The Potential Role of Plant Polysaccharides in Treatment of Ulcerative Colitis
Abstract
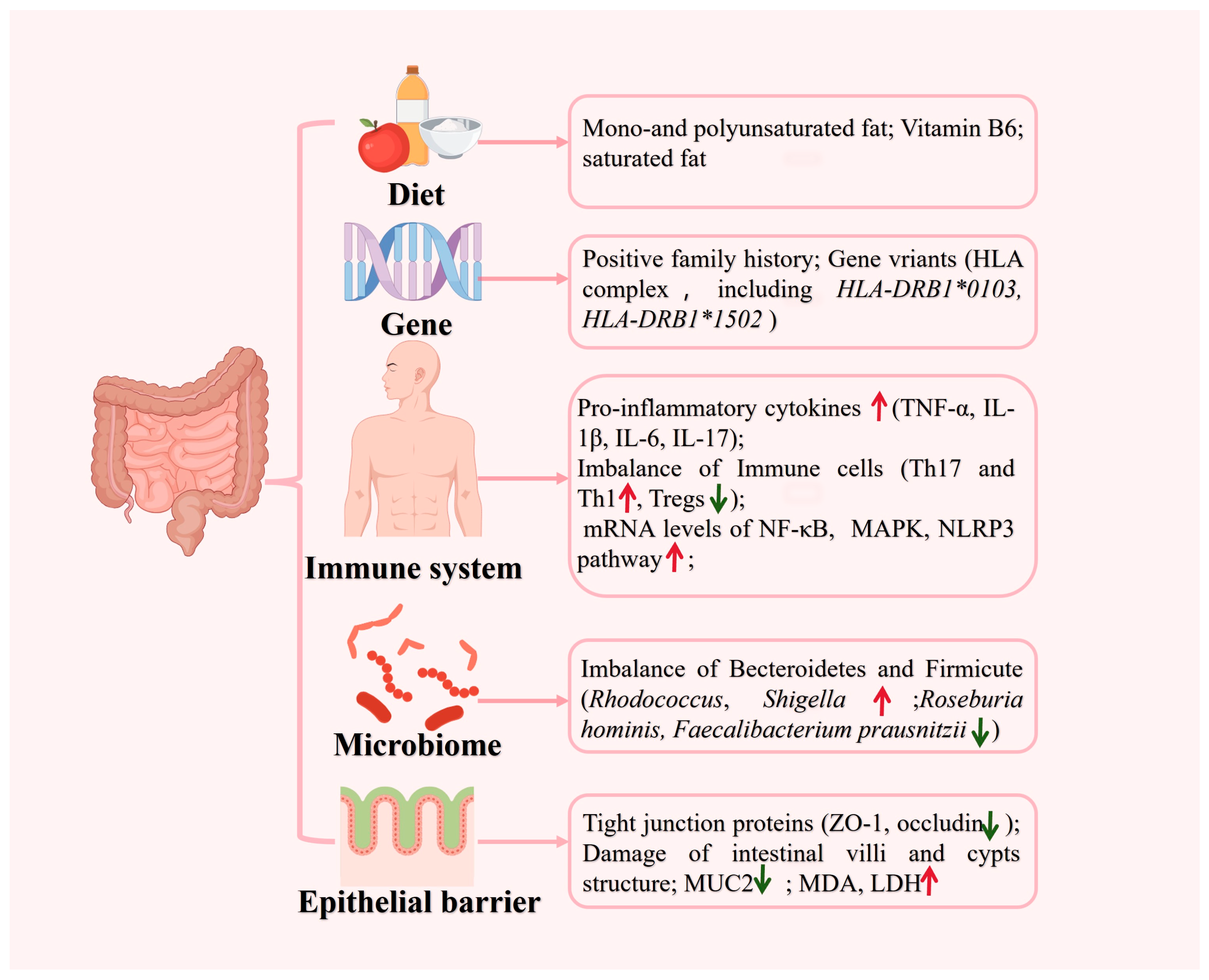
:1. Introduction
2. Etiology and Pathogenesis of UC
3. The Role of Natural Polysaccharides in UC
3.1. Regulating the Expression of Cytokines
3.2. Regulating the Balance of Immune Cells
3.3. Regulating the Function of Intestinal Mucus
3.4. Protecting the Intestinal Barrier Integrity
3.5. Regulating the Intestinal Microbiota
3.6. As a Drug Carrier
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative Colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Ma, R.; Li, L.; Wu, W.; Cai, D.; Lu, Q. Natural-Derived Alkaloids Exhibit Great Potential in the Treatment of Ulcerative Colitis. Pharmacol. Res. 2022, 175, 105972. [Google Scholar] [CrossRef]
- Panés, J.; Alfaro, I. New Treatment Strategies for Ulcerative Colitis. Expert Rev. Clin. Immunol. 2017, 13, 963–973. [Google Scholar] [CrossRef]
- Kweon, D.Y.; Song, H.J.; Kim, J.E.; Jin, Y.J.; Roh, Y.J.; Seol, A.; Park, J.M.; Lee, E.S.; Choi, W.S.; Hwang, D.Y. Therapeutic Effects of Aloe Saponaria against Ulcerative Colitis Induced by Dextran Sulfate Sodium. Curr. Issues Mol. Biol. 2023, 45, 1483–1499. [Google Scholar] [CrossRef]
- Ben-Arye, E.; Goldin, E.; Wengrower, D.; Stamper, A.; Kohn, R.; Berry, E. Wheat Grass Juice in the Treatment of Active Distal Ulcerative Colitis: A Randomized Double-Blind Placebo-Controlled Trial. Scand. J. Gastroenterol. 2002, 37, 444–449. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Targan, S.R.; Byers, V.S.; Rutty, D.A.; Mu, H.; Zhang, X.; Tang, T. Andrographis paniculataExtract (HMPL-004) for Active Ulcerative Colitis. Off. J. Am. Coll. Gastroenterol. ACG 2013, 108, 90. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia Serrata. Clin. Pharmacokinet. 2011, 50, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Hooda, P.; Malik, R.; Bhatia, S.; Al-Harrasi, A.; Najmi, A.; Zoghebi, K.; Halawi, M.A.; Makeen, H.A.; Mohan, S. Phytoimmunomodulators: A Review of Natural Modulators for Complex Immune System. Heliyon 2024, 10, e23790. [Google Scholar] [CrossRef] [PubMed]
- Shi, L. Bioactivities, Isolation and Purification Methods of Polysaccharides from Natural Products: A Review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological Activities and Pharmaceutical Applications of Polysaccharide from Natural Resources: A Review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Li, Q.-M.; Zha, X.-Q.; Luo, J.-P. Intervention and Potential Mechanism of Non-Starch Polysaccharides from Natural Resources on Ulcerative Colitis: A Review. Int. J. Biol. Macromol. 2022, 210, 545–564. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Wan, D.-L.; Li, Q.-M.; Zha, X.-Q.; Luo, J.-P. Structural Characteristics and Immunostimulatory Activities of a New Polysaccharide from Dendrobium fimbriatum Hook. Food Funct. 2021, 12, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, J.; Gao, F.; Chen, W.; Zong, Y.; Li, J.; He, Z.; Du, R. Extraction, Purification, and Structural Characterization of Polysaccharides from Sanghuangporus Vaninii with Anti-Inflammatory Activity. Molecules 2023, 28, 6081. [Google Scholar] [CrossRef]
