Exploring Protein-Based Carriers in Drug Delivery: A Review
Abstract
1. Introduction
2. The Crucial Role of Proteins in Advancing Drug Delivery Systems
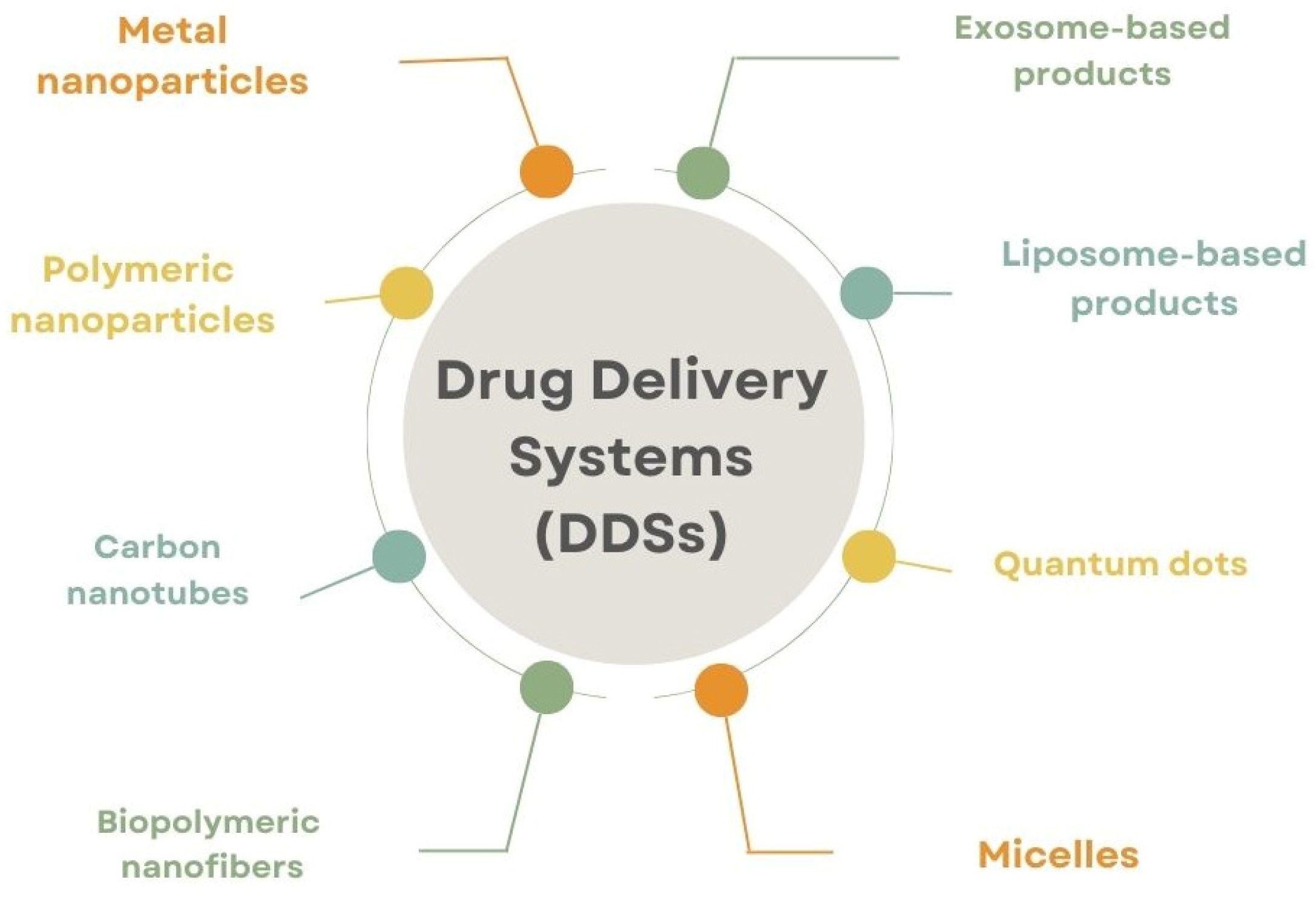
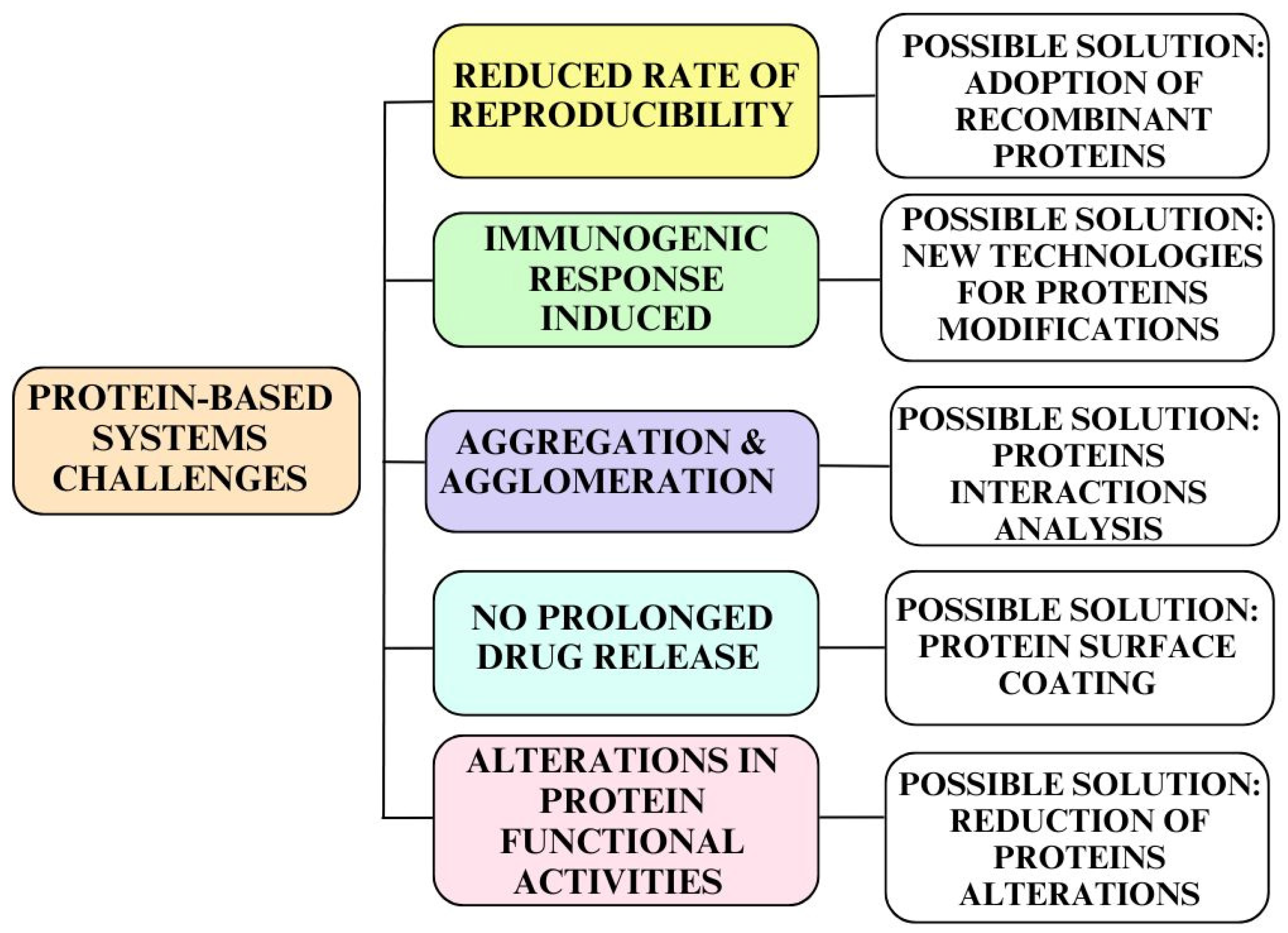
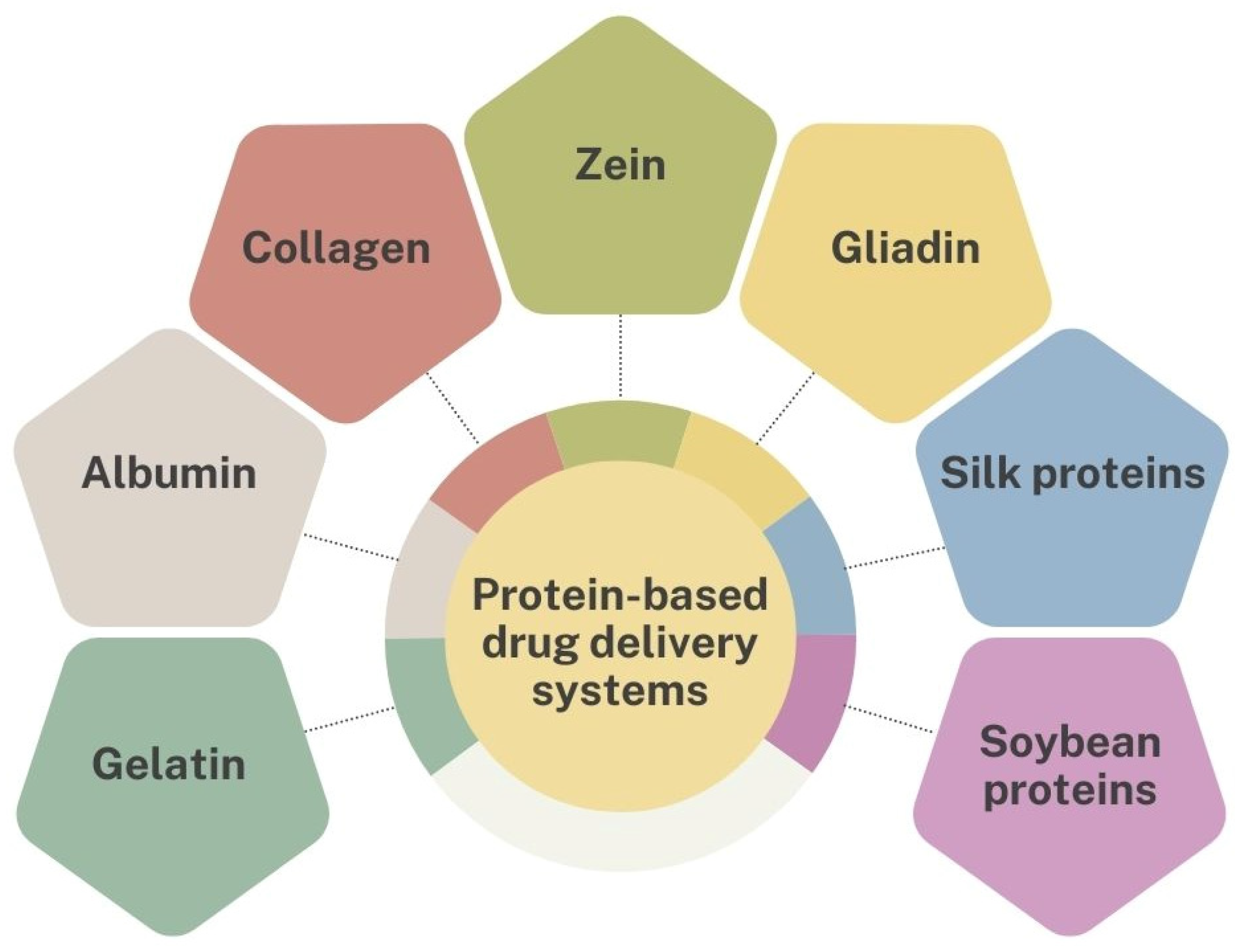
3. Protein-Based Drug Carriers
3.1. Gelatin-Based Drug Carriers
3.2. Albumin-Based Drug Carriers
3.3. Collagen-Based Drug Carriers
3.4. Zein-Based Drug Carriers
3.5. Gliadin-Based Drug Carriers
3.6. Silk Protein-Based Drug Carriers
3.7. Soybean Protein-Based Drug Carriers
4. A Comparative Analysis of Protein-Based Drug Carriers with Other Types of Carriers
- liposome-based systems (a challenging aspect is that liposomes could break down and interact with digestive enzymes, so we must focus our research on their stability, release mechanisms, and interactions with the immune system) [96];
- lipid nanoemulsions (the proper choice of lipid types and emulsifiers significantly influences the stability and effectiveness of the carriers) [97];
- solid lipid nanoparticles (which are highly stable and able to provide an effective drug controlled release but, on the other hand, they also present several challenges as drug delivery systems; for example, they have a restricted encapsulation ability for hydrophilic drugs that may represent a limiting factor, considering all the drugs employed in numerous therapies and affected by poor bioavailability) [98];
- lipid-based nanocarriers (with the development of different nanoformulations whose stability can hardly be controlled in harsh environmental conditions) [99].
