden of Disease Study, 1990–2017. JAAD Int. 2021, 2, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Rundle, C.W.; Militello, M.; Barber, C.; Presley, C.L.; Rietcheck, H.R.; Dellavalle, R.P. Epidemiologic Burden of Skin Cancer in the US and Worldwide. Curr. Dermatol. Rep. 2020, 9, 309–322. [Google Scholar] [CrossRef]
- Llorente, X.; Esteruelas, G.; Bonilla, L.; Agudelo, M.G.; Filgaira, I.; Lopez-Ramajo, D.; Gong, R.C.; Soler, C.; Espina, M.; García, M.L.; et al. Riluzole-Loaded Nanostructured Lipid Carriers for Hyperproliferative Skin Diseases. Int. J. Mol. Sci. 2023, 24, 8053. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.A.; Pereira, T.A.; Ramos, D.N.; Rezende, L.C.D.; Emery, F.S.; Sobral, L.M.; Leopoldino, A.M.; Lopez, R.F.V. Topical Skin Cancer Therapy Using Doxorubicin-Loaded Cationic Lipid Nanoparticles and Iontophoresis. J. Biomed. Nanotechnol. 2015, 11, 1975–1988. [Google Scholar] [CrossRef] [PubMed]
- Venâncio, J.H.; Andrade, L.M.; Esteves, N.L.S.; Brito, L.B.; Valadares, M.C.; Oliveira, G.A.R.; Lima, E.M.; Marreto, R.N.; Gratieri, T.; Taveira, S.F. Topotecan-Loaded Lipid Nanoparticles as a Viable Tool for the Topical Treatment of Skin Cancers. J. Pharm. Pharmacol. 2017, 69, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, M.C.O.; Da Silva, P.B.; Radicchi, M.A.; Andrade, B.Y.G.; De Oliveira, J.V.; Venus, T.; Merker, C.; Estrela-Lopis, I.; Longo, J.P.F.; Báo, S.N. Docetaxel-Loaded Solid Lipid Nanoparticles Prevent Tumor Growth and Lung Metastasis of 4T1 Murine Mammary Carcinoma Cells. J. Nanobiotechnol. 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Park, J.W.; Yoon, I.S.; Kim, D.D. Surface-Modified Solid Lipid Nanoparticles for Oral Delivery of Docetaxel: Enhanced Intestinal Absorption and Lymphatic Uptake. Int. J. Nanomed. 2014, 9, 495–504. [Google Scholar] [CrossRef]
- Thambiraj, S.; Vijayalakshmi, R.; Ravi Shankaran, D. An Effective Strategy for Development of Docetaxel Encapsulated Gold Nanoformulations for Treatment of Prostate Cancer. Sci. Rep. 2021, 11, 2808. [Google Scholar] [CrossRef] [PubMed]
- Thambiraj, S.; Shruthi, S.; Vijayalakshmi, R.; Ravi Shankaran, D. Evaluation of Cytotoxic Activity of Docetaxel Loaded Gold Nanoparticles for Lung Cancer Drug Delivery. Cancer Treat. Res. Commun. 2019, 21, 100157. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Tang, B.; Chao, Y.; Xu, H.; Gou, J.; Zhang, Y.; Xu, H.; Tang, X. Cysteine-Functionalized Nanostructured Lipid Carriers for Oral Delivery of Docetaxel: A Permeability and Pharmacokinetic Study. Mol. Pharm. 2015, 12, 2384–2395. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Keum, C.G.; Song, J.Y.; Kim, D.; Cho, C.W. A Novel Aqueous Parenteral Formulation of Docetaxel Using Prodrugs. Int. J. Pharm. 2014, 462, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Engels, F.K.; Mathot, R.A.A.; Verweij, J. Alternative Drug Formulations of Docetaxel: A Review. Anti-Cancer Drugs 2007, 18, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.G.; Kim, D.W.; Yeo, W.H.; Ramasamy, T.; Oh, Y.K.; Park, Y.J.; Kim, J.A.; Oh, D.H.; Ku, S.K.; Kim, J.K.; et al. Docetaxel-Loaded Thermosensitive and Bioadhesive Nanomicelles as a Rectal Drug Delivery System for Enhanced Chemotherapeutic Effect. Pharm. Res. 2013, 30, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- FDA. Taxotere (Docetaxel) Injection Drug Approval Package; FDA: Silver Spring, MD, USA, 2004. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/20-449s028_Taxotere.cfm (accessed on 15 April 2024).
- EMA-Docefrez. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/docefrez-epar-product-information_en.pdf (accessed on 15 April 2024).
