From Field to Pharmacy: Isolation, Characterization and Tableting Behaviour of Microcrystalline Cellulose from Wheat and Corn Harvest Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Isolation of MCC from HRs
2.2.2. Compositional Analysis of HRs before and after Treatment
Determination of Hemicellulose Content
Determination of Cellulose and Lignin Content
Determination of the Ash Content
2.2.3. Physicochemical Characterization of MCC
Particle Size Analysis
Fourier-Transform Infrared (FT-IR) Spectroscopy
Powder X-ray Diffraction (PXRD) Analysis
Differential Scanning Calorimetry (DSC)
2.2.4. Functional Characterization of MCC
Determination of Bulk and Tapped Density
Evaluation of Powder Behaviour in Tableting Process
3. Results and Discussion
3.1. Compositional Analysis of HRs before and after Treatment
3.2. Physicochemical Characterization of MCC
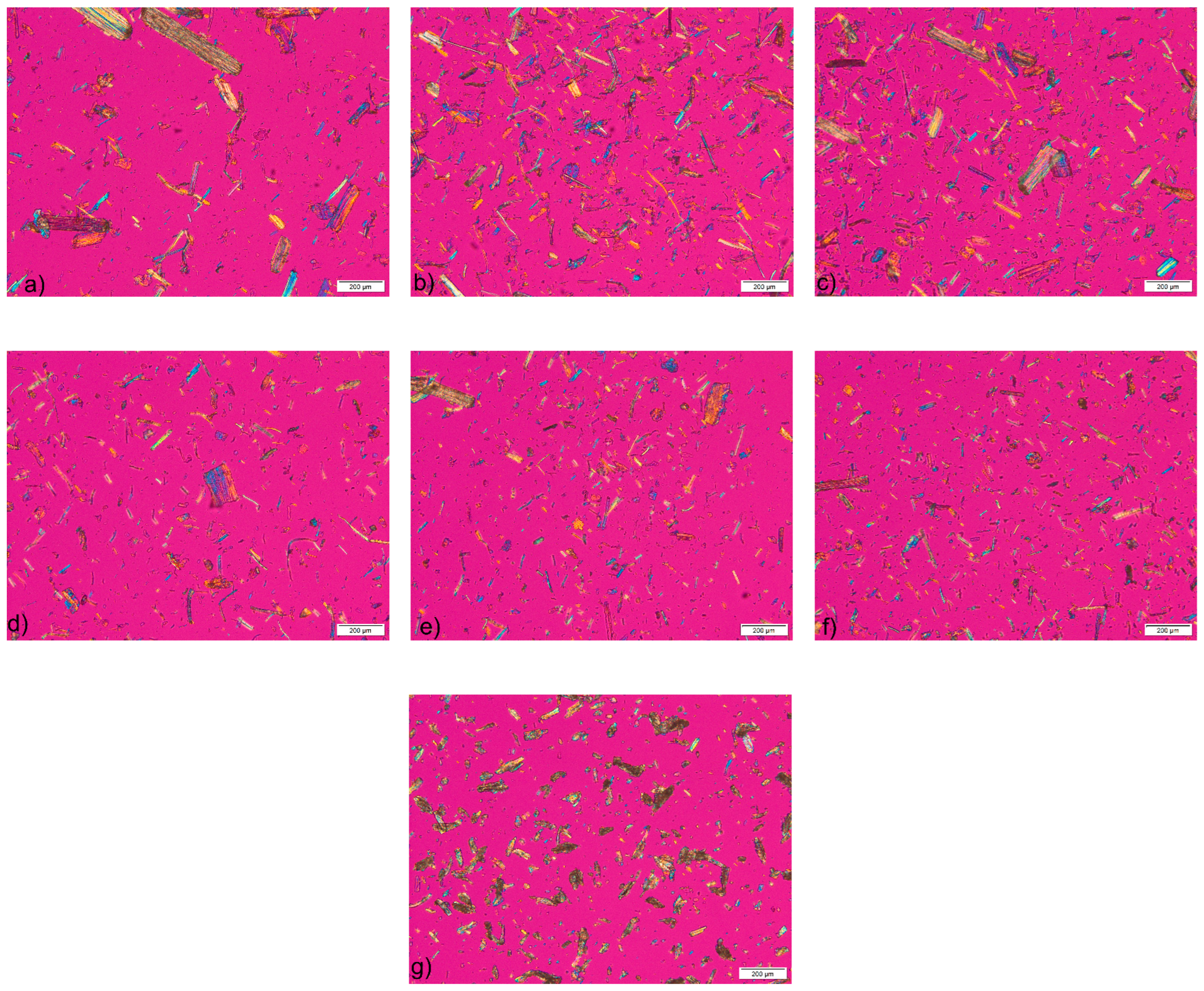
3.2.1. Microscopic Analysis
3.2.2. FT-IR Spectroscopy
3.2.3. PXRD Analysis
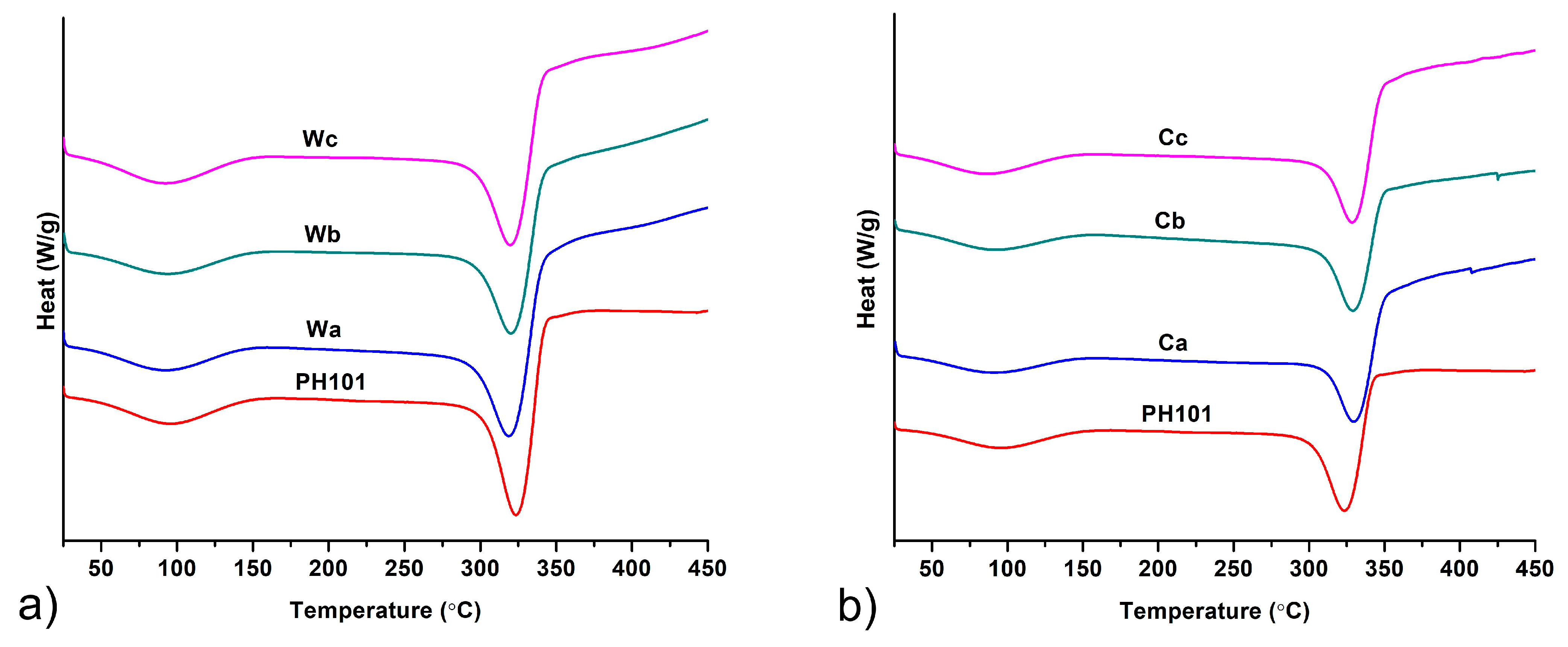
3.2.4. DSC Analysis
3.3. Functional Characterization of MCC
3.3.1. Powder Flow Properties
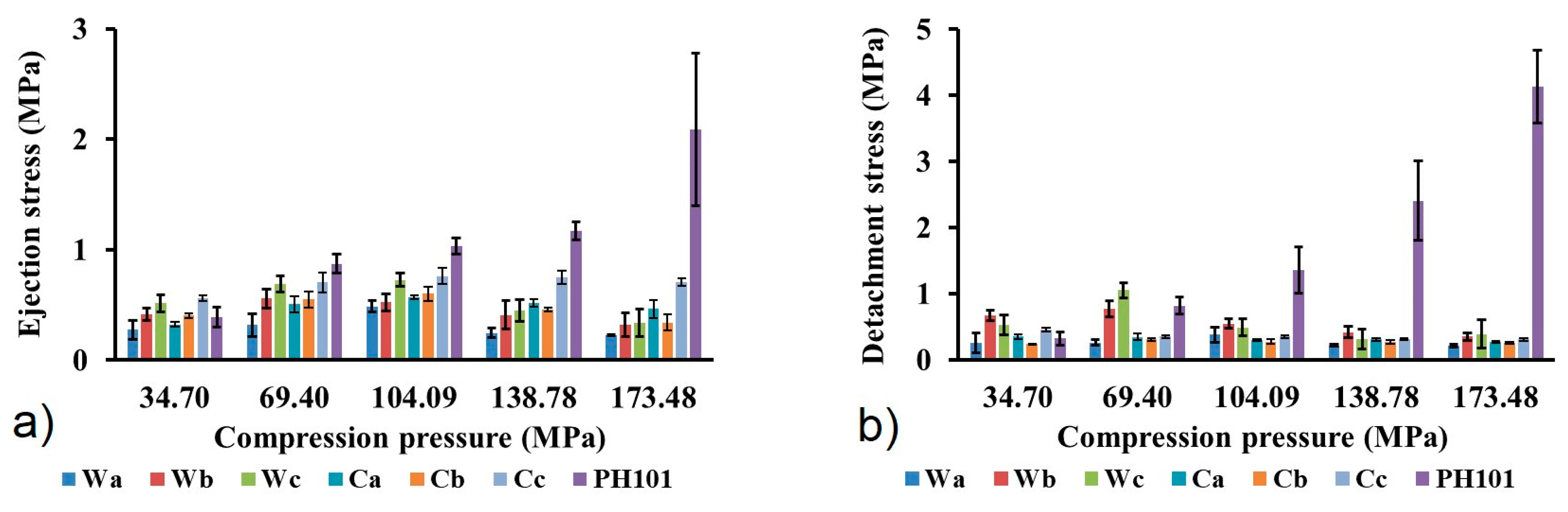
3.3.2. Evaluation of Powder Behaviour in Tableting Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). World Food and Agriculture—Statistical Yearbook 2023; FAO: Rome, Italy, 2023. [Google Scholar]
- Porter, C. World Agricultural Prospects. The Road to 2050; Supply Intelligence: Tunbridge Wells, UK, 2016. [Google Scholar]
- Phiri, R.; Rangappa, S.M.; Siengchin, S. Agro-waste for renewable and sustainable green production: A review. J. Clean. Prod. 2024, 434, 139989. [Google Scholar] [CrossRef]
- Bhuvaneshwari, S.; Hettiarachchi, H.; Meegoda, J.N. Crop Residue Burning in India: Policy Challenges and Potential Solutions. Int. J. Environ. Res. Public Health 2019, 16, 832. [Google Scholar] [CrossRef]