- alginate-based drug delivery systems (many carriers have been developed for curcumin delivery but also for the controlled release of tuberculosis drugs; however, a significant challenge with these systems is the physicochemical changes they can undergo in the biological environment, which can alter their drug release capabilities) [100];
- cellulose-based drug delivery systems (based on cellulose’s ability to create compounds with a large surface, they are useful for drugs loading and targeting; different studies show the synthesis of cellulose-containing nanocomposites adopted in anticancer treatments. The main problem with these systems is related to their limited rate of drug controlled release due to changes in the biological environment. Moreover, some polysaccharides show poor mechanical properties and are not compatible with hydrophobic polymers; these disadvantages indicate the need to make surface modifications in order to enhance polysaccharides’ features and use them as effective drug delivery systems) [101].
5. Clinical Development of Protein-Based Drug Delivery Systems
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Yan, Y.; Palanisamy, C.P.; Jayaraman, S.; Natarajan, P.M.; Umapathy, V.R.; Gopathy, S.; Roy, J.R.; Sadagopan, J.C.; Thalamati, D. Materials-based drug delivery approaches: Recent advances and future perspectives. Green Process. Synth. 2024, 13, 20230094. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Mora-Boza, A.; García-Fernández, L. Emerging Biofabrication Techniques: A Review on Natural Polymers for Biomedical Applications. Polymers 2021, 13, 1209. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymersbased nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Cheng, Z. Research on the Application of Synthetic Polymer Materials in Contemporary Public Art. Polymers 2022, 14, 1208. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M.; Bejenaru, L.E.; Bejenaru, C. Chapter 8—Natural and synthetic polymers for drug delivery and targeting. In Nanobiomaterials in Drug Delivery. Applications of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 229–284. [Google Scholar] [CrossRef]
- Bhatia, S. Natural polymers vs. synthetic polymer. In Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Bhatia, S., Ed.; Springer: New York, NY, USA, 2016; pp. 95–118. [Google Scholar] [CrossRef]
- Chandra, P.; Shahjad; Sharma, K.K.; Verma, A. Role of Macromolecules in Medical Application: Challenges and Opportunities. Macromol. Symp. 2024, 413, 2300110. [Google Scholar] [CrossRef]
- Martínez-López, A.L.; Pangua, C.; Reboredo, C.; Campión, R.; Morales-Gracia, J.; Irache, J.M. Protein-based nanoparticles for drug delivery purposes. Int. J. Pharm. 2020, 581, 119289. [Google Scholar] [CrossRef]
- Tang, J.; Yao, D.; Xia, S.; Cheong, L.-Z.; Tu, M. Recent progress in plant-based proteins: From extraction and modification methods to applications in the food industry. Food Chem. X 2024, 23, 101540. [Google Scholar] [CrossRef]
- Bouchard, J.; Malalgoda, M.; Storsley, J.; Malunga, L.; Netticadan, T.; Thandapilly, S.J. Health benefits of cereal grain-and pulse-derived proteins. Molecules 2022, 27, 3746. [Google Scholar] [CrossRef]
- Georgilis, E.; Abdelghani, M.; Pille, J.; Aydinlioglu, E.; van Hest, J.C.; Lecommandoux, S.; Garanger, E. Nanoparticles based on natural, engineered or synthetic proteins and polypeptides for drug delivery applications. Int. J. Pharm. 2020, 586, 119537. [Google Scholar] [CrossRef]
