Dry Powder Inhaler Formulation of Lactobacillus rhamnosus GG Targeting Pseudomonas aeruginosa Infection in Bronchiectasis Maintenance Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of DPI of LGG by SFD
2.2.2. LGG Cell Viability after SFD
2.2.3. Aerosolization Efficiency
2.2.4. Physical Characterizations of the Optimal Formulation of DPI of LGG
2.2.5. Cytotoxicity of DPI of LGG toward A549 and HBE cells
2.2.6. Adhesion of DPI of LGG to A549 and HBE Cells
2.2.7. P. aeruginosa Growth Inhibition by DPI of LGG
2.2.8. Inhibition of P. aeruginosa Adhesion to HBE Cells by DPI of LGG
2.2.9. LGG Cell Viability and DPI Stability after Storage
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Optimal Excipient Compositions for DPI of LGG
3.1.1. LGG Cell Viability after SFD and Aerosolization Efficiency
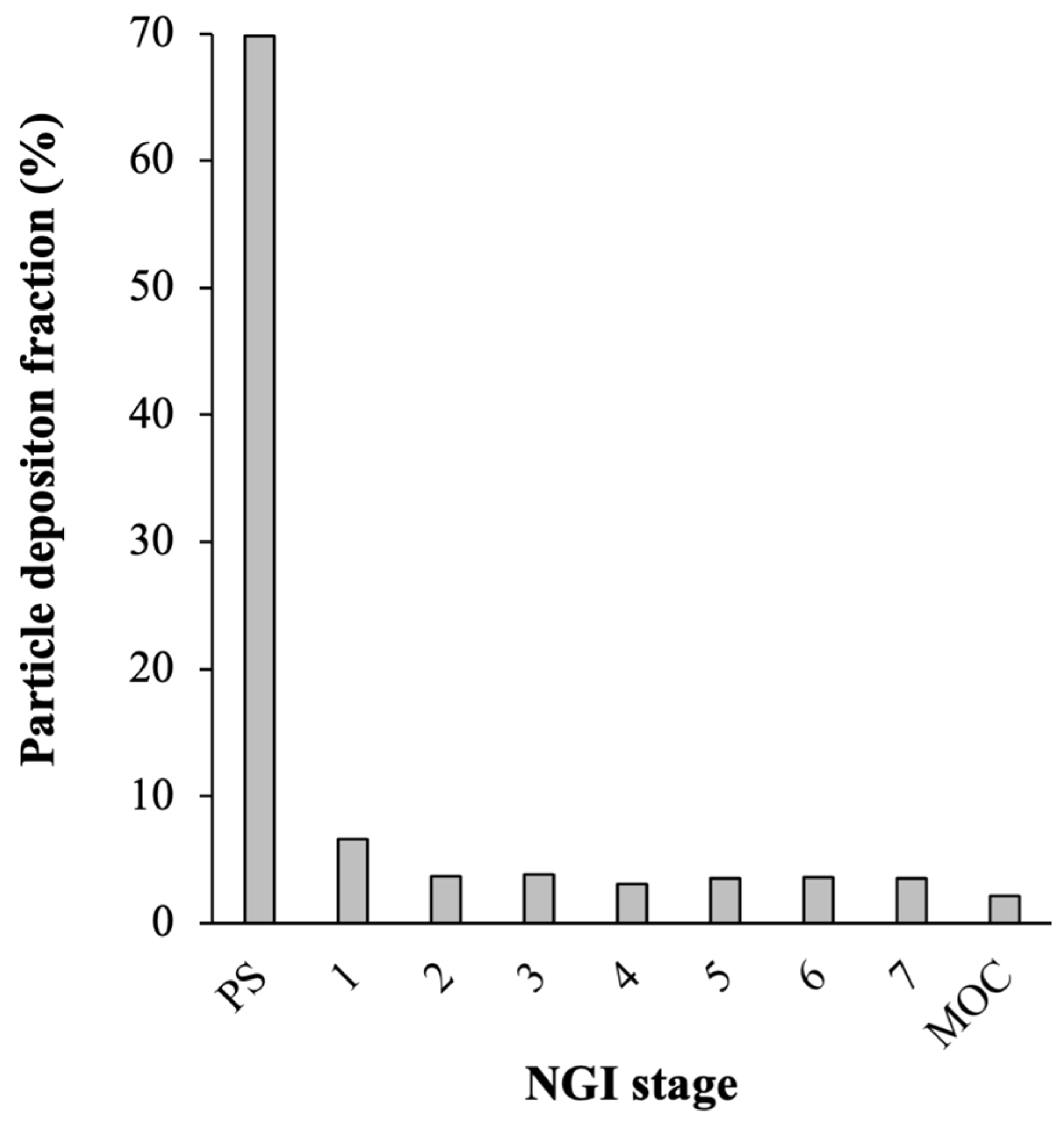
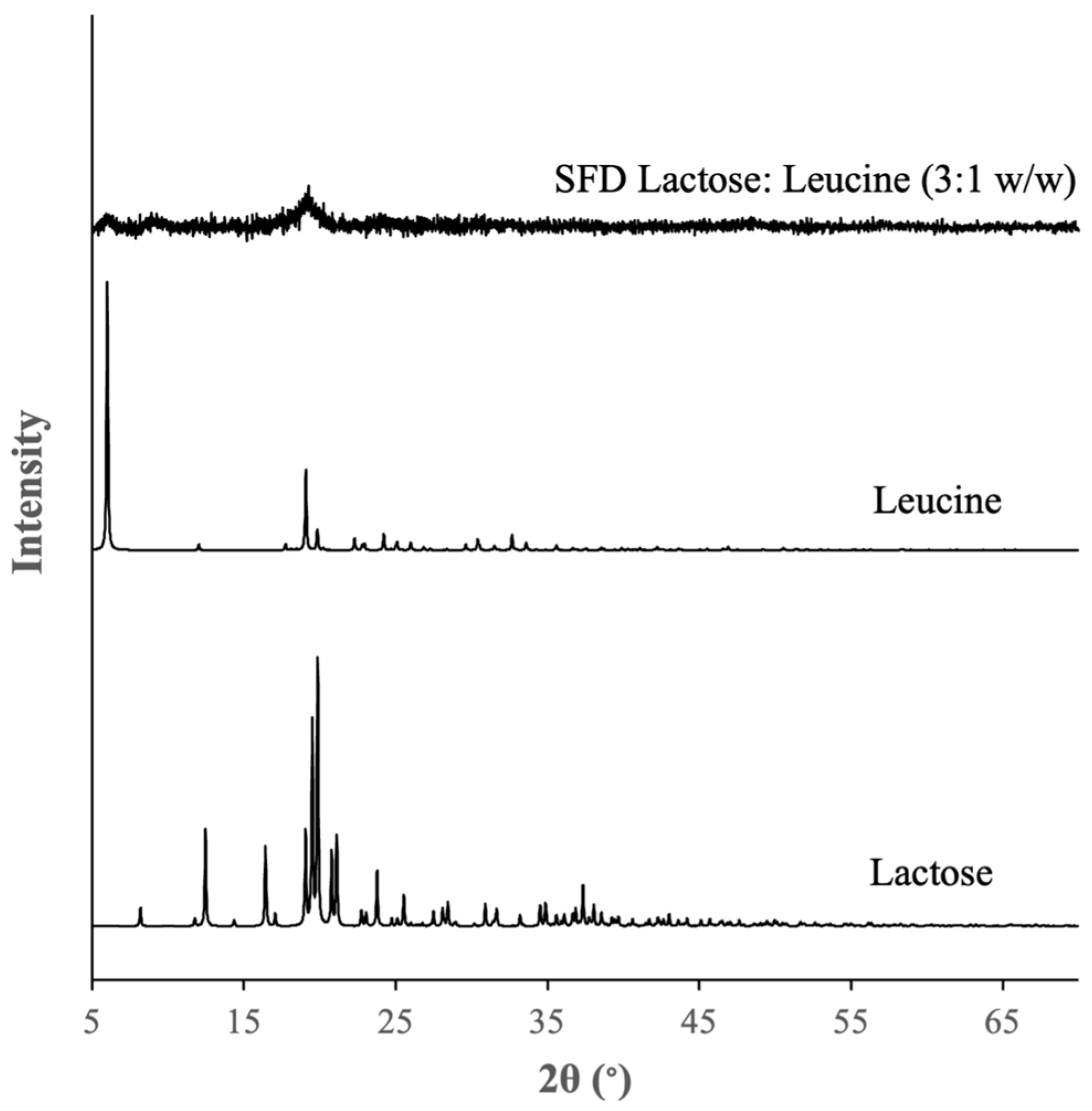
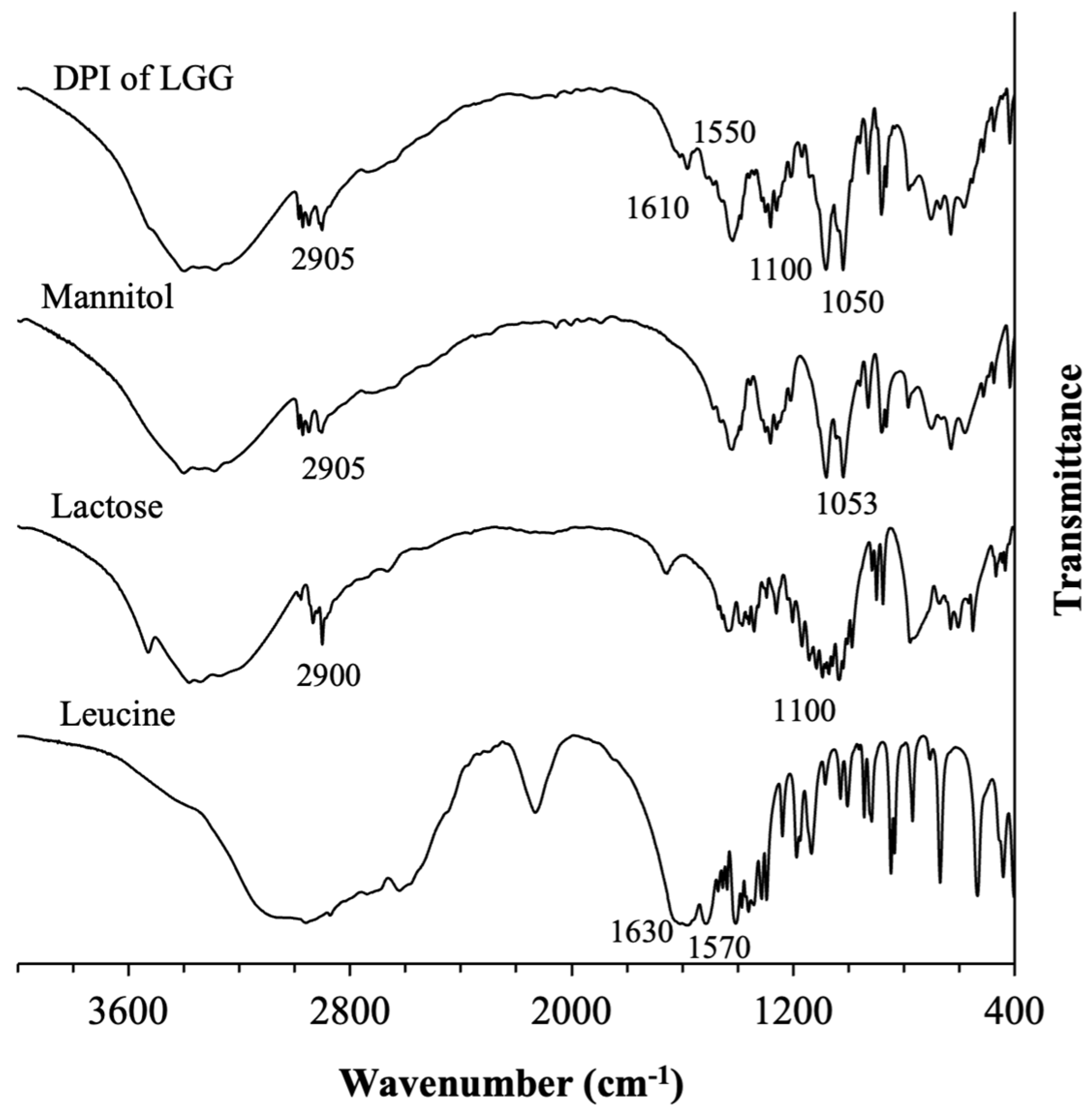
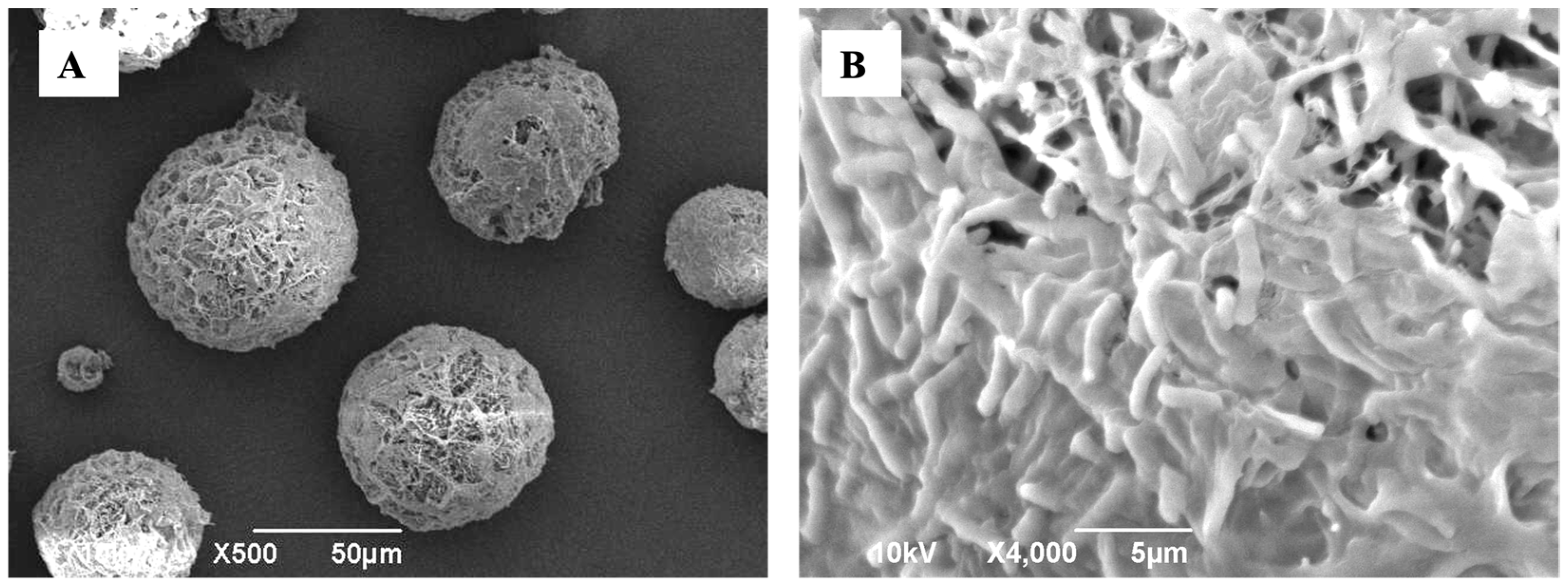
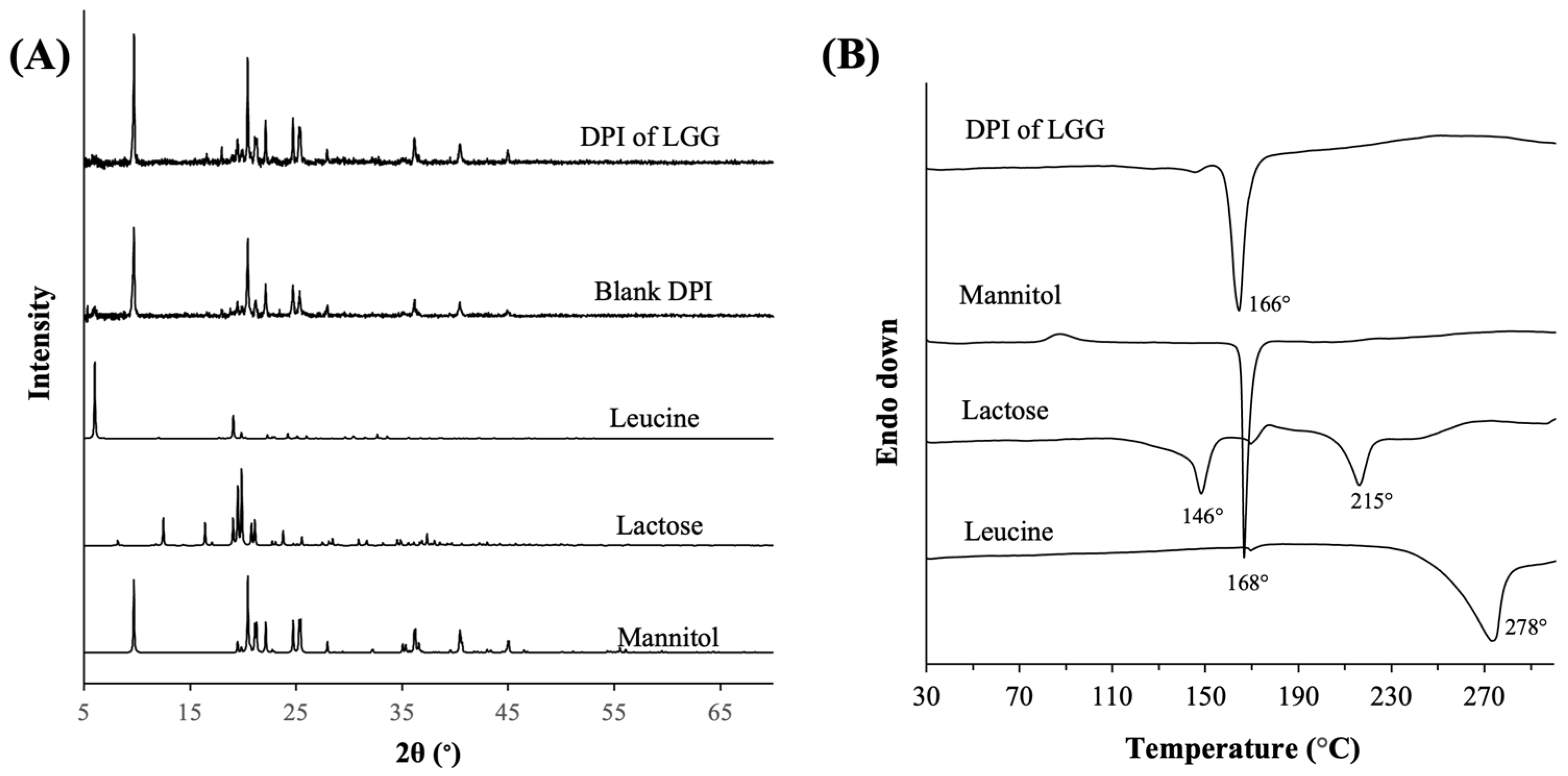
3.1.2. Physical Characteristics of the Optimal Formulation of DPI of LGG
3.2. Performances of the Optimal Formulation of DPI of LGG
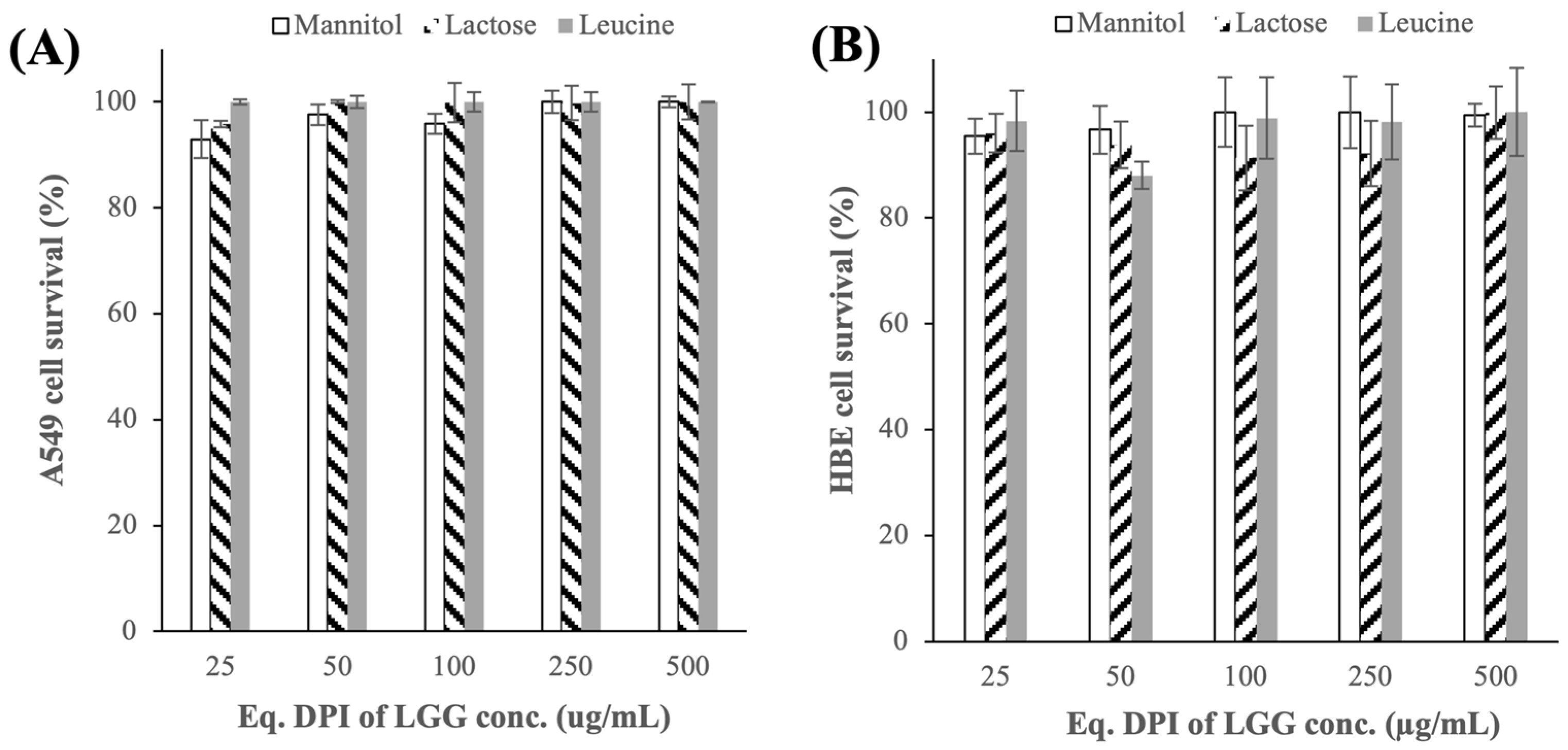
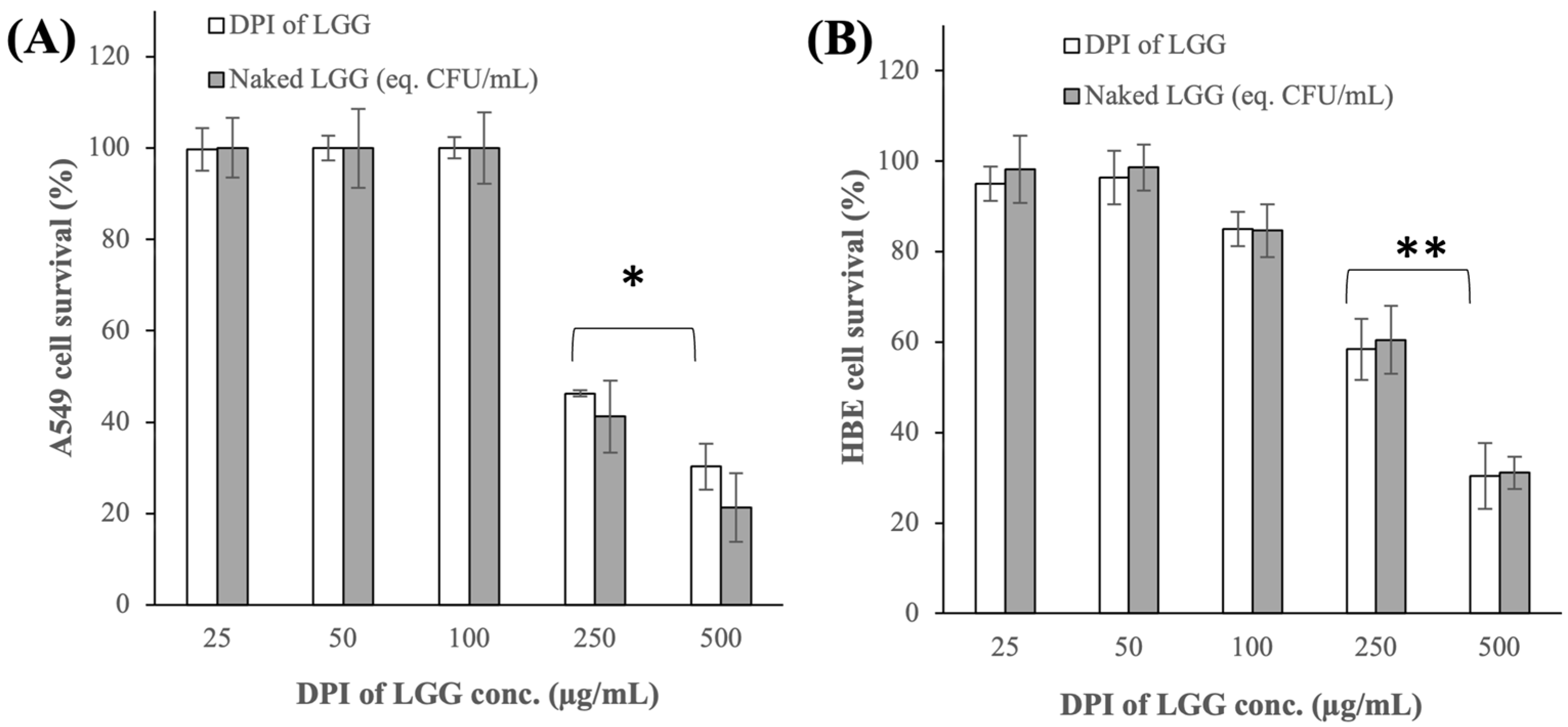
3.2.1. Cytotoxicity Toward A549 and HBE Cells
3.2.2. Adhesion Capacity to A549 and HBE Cells
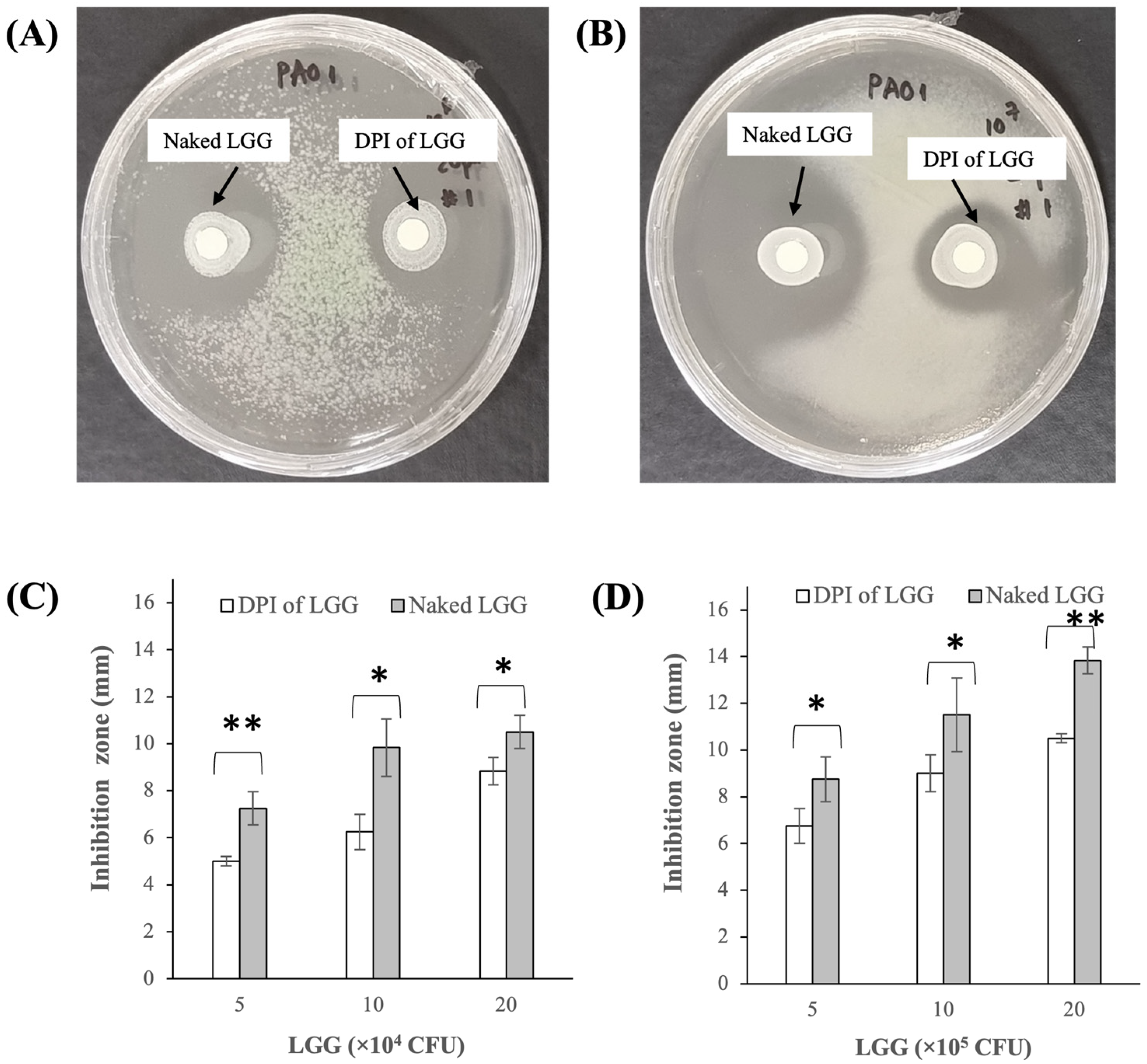
3.2.3. Growth Inhibition of P. aeruginosa PAO1
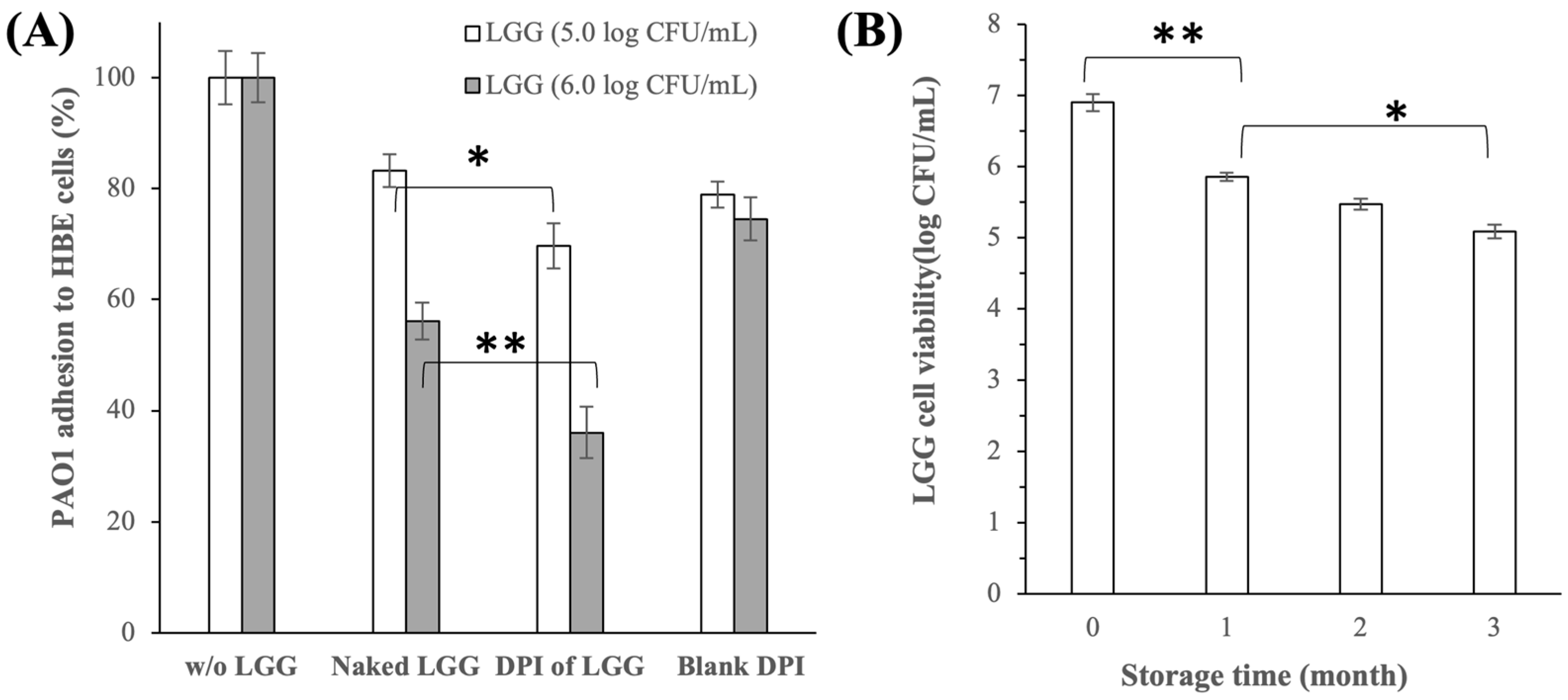
3.2.4. Inhibition of P. aeruginosa PAO1 Adhesion to HBE Cells
3.2.5. LGG Cell Viability and DPI of LGG Stability after Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Yuksel, N.; Gelmez, B.; Yildiz-Pekoz, A. Lung Microbiota: Its Relationship to Respiratory System Diseases and Approaches for Lung-Targeted Probiotic Bacteria Delivery. Mol. Pharm. 2023, 20, 3320–3337. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.L.; Miranda, M.; Fialho, A.K.; Castro-Faria-Neto, H.; Anatriello, E.; Keller, A.C.; Aimbire, F. Oral Feeding with Probiotic Lactobacillus Rhamnosus Attenuates Cigarette Smoke-Induced COPD in C57Bl/6 Mice: Relevance to Inflammatory Markers in Human Bronchial Epithelial Cells. PLoS ONE 2020, 15, e0225560. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Soni, K.D.; Mathur, P. Efficacy of Probiotics in the Prevention of VAP in Critically Ill ICU Patients: An Updated Systematic Review and Meta-Analysis of Randomized Control Trials. J. Intensive Care 2020, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Sadrifar, S.; Abbasi-Dokht, T.; Forouzandeh, S.; Malek, F.; Yousefi, B.; Salek Farrokhi, A.; Karami, J.; Baharlou, R. Immunomodulatory Effects of Probiotic Supplementation in Patients with Asthma: A Randomized, Double-Blind, Placebo-Controlled Trial. Allergy Asthma Clin. Immunol. 2023, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; He, F.; Kubota, A.; Harata, G.; Hiramatsu, M. Oral Administration of Lactobacilli from Human Intestinal Tract Protects Mice against Influenza Virus Infection. Lett. Appl. Microbiol. 2010, 51, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.; Cheema, H.A.; Mithani, M.S.; Shahid, A.; Nawaz, A.; Hermis, A.H.; Chinnam, S.; Nashwan, A.J.; Cherrez-Ojeda, I.; Awan, R.U.; et al. Probiotics for the Prevention and Treatment of COVID-19: A Rapid Systematic Review and Meta-Analysis. Front. Nutr. 2023, 10, 1274122. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Ahrari, I.; Ghanei, M.; Jamalkandi, S.A.; Karimi, M.; Salimian, J. Oral and Nasal Probiotic Administration for the Prevention and Alleviation of Allergic Diseases, Asthma and Chronic Obstructive Pulmonary Disease. Nutr. Res. Rev. 2021, 34, 1–16. [Google Scholar] [CrossRef]
- Jung, Y.-J.; Lee, Y.-T.; Ngo, V.; Cho, Y.-H.; Ko, E.-J.; Hong, S.-M.; Kim, K.-H.; Jang, J.-H.; Oh, J.-S.; Park, M.-K.; et al. Heat-Killed Lactobacillus Casei Confers Broad Protection against Influenza A Virus Primary Infection and Develops Heterosubtypic Immunity against Future Secondary Infection. Sci. Rep. 2017, 7, 17360. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-K.; Ngo, V.; Kwon, Y.