- Singh, D.; Dhiman, S.K.; Kumar, V.; Babu, R.; Shree, K.; Priyadarshani, A.; Singh, A.; Shakya, L.; Nautiyal, A.; Saluja, S. Crop Residue Burning and Its Relationship between Health, Agriculture Value Addition, and Regional Finance. Atmosphere 2022, 13, 1405. [Google Scholar] [CrossRef]
- Debnath, B.; Haldar, D.; Purkait, M.K. A critical review on the techniques used for the synthesis and applications of crystalline cellulose derived from agricultural wastes and forest residues. Carbohydr. Polym. 2021, 273, 118537. [Google Scholar] [CrossRef]
- Krivokapić, J.; Ivanović, J.; Djuriš, J.; Medarević, D.; Potpara, Z.; Maksimović, Z.; Ibrić, S. Tableting properties of microcrystalline cellulose obtained from wheat straw measured with a single punch bench top tablet press. Saudi Pharm. J. 2020, 28, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Patil, T.; Vineeth, S.; Das, S.; Pramanik, A.; Mhaske, S. Isolation of microcrystalline cellulose from corn stover with emphasis on its constituents: Corn cover and corn cob. Mater. Today Proc. 2019, 27, 589–594. [Google Scholar] [CrossRef]
- Li, J.B.; Dong, H.L.; Xiu, H.J.; Zhang, M.Y.; Reddy, K.S.; Zhang, X.F.; Ji, Y. Extraction, separation and refining of microcrys-talline cellulose from wheat straw using various pretreatments. Int. J. Agric. Biol. Eng. 2016, 9, 137. [Google Scholar] [CrossRef]
- Azubuike, C.P.; Okhamafe, A.O. Physicochemical, spectroscopic and thermal properties of microcrystalline cellulose derived from corn cobs. Int. J. Recycl. Org. Waste Agric. 2012, 1, 9. [Google Scholar] [CrossRef]
- Harini, K.; Mohan, C.C. Isolation and characterization of micro and nanocrystalline cellulose fibers from the walnut shell, corncob and sugarcane bagasse. Int. J. Biol. Macromol. 2020, 163, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Barbash, V.A.; Yashchenko, O.V.; Yakymenko, O.S.; Myshak, V.D. Extraction, properties and use of nanocellulose from corn crop residues. Appl. Nanosci. 2023, 13, 7455–7468. [Google Scholar] [CrossRef]