- Ahmed, A.U. An Overview of Inflammation: Mechanism and Consequences. Front. Biol. 2011, 6, 274–281. [Google Scholar] [CrossRef]
- Yeshi, K.; Ruscher, R.; Hunter, L.; Daly, N.L.; Loukas, A.; Wangchuk, P. Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products. J. Clin. Med. 2020, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, R.; Gao, H.; Jung, S.; Gao, X.; Sun, R.; Liu, X.; Kim, Y.; Lee, H.-S.; Kawai, Y.; et al. Genetic Architecture of the Inflammatory Bowel Diseases across East Asian and European Ancestries. Nat. Genet. 2023, 55, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Brant, S.R. Recent Insights Into the Genetics of Inflammatory Bowel Disease. Gastroenterology 2011, 140, 1704–1712.e2. [Google Scholar] [CrossRef]
- El Hadad, J.; Schreiner, P.; Vavricka, S.R.; Greuter, T. The Genetics of Inflammatory Bowel Disease. Mol. Diagn. Ther. 2024, 28, 27–35. [Google Scholar] [CrossRef]
- Hirano, A.; Umeno, J.; Torisu, T. P838 Characteristics of Mucosal Microbial Composition of Patients with Inflammatory Bowel Disease Susceptibility HLA Genotype. J. Crohn’s Colitis 2020, 14, S649–S650. [Google Scholar] [CrossRef]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Takahashi, A.; Kondoh, M.; Suzuki, H.; Watari, A.; Yagi, K. Pathological Changes in Tight Junctions and Potential Applications into Therapies. Drug Discov. Today 2012, 17, 727–732. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus Barrier, Mucins and Gut Microbiota: The Expected Slimy Partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Mesmin, L.; Chassaing, B.; Desvaux, M.; De Paepe, K.; Gresse, R.; Sauvaitre, T.; Forano, E.; de Wiele, T.V.; Schüller, S.; Juge, N.; et al. Experimental Models to Study Intestinal Microbes–Mucus Interactions in Health and Disease. FEMS Microbiol. Rev. 2019, 43, 457–489. [Google Scholar] [CrossRef] [PubMed]
- Niv, Y. Mucin Gene Expression in the Intestine of Ulcerative Colitis Patients: A Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1241. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Zhou, Y.; Zhang, H.; Zhou, H.; Zhang, X. Slimy Partners: The Mucus Barrier and Gut Microbiome in Ulcerative Colitis. Exp. Mol. Med. 2021, 53, 772–787. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, Y.; Ogino, H.; Tanaka, M.; Ihara, E.; Fukaura, K.; Nishioka, K.; Chinen, T.; Tanaka, Y.; Nakayama, J.; Kang, D.; et al. Mucosa-Associated Gut Microbiota Reflects Clinical Course of Ulcerative Colitis. Sci. Rep. 2021, 11, 13743. [Google Scholar] [CrossRef]
- Sommer, F.; Rühlemann, M.C.; Bang, C.; Höppner, M.; Rehman, A.; Kaleta, C.; Schmitt-Kopplin, P.; Dempfle, A.; Weidinger, S.; Ellinghaus, E.; et al. Microbiomarkers in Inflammatory Bowel Diseases: Caveats Come with Caviar. Gut 2017, 66, 1734–1738. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Leontiadis, G.I.; Tse, F.; Yuan, Y.; Surette, M.; Moayyedi, P. Differences in Gut Microbiota in Patients With vs Without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology 2020, 158, 930–946.e1. [Google Scholar] [CrossRef]
- Glick, L.R.; Cifu, A.S.; Feld, L. Ulcerative Colitis in Adults. J. Am. Med. Assoc. 2020, 324, 1205–1206. [Google Scholar] [CrossRef] [PubMed]