-M.; Lee, Y.-T.; Yoo, S.; Cho, Y.-H.; Hong, S.-M.; Hwang, H.S.; Ko, E.-J.; Jung, Y.-J.; et al. Lactobacillus Plantarum DK119 as a Probiotic Confers Protection against Influenza Virus by Modulating Innate Immunity. PLoS ONE 2013, 8, e75368. [Google Scholar] [CrossRef]
- Tomosada, Y.; Chiba, E.; Zelaya, H.; Takahashi, T.; Tsukida, K.; Kitazawa, H.; Alvarez, S.; Villena, J. Nasally Administered Lactobacillus Rhamnosus Strains Differentially Modulate Respiratory Antiviral Immune Responses and Induce Protection against Respiratory Syncytial Virus Infection. BMC Immunol. 2013, 14, 40. [Google Scholar] [CrossRef]
- Jokicevic, K.; Kiekens, S.; Byl, E.; De Boeck, I.; Cauwenberghs, E.; Lebeer, S.; Kiekens, F. Probiotic Nasal Spray Development by Spray Drying. Eur. J. Pharm. Biopharm. 2021, 159, 211–220. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Ghanavati, R.; Afifirad, R.; Darb Emamie, A.; kakanj, M.; Talebi, M. The Effect of Probiotics on Respiratory Tract Infection with Special Emphasis on COVID-19: Systemic Review 2010–20. Int. J. Infect. Dis. 2021, 105, 91–104. [Google Scholar] [CrossRef]
- Spacova, I.; Petrova, M.I.; Fremau, A.; Pollaris, L.; Vanoirbeek, J.; Ceuppens, J.L.; Seys, S.; Lebeer, S. Intranasal Administration of Probiotic Lactobacillus Rhamnosus GG Prevents Birch Pollen-Induced Allergic Asthma in a Murine Model. Allergy 2019, 74, 100–110. [Google Scholar] [CrossRef]
- Byun, A.S.; Vitetta, L.; Chan, H.-K.; Kwok, P.C.L. Respiratory Delivery of Probiotics to Improve Lung Health. In Respiratory Delivery of Biologics, Nucleic Acids, and Vaccines; Lam, J., Kwok, P.C.L., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 149–172. ISBN 978-3-031-47567-2. [Google Scholar]
- Le Noci, V.; Guglielmetti, S.; Arioli, S.; Camisaschi, C.; Bianchi, F.; Sommariva, M.; Storti, C.; Triulzi, T.; Castelli, C.; Balsari, A.; et al. Modulation of Pulmonary Microbiota by Antibiotic or Probiotic Aerosol Therapy: A Strategy to Promote Immunosurveillance against Lung Metastases. Cell Rep. 2018, 24, 3528–3538. [Google Scholar] [CrossRef] [PubMed]