- EMA-Taxotere. Docefrez Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/taxotere-epar-product-information_en.pdf (accessed on 15 April 2024).
- Zhang, H.; Dou, J.; Zhai, Y.; Liu, A.; Zhai, G. Advances in the Formulations of Non-Injection Administration of Docetaxel. J. Drug Target. 2014, 22, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Sumera; Anwar, A.; Ovais, M.; Khan, A.; Raza, A. Docetaxel-Loaded Solid Lipid Nanoparticles: A Novel Drug Delivery System. IET Nanobiotechnol. 2017, 11, 621–629. [Google Scholar] [CrossRef]
- Majumdar, A.; Dubey, N.; Dubey, N. Dermal Delivery of Docetaxel Loaded Nano Liquid Crystals for the Treatment of Skin Cancer. Int. J. Appl. Pharm. 2019, 11, 188–193. [Google Scholar] [CrossRef]
- Makoni, P.A.; Ranchhod, J.; WaKasongo, K.; Khamanga, S.M.; Walker, R.B. The Use of Quantitative Analysis and Hansen Solubility Parameter Predictions for the Selection of Excipients for Lipid Nanocarriers to Be Loaded with Water Soluble and Insoluble Compounds. Saudi Pharm. J. 2020, 28, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, N.V.; Khatri, H.N.; Patel, M.M. Formulation, Optimization, and Characterization of Rifampicin-Loaded Solid Lipid Nanoparticles for the Treatment of Tuberculosis. Drug Dev. Ind. Pharm. 2018, 44, 1975–1989. [Google Scholar] [CrossRef] [PubMed]
- Sinhmar, G.K.; Shah, N.N.; Chokshi, N.V.; Khatri, H.N.; Patel, M.M. Process, Optimization, and Characterization of Budesonide-Loaded Nanostructured Lipid Carriers for the Treatment of Inflammatory Bowel Disease. Drug Dev. Ind. Pharm. 2018, 44, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Nerli, G.; Gonçalves, L.M.D.; Cirri, M.; Almeida, A.J.; Maestrelli, F.; Mennini, N.; Mura, P.A. Design, Evaluation and Comparison of Nanostructured Lipid Carriers and Chitosan Nanoparticles as Carriers of Poorly Soluble Drugs to Develop Oral Liquid Formulations Suitable for Pediatric Use. Pharmaceutics 2023, 15, 1350. [Google Scholar] [CrossRef] [PubMed]
- ICH-International Conference on Harmonization International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use-ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology Q2(R1) Step 4 Version. 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 12 June 2024).
- Carvalho, F.V.D.; Ribeiro, L.N.D.M.; Moura, L.D.D.; Rodrigues da Silva, G.H.; Mitsutake, H.; Mendonça, T.C.; Geronimo, G.; Breitkreitz, M.C.; de Paula, E. Docetaxel Loaded in Copaiba Oil-Nanostructured Lipid Carriers as a Promising DDS for Breast Cancer Treatment. Molecules 2022, 27, 8838. [Google Scholar] [CrossRef] [PubMed]
- Adame-Campos, R.L.; Ghilardi, A.; Gao, Y.; Paneque-Gálvez, J.; Mas, J.F. Variables Selection for Aboveground Biomass Estimations Using Satellite Data: A Comparison between Relative Importance Approach and Stepwise Akaike’s Information Criterion. ISPRS Int. J. Geoinf. 2019, 8, 245. [Google Scholar] [CrossRef]
- Aubrit, F.; Jacob, D.; Boj, S. Application Note AN11-NIR DLS for Quantum Dots Size Measurement NIR Dynamic Light Scattering for Quantum Dots Size Measurement. Available online: https://www.cordouan-tech.com/wp-content/uploads/2020/03/DLS-applied-to-the-fluorescent-samples-using-NIR-Laser-quantum-dots.pdf (accessed on 16 April 2024).