- Mahid, S.S.; Minor, K.S.; Soto, R.E.; Hornung, C.A.; Galandiuk, S. Smoking and Inflammatory Bowel Disease: A Meta-Analysis. Mayo Clin. Proc. 2006, 81, 1462–1471. [Google Scholar] [CrossRef]
- Orholm, M.; Binder, V.; Sørensen, T.I.; Rasmussen, L.P.; Kyvik, K.O. Concordance of Inflammatory Bowel Disease among Danish Twins: Results of a Nationwide Study. Scand. J. Gastroenterol. 2000, 35, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.-C.; Sollano, J.; Hui, Y.T.; Yu, W.; Santos Estrella, P.V.; Llamado, L.J.Q.; Koram, N. Epidemiology, Burden of Disease, and Unmet Needs in the Treatment of Ulcerative Colitis in Asia. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; He, D.; Xie, J.; Zhou, Q.; Zeng, H.; Li, H.; Huang, L. The Synergistic Effects of Polysaccharides and Ginsenosides From American Ginseng (Panax quinquefolius L.) Ameliorating Cyclophosphamide-Induced Intestinal Immune Disorders and Gut Barrier Dysfunctions Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2021, 12, 665901. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Chan, S.S.M.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The Role of Diet in the Aetiopathogenesis of Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-Fat-Induced Taurocholic Acid Promotes Pathobiont Expansion and Colitis in Il10−/− Mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Fuchs, C.S.; Richter, J.M.; Schernhammer, E.S.; Chan, A.T. Sleep Duration Affects Risk for Ulcerative Colitis: A Prospective Cohort Study. Clin. Gastroenterol. Hepatol. 2014, 12, 1879–1886. [Google Scholar] [CrossRef]
- Niu, W.; Chen, X.; Xu, R.; Dong, H.; Yang, F.; Wang, Y.; Zhang, Z.; Ju, J. Polysaccharides from Natural Resources Exhibit Great Potential in the Treatment of Ulcerative Colitis: A Review. Carbohydr. Polym. 2021, 254, 117189. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Dou, P.; Wu, Z.; Zheng, Z.; Pan, X.; Zhou, T.; Wang, K. Oral Absorption Characteristics and Mechanisms of a Pectin-Type Polysaccharide from Smilax china L. across the Intestinal Epithelium. Carbohydr. Polym. 2021, 270, 118383. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Verstrepen, L.; Ghyselinck, J.; Albers, R.; Marzorati, M.; Mercenier, A. A Novel Non-Digestible, Carrot-Derived Polysaccharide (cRG-I) Selectively Modulates the Human Gut Microbiota While Promoting Gut Barrier Integrity: An Integrated In Vitro Approach. Nutrients 2020, 12, 1917. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.-H.; Duan, J.-A.; Zhang, W.; Jiang, S.; Guo, J.-M.; Wei, D.-D. Polysaccharides From Chrysanthemum Morifolium Ramat Ameliorate Colitis Rats via Regulation of the Metabolic Profiling and NF-κ B/TLR4 and IL-6/JAK2/STAT3 Signaling Pathways. Front. Pharmacol. 2018, 9, 746. [Google Scholar] [CrossRef]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria Baicalensis Georgi Polysaccharide Ameliorates DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, Y.; Tian, Z.; Liu, F.; Shi, Y.; Liu, Y.; Xia, P. Astragalus Polysaccharides Protect against Dextran Sulfate Sodium-Induced Colitis by Inhibiting NF-κB Activation. Int. J. Biol. Macromol. 2017, 98, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, H.; Wen, Y.; Jiang, D.; Zhu, S.; He, X.; Xiong, Q.; Gao, J.; Hou, S.; Huang, S.; et al. Tremella Fuciformis Polysaccharides Ameliorated Ulcerative Colitis via Inhibiting Inflammation and Enhancing Intestinal Epithelial Barrier Function. Int. J. Biol. Macromol. 