- Lal, V.; Ambalavanan, N.; Gaggar, A.; Morrow, C. Inhaled Respiratory Probiotics for Lung Diseases of Infancy, Childhood and Adulthood 2021. U.S. Patent 11,141,443, 12 October 2021. [Google Scholar]
- Glieca, S.; Quarta, E.; Bottari, B.; Bancalari, E.; Monica, S.; Scaltriti, E.; Tambassi, M.; Flammini, L.; Bertoni, S.; Bianchera, A.; et al. Development of Inhalation Powders Containing Lactic Acid Bacteria with Antimicrobial Activity against Pseudomonas Aeruginosa. Int. J. Antimicrob. Agents 2024, 63, 107001. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Chang, A.B.; Chotirmall, S.H.; Dhar, R.; McShane, P.J. Bronchiectasis. Nat. Rev. Dis. Primers 2018, 4, 45. [Google Scholar] [CrossRef]
- Conceição, M.; Shteinberg, M.; Goeminne, P.; Altenburg, J.; Chalmers, J.D. Eradication Treatment for Pseudomonas aeruginosa Infection in Adults with Bronchiectasis: A Systematic Review and Meta-Analysis. Eur. Respir. Rev. 2024, 33, 230178. [Google Scholar] [CrossRef] [PubMed]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Leucine as an Excipient in Spray Dried Powder for Inhalation. Drug Discov. Today 2021, 26, 2384–2396. [Google Scholar] [CrossRef] [PubMed]
- Trisopon, K.; Kittipongpatana, O.S.; Saokham, P. Optimizing Large Porous Mannitol-Leucine Microparticles via Spray Drying Technique. Adv. Powder Technol. 2024, 35, 104512. [Google Scholar] [CrossRef]
- Moreira, M.T.C.; Martins, E.; Perrone, Í.T.; de Freitas, R.; Queiroz, L.S.; de Carvalho, A.F. Challenges Associated with Spray Drying of Lactic Acid Bacteria: Understanding Cell Viability Loss. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3267–3283. [Google Scholar] [CrossRef]