- Maguire, C.M.; Rösslein, M.; Wick, P.; Prina-Mello, A. Characterisation of Particles in Solution–a Perspective on Light Scattering and Comparative Technologies. Sci. Technol. Adv. Mater. 2018, 19, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Farhadian, N.; Karimi, M.; Ebrahimi, M. Enhanced Bactericidal Effect of Ceftriaxone Drug Encapsulated in Nanostructured Lipid Carrier against Gram-Negative Escherichia Coli Bacteria: Drug Formulation, Optimization, and Cell Culture Study. Antimicrob. Resist. Infect. Control 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Hydrodynamic Diameter and Zeta Potential of Nanostructured Lipid Carriers: Emphasizing Some Parameters for Correct Measurements. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126610. [Google Scholar] [CrossRef]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to Improve the Scientific Value of Zeta-Potential Measurements in NanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Marques, S.S.; Ramos, I.I.; Fernandes, S.R.; Barreiros, L.; Lima, S.A.C.; Reis, S.; Domingues, M.R.M.; Segundo, M.A. Insights on Ultrafiltration-Based Separation for the Purification and Quantification of Methotrexate in Nanocarriers. Molecules 2020, 25, 1879. [Google Scholar] [CrossRef] [PubMed]
- Tupal, A.; Sabzichi, M.; Ramezani, F.; Kouhsoltani, M.; Hamishehkar, H. Dermal Delivery of Doxorubicin-Loaded Solid Lipid Nanoparticles for the Treatment of Skin Cancer. J. Microencapsul. 2016, 33, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, E.J.; Kim, T.W.; Na, D.H. Recent Progress in Drug Release Testing Methods of Biopolymeric Particulate System. Pharmaceutics 2021, 13, 1313. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Wang, X.; Zou, Y.; Wang, Y.; Burgess, D.J. In Vitro Release Testing Method Development for Long-Acting Injectable Suspensions. Int. J. Pharm. 2022, 622, 121840. [Google Scholar] [CrossRef] [PubMed]
- Losito, D.W.; Lopes, P.S.; Ueoka, A.R.; Fantini, M.C.A.; Oseliero Filho, P.L.; Andréo-Filho, N.; Martins, T.S. Biocomposites Based on SBA-15 and Papain: Characterization, Enzymatic Activity and Cytotoxicity Evaluation. Microporous Mesoporous Mater. 2021, 325, 111316. [Google Scholar] [CrossRef]
- Scioli-Montoto, S.; Sbaraglini, M.L.; Cisneros, J.S.; Chain, C.Y.; Ferretti, V.; León, I.E.; Alvarez, V.A.; Castro, G.R.; Islan, G.A.; Talevi, A.; et al. Novel Phenobarbital-Loaded Nanostructured Lipid Carriers for Epilepsy Treatment: From QbD to In Vivo Evaluation. Front. Chem. 2022, 10, 908386. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, C.T.; Sales, C.C.; Andréo-Filho, N.; Martins, T.S.; Ferraz, H.O.; Santos, Y.R.; Lopes, P.S.; Grice, J.E.; Benson, H.A.E.; Leite-Silva, V.R. Development of a Nanotechnology Matrix-Based Citronella Oil Insect Repellent to Obtain a Prolonged Effect and Evaluation of the Safety and Efficacy. Life 2023, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Ivan, B.C.; Barbuceanu, S.F.; Hotnog, C.M.; Anghel, A.I.; Ancuceanu, R.V.; Mihaila, M.A.; Brasoveanu, L.I.; Shova, S.; Draghici, C.; Olaru, O.T.; et al. New Pyrrole Derivatives as Promising Biological Agents: Design, Synthesis, Characterization, In Silico, and Cytotoxicity Evaluation. Int. J. Mol. Sci. 2022, 23, 8854. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, M.; Bostan, M.; Hotnog, D.; Ferdes, M.; Brasoveanu, L.I.; Nicolau, S.S. Real-Time Analysis of Quercetin, Resveratrol and/or Doxorubicin Effects In Mcf-7 Cells. Rom. Biotechnol. Lett. 2013, 18, 8106–8114. [Google Scholar]
- Iancu, I.V.; Botezatu, A.; Plesa, A.; Huica, I.; Fudulu, A.; Albulescu, A.; Bostan, M.; Mihaila, M.; Grancea, C.; Manda, D.A.; et al. Alterations of Regulatory Factors and DNA Methylation Pattern in Thyroid Cancer. Cancer Biomark. 2020, 28, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Hotnog, C.M.; Mihaila, M.; Puiu, L.; Botezatu, A.; Roman, V.; Popescu, I.D.; Bostan, M.; Brasoveanu, L.I. Modulation of the Interplay between P53, ICAM-1 and VEGF in Drug-Treated LoVo Colon Cancer Cells. Rom. Biotechnol. Lett. 2019, 24, 261–270. [Google Scholar] [CrossRef]
- Hotnog, D.; Mihaila, M.; Botezatu, A.; Matei, G.; Hotnog, C.; Anton, G.; Bostan, M.; Brasoveanu, L.I. Genistein Potentiates the Apoptotic Effect of 5-Fluorouracyl in Colon Cancer Cell Lines. Rom. Biotechnol. Lett. 2013, 18, 7151–7160. [Google Scholar]
- Panda, M.; Rao, M.E.B.; Panda, J.; Patra, C.N.; Patro, G. Gelucire: A Flexible Formulation Excipients. Res. J. Pharm. Technol. 2023, 16, 955–961. [Google Scholar] [CrossRef]
- Panigrahi, K.C.; Patra, C.N.; Jena, G.K.; Ghose, D.; Jena, J.; Panda, S.K.; Sahu, M. Gelucire: A Versatile Polymer for Modified Release Drug Delivery System. Futur. J. Pharm. Sci. 2018, 4, 102–108. [Google Scholar] [CrossRef]
- Kumar, A.; Kanwar, R.; Mehta, S.K. Development of Phosphatidylcholine/Tween 80 Based Biocompatible Clove Oil-in-Water Nanoemulsion as a Green Nanocarrier for Controlled Herbicide Delivery. Environ. Pollut. 2022, 293, 118558. [Google Scholar] [CrossRef] [PubMed]
- Karn-Orachai, K.; Smith, S.M.; Phunpee, S.; Treethong, A.; Puttipipatkhachorn, S.; Pratontep, S.; Ruktanonchai, U.R. The Effect of Surfactant Composition on the Chemical and Structural Properties of Nanostructured Lipid Carriers. J. Microencapsul. 2014, 31, 609–618. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Akhter, M.H.; Rizwanullah, M.; Ahmad, J.; Ahsan, M.J.; Mujtaba, M.A.; Amin, S. Nanocarriers in Advanced Drug Targeting: Setting Novel Paradigm in Cancer Therapeutics. Artif. Cells Nanomed. Biotechnol. 2018, 46, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Talianu, M.T.; Dinu-Pîrvu, C.E.; Ghica, M.V.; Anuţa, V.; Prisada, R.M.; Popa, L. Development and Characterization of New Miconazole-Based Microemulsions for Buccal Delivery by Implementing a Full Factorial Design Modeling. Pharmaceutics 2024, 16, 271. [Google Scholar] [CrossRef]