- Sihag, S.S.; Pal, J.; Yadav, M. Extraction and Characterization of Nanocellulose from Wheat Straw: Facile Approach. J. Water Environ. Nanotechnol. 2022, 7, 317–331. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Zhang, Y.; Chi, F.; Chen, C.; Wan, W.; Feng, Y.; Song, X.; Cui, Q. Integrated lactic acid production from lignocellulosic agricultural wastes under thermal conditions. J. Environ. Manag. 2023, 342, 118281. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, S.; Le Bras, J.; Nardello-Rataj, V.; Agach, M.; Estrine, B. Acidic Pretreatment of Wheat Straw in Decanol for the Production of Surfactant, Lignin and Glucose. Int. J. Mol. Sci. 2012, 13, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-L.; Liu, H.; Feng, Q.-H.; Xie, Y.-M.; Zhan, H.-Y. Preparation, characterization and in vitro anticoagulant activity of corn stover xylan sulfates. J. Biomater. Sci. Polym. Ed. 2017, 28, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Mondal, I.H.; Yeasmin, M.S.; Rahman, S. Preparation of food grade carboxymethyl cellulose from corn husk agrowaste. Int. J. Biol. Macromol. 2015, 79, 144–150. [Google Scholar] [CrossRef]
- Zhong, Y.; Frost, H.; Bustamante, M.; Li, S.; Liu, Y.S.; Liao, W. A mechano-biocatalytic one-pot approach to release sugars from lignocellulosic materials. Renew. Sustain. Energy Rev. 2020, 121, 109675. [Google Scholar] [CrossRef]
- Wang, S.; Gao, W.; Li, H.; Xiao, L.; Sun, R.; Song, G. Selective Fragmentation of Biorefinery Corncob Lignin into p-Hydroxycinnamic Esters with a Supported Zinc Molybdate Catalyst. ChemSusChem 2018, 11, 2114–2123. [Google Scholar] [CrossRef] [PubMed]
