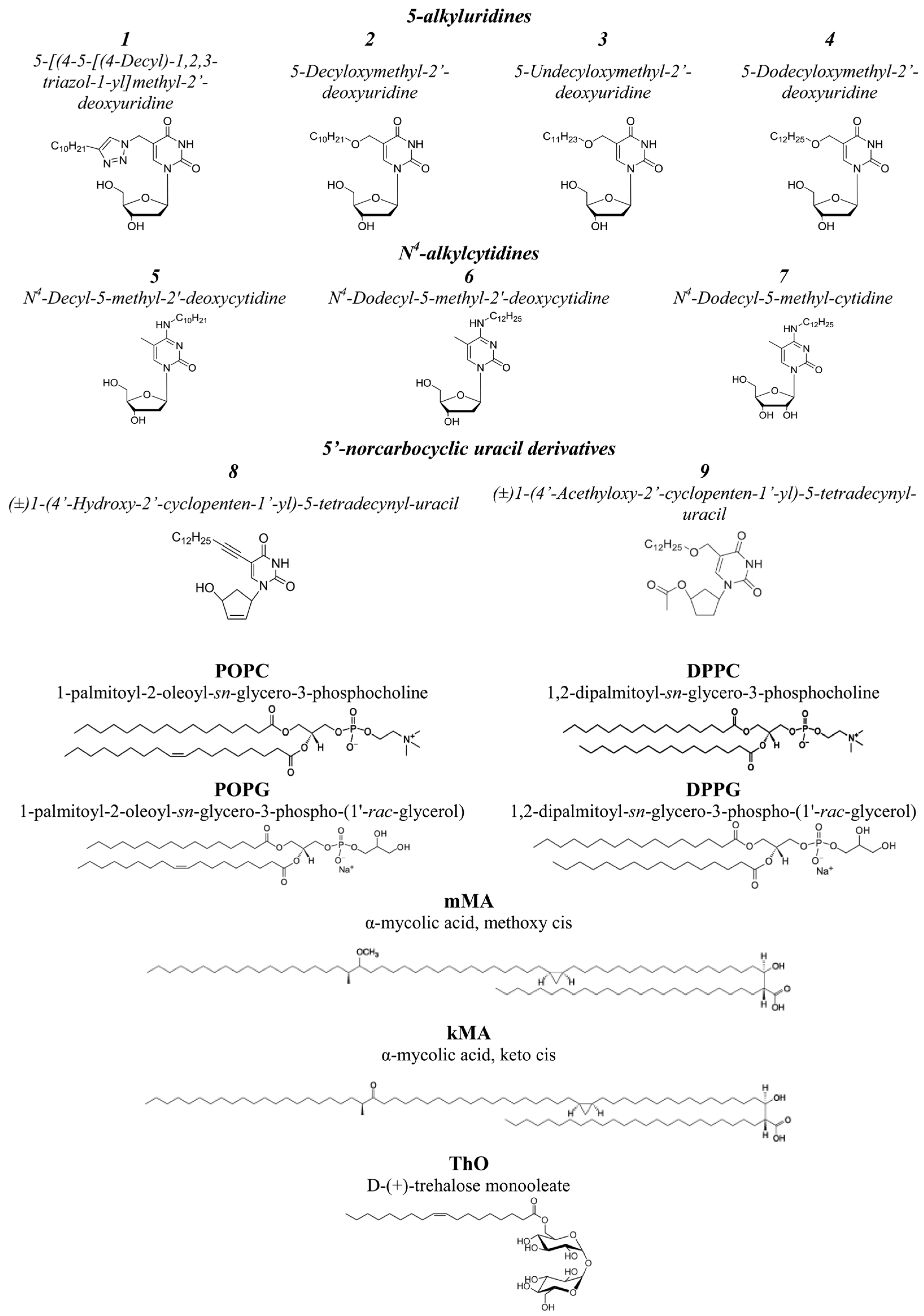
Derivatives of Pyrimidine Nucleosides Affect Artificial Membranes Enriched with Mycobacterial Lipids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Differential Scanning Microcalorimetry of Lipid Phase Transitions
2.2. Preparation of Planar Lipid Bilayers and Recording of Current
2.3. Calcein Release from Unilamellar Lipid Vesicles
3. Results and Discussion
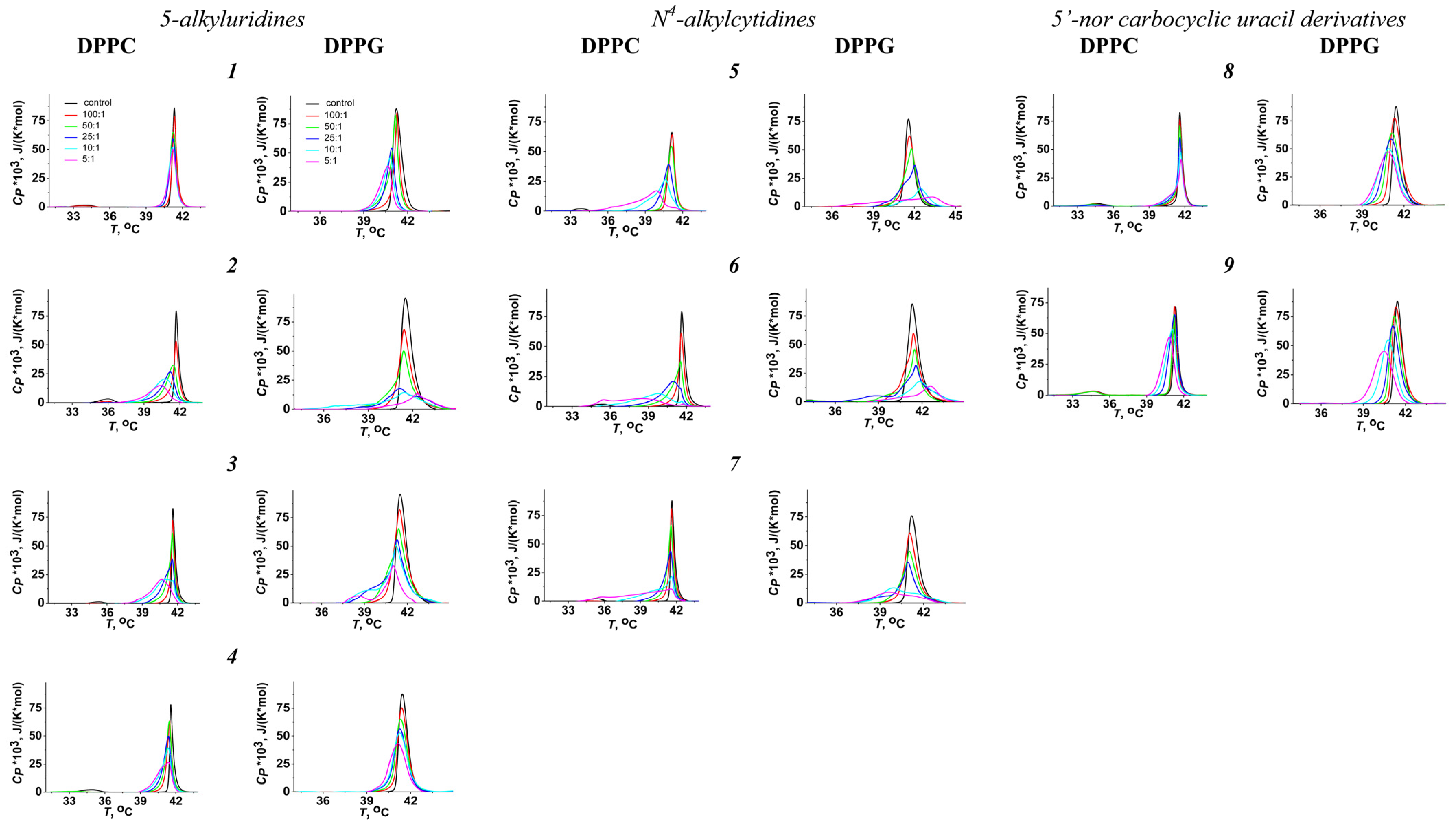
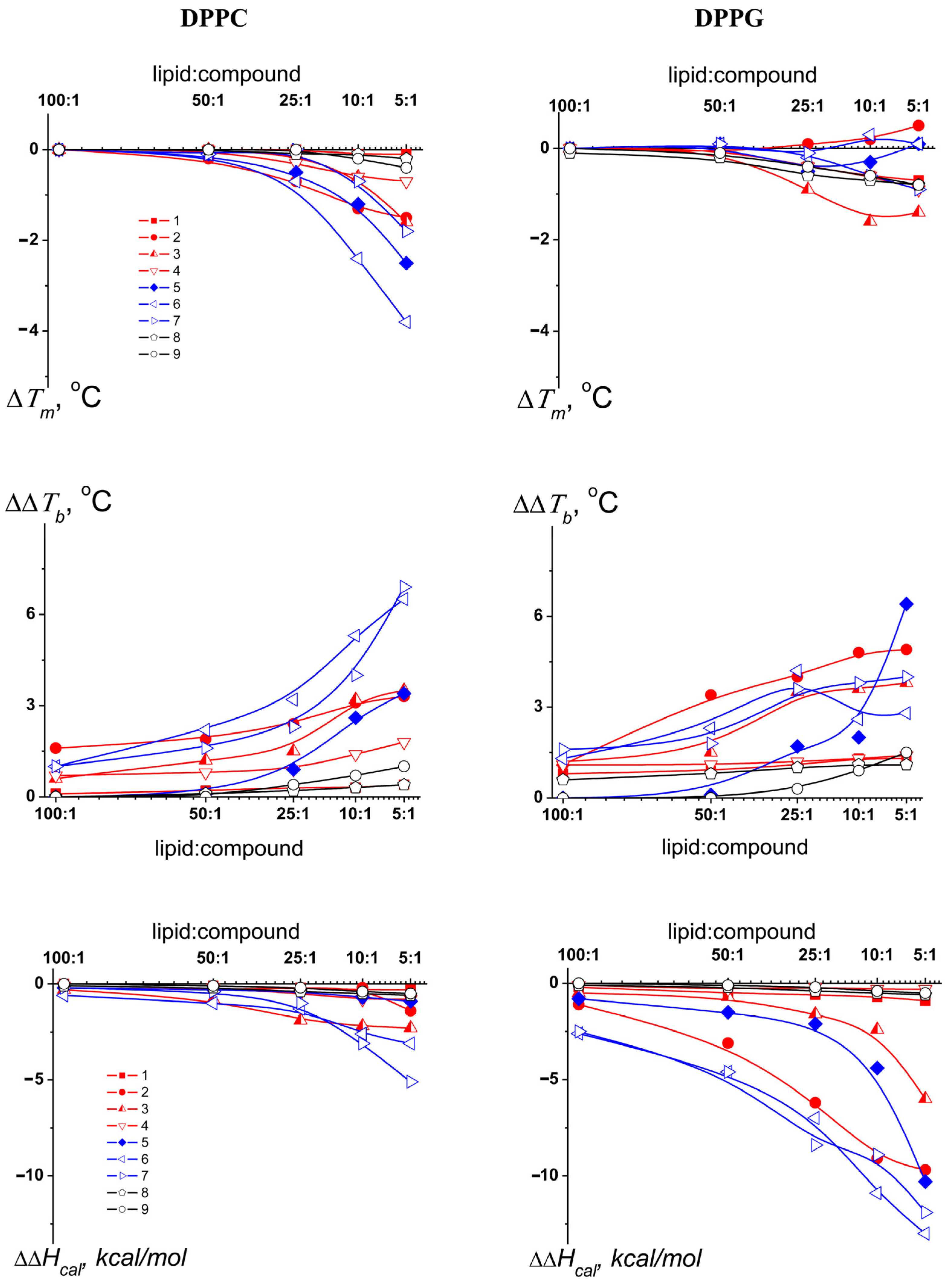
3.1. Effects of Pyrimidine Nucleoside Derivatives on Lipid Packing
3.2. Effects of Nucleoside Derivatives on Membrane Permeability
3.2.1. Phospholipid Bilayers
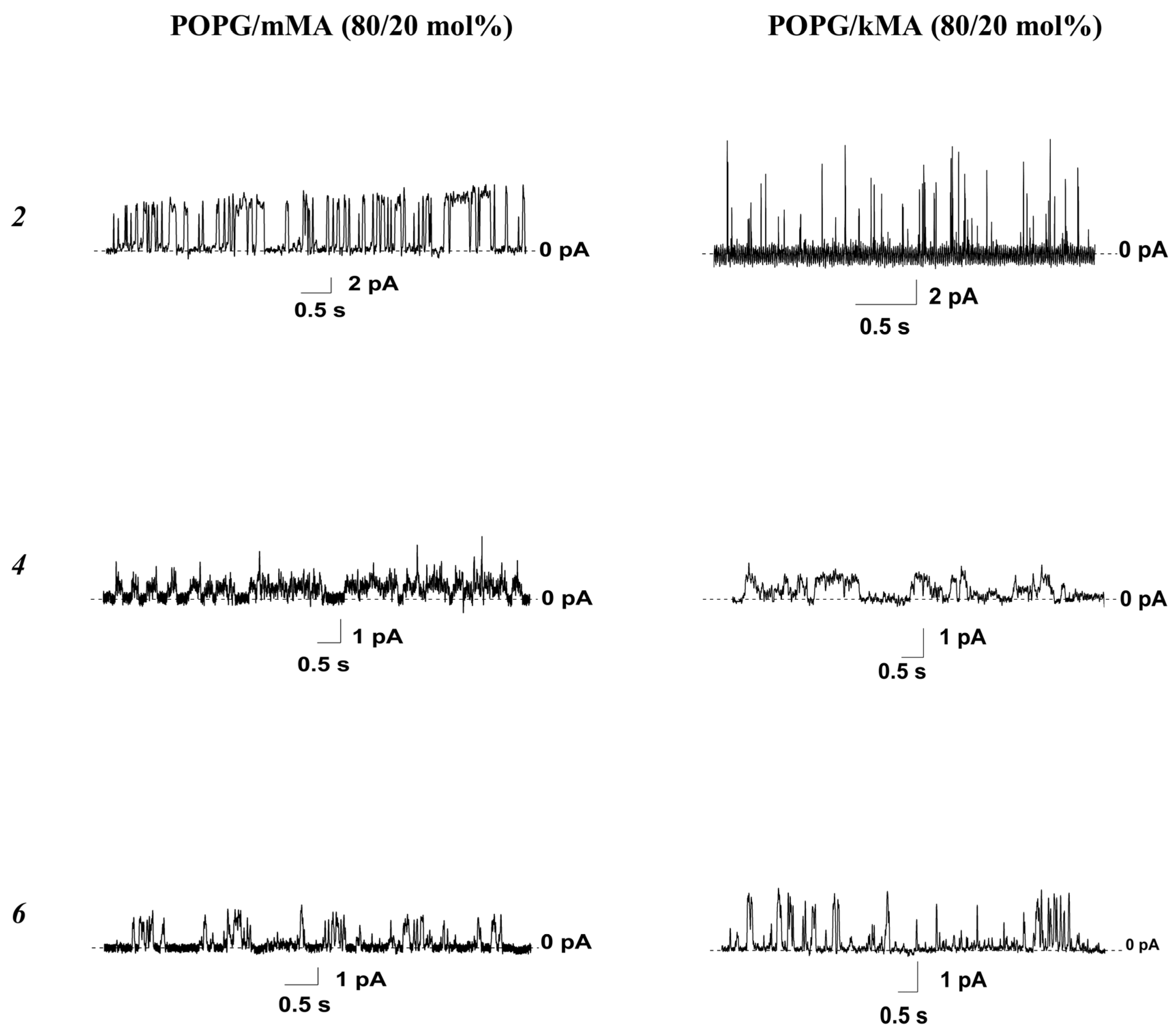
3.2.2. Lipid Bilayers Enriched with Specific Mycobacterial Lipids
- (1)
- Addition of mMA into POPG bilayers potentiated the disintegrating activity of all tested nucleoside derivatives and the pore-forming ability of compounds 2, 4, and 6; it also inhibited the induction of ion-permeable pores by the 5-alkyluridine compound 1;
- (2)
- Introduction of kMA into POPG membranes facilitated the disordering activity of derivatives 1, 6, 8, and 9 and the pore-forming ability of compounds 2, 4, and 6;
- (3)
- Inclusion of ThO into POPG bilayers led to an increase in the ability of 5-alkyluridines 1 and 4 to cause membrane collapse and form ion-permeable pores, as well as inhibit and potentiate the disintegrating action of compounds 8 and 9, respectively.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Liu, Y.; Matsumoto, M.; Ishida, H.; Ohguro, K.; Yoshitake, M.; Gupta, R.; Geiter, L.; Hafkin, J. Delamanid: From discovery to its use for pulmonary multidrug-resistant tuberculosis (MDR-TB). Tuberculosis 2018, 111, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, F.; Zhang, W. Bedaquiline and delamanid in the treatment of multidrug-resistant tuberculosis: Promising but challenging. Drug Dev. Res. 2019, 80, 98–105. [Google Scholar] [CrossRef]