- Sharma, A.; Jangra, N.; Dheer, D.; Jha, S.K.; Gupta, G.; Puri, V.; Kesharwani, P. Understanding the journey of biopolymeric nanoformulations for oral drug delivery: Conventional to advanced treatment approaches. Eur. Polym. J. 2024, 218, 113338. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.; Rezigue, M.; Alsharedeh, R.H.; Obeid, M.A.; Mishra, V.; Serrano-Aroca, A.; El-Tanani, M.; Tambuwala, M.M. Protein-based nanomaterials: A new tool for targeted drug delivery. Ther. Deliv. 2022, 13, 321–338. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle Therapeutics: An Emerging Treatment Modality for Cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, shape, charge and “stealthy” surface: Carrier properties affect the drug circulation time in vivo. Asian J. Pharm. Sci. 2021, 16, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Oh, W.-K.; Jeong, Y.S.; Hong, J.-Y.; Cho, B.-R.; Hahn, J.-S.; Jang, J. Cytotoxicity of, and innate immune response to, size-controlled polypyrrole nanoparticles in mammalian cells. Biomaterials 2011, 32, 2342–2350. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Sadeghi, S.; Lee, W.K.; Kong, S.N.; Shetty, A.; Drum, C.L. Oral administration of protein nanoparticles: An emerging route to disease treatment. Pharmacol. Res. 2020, 158, 104685. [Google Scholar] [CrossRef]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnology 2021, 19, 159. [Google Scholar] [CrossRef]
- Pang, X.; Gong, K.; Zhang, X.; Wu, S.; Cui, Y.; Qian, B.-Z. Osteopontin as a multifaceted driver of bone metastasis and drug resistance. Pharmacol. Res. 2019, 144, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Gao, W.; Su, M.; Nice, E.C.; Zhang, W.; Lin, J.; Xie, N. Adaptive mechanisms of tumor therapy resistance driven by tumor microenvironment. Front. Cell Dev. Biol. 2021, 9, 641469. [Google Scholar] [CrossRef] [PubMed]
- Jevševar, S.; Kunstelj, M.; Porekar, V.G. PEGylation of therapeutic proteins. Biotechnol. J. Healthc. Nutr. Technol. 2010, 5, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Alzahrani, N.; Alzahrani, R.; Alshamrani, W.; Aloufi, W.; Ali, A.; Najib, S.; Siddiqui, N.A. Stability issues and approaches to stabilized nanoparticles based drug delivery system. J. Drug Target. 2020, 28, 468–486. [Google Scholar] [CrossRef]
- Soane, D.S.; Mahoney, R.P.; Wuthrich, P.; Greene, D.G. Stabilizing Excipients for Therapeutic Protein Formulations. U.S. Patent US10279048B2, 7 May 2019. [Google Scholar]
- Ghosh, R.; Calero-Rubio, C.; Saluja, A.; Roberts, C.J. Relating protein–protein interactions and aggregation rates from low to high concentrations. J. Pharm. Sci. 2016, 105, 1086–1096. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Rahban, M.; Ahmad, F.; Piatyszek, M.A.; Haertlé, T.; Saso, L.; Saboury, A.A. Stabilization challenges and aggregation in protein-based therapeutics in the pharmaceutical industry. RSC Adv. 2023, 13, 35947–35963. [Google Scholar] [CrossRef]
- Warwar Damouny, C.; Martin, P.; Vasilyev, G.; Vilensky, R.; Fadul, R.; Redenski, I.; Srouji, S.; Zussman, E. Injectable hydrogels based on inter-polyelectrolyte interactions between hyaluronic acid, gelatin, and cationic cellulose nanocrystals. Biomacromolecules 2022, 23, 3222–3234. [Google Scholar] [CrossRef]
- Jamroży, M.; Kudłacik-Kramarczyk, S.; Drabczyk, A.; Krzan, M. Advanced Drug Carriers: A Review of Selected Protein, Polysaccharide, and Lipid Drug Delivery Platforms. Int. J. Mol. Sci. 2024, 25, 786. [Google Scholar] [CrossRef]
- Raj, V.; Prabha, G. Synthesis, characterization and in vitro drug release of cisplatin loaded Cassava starch acetate–PEG/gelatin nanocomposites. J. Assoc. Arab. Univ. Basic Appl. Sci. 2016, 21, 10–16. [Google Scholar] [CrossRef]
- Ahmad, A.; Ansari, M.M.; Mishra, R.K.; Kumar, A.; Vyawahare, A.; Verma, R.K.; Raza, S.S.; Khan, R. Enteric-coated gelatin nanoparticles mediated oral delivery of 5-aminosalicylic acid alleviates severity of DSS-induced ulcerative colitis. Mater. Sci. Eng. C 2021, 119, 111582. [Google Scholar] [CrossRef] [PubMed]