- Dahliyanti, A.; Widharyanti, I.D.; Curie, C.A. Recovery and characterization of naturally occurring silicon dioxide from corn wastes. AIP Conf. Proc. 2019, 2085, 020048. [Google Scholar] [CrossRef]
- Ahmed, M.; Hameed, B.; Hummadi, E. Insight into the chemically modified crop straw adsorbents for the enhanced removal of water contaminants: A review. J. Mol. Liq. 2021, 330, 115616. [Google Scholar] [CrossRef]
- Wei, S.; Zhu, M.; Song, J.; Peng, P. Comprehensive Characterization of Biochars Produced from Three Major Crop Straws of China. BioResources 2017, 12, 3316–3330. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Xie, W.; Deng, H. Extraction, isolation and characterization of nanocrystalline cellulose from industrial kelp (Laminaria japonica) waste. Carbohydr. Polym. 2017, 173, 353–359. [Google Scholar] [CrossRef]
- Adel, A.M.; El–Wahab, Z.H.A.; Ibrahim, A.A.; Al–Shemy, M.T. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part I. Acid catalyzed hydrolysis. Bioresour. Technol. 2010, 101, 4446–4455. [Google Scholar] [CrossRef] [PubMed]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.R.N.; Taiwo, O.F.A.; Hassan, T.M.; Haafiz, M.K.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Shao, X.; Wang, J.; Liu, Z.; Hu, N.; Liu, M.; Xu, Y. Preparation and Characterization of Porous Microcrystalline Cellulose from Corncob. Ind. Crop. Prod. 2020, 151, 112457. [Google Scholar] [CrossRef]
- Katakojwala, R.; Mohan, S.V. Microcrystalline cellulose production from sugarcane bagasse: Sustainable process development and life cycle assessment. J. Clean. Prod. 2020, 249, 119342. [Google Scholar] [CrossRef]