- Pym, A.S.; Diacon, A.H.; Tang, S.J.; Conradie, F.; Danilovits, M.; Chuchottaworn, C.; Vasilyeva, I.; Andries, K.; Bakare, N.; De Marez, T.; et al. TMC207-C209 Study Group. Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur. Respir. J. 2016, 47, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Pieterman, E.D.; Keutzer, L.; van der Meijden, A.; van den Berg, S.; Wang, H.; Zimmerman, M.D.; Simonsson, U.S.H.; Bax, H.I.; de Steenwinkel, J.E.M. Superior Efficacy of a Bedaquiline, Delamanid, and Linezolid Combination Regimen in a Mouse Tuberculosis Model. J. Infect. Dis. 2021, 224, 1039–1047. [Google Scholar] [CrossRef]
- Lupien, A.; Vocat, A.; Foo, C.S.; Blattes, E.; Gillon, J.Y.; Makarov, V.; Cole, S.T. Optimized Background Regimen for Treatment of Active Tuberculosis with the Next-Generation Benzothiazinone Macozinone (PBTZ169). Antimicrob. Agents Chemother. 2018, 62, e00840-18. [Google Scholar] [CrossRef]
- Onajole, O.K.; Govender, P.; van Helden, P.D.; Kruger, H.G.; Maguire, G.E.; Wiid, I.; Govender, T. Synthesis and evaluation of SQ109 analogues as potential anti-tuberculosis candidates. Eur. J. Med. Chem. 2010, 45, 2075–2079. [Google Scholar] [CrossRef]
- de Jager, V.R.; Dawson, R.; van Niekerk, C.; Hutchings, J.; Kim, J.; Vanker, N.; van der Merwe, L.; Choi, J.; Nam, K.; Diacon, A.H. Telacebec (Q203), a New Antituberculosis Agent. N. Engl. J. Med. 2020, 382, 1280–1281. [Google Scholar] [CrossRef] [PubMed]