- Tran, T.-T.; Vidaillac, C.; Yu, H.; Yong, V.F.L.; Roizman, D.; Chandrasekaran, R.; Lim, A.Y.H.; Low, T.B.; Tan, G.L.; Abisheganaden, J.A.; et al. A New Therapeutic Avenue for Bronchiectasis: Dry Powder Inhaler of Ciprofloxacin Nanoplex Exhibits Superior Ex Vivo Mucus Permeability and Antibacterial Efficacy to Its Native Ciprofloxacin Counterpart. Int. J. Pharm. 2018, 547, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Lei, A.; Zhang, N.; Zhu, C. The Beneficial Role of Probiotic Lactobacillus in Respiratory Diseases. Front. Immunol. 2022, 13, 908010. [Google Scholar] [CrossRef] [PubMed]
- Harata, G.; He, F.; Hiruta, N.; Kawase, M.; Kubota, A.; Hiramatsu, M.; Yausi, H. Intranasal Administration of Lactobacillus Rhamnosus GG Protects Mice from H1N1 Influenza Virus Infection by Regulating Respiratory Immune Responses. Lett. Appl. Microbiol. 2010, 50, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-S.; Quan Toh, Z.; Dunne, E.M.; Mulholland, E.K.; Tang, M.L.; Robins-Browne, R.M.; Licciardi, P.V.; Satzke, C. Inhibition of Streptococcus Pneumoniae Adherence to Human Epithelial Cells in Vitro by the Probiotic Lactobacillus Rhamnosus GG. BMC Res. Notes 2013, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fu, N.; Woo, M.W.; Chen, X.D. Heat Stability of Lactobacillus Rhamnosus GG and Its Cellular Membrane during Droplet Drying and Heat Treatment. Food Res. Int. 2018, 112, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tran, T.; Teo, J.; Hadinoto, K. Dry Powder Aerosols of Curcumin-Chitosan Nanoparticle Complex Prepared by Spray Freeze Drying and Their Antimicrobial Efficacy against Common Respiratory Bacterial Pathogens. Colloids Surf. A-Physicochem. Eng. Asp. 2016, 504, 34–42. [Google Scholar] [CrossRef]
- Farinha, S.; Sá, J.V.; Lino, P.R.; Galésio, M.; Pires, J.; Rodrigues, M.Â.; Henriques, J. Spray Freeze Drying of Biologics: A Review and Applications for Inhalation Delivery. Pharm. Res. 2023, 40, 1115–1140. [Google Scholar] [CrossRef]
- Gurram, S.; Jha, D.K.; Shah, D.S.; Kshirsagar, M.M.; Amin, P.D. Insights on the Critical Parameters Affecting the Probiotic Viability During Stabilization Process and Formulation Development. AAPS PharmSciTech 2021, 22, 156. [Google Scholar] [CrossRef] [PubMed]