- Najafabadi, A.P.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Rahdar, A.; Díez-Pascual, A.M. pH-sensitive ameliorated quercetin delivery using graphene oxide nanocarriers coated with potential anticancer gelatin-polyvinylpyrrolidone nanoemulsion with bitter almond oil. J. Drug Deliv. Sci. Technol. 2023, 82, 104339. [Google Scholar] [CrossRef]
- Ostovar, S.; Pourmadadi, M.; Shamsabadipour, A.; Mashayekh, P. Nanocomposite of chitosan/gelatin/carbon quantum dots as a biocompatible and efficient nanocarrier for improving the Curcumin delivery restrictions to treat brain cancer. Int. J. Biol. Macromol. 2023, 242, 124986. [Google Scholar] [CrossRef] [PubMed]
- Jaberifard, F.; Arsalani, N.; Ghorbani, M.; Mostafavi, H. Incorporating halloysite nanotube/carvedilol nanohybrids into gelatin microsphere as a novel oral pH-sensitive drug delivery system. Colloids Surf. A 2022, 637, 128122. [Google Scholar] [CrossRef]
- Milano, F.; Masi, A.; Madaghiele, M.; Sannino, A.; Salvatore, L.; Gallo, N. Current trends in gelatin-based drug delivery systems. Pharmaceutics 2023, 15, 1499. [Google Scholar] [CrossRef]
- Cascone, M.G.; Lazzeri, L.; Carmignani, C.; Zhu, Z. Gelatin nanoparticles produced by a simple W/O emulsion as delivery system for methotrexate. J. Mater. Sci. Mater. Med. 2002, 13, 523–526. [Google Scholar] [CrossRef]
- Hoffmann, H.; Reger, M. Emulsions with unique properties from proteins as emulsifiers. Adv. Colloid Interface Sci. 2014, 205, 94–104. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Zhang, Y.; Wang, X.; Lorenzo, J.M.; Zhong, J. Gelatins as emulsifiers for oil-in-water emulsions: Extraction, chemical composition, molecular structure, and molecular modification. Trends Food Sci. Technol. 2020, 106, 113–131. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Heuzey, M.-C. Pickering emulsion gels based on insoluble chitosan/gelatin electrostatic complexes. RSC Adv. 2016, 6, 89776–89784. [Google Scholar] [CrossRef]
- Jin, W.; Zhu, J.; Jiang, Y.; Shao, P.; Li, B.; Huang, Q. Gelatin-based nanocomplex-stabilized Pickering emulsions: Regulating droplet size and wettability through assembly with glucomannan. J. Agric. Food Chem. 2017, 65, 1401–1409. [Google Scholar] [CrossRef]
- Leiva-Vega, J.; Villalobos-Carvajal, R.; Ferrari, G.; Donsì, F.; Zúñiga, R.N.; Shene, C.; Beldarraín-Iznaga, T. Beldarraín-Iznaga, T. Influence of interfacial structure on physical stability and antioxidant activity of curcumin multilayer emulsions. Food Bioprod. Process. 2020, 121, 65–75. [Google Scholar] [CrossRef]
- Morimoto, K.; Chono, S.; Kosai, T.; Seki, T.; Tabata, Y. Design of novel injectable cationic microspheres based on aminated gelatin for prolonged insulin action. J. Pharm. Pharmacol. 2005, 57, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zhang, R.; Han, L.; Zhang, T.; Ngai, T. Pickering emulsions stabilized by aminated gelatin nanoparticles: Are gelatin nanoparticles acting as genuine Pickering stabilizers or structuring agents? Food Hydrocoll. 2022, 123, 107151. [Google Scholar] [CrossRef]
- Lee, E.J.; Khan, S.A.; Park, J.K.; Lim, K.H. Studies on the characteristics of drug-loaded gelatin nanoparticles prepared by nanoprecipitation. Bioprocess Biosyst. Eng. 2012, 35, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Das, R.P.; Chakravarti, S.; Patel, S.S.; Lakhamje, P.; Gurjar, M.; Gota, V.; Kunwar, A. Tuning the pharmacokinetics and efficacy of irinotecan (IRI) loaded gelatin nanoparticles through folate conjugation. Int. J. Pharm. 2020, 586, 119522. [Google Scholar] [CrossRef]