- Degiacomi, G.; Sammartino, J.C.; Sinigiani, V.; Marra, P.; Urbani, A.; Pasca, M.R. In vitro Study of Bedaquiline Resistance in Mycobacterium tuberculosis Multi-Drug Resistant Clinical Isolates. Front. Microbiol. 2020, 11, 559469. [Google Scholar] [CrossRef]
- Vilchèze, C. Mycobacterial Cell Wall: A Source of Successful Targets for Old and New Drugs. Appl. Sci. 2020, 10, 2278. [Google Scholar] [CrossRef]
- Modak, B.; Girkar, S.; Narayan, R.; Kapoor, S. Mycobacterial Membranes as Actionable Targets for Lipid-Centric Therapy in Tuberculosis. J. Med. Chem. 2022, 65, 3046–3065. [Google Scholar] [CrossRef]
- Pal, R.; Hameed, S.; Kumar, P.; Singh, S.; Fatima, Z. Comparative lipidomics of drug sensitive and resistant Mycobacterium tuberculosis reveals altered lipid imprints. 3 Biotech. 2017, 7, 325. [Google Scholar] [CrossRef]
- Yang, T.; Moreira, W.; Nyantakyi, S.A.; Chen, H.; Aziz, D.B.; Go, M.L.; Dick, T. Amphiphilic Indole Derivatives as Antimycobacterial Agents: Structure-Activity Relationships and Membrane Targeting Properties. J. Med. Chem. 2017, 60, 2745–2763. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, L.P.; Lin, G.; Jiang, X.; Nathan, C. Nitazoxanide kills replicating and nonreplicating Mycobacterium tuberculosis and evades resistance. J. Med. Chem. 2009, 52, 5789–5792. [Google Scholar] [CrossRef]
- de Carvalho, L.P.; Darby, C.M.; Rhee, K.Y.; Nathan, C. Nitazoxanide Disrupts Membrane Potential and Intrabacterial pH Homeostasis of Mycobacterium tuberculosis. ACS Med. Chem. Lett. 2011, 2, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nyantakyi, S.A.; Li, M.; Gopal, P.; Aziz, D.B.; Yang, T.; Moreira, W.; Gengenbacher, M.; Dick, T.; Go, M.L. The Mycobacterial Membrane: A Novel Target Space for Anti-tubercular Drugs. Front. Microbiol. 2018, 9, 1627. [Google Scholar] [CrossRef]