2021, 180, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, S.; Chen, J.; Lin, J.; Xiong, Q.; Yang, Y.; Yuan, J.; Zhou, L.; He, L.; Hou, S.; et al. Therapeutic Roles of Polysaccharides from Dendrobium Officinaleon Colitis and Its Underlying Mechanisms. Carbohydr. Polym. 2018, 185, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Honglang, L.; Weifeng, L.; Junmin, C.; Jiantao, Y.; Junjing, G. The Mechanism of Alopolysaccharide Protecting Ulceralive Colitis. Biomed. Pharmacother. 2017, 88, 145–150. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, H.; Zhao, A.; Lu, H.; Sun, W.; Ma, C.; Yang, Y.; Xin, X.; Zou, H.; Qiu, M.; et al. The in Vivo and in Vitro Study of Polysaccharides from a Two-Herb Formula on Ulcerative Colitis and Potential Mechanism of Action. J. Ethnopharmacol. 2014, 153, 151–159. [Google Scholar] [CrossRef]
- Su, L.; Mao, C.; Wang, X.; Li, L.; Tong, H.; Mao, J.; Ji, D.; Lu, T.; Hao, M.; Huang, Z.; et al. The Anti-Colitis Effect of Schisandra Chinensis Polysaccharide Is Associated With the Regulation of the Composition and Metabolism of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 519479. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wang, Y.; Yang, X.; Yin, H.; Nie, S.; Wu, X. Structure Characterization of a Polysaccharide Extracted from Noni (Morinda citrifolia L.) and Its Protective Effect against DSS-Induced Bowel Disease in Mice. Food Hydrocoll. 2019, 90, 189–197. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.; Kan, J.; Zhang, X.; Wu, X.; Sun, R.; Tang, S.; Liu, J.; Qian, C.; Jin, C. Structural Characterization of Water-Soluble Polysaccharide from Arctium Lappa and Its Effects on Colitis Mice. Carbohydr. Polym. 2019, 213, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-N.; Mei, Q.-B.; Liu, L.; Zhang, F.; Liu, Z.-G.; Wang, Z.-P.; Wang, R.-T. Protective Effects of Rheum Tanguticum Polysaccharide against Hydrogen Peroxide-Induced Intestinal Epithelial Cell Injury. World J. Gastroenterol. 2005, 11, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-J.; Huang, X.-J.; Shi, X.-D.; Chen, H.-H.; Cui, S.W.; Nie, S.-P. Protective Effect of Three Glucomannans from Different Plants against DSS Induced Colitis in Female BALB/c Mice. Food Funct. 2019, 10, 1928–1939. [Google Scholar] [CrossRef]
- Tao, J.-H.; Duan, J.-A.; Jiang, S.; Feng, N.-N.; Qiu, W.-Q.; Ling, Y. Polysaccharides from Chrysanthemum Morifolium Ramat Ameliorate Colitis Rats by Modulating the Intestinal Microbiota Community. Oncotarget 2017, 8, 80790–80803. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Wang, L.; Zhao, Z.; You, L.; Pedisić, S.; Kulikouskaya, V.; Lin, Z. Polysaccharide from Gracilaria Lemaneiformis Prevents Colitis in Balb/c Mice via Enhancing Intestinal Barrier Function and Attenuating Intestinal Inflammation. Food Hydrocoll. 2020, 109, 106048. [Google Scholar] [CrossRef]