- Mahar, R.; Chakraborty, A.; Nainwal, N. The Influence of Carrier Type, Physical Characteristics, and Blending Techniques on the Performance of Dry Powder Inhalers. J. Drug Deliv. Sci. Technol. 2022, 76, 103759. [Google Scholar] [CrossRef]
- Warren, E.; Morgan, K.; Toward, T.J.; Schwenkglenks, M.; Leadbetter, J. Cost Effectiveness of Inhaled Mannitol (Bronchitol®) in Patients with Cystic Fibrosis. PharmacoEconomics 2019, 37, 435–446. [Google Scholar] [CrossRef]
- Nevitt, S.; Thornton, J.; Murray, C.; Dwyer, T. Inhaled Mannitol for Cystic Fibrosis. Cochrane Database Syst. Rev. 2020, 1–115. [Google Scholar] [CrossRef] [PubMed]
- Louey, M.D.; Van Oort, M.; Hickey, A.J. Standardized Entrainment Tubes for the Evaluation of Pharmaceutical Dry Powder Dispersion. J. Aerosol Sci. 2006, 37, 1520–1531. [Google Scholar] [CrossRef]
- Yang, Y.; Cheow, W.; Hadinoto, K. Dry Powder Inhaler Formulation of Lipid-Polymer Hybrid Nanoparticles via Electrostatically-Driven Nanoparticle Assembly onto Microscale Carrier Particles. Int. J. Pharm. 2012, 434, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, I.; van den Broek, M.F.L.; Allonsius, C.N.; Spacova, I.; Wittouck, S.; Martens, K.; Wuyts, S.; Cauwenberghs, E.; Jokicevic, K.; Vandenheuvel, D.; et al. Lactobacilli Have a Niche in the Human Nose. Cell Rep. 2020, 31, 107674. [Google Scholar] [CrossRef]
- Listiohadi, Y.; Hourigan, J.A.; Sleigh, R.W.; Steele, R.J. Moisture Sorption, Compressibility and Caking of Lactose Polymorphs. Int. J. Pharm. 2008, 359, 123–134. [Google Scholar] [CrossRef]
- Kho, K.; Hadinoto, K. Dry Powder Inhaler Delivery of Amorphous Drug Nanoparticles: Effects of the Lactose Carrier Particle Shape and Size. Powder Technol. 2013, 233, 303–311. [Google Scholar] [CrossRef]
- Jofré, A.; Aymerich, T.; Garriga, M. Impact of Different Cryoprotectants on the Survival of Freeze-Dried Lactobacillus Rhamnosus and Lactobacillus Casei/Paracasei during Long-Term Storage. Benef. Microbes 2015, 6, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Mills, S.; Stanton, C.; Fitzgerald, G.F.; Roos, Y.; Ross, R. Paul Effect of Disaccharides on Survival during Storage of Freeze Dried Probiotics. Dairy Sci. Technol. 2008, 88, 19–30. [Google Scholar] [CrossRef]