- Tang, X.; Huston, K.J.; Larson, R.G. Molecular Dynamics Simulations of Structure-Property Relationships of Tween 80 Surfactants in Water and at Interfaces. J. Phys. Chem. B 2014, 118, 12907–12918. [Google Scholar] [CrossRef]
- Lúcio, M.; Giannino, N.; Barreira, S.; Catita, J.; Gonçalves, H.; Ribeiro, A.; Fernandes, E.; Carvalho, I.; Pinho, H.; Cerqueira, F.; et al. Nanostructured Lipid Carriers Enriched Hydrogels for Skin Topical Administration of Quercetin and Omega-3 Fatty Acid. Pharmaceutics 2023, 15, 2078. [Google Scholar] [CrossRef]
- Elsewedy, H.S.; Shehata, T.M.; Almostafa, M.M.; Soliman, W.E. Hypolipidemic Activity of Olive Oil-Based Nanostructured Lipid Carrier Containing Atorvastatin. Nanomaterials 2022, 12, 2160. [Google Scholar] [CrossRef]
- Kamel, A.E.; Fadel, M.; Louis, D. Curcumin-Loaded Nanostructured Lipid Carriers Prepared Using PeceolTM and Olive Oil in Photodynamic Therapy: Development and Application in Breast Cancer Cell Line. Int. J. Nanomed. 2019, 14, 5073–5085. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for Characterization of Nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 44–58. ISBN 9780081005637. [Google Scholar]
- Kim, C.H.; Sung, S.W.; Lee, E.S.; Kang, T.H.; Yoon, H.Y.; Goo, Y.T.; Cho, H.R.; Kim, D.Y.; Kang, M.J.; Choi, Y.S.; et al. Sterically Stabilized RIPL Peptide-Conjugated Nanostructured Lipid Carriers: Characterization, Cellular Uptake, Cytotoxicity, and Biodistribution. Pharmaceutics 2018, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lee, S.; Choi, J.Y.; Lyu, M.J.; Jung, H.M.; Goo, Y.T.; Kang, M.J.; Choi, Y.W. Functionalized Lipid Nanocarriers for Simultaneous Delivery of Docetaxel and Tariquidar to Chemoresistant Cancer Cells. Pharmaceuticals 2023, 16, 349. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Patel, B.; Patel, M.M. Fabrication, Optimisation and in Vitro Evaluation of Docetaxel and Curcumin Co-Loaded Nanostructured Lipid Carriers for Improved Antitumor Activity against Non-Small Cell Lung Carcinoma. J. Microencapsul. 2020, 37, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Artyukhov, A.A.; Nechaeva, A.M.; Shtilman, M.I.; Chistyakov, E.M.; Svistunova, A.Y.; Bagrov, D.V.; Kuskov, A.N.; Docea, A.O.; Tsatsakis, A.M.; Gurevich, L.; et al. Nanoaggregates of Biphilic Carboxyl-Containing Copolymers as Carriers for Ionically Bound Doxorubicin. Materials 2022, 15, 7136. [Google Scholar] [CrossRef] [PubMed]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Alsaidan, O.A.; Zafar, A.; Dodle, T.; Gupta, J.K.; Yasir, M.; Mohanty, A.; Khalid, M. Development of Atomoxetine-Loaded NLC In Situ Gel for Nose-to-Brain Delivery: Optimization, In Vitro, and Preclinical Evaluation. Pharmaceutics 2023, 15, 1985. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P. Drug Carriers: A Review on the Most Used Mathematical Models for Drug Release. Processes 2022, 10, 1094. [Google Scholar] [CrossRef]