- He, C.; Li, H.; Huan, O.; Wei, H.; Xiong, H.; Ni, H.; Zheng, M. Physicochemical properties and structure characterization of microcrystalline cellulose from pomelo fruitlets. J. Food Process. Preserv. 2022, 46, e17071. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Chen, M.; Goff, H.D.; Zhong, F.; Sharif, H.R.; Li, Y. Functionality and nutritional aspects of microcrystalline cellulose in food. Carbohydr. Polym. 2017, 172, 159–174. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications). In Agriculture Handbook; U.S. Agricultural Research Service: Washington, DC, USA, 1970. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Kirk, T.K.; Obst, J.R. Lignin Determination. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 161, pp. 87–101. ISBN 978-0-12-182062-6. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Health Care. European Pharmacopoeia, 11th ed.; European Directorate for the Quality of Medicines & Health Care: Strasbourg, France, 2022. [Google Scholar]
- Fell, J.T.; Newton, J.M. Determination of Tablet Strength by the Diametral-Compression Test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef]
- Sun, R.; Tomkinson, J.; Wang, Y.; Xiao, B. Physico-chemical and structural characterization of hemicelluloses from wheat straw by alkaline peroxide extraction. Polymer 2000, 41, 2647–2656. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2007, 99, 1664–1671. [Google Scholar] [CrossRef]
- Kambli, N.; Basak, S.; Samanta, K.K.; Deshmukh, R.R. Extraction of natural cellulosic fibers from cornhusk and its physico-chemical properties. Fibers Polym. 2016, 17, 687–694. [Google Scholar] [CrossRef]
- Woźniak, M.; Ratajczak, I.; Wojcieszak, D.; Waśkiewicz, A.; Szentner, K.; Przybył, J.; Borysiak, S.; Goliński, P. Chemical and Structural Characterization of Maize Stover Fractions in Aspect of Its Possible Applications. Materials 2021, 14, 1527. [Google Scholar] [CrossRef]
- Asahi Kasei: Ceolus™ PH Product Information. Available online: https://www.ceolus.com/en/mcc/grade_lineup/ceolus_ph/ (accessed on 10 May 2024).