- Koh, J.J.; Zou, H.; Mukherjee, D.; Lin, S.; Lim, F.; Tan, J.K.; Tan, D.Z.; Stocker, B.L.; Timmer, M.S.M.; Corkran, H.M.; et al. Amphiphilic xanthones as a potent chemical entity of anti-mycobacterial agents with membrane-targeting properties. Eur. J. Med. Chem. 2016, 123, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Moreira, W.; Aziz, D.B.; Dick, T. Boromycin Kills Mycobacterial Persisters without Detectable Resistance. Front. Microbiol. 2016, 7, 199. [Google Scholar] [CrossRef]
- Harland, C.W.; Botyanszki, Z.; Rabuka, D.; Bertozzi, C.R.; Parthasarathy, R. Synthetic trehalose glycolipids confer desiccation resistance to supported lipid monolayers. Langmuir 2009, 25, 5193–5198. [Google Scholar] [CrossRef]
- Adhyapak, P.; Srivatsav, A.T.; Mishra, M.; Singh, A.; Narayan, R.; Kapoor, S. Dynamical Organization of Compositionally Distinct Inner and Outer Membrane Lipids of Mycobacteria. Biophys. J. 2020, 118, 1279–1291. [Google Scholar] [CrossRef]
- Langford, K.W.; Penkov, B.; Derrington, I.M.; Gundlach, J.H. Unsupported planar lipid membranes formed from mycolic acids of Mycobacterium tuberculosis. J. Lipid Res. 2011, 52, 272–277. [Google Scholar] [CrossRef]
- Vasyankin, A.V.; Panteleev, S.V.; Steshin, I.S.; Shirokova, E.A.; Rozhkov, A.V.; Livshits, G.D.; Radchenko, E.V.; Ignatov, S.K.; Palyulin, V.A. Temperature-Induced Restructuring of Mycolic Acid Bilayers Modeling the Mycobacterium tuberculosis Outer Membrane: A Molecular Dynamics Study. Molecules 2024, 29, 696. [Google Scholar] [CrossRef] [PubMed]