- Jain, A.; Thakur, K.; Kush, P.; Jain, U.K. Docetaxel Loaded Chitosan Nanoparticles: Formulation, Characterization and Cytotoxicity Studies. Int. J. Biol. Macromol. 2014, 69, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Controlled Drug Release from Nanoengineered Polysaccharides. Pharmaceutics 2023, 15, 1364. [Google Scholar] [CrossRef] [PubMed]
- Concha, L.; Pires, A.L.R.; Moraes, A.M.; Mas-Hernández, E.; Berres, S.; Hernandez-Montelongo, J. Cost Function Analysis Applied to Different Kinetic Release Models of Arrabidaea Chica Verlot Extract from Chitosan/Alginate Membranes. Polymers 2022, 14, 1109. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, I.; Kaszkur, Z.; Hoell, A. Development of Nanoparticle Bulk Morphology Analysis: A Multidomain XRD Approach. Nanoscale 2023, 15, 8633–8642. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, F.C.; Fantini, M.C.A.; Carollo, A.R.H.; Tedesco, A.C.; Lopes Badra Bentley, M.V. Analysis of Liquid Crystalline Nanoparticles by Small Angle X-Ray Diffraction: Evaluation of Drug and Pharmaceutical Additives Influence on the Internal Structure. J. Pharm. Sci. 2011, 100, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Nogueira Caio, T.; Gonçalves, N.; Wypych, F.; Lona, L.M.F. Enhancement of Mechanical and Thermal Properties of Poly(L-Lactide) Nanocomposites Filled with Synthetic Layered Compounds. Int. J. Polym. Sci. 2017, 2017, 1985078. [Google Scholar] [CrossRef]
- Fornasier, M.; Murgia, S. Non-Lamellar Lipid Liquid Crystalline Nanoparticles: A Smart Platform for Nanomedicine Applications. Front. Soft Matter 2023, 3, 1109508. [Google Scholar] [CrossRef]
- Mohammad, Y.; Prentice, R.N.; Boyd, B.J.; Rizwan, S.B. Comparison of Cubosomes and Hexosomes for the Delivery of Phenytoin to the Brain. J. Colloid Interface Sci. 2022, 605, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Noro, M.G.; Olmsted, P.D. Lamellar and Inverse Micellar Structures of Skin Lipids: Effect of Templating. Phys. Rev. Lett. 2013, 111, 148101. [Google Scholar] [CrossRef]
- Maciuca, A.M.; Munteanu, A.C.; Mihaila, M.; Badea, M.; Olar, R.; Nitulescu, G.M.; Munteanu, C.V.A.; Bostan, M.; Uivarosi, V. Rare-Earth Metal Complexes of the Antibacterial Drug Oxolinic Acid: Synthesis, Characterization, DNA/Protein Binding and Cytotoxicity Studies. Molecules 2020, 25, 5418. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, M.; Hotnog, C.M.; Bostan, M.; Munteanu, A.C.; Vacaroiu, I.A.; Brasoveanu, L.I.; Uivarosi, V. Anticancer Activity of Some Ruthenium (Iii) Complexes with Quinolone Antibiotics: In Vitro Cytotoxicity, Cell Cycle Modulation, and Apoptosis-Inducing Properties in Lovo Colon Cancer Cell Line. Appl. Sci. 2021, 11, 8594. [Google Scholar] [CrossRef]
- Bostan, M.; Mihaila, M.; Petrica-Matei, G.G.; Radu, N.; Hainarosie, R.; Stefanescu, C.D.; Roman, V.; Diaconu, C.C. Resveratrol Modulation of Apoptosis and Cell Cycle Response to Cisplatin in Head and Neck Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 6322. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.; Wang, R. Rapid Profiling of G2 Phase to Mitosis Progression by Flow Cytometry in Asynchronous Cells. Cell Cycle 2020, 19, 2897–2905. [Google Scholar] [CrossRef]