- Pehkonen, K.S.; Roos, Y.H.; Miao, S.; Ross, R.P.; Stanton, C. State Transitions and Physicochemical Aspects of Cryoprotection and Stabilization in Freeze-drying of Lactobacillus Rhamnosus GG (LGG). J. Appl. Microbiol. 2008, 104, 1732–1743. [Google Scholar] [CrossRef]
- Van Holsbeke, C.; Marshall, J.; De Backer, J.; Vos, W. Median Mass Aerodynamic Diameter (MMAD) and Fine Particle Fraction (FPF): Influence on Lung Deposition? Eur. Respir. J. 2014, 44, P912. [Google Scholar]
- GombÁs, Á.; Szabó-Révész, P.; Kata, M.; Regdon, G.; Erős, I. Quantitative Determination of Crystallinity of α-Lactose Monohydrate by DSC. J. Therm. Anal. Calorim. 2002, 68, 503–510. [Google Scholar] [CrossRef]
- Balieiro, A.; Santos, R.; Pereira, M.; Figueiredo, R.; Freitas, L.; Alsina, O.; Lima, Á.; Soares, C. Adsorption Process of Molecularly Imprinted Silica for Extraction of Lactose from Milk. Braz. J. Chem. Eng. 2016, 33, 361–372. [Google Scholar] [CrossRef]
- Němec, I.; Mička, Z. FTIR and FT Raman Study of L-Leucine Addition Compound with Nitric Acid. J. Mol. Struct. 1999, 482, 23–28. [Google Scholar] [CrossRef]
- Knowles, M.R.; Boucher, R.C. Mucus Clearance as a Primary Innate Defense Mechanism for Mammalian Airways. J. Clin. Investig. 2002, 109, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Kaya, E.; Catelli, E.; Quinti, S.; Botti, M.; De Carli, A.; Bianchi, M.; Maisetta, G.; Esin, S. Lactobacillus Probiotic Strains Differ in Their Ability to Adhere to Human Lung Epithelial Cells and to Prevent Adhesion of Clinical Isolates of Pseudomonas Aeruginosa from Cystic Fibrosis Lung. Microorganisms 2023, 11, 1707. [Google Scholar] [CrossRef] [PubMed]
- Jamalifar, H.; Rahimi, H.R.; Samadi, N.; Shahverdi, A.R.; Sharifian, Z.; Hosseini, F.; Eslahi, H.; Fazeli, M.R. Antimicrobial Activity of Different Lactobacillus Species against Multi-Drug Resistant Clinical Isolates of Pseudomonas Aeruginosa. Iran. J. Microbiol. 1970, 3, 21. [Google Scholar]