- Neres, J.; Labello, N.P.; Somu, R.V.; Boshoff, H.I.; Wilson, D.J.; Vannada, J.; Chen, L.; Barry CE3rd Bennett, E.M.; Aldrich, C.C. Inhibition of siderophore biosynthesis in Mycobacterium tuberculosis with nucleoside bisubstrate analogues: Structure-activity relationships of the nucleobase domain of 5’-O-[N-(salicyl)sulfamoyl]adenosine. J. Med. Chem. 2008, 51, 5349–5370. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, N.C.; Rai, D.; Tse, C.; Agrawal, B.; Kunimoto, D.Y.; Kumar, R. Inhibition of mycobacterial replication by pyrimidines possessing various C-5 functionalities and related 2’-deoxynucleoside analogues using in vitro and in vivo models. J. Med. Chem. 2010, 53, 6180–6187. [Google Scholar] [CrossRef] [PubMed]
- Shmalenyuk, E.R.; Chernousova, L.N.; Karpenko, I.L.; Kochetkov, S.N.; Smirnova, T.G.; Andreevskaya, S.N.; Chizhov, A.O.; Efremenkova, O.V.; Alexandrova, L.A. Inhibition of Mycobacterium tuberculosis strains H37Rv and MDR MS-115 by a new set of C5 modified pyrimidine nucleosides. Bioorg. Med. Chem. 2013, 21, 4874–4884. [Google Scholar] [CrossRef]
- Matyugina, E.; Khandazhinskaya, A.; Chernousova, L.; Andreevskaya, S.; Smirnova, T.; Chizhov, A.; Karpenko, I.; Kochetkov, S.; Alexandrova, L. The synthesis and antituberculosis activity of 5’-nor carbocyclic uracil derivatives. Bioorg. Med. Chem. 2012, 20, 6680–6686. [Google Scholar] [CrossRef]
- Khandazhinskaya, A.L.; Alexandrova, L.A.; Matyugina, E.S.; Solyev, P.N.; Efremenkova, O.V.; Buckheit, K.W.; Wilkinson, M.; Buckheit, R.W., Jr.; Chernousova, L.N.; Smirnova, T.G.; et al. Novel 5’-Norcarbocyclic Pyrimidine Derivatives as Antibacterial Agents. Molecules 2018, 23, 3069. [Google Scholar] [CrossRef]
- Alexandrova, L.A.; Jasko, M.V.; Negrya, S.D.; Solyev, P.N.; Shevchenko, O.V.; Solodinin, A.P.; Kolonitskaya, D.P.; Karpenko, I.L.; Efremenkova, O.V.; Glukhova, A.A.; et al. Discovery of novel N4-alkylcytidines as promising antimicrobial agents. Eur. J. Med. Chem. 2021, 215, 113212. [Google Scholar] [CrossRef]