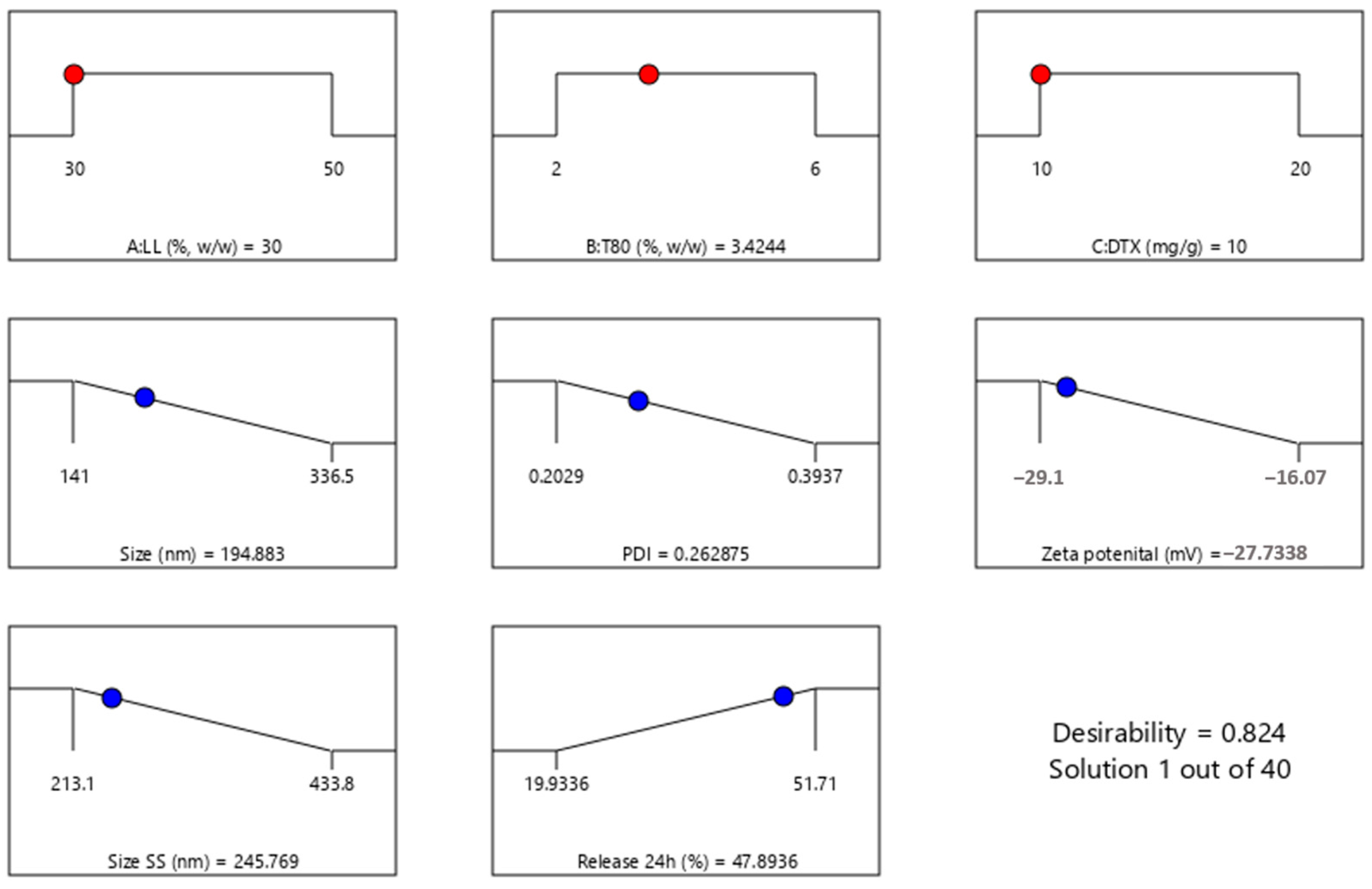





| Variables | Level | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| LL (% w/w) | 30 | 40 | 50 |
| T80% | 2 | 4 | 6 |
| DTX (mg/g) | 10 | 15 | 20 |
| Responses | Optimization | ||
| Size (nm) | minimum | ||
| Size SS (nm) | minimum | ||
| PDI | minimum | ||
| ZP (mV) | maximum (in absolute value) | ||
| Release 24 h (%) | maximum | ||
| Variables | Responses | |||||||
|---|---|---|---|---|---|---|---|---|
| Formulation | A: LL (%, w/w) | B: T80 (% w/w) | C: DTX (mg/g) | Size (nm) | PDI | Size SS (nm) | ZP (mV) | Release 24 h (%) |
| NLC_1 | 30 | 4 | 20 | 141 | 0.2993 | 434 | −20.68 | 34.54 |
| NLC_2 | 40 | 2 | 10 | 291 | 0.2083 | 239 | −28.93 | 41.96 |
| NLC_3 | 30 | 2 | 15 | 287 | 0.2189 | 328 | −29.01 | 39.21 |
| NLC_4 | 40 | 6 | 20 | 179 | 0.2804 | 310 | −16.07 | 40.07 |
| NLC_5 | 40 | 4 | 15 | 193 | 0.2162 | 282 | −24.22 | 38.86 |
| NLC_6 | 40 | 4 | 15 | 200 | 0.2174 | 267 | −22.42 | 41.21 |
| NLC_7 | 50 | 4 | 10 | 217 | 0.2873 | 226 | −17.94 | 47.67 |
| NLC_8 | 40 | 2 | 20 | 337 | 0.2987 | 334 | −20.63 | 19.93 |
| NLC_9 | 40 | 6 | 10 | 158 | 0.3937 | 248 | −22.98 | 39.83 |
| NLC_10 | 30 | 4 | 10 | 168 | 0.2463 | 270 | −27.36 | 44.26 |
| NLC_11 | 50 | 2 | 15 | 323 | 0.2029 | 286 | −25.40 | 37.30 |
| NLC_12 | 30 | 6 | 15 | 176 | 0.2276 | 300 | −23.32 | 51.71 |
| NLC_13 | 50 | 4 | 20 | 220 | 0.2843 | 213 | −16.26 | 38.07 |
| NLC_14 | 50 | 6 | 15 | 186 | 0.2041 | 242 | −19.73 | 42.23 |
| NLC_15 | 40 | 4 | 15 | 214 | 0.2155 | 268 | −24.91 | 35.86 |
| Dependent Variable | Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Size (nm) | Model | 48,977.18 | 3 | 16,325.73 | 51.00 | <0.0001 |
| A-LL (%, w/w) | 3806.28 | 1 | 3806.28 | 11.89 | 0.0054 | |
| B-T80 (%, w/w) | 36,301.65 | 1 | 36,301.65 | 113.41 | <0.0001 | |
| B² | 8869.25 | 1 | 8869.25 | 27.71 | 0.0003 | |
| Residual | 3521.11 | 11 | 320.10 | - | - | |
| Cor Total | 52,498.29 | 14 | - | - | - | |
| PDI | Model | 0.0341 | 4 | 0.0085 | 15.11 | 0.0003 |
| B-T80 (%, w/w) | 0.0039 | 1 | 0.0039 | 6.95 | 0.0249 | |
| C-DTX (mg/g) | 0.0001 | 1 | 0.0001 | 0.1628 | 0.6951 | |
| BC | 0.0104 | 1 | 0.0104 | 18.40 | 0.0016 | |
| C² | 0.0197 | 1 | 0.0197 | 34.93 | 0.0001 | |
| Residual | 0.0056 | 10 | 0.0006 | |||
| Cor Total | 0.0397 | 14 | - | - | - | |
| Zeta potential (mV) | Model | 214.82 | 4 | 53.71 | 19.93 | <0.0001 |
| A-LL (%, w/w) | 55.81 | 1 | 55.81 | 20.71 | 0.0011 | |
| B-T80 (%, w/w) | 60.28 | 1 | 60.28 | 22.37 | 0.0008 | |
| C-DTX (mg/g) | 69.44 | 1 | 69.44 | 25.77 | 0.0005 | |
| C² | 29.29 | 1 | 29.29 | 10.87 | 0.0081 | |
| Residual | 26.95 | 10 | 2.69 | - | - | |
| Cor Total | 241.77 | 14 | - | - | - | |
| Size SS (nm) | Model | 36,456.67 | 3 | 12,152.22 | 23.28 | <0.0001 |