- Periasamy, S.; Lin, C.-H.; Nagarajan, B.; Sankaranarayanan, N.V.; Desai, U.R.; Liu, M.-Y. Mucoadhesive Role of Tamarind Xyloglucan on Inflammation Attenuates Ulcerative Colitis. J. Funct. Foods 2018, 47, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Zhang, Y.; Li, Q.; Zeng, F.; Wang, K. Inhibition of Dextran Sodium Sulfate-Induced Experimental Colitis in Mice by Angelica Sinensis Polysaccharide. J. Med. Food 2020, 23, 584–592. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Liu, X.; Xu, G.; Ye, M.; Bai, L.; Lin, R.; Sha, X.; Liang, L.; Huang, J.; Zhou, C.; et al. Identification of Bioactive Polysaccharide from Pseudostellaria Heterophylla with Its Anti-Inflammatory Effects. J. Funct. Foods 2021, 78, 104353. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Q.; Taha, R.; Abdelmotalab, M.I.; Wen, Q.; Yuan, Y.; Zhao, Y.; Li, Q.; Liao, C.; Huang, X.; et al. Polysaccharide from Atractylodes Macrocephala Koidz. Ameliorates DSS-Induced Colitis in Mice by Regulating the Th17/Treg Cell Balance. Front. Immunol. 2022, 13, 1021695. [Google Scholar] [CrossRef]
- Feng, W.; Liu, J.; Tan, Y.; Ao, H.; Wang, J.; Peng, C. Polysaccharides from Atractylodes macrocephala Koidz. Ameliorate Ulcerative Colitis via Extensive Modification of Gut Microbiota and Host Metabolism. Food Res. Int. 2020, 138, 109777. [Google Scholar] [CrossRef] [PubMed]
- Leppkes, M.; Neurath, M.F. Cytokines in Inflammatory Bowel Diseases—Update 2020. Pharmacol. Res. 2020, 158, 104835. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, Y.; Hu, X.; Wang, J. Structural Characterization and Anti-Inflammatory Activity of a Polysaccharide from the Lignified Okra. Carbohydr. Polym. 2021, 265, 118081. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yan, H.; Han, L.; Cui, L.; Hussain, H.; Feng, Q.; Zhao, Y.; Zhang, Z.; Li, J.; Aziz, S.; et al. Structural Characterization and Anti-Inflammatory Activity of Neutral Polysaccharides from American Ginseng. Int. J. Biol. Macromol. 2023, 248, 125586. [Google Scholar] [CrossRef]
- Jen, C.; Su, C.; Lu, M.; Lai, M.; Ng, L. Synergistic Anti-inflammatory Effects of Different Polysaccharide Components from Xylaria nigripes. J. Food Biochem. 2021, 45, e13694. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Feng, J. Signaling Pathways Associated with Inflammatory Bowel Disease. Recent Pat. Inflamm. Allergy Drug Discov. 2010, 4, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Gareb, B.; Otten, A.T.; Frijlink, H.W.; Dijkstra, G.; Kosterink, J.G.W. Review: Local Tumor Necrosis Factor-α Inhibition in Inflammatory Bowel Disease. Pharmaceutics 2020, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Tindemans, I.; Joosse, M.E.; Samsom, J.N. Dissecting the Heterogeneity in T-Cell Mediated Inflammation in IBD. Cells 2020, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Huang, J.; Sun, M.; Jiang, Y.; Wang, S.; Wang, L.; Yu, N.; Peng, D.; Wang, Y.; Chen, W.; et al. Poria cocos Polysaccharide Improves Intestinal Barrier Function and Maintains Intestinal Homeostasis in Mice. Int. J. Biol. Macromol. 2023, 249, 125953. [Google Scholar] [CrossRef]