- Kian, L.; Saba, N.; Jawaid, M.; Fouad, H. Characterization of microcrystalline cellulose extracted from olive fiber. Int. J. Biol. Macromol. 2020, 156, 347–353. [Google Scholar] [CrossRef]
- Gichuki, J.; Kareru, P.G.; Gachanja, A.N.; Ngamau, C. Characteristics of Microcrystalline Cellulose from Coir Fibers. J. Nat. Fibers 2020, 19, 915–930. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; El-Zawawy, W.K.; Jüttke, Y.; Koschella, A.; Heinze, T. Cellulose and microcrystalline cellulose from rice straw and banana plant waste: Preparation and characterization. Cellulose 2013, 20, 2403–2416. [Google Scholar] [CrossRef]
- Abdullah, M.; Nazir, M.; Raza, M.; Wahjoedi, B.; Yussof, A. Autoclave and ultra-sonication treatments of oil palm empty fruit bunch fibers for cellulose extraction and its polypropylene composite properties. J. Clean. Prod. 2016, 126, 686–697. [Google Scholar] [CrossRef]
- Mahmoud, Y.; Safidine, Z.; Belhaneche-Bensemra, N. Characterization of microcrystalline cellulose extracted from walnut and apricots shells by alkaline treatment. J. Serbian Chem. Soc. 2021, 86, 521–532. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M. Microcrystalline cellulose from Posidonia oceanica brown algae: Extraction and characterization. Int. J. Biol. Macromol. 2019, 138, 837–845. [Google Scholar] [CrossRef]
- Landín, M.; Martínez-Pacheco, R.; Gómez-Amoza, J.; Souto, C.; Concheiro, A.; Rowe, R. Effect of batch variation and source of pulp on the properties of microcrystalline cellulose. Int. J. Pharm. 1993, 91, 133–141. [Google Scholar] [CrossRef]
- Shlieout, G.; Arnold, K.; Müller, G. Powder and mechanical properties of microcrystalline cellulose with different degrees of polymerization. AAPS Pharmscitech 2002, 3, 45–54. [Google Scholar] [CrossRef]
- Ventura-Cruz, S.; Flores-Alamo, N.; Tecante, A. Preparation of microcrystalline cellulose from residual Rose stems (Rosa spp.) by successive delignification with alkaline hydrogen peroxide. Int. J. Biol. Macromol. 2020, 155, 324–329. [Google Scholar] [CrossRef]
- Jantip, S.; Suwanruji, P. Preparation and Properties of Microcrystalline Cellulose from Corn Residues. Adv. Mater. Res. 2011, 332–334, 1781–1784. [Google Scholar] [CrossRef]
- Kambli, N.D.; Mageshwaran, V.; Patil, P.G.; Saxena, S.; Deshmukh, R.R. Synthesis and characterization of microcrystalline cellulose powder from corn husk fibres using bio-chemical route. Cellulose 2017, 24, 5355–5369. [Google Scholar] [CrossRef]
- Taye, M.; Chaudhary, B.U.; Kale, R.D. Extraction and Analysis of Microcrystalline Cellulose from Delignified Serte Leaf Fiber Wastes. J. Nat. Fibers 2019, 18, 1729–1741. [Google Scholar] [CrossRef]
- Neves, R.M.; Ornaghi, H.L.; Zattera, A.J.; Amico, S.C. The influence of silane surface modification on microcrystalline cellulose characteristics. Carbohydr. Polym. 2019, 230, 115595. [Google Scholar] [CrossRef] [PubMed]
- Medarević, D.; Djuriš, J.; Krkobabić, M.; Ibrić, S. Improving Tableting Performance of Lactose Monohydrate by Fluid-Bed Melt Granulation Co-Processing. Pharmaceutics 2021, 13, 2165. [Google Scholar] [CrossRef] [PubMed]
- Krstic, M.; Maksimović, Z.; Ibrić, S.; Bakic, T.; Prodanovic, J.; Ražić, S. Lignocellulosic biomass as a source of microcrystalline cellulose-chemical and technological characterization and future perspectives. Cellul. Chem. Technol. 2018, 52, 577–588. [Google Scholar]
- Anuar, M.; Briscoe, B. The elastic relaxation of starch tablets during ejection. Powder Technol. 2009, 195, 96–104. [Google Scholar] [CrossRef]