- Khandazhinskaya, A.L.; Matyugina, E.S.; Alexandrova, L.A.; Kezin, V.A.; Chernousova, L.N.; Smirnova, T.G.; Andreevskaya, S.N.; Popenko, V.I.; Leonova, O.G.; Kochetkov, S.N. Interaction of 5-substituted pyrimidine nucleoside analogues and M. Tuberculosis: A view through an electron microscope. Biochimie 2020, 171–172, 170–177. [Google Scholar] [CrossRef]
- Montal, M.; Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA. 1972, 69, 3561–3566. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859 Pt B, 1558–1572. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Geiger, O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef]
- Parsons, J.B.; Rock, C.O. Bacterial lipids: Metabolism and membrane homeostasis. Prog. Lipid Res. 2013, 52, 249–276. [Google Scholar] [CrossRef] [PubMed]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta 1998, 1376, 91–145. [Google Scholar] [CrossRef] [PubMed]
- Durvasula, R.V.; Huang, C.H. Thermotropic phase behavior of mixed-chain phosphatidylglycerols: Implications for acyl chain packing in fully hydrated bilayers. Biochim. Biophys. Acta 1999, 1417, 111–121. [Google Scholar] [CrossRef]
- Escobar, J.F.B.; Restrepo, M.H.P.; Fernández, D.M.M.; Martínez, A.M.; Giordani, C.; Castelli, F.; Sarpietro, M.G. Synthesis and interaction of sterol-uridine conjugate with DMPC liposomes studied by differential scanning calorimetry. Colloids Surf. B Biointerfaces 2018, 166, 203–209. [Google Scholar] [CrossRef]
- Berrío Escobar, J.F.; Márquez Fernández, D.M.; Giordani, C.; Castelli, F.; Sarpietro, M.G. Anomalous interaction of tri-acyl ester derivatives of uridine nucleoside with a l-α-dimyristoylphosphatidylcholine biomembrane model: A differential scanning calorimetry study. J. Pharm. Pharmacol. 2019, 71, 329–337. [Google Scholar] [CrossRef]