- Shokri, D.; Khorasgani, M.R.; Mohkam, M.; Fatemi, S.M.; Ghasemi, Y.; Taheri-Kafrani, A. The Inhibition Effect of Lactobacilli Against Growth and Biofilm Formation of Pseudomonas Aeruginosa. Probiotics Antimicrob. Proteins 2018, 10, 34–42. [Google Scholar] [CrossRef]

| Formulation | LGG (Dry Mass) (wt.%) | Mannitol (wt.%) | Lactose (wt.%) | Leucine (wt.%) |
|---|---|---|---|---|
| DPI_1 | 35 | 55 | 5 | 5 |
| DPI_2 | 35 | 50 | 10 | 5 |
| DPI_3 | 35 | 45 | 15 | 5 |
| DPI_4 | 35 | 40 | 15 | 10 |
| DPI_5 | 35 | 35 | 15 | 15 |
| DPI_6 | 35 | 45 | 10 | 10 |
| DPI_7 | 12.5 | 75 | 0 | 12.5 |
| Formulation | Cell Viability Reduction (log CFU/mL) | LGG Survival Rate (%) | ED (wt.%) | FPF (wt.%) | MMAD ) |
|---|---|---|---|---|---|
| DPI_1 | 0.2 | 1.9 | 2 | 3 | 0.2 |
| DPI_2 | 0.2 | 5.5 | 3 | 3 | 0.3 |
| DPI_3 | 0.1 | 12.4 | 2 | 4 | 0.2 |
| DPI_4 | 0.1 | 7.8 | 1 | 3 | 0.4 |
| DPI_5 | 0.2 | 3.8 | 3 | 2 | 0.3 |
| DPI_6 | 0.2 | 5.5 | 2 | 2 | 0.1 |
| DPI_7 | 0.1 | 0.8 | 1 | 4 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.-T.; Cheow, W.S.; Pu, S.; Park, J.-W.; Hadinoto, K. Dry Powder Inhaler Formulation of Lactobacillus rhamnosus GG Targeting Pseudomonas aeruginosa Infection in Bronchiectasis Maintenance Therapy. Pharmaceutics 2024, 16, 980. https://doi.org/10.3390/pharmaceutics16080980
Tran T-T, Cheow WS, Pu S, Park J-W, Hadinoto K. Dry Powder Inhaler Formulation of Lactobacillus rhamnosus GG Targeting Pseudomonas aeruginosa Infection in Bronchiectasis Maintenance Therapy. Pharmaceutics. 2024; 16(8):980. https://doi.org/10.3390/pharmaceutics16080980
Chicago/Turabian StyleTran, The-Thien, Wean Sin Cheow, Siyu Pu, Jin-Won Park, and Kunn Hadinoto. 2024. "Dry Powder Inhaler Formulation of Lactobacillus rhamnosus GG Targeting Pseudomonas aeruginosa Infection in Bronchiectasis Maintenance Therapy" Pharmaceutics 16, no. 8: 980. https://doi.org/10.3390/pharmaceutics16080980