- Pitt, K.G.; Webber, R.J.; Hill, K.A.; Dey, D.; Gamlen, M.J. Compression prediction accuracy from small scale compaction studies to production presses. Powder Technol. 2015, 270, 490–493. [Google Scholar] [CrossRef]
- Osamura, T.; Takeuchi, Y.; Onodera, R.; Kitamura, M.; Takahashi, Y.; Tahara, K.; Takeuchi, H. Characterization of tableting properties measured with a multi-functional compaction instrument for several pharmaceutical excipients and actual tablet formulations. Int. J. Pharm. 2016, 510, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Schaller, B.E.; Moroney, K.M.; Castro-Dominguez, B.; Cronin, P.; Belen-Girona, J.; Ruane, P.; Croker, D.M.; Walker, G.M. Systematic development of a high dosage formulation to enable direct compression of a poorly flowing API: A case study. Int. J. Pharm. 2019, 566, 615–630. [Google Scholar] [CrossRef] [PubMed]




| Sample | CEL (%) | HEM (%) | LIG (%) | ASH (%) |
|---|---|---|---|---|
| W | 54.09 | 23.26 | 14.11 | 6.51 |
| C | 41.01 | 37.43 | 13.17 | 7.87 |
| W 1% | 78.52 | 10.21 | 4.62 | 2.34 |
| W 2% | 79.07 | 11.27 | 3.14 | 2.16 |
| W 4% | 81.14 | 8.11 | 4.2 | 2.62 |
| W 8% | 77.21 | 8.24 | 5.58 | 3.62 |
| C 1% | 75.67 | 13.60 | 4.12 | 5.29 |
| C 2% | 75.81 | 12.41 | 3.71 | 8.06 |
| C 4% | 73.26 | 15.14 | 2.62 | 5.90 |
| C 8% | 78.55 | 12.92 | 2.56 | 5.87 |
| Sample | Source of HRs | HCl Concentration Used for Hydrolysis |
|---|---|---|
| Wa | Wheat | 1 M |
| Wb | Wheat | 1.5 M |
| Wc | Wheat | 2 M |
| Ca | Corn | 1 M |
| Cb | Corn | 1.5 M |
| Cc | Corn | 2 M |
| Wa | Wb | Wc | Ca | Cb | Cc | PH101 | |
|---|---|---|---|---|---|---|---|
| Mean particle size (μm) | 58.47 | 49.48 | 42.97 | 60.95 | 45.06 | 39.05 | 54.44 |
| D10 (μm) | 14.92 | 13.80 | 9.89 | 20.47 | 14.81 | 14.42 | 16.07 |
| D50 (μm) | 35.62 | 34.89 | 26.55 | 50.69 | 35.59 | 29.52 | 43.94 |
| D90 (μm) | 140.17 | 99.33 | 86.13 | 116.67 | 81.78 | 73.75 | 106.43 |
| Span | 3.52 | 2.45 | 2.87 | 1.90 | 1.88 | 2.01 | 2.06 |
| MCC Sample | Bulk Density (g/cm3) | Tapped Density (g/cm3) | CI (%) | Hausner Ratio | Flowability (Ph. Eur 11.0 (2.9.36)) |
|---|---|---|---|---|---|
| Wa | 0.230 ± 0.009 | 0.307 ± 0.006 | 25.01 ± 2.10 | 1.33 ± 0.04 | Poor |
| Wb | 0.209 ± 0.010 | 0.289 ± 0.017 | 27.62 ± 0.98 | 1.38 ± 0.02 | Poor |
| Wc | 0.230 ± 0.003 | 0.361 ± 0.009 | 36.33 ± 1.56 | 1.57 ± 0.04 | Very poor |
| Ca | 0.236 ± 0.009 | 0.358 ± 0.015 | 33.91 ± 0.50 | 1.51 ± 0.01 | Very poor |
| Cb | 0.289 ± 0.001 | 0.407 ± 0.001 | 29.13 ± 0.25 | 1.41 ± 0.01 | Poor |
| Cc | 0.324 ± 0.012 | 0.485 ± 0.030 | 32.60 ± 1.57 | 1.48 ± 0.03 | Very poor |
| PH101 | 0.352 ± 0.016 | 0.456 ± 0.029 | 22.67 ± 2.00 | 1.29 ± 0.03 | Passable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medarević, D.; Čežek, M.; Knežević, A.; Turković, E.; Barudžija, T.; Samardžić, S.; Maksimović, Z. From Field to Pharmacy: Isolation, Characterization and Tableting Behaviour of Microcrystalline Cellulose from Wheat and Corn Harvest Residues. Pharmaceutics 2024, 16, 1090. https://doi.org/10.3390/pharmaceutics16081090
Medarević D, Čežek M, Knežević A, Turković E, Barudžija T, Samardžić S, Maksimović Z. From Field to Pharmacy: Isolation, Characterization and Tableting Behaviour of Microcrystalline Cellulose from Wheat and Corn Harvest Residues. Pharmaceutics. 2024; 16(8):1090. https://doi.org/10.3390/pharmaceutics16081090
Chicago/Turabian StyleMedarević, Djordje, Maša Čežek, Aleksandar Knežević, Erna Turković, Tanja Barudžija, Stevan Samardžić, and Zoran Maksimović. 2024. "From Field to Pharmacy: Isolation, Characterization and Tableting Behaviour of Microcrystalline Cellulose from Wheat and Corn Harvest Residues" Pharmaceutics 16, no. 8: 1090. https://doi.org/10.3390/pharmaceutics16081090