- Ren, X.; Zhu, Y.; Gamallat, Y.; Ma, S.; Chiwala, G.; Meyiah, A.; Xin, Y.E. Coli O124 K72 Alters the Intestinal Barrier and the Tight Junctions Proteins of Guinea Pig Intestine. Biomed. Pharmacother. 2017, 94, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora. Int. J. Mol. Sci. 2019, 20, 5751. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Kazmierczak, C.; Duchêne, B.; Jonckheere, N.; Leteurtre, E.; Van Seuningen, I. Cryosectioning the Intestinal Crypt-Villus Axis: An Ex Vivo Method to Study the Dynamics of Epigenetic Modifications from Stem Cells to Differentiated Cells. Stem Cell Res. 2015, 14, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, W.; Xu, B.; Wu, E.; Cui, Y.; Hao, K.; Zhang, G.; Zhou, C.; Xu, Y.; Li, J.; et al. Polysaccharides From the Roots of Millettia Speciosa Champ Modulate Gut Health and Ameliorate Cyclophosphamide-Induced Intestinal Injury and Immunosuppression. Front. Immunol. 2021, 12, 766296. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Park, H.; Choe, B.-H.; Kang, B. The Role and Function of Mucins and Its Relationship to Inflammatory Bowel Disease. Front. Med. 2022, 9, 848344. [Google Scholar] [CrossRef] [PubMed]
- Der Sluis, M.V.; Koning, B.A.E.D.; Bruijn, A.C.J.M.D.; Velcich, A.; Meijerink, J.P.P.; Goudoever, J.B.V.; Büller, H.A.; Dekker, J.; Seuningen, I.V.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Gentile, M.; Yeiser, J.R.; Walland, A.C.; Bornstein, V.U.; Chen, K.; He, B.; Cassis, L.; Bigas, A.; Cols, M.; et al. Mucus Enhances Gut Homeostasis and Oral Tolerance by Delivering Immunoregulatory Signals. Science 2013, 342, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Batista, J.A.; Magalhães, D.D.A.; Sousa, S.G.; Ferreira, J.D.S.; Pereira, C.M.C.; Lima, J.V.D.N.; De Albuquerque, I.F.; Bezerra, N.L.S.D.; De Brito, T.V.; Monteiro, C.E.D.S.; et al. Polysaccharides Derived from Morinda citrifolia Linn Reduce Inflammatory Markers during Experimental Colitis. J. Ethnopharmacol. 2020, 248, 112303. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, P.; Li, C.; Xu, F.; Chen, J. A Polysaccharide from Rosa Roxburghii Tratt Fruit Attenuates High-Fat Diet-Induced Intestinal Barrier Dysfunction and Inflammation in Mice by Modulating the Gut Microbiota. Food Funct. 2022, 13, 530–547. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Vila, A.V.; Bonder, M.J.; Fu, J.; Gevers, D.; Visschedijk, M.C.; Spekhorst, L.M.; Alberts, R.; Franke, L.; van Dullemen, H.M.; et al. Interplay of Host Genetics and Gut Microbiota Underlying the Onset and Clinical Presentation of Inflammatory Bowel Disease. Gut 2018, 67, 108–119. [Google Scholar] [CrossRef]
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. In-Depth Analysis of the Mechanisms of Aloe Polysaccharides on Mitigating Subacute Colitis in Mice via Microbiota Informatics. Carbohydr. Polym. 2021, 265, 118041. [Google Scholar] [CrossRef]
- Varela, E.; Manichanh, C.; Gallart, M.; Torrejón, A.; Borruel, N.; Casellas, F.; Guarner, F.; Antolin, M. Colonisation by Faecalibacterium Prausnitzii and Maintenance of Clinical Remission in Patients with Ulcerative Colitis. Aliment. Pharmacol. Ther. 2013, 38, 151–161. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, Q.; Kang, X.; Tian, G.; Ming, D.; Yang, J. Protective Role of a New Polysaccharide Extracted from Lonicera Japonica Thunb in Mice with Ulcerative Colitis Induced by Dextran Sulphate Sodium. BioMed Res. Int. 2021, 2021, e8878633. [Google Scholar] [CrossRef]