- Marrink, S.J.; Risselada, J.; Mark, A.E. Simulation of gel phase formation and melting in lipid bilayers using a coarse grained model. Chem. Phys. Lipids 2005, 35, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, L.A.; Oskolsky, I.A.; Makarov, D.A.; Jasko, M.V.; Karpenko, I.L.; Efremenkova, O.V.; Vasilyeva, B.F.; Avdanina, D.A.; Ermolyuk, A.A.; Benko, E.E.; et al. New Biocides Based on N4-Alkylcytidines: Effects on Microorganisms and Application for the Protection of Cultural Heritage Objects of Painting. Int. J. Mol. Sci. 2024, 25, 3053. [Google Scholar] [CrossRef]
- Thanna, S.; Sucheck, S.J. Targeting the trehalose utilization pathways of Mycobacterium tuberculosis. Medchemcomm 2016, 7, 69–85. [Google Scholar] [CrossRef]
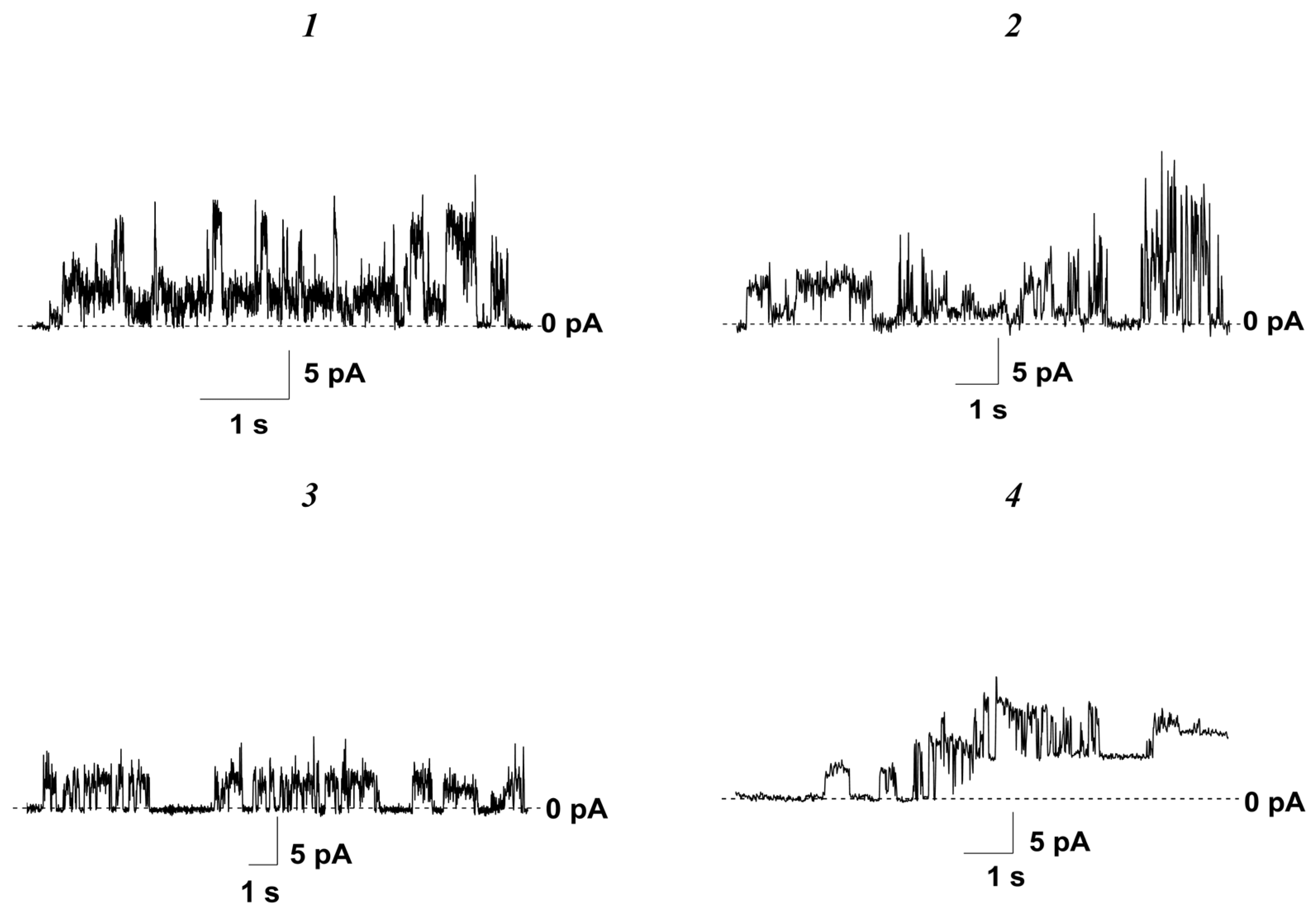

| Compound | LogP | POPC | POPG | ||||
|---|---|---|---|---|---|---|---|
| Ctr, μM | IFmax, % | Ctr, μM | Csc, μM | gsc, pS | IFmax, % | ||
| 1 | 1.92 | 355 ± 10 | 8 ± 4 | 220 ± 15 | 175 ± 10 | 5–40 | 47 ± 5 |
| 2 | 1.86 | 360 ± 15 | 5 ± 3 | 250 ± 10 | 140 ± 15 | 5–10 | 38 ± 7 |
| 3 | 2.26 | 375 ± 10 | 10 ± 3 | 240 ± 15 | 145 ± 10 | 5–50 | 26 ± 4 |
| 4 | 2.66 | 350 ± 25 | 18 ± 4 | 210 ± 10 | 150 ± 10 | 30–210 | 39 ± 3 |
| 5 | 3.14 | 375 ± 10 | 7 ± 3 | 275 ± 15 | – # | – # | 5 ± 3 |
| 6 | 3.93 | 405 ± 15 | 6 ± 2 | 280 ± 10 | – # | – # | 11 ± 4 |
| 7 | 3.02 | 340 ± 20 | 5 ± 3 | 295 ± 15 | – # | – # | 10 ± 5 |
| 8 | 4.58 | 355 ± 10 | 4 ± 2 | 350 ± 10 | – # | – # | 14 ± 3 |
| 9 | 4.00 | 380 ± 20 | 3 ± 1 | 340 ± 15 | – # | – # | 4 ± 2 |