| A-LL (%, w/w) | 16,644.00 | 1 | 16,644.00 | 31.88 | 0.0001 | |
| C-DTX (mg/g) | 11,989.26 | 1 | 11,989.26 | 22.97 | 0.0006 | |
| AC | 7823.40 | 1 | 7823.40 | 14.99 | 0.0026 | |
| Residual | 5742.60 | 11 | 522.05 | |||
| Cor Total | 42,199.27 | 14 | - | - | - | |
| Released 24 h (%) | Model | 590.00 | 5 | 118.00 | 11.37 | 0.0011 |
| A-LL (%, w/w) | 2.48 | 1 | 2.48 | 0.2384 | 0.6370 | |
| B-T80 (%, w/w) | 157.02 | 1 | 157.02 | 15.13 | 0.0037 | |
| C-DTX (mg/g) | 211.19 | 1 | 211.19 | 20.34 | 0.0015 | |
| BC | 123.88 | 1 | 123.88 | 11.93 | 0.0072 | |
| A² | 95.43 | 1 | 95.43 | 9.19 | 0.0142 | |
| Residual | 93.43 | 9 | 10.38 | |||
| Cor Total | 683.42 | 14 | - | - | - |
| Formulation | Size (nm) | PDI | Size SS (nm) | PDI SS | ZP (mV) | EE % | Release 24 h |
|---|---|---|---|---|---|---|---|
| NLC-DTX | 199 ± 7.21 | 0.2793 ± 0.0139 | 277 ± 14.00 | 0.2734 ± 0.0220 | −28.73 ± 3.55 | 99.16 ± 2.28 | 45.62 ± 3.19 |
| NLC-Blank | 187 ± 8.53 | 0.1955 ± 0.0379 | 327 ± 9.26 | 0.2327 ± 0.03646 | −35.63 ± 3.70 | - | - |
| Sample | First order | Higuchi | Hixson-Crowell | Korsmeyer-Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | k1 | R2 | KH | R2 | k3 | R2 | kKP | n | |
| NLC-DTX | 0.9890 | 0.034 | 0.9939 | 11.734 | 0.9814 | 0.009 | 0.9971 | 9.289 | 0.564 |
| Excipient | Temperature Interval(s) and Mass Loss | Residue | |||
|---|---|---|---|---|---|
| Gelucire 43/01 (G43/01) | 270–450 °C | not present | |||
| 99.75% | |||||
| Glyceryl caprylate | 50–120 °C | 120–280 °C | 280–330 °C | not present | |
| 1.39% | 81.96% | 16.58% | |||
| MCT | 180–350 °C | not present | |||
| 99.97% | |||||
| S-PC-3 | 50–180 °C | 180–270 °C | 270–370 °C | 370–540 °C | 7.20% |
| 4.50% | 9.19% | 73.69% | 4.50% | ||
| Tween 80 (T80) | 300–500 °C | not present | |||
| 95.51% | |||||
| Mannitol | 220–500 °C | not present | |||
| 98.95% | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocoș, F.-I.; Anuța, V.; Popa, L.; Ghica, M.V.; Nica, M.-A.; Mihăilă, M.; Fierăscu, R.C.; Trică, B.; Nicolae, C.A.; Dinu-Pîrvu, C.-E. Development and Evaluation of Docetaxel-Loaded Nanostructured Lipid Carriers for Skin Cancer Therapy. Pharmaceutics 2024, 16, 960. https://doi.org/10.3390/pharmaceutics16070960
Cocoș F-I, Anuța V, Popa L, Ghica MV, Nica M-A, Mihăilă M, Fierăscu RC, Trică B, Nicolae CA, Dinu-Pîrvu C-E. Development and Evaluation of Docetaxel-Loaded Nanostructured Lipid Carriers for Skin Cancer Therapy. Pharmaceutics. 2024; 16(7):960. https://doi.org/10.3390/pharmaceutics16070960
Chicago/Turabian StyleCocoș, Florentina-Iuliana, Valentina Anuța, Lăcrămioara Popa, Mihaela Violeta Ghica, Mihaela-Alexandra Nica, Mirela Mihăilă, Radu Claudiu Fierăscu, Bogdan Trică, Cristian Andi Nicolae, and Cristina-Elena Dinu-Pîrvu. 2024. "Development and Evaluation of Docetaxel-Loaded Nanostructured Lipid Carriers for Skin Cancer Therapy" Pharmaceutics 16, no. 7: 960. https://doi.org/10.3390/pharmaceutics16070960