- Li, X.; Lu, C.; Yang, Y.; Yu, C.; Rao, Y. Site-Specific Targeted Drug Delivery Systems for the Treatment of Inflammatory Bowel Disease. Biomed. Pharmacother. 2020, 129, 110486. [Google Scholar] [CrossRef]
- Lima, I.B.C.; Moreno, L.; Clara, G.A.I.; Silva-Filho, E.C.; Irache, J.M.; Veiga, F.J.B.; Rolim, H.M.L.; Nunes, L.C.C. Development of Nanostructured Systems Using Natural Polymers to Optimize the Treatment of Inflammatory Bowel Diseases: A Prospective Study. J. Drug Deliv. Sci. Technol. 2021, 64, 102590. [Google Scholar] [CrossRef]
- Gamboa, A.; Araujo, V.; Caro, N.; Gotteland, M.; Abugoch, L.; Tapia, C. Spray Freeze-Drying as an Alternative to the Ionic Gelation Method to Produce Chitosan and Alginate Nano-Particles Targeted to the Colon. J. Pharm. Sci. 2015, 104, 4373–4385. [Google Scholar] [CrossRef]
- Shah, A.; Aftab, S.; Nisar, J.; Ashiq, M.N.; Iftikhar, F.J. Nanocarriers for Targeted Drug Delivery. J. Drug Deliv. Sci. Technol. 2021, 62, 102426. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, B.-W.; Sun, R.; Li, X.; Wu, D.; Hu, J.-N. Colon-Targeting Angelica Sinensis Polysaccharide Nanoparticles with Dual Responsiveness for Alleviation of Ulcerative Colitis. ACS Appl. Mater. Interfaces 2023, 15, 26298–26315. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wu, C.; Chen, H.; Zheng, T.; Ye, H.; Wang, J.; Zhang, Y.; Gao, J.; Li, Y.; Dong, Z. Rhubarb Polysaccharide and Berberine Co-Assembled Nanoparticles Ameliorate Ulcerative Colitis by Regulating the Intestinal Flora. Front. Pharmacol. 2023, 14, 1184183. [Google Scholar] [CrossRef]
- Cui, M.; Fang, Z.; Song, M.; Zhou, T.; Wang, Y.; Liu, K. Phragmites Rhizoma Polysaccharide-Based Nanocarriers for Synergistic Treatment of Ulcerative Colitis. Int. J. Biol. Macromol. 2022, 220, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, S.; Song, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Chen, T.; Zheng, W.; Huang, Z. Selenium Nanoparticles Decorated with Ulva Lactuca Polysaccharide Potentially Attenuate Colitis by Inhibiting NF-κB Mediated Hyper Inflammation. J. Nanobiotechnol. 2017, 15, 20. [Google Scholar] [CrossRef]
- Shi, R.; Dan, B.; Lü, L. Bioactive Effects Advances of Natural Polysaccharides. J. Future Foods 2023, 3, 234–239. [Google Scholar] [CrossRef]


| Source | PS | The Role in UC Treatment | Refs. |
|---|---|---|---|
| Chrysanthemum | CP | Decreased inflammatory cytokines Decreased mRNA levels of TLR4, NF-κB, IL-6, STAT3, and JAK2 Decreased The expression of p-P65, TLR4, p-STAT3, and p-JAK2 | [42] |
| Scutellaria baicalensis | SP1-1 | Inhibited expression of TNF-α, IL-1β, IL-6, IL-17 Decreased CD11b+ macrophage infiltration in colons Decreased Cle-caspase-1 Decreased Bacteroides, Proteobacteria and Staphylococcus | [43] |
| Astragalus | APS | Inhibited NF-κB phosphorylation Decreased e and MPO | [44] |
| Tremella fuciformis | TFP | Decreased inflammatory cells infiltration Restored intestinal epithelial barrier integrity Decreased TNF-α, IL-1β and IL-6 Improved mRNA and protein expression of ZO-1 and OCLN | [45] |
| Dendrobium officinale | DOPS | Improved clinical signs and symptoms Decreased mortality Alleviated colonic pathological damage Reduced the level of IL-1β, IL-6, IL-18, TNF-α and IFN-γ | [46] |