| POPG/mMA (80/20 mol%) | POPG/kMA (80/20 mol%) | POPG/ThO (80/20 mol%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Ctr, μM | Csc, μM | gsc, pS | Ctr, μM | Csc, μM | gsc, pS | Ctr, μM | Csc, μM | gsc, pS |
| 1 | 80 ± 30 | – # | – # | 83 ± 28 | – # | – # | 20 ± 10 | 8 ± 3 | 5–24 |
| 2 | 105 ± 55 | 58 ± 32 | 10–40 | 250 ± 50 | 65 ± 35 | 5–100 | nt * | nt * | nt * |
| 4 | 105 ± 73 | 22 ± 12 | 5–20 | 300 ± 50 | 20 ± 3 | 5–50 | 23 ± 3 | 18 ± 5 | 5–50 |
| 6 | 16 ± 7 | 3 ± 1 | 5–100 | 33 ± 18 | 9 ± 2 | 5–70 | nt * | nt * | nt * |
| 8 | 21 ± 4 | – # | – # | 90 ± 7 | – # | – # | 525 ± 140 | – # | – # |
| 9 | 62 ± 10 | – # | – # | 44 ± 12 | – # | – # | 80 ± 25 | – # | – # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostroumova, O.S.; Efimova, S.S.; Zlodeeva, P.D.; Alexandrova, L.A.; Makarov, D.A.; Matyugina, E.S.; Sokhraneva, V.A.; Khandazhinskaya, A.L.; Kochetkov, S.N. Derivatives of Pyrimidine Nucleosides Affect Artificial Membranes Enriched with Mycobacterial Lipids. Pharmaceutics 2024, 16, 1110. https://doi.org/10.3390/pharmaceutics16091110
Ostroumova OS, Efimova SS, Zlodeeva PD, Alexandrova LA, Makarov DA, Matyugina ES, Sokhraneva VA, Khandazhinskaya AL, Kochetkov SN. Derivatives of Pyrimidine Nucleosides Affect Artificial Membranes Enriched with Mycobacterial Lipids. Pharmaceutics. 2024; 16(9):1110. https://doi.org/10.3390/pharmaceutics16091110
Chicago/Turabian StyleOstroumova, Olga S., Svetlana S. Efimova, Polina D. Zlodeeva, Liudmila A. Alexandrova, Dmitry A. Makarov, Elena S. Matyugina, Vera A. Sokhraneva, Anastasia L. Khandazhinskaya, and Sergey N. Kochetkov. 2024. "Derivatives of Pyrimidine Nucleosides Affect Artificial Membranes Enriched with Mycobacterial Lipids" Pharmaceutics 16, no. 9: 1110. https://doi.org/10.3390/pharmaceutics16091110