| Aloe | AP | Down-regulated IL-6, JAK2, STAT-3 and cell apoptosis | [47] |
| Lycium barbarum, Astragalus membranaceus | QHPS | Inhibited levels of DAO, DLA and EDT Protected the integrity of the intestinal mucosa Repaired the injury of intestinal tract | [48] |
| Schisandra chinensis | SCP | Decreased IL-6, IL-10, IL-17, IL-23, and TNF-α levels Returned abundance of Firmicutes, Proteobacteria, and Bacteroidetes Increased the content of SCFAs | [49] |
| Noni fruit | NFP | Promoted the expression of zonula, occludens-1 | [50] |
| Arctium lappa | ALP-1 | Decreased IL-1β, IL-6 and TNF-α and IL-10 Increased the abundance of Firmicutes, Ruminococcaceae, Lachnospiraceae, Lactobacillus Inhibited the level of Proteobacteria, Alcaligenaceae, Staphylococcus and and Bacteroidetes | [51] |
| Scutellaria baicalensis | SP2-1 | Suppressed proinflammatory cytokines. Up-regulated expressions of ZO-1, OCLN and Claudin-5; Enhanced the levels of acetic acid, propionic acid, and butyric acid Increased the abundance of Firmicutes, Bifidobacterium, Lactobacillus, and Roseburia Inhibited the levels of Bacteroides, Proteobacteria and Staphylococcus | [43] |
| Rheum tanguticum | RTP | Elevated cell survival, SOD activity Decreased MDA, LDH activity and cell apoptosis | [52] |
| Glucomannans | GMs | Reduced IL-1β, IL-6 and TNF-α and IL-10 Regulated the expressions of TLR-2, TLR-4, TLR-6, and TLR-9 | [53] |
| Chrysanthemum morifolium | - | Decreased the abundances of Enterococcus, Escherichia and prevotella Eelevated Bifidobacterium, Butyricicoccus, Clostridium, Lachnospiraceae, Lactobacillus and Rikenellaceae | [54] |
| Gracilaria Lemaneiformis | SP | Suppressed the secretion of TNF-α, IL-6 and IL-1β Promoted Claudin-1, ZO-1, and MUC-2 | [55] |
| Tamarind xyloglucan | TCG | Decreased IL-1β and IL-6 levels Decreased the expression of TLR4, MyD88, I-κB and NF-κB | [56] |
| Angelica sinensis | ASP | Reduced IL-1β, IL-6 levels Improved occludens 1, occludin, and claudin-1 | [57] |
| Pseudostellaria heterophylla | PAMK | Decreased IL-1β and TNF-α Increased the abundance of Bacteroides | [58] |
| Atractylodes macrocephala | AMP | Regulated the balance between Th 17 and the Treg cells; Increased the content of SCFAs; Increased Butyricicoccus, Lactobacillus, Decreased Actinobacteria, Akkermansia, Anaeroplasma, Bifidobacterium, Erysipelatoclostridium, Faecalibaculum, Parasutterella, Parvibacter, Tenericutes, Verrucomicrobia | [59,60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dilixiati, Y.; Aipire, A.; Song, M.; Nijat, D.; Wubuli, A.; Cao, Q.; Li, J. The Potential Role of Plant Polysaccharides in Treatment of Ulcerative Colitis. Pharmaceutics 2024, 16, 1073. https://doi.org/10.3390/pharmaceutics16081073
Dilixiati Y, Aipire A, Song M, Nijat D, Wubuli A, Cao Q, Li J. The Potential Role of Plant Polysaccharides in Treatment of Ulcerative Colitis. Pharmaceutics. 2024; 16(8):1073. https://doi.org/10.3390/pharmaceutics16081073
Chicago/Turabian StyleDilixiati, Yilizilan, Adila Aipire, Ming Song, Dilaram Nijat, Abudukahaer Wubuli, Qi Cao, and Jinyao Li. 2024. "The Potential Role of Plant Polysaccharides in Treatment of Ulcerative Colitis" Pharmaceutics 16, no. 8: 1073. https://doi.org/10.3390/pharmaceutics16081073