- Suresh, D.; Suresh, A.; Kannan, R. Engineering biomolecular systems: Controlling the self-assembly of gelatin to form ultra-small bioactive nanomaterials. Bioact. Mater. 2022, 18, 321–336. [Google Scholar] [CrossRef]
- van de Wouw, J.; Joles, J.A. Albumin is an Interface between Blood Plasma and Cell Membrane, and Not Just a Sponge. Clin. Kidney J. 2022, 15, 624–634. [Google Scholar] [CrossRef]
- Jalali, E.S.; Shojaosadati, S.A.; Hamedi, S. Green synthesis of bovine serum albumin/oxidized gum Arabic nanocomposite as pH-responsive carrier for controlled release of piperine and the molecular docking study. Int. J. Biol. Macromol. 2023, 225, 51–62. [Google Scholar] [CrossRef]
- Ma, N.; Liu, J.; He, W.; Li, Z.; Luan, Y.; Song, Y.; Garg, S. Folic acid-grafted bovine serum albumin decorated graphene oxide: An efficient drug carrier for targeted cancer therapy. J. Colloid Interface Sci. 2017, 490, 598–607. [Google Scholar] [CrossRef]
- Duarte, M.M.V.M. Development and Characterization of Niosomes as New Drug Delivery Systems. Ph.D. Thesis, Universidade de Lisboa, Lisbon, Portugal, 2020. [Google Scholar]
- Durak, S.; Rad, M.E.; Yetisgin, A.A.; Sutova, H.E.; Kutlu, O.; Cetinel, S.; Zarrabi, A. Niosomal drug delivery systems for ocular disease—Recent advances and future prospects. Nanomaterials 2020, 10, 1191. [Google Scholar] [CrossRef]
- Hanafy, N.A.; Abdelbadea, R.H.; Abdelaziz, A.E.; Mazyed, E.A. Formulation and Optimization of Folate-Bovine Serum Albumin-Coated Ethoniosomes of Pterostilbene as a Targeted Drug Delivery System for Lung Cancer: In Vitro and In Vivo Demonstrations. Cancer Nanotechnol. 2023, 14, 49. [Google Scholar] [CrossRef]
- Xiong, B.; Liu, H.; Yi, M.; Li, Y.; Huang, Y.; Guo, W.; Lu, B. A pH/GSH Dual-Responsive, Folic Acid Targeted Drug Delivery System Based on Bovine Serum Albumin Nanoparticles for Cancer Therapy. Part. Part. Syst. Charact. 2024, 41, 2300184. [Google Scholar] [CrossRef]
- Jiang, H.; Liang, G.; Dai, M.; Dong, Y.; Wu, Y.; Zhang, L.; Qi, L. Preparation of Doxorubicin-Loaded Collagen-PAPBA Nanoparticles and Their Anticancer Efficacy in Ovarian Cancer. Ann. Transl. Med. 2020, 8, 880. [Google Scholar] [CrossRef] [PubMed]
- Siaghi, M.; Karimizade, A.; Mellati, A.; Saeedi, M.; Amiri, F.T.; Kalhori, S.; Shahani, S. Luteolin-incorporated fish collagen hydrogel scaffold: An effective drug delivery strategy for wound healing. Int. J. Pharm. 2024, 657, 124138. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Ding, C.; Hu, M.; Zhang, R.; Cheng, B. Collagen/functionalized cellulose nanofibril composite aerogels with pH-responsive characteristics for drug delivery system. Int. J. Biol. Macromol. 2024, 261, 129650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.-C.; Wang, J.-j. Drugs adsorption and release behavior of collagen/bacterial cellulose porous microspheres. Int. J. Biol. Macromol. 2019, 140, 196–205. [Google Scholar] [CrossRef]
- Rathore, P.; Arora, I.; Rastogi, S.; Akhtar, M.; Singh, S.; Samim, M. Collagen nanoparticle-mediated brain silymarin delivery: An approach for treating cerebral ischemia and reperfusion-induced brain injury. Front. Neurosci. 2020, 14, 538404. [Google Scholar] [CrossRef]
- Luo, X.; Wu, S.; Xiao, M.; Gu, H.; Zhang, H.; Chen, J.; Zhang, J. Advances and Prospects of Prolamine Corn Protein Zein as a Promising Multifunctional Drug Delivery System for Cancer Treatment. Int. J. Nanomed. 2023, 18, 2589–2621. [Google Scholar] [CrossRef]
- Dong, F.; Dong, X.; Zhou, L.; Xiao, H.; Ho, P.Y.; Wong, M.S.; Wang, Y. Doxorubicin-Loaded Biodegradable Self-Assembly Zein Nanoparticles and Their Anti-Cancer Effect: Preparation, In Vitro Evaluation, and Cellular Uptake. Colloids Surf. B Biointerfaces 2016, 140, 324–331. [Google Scholar] [CrossRef]
- Yu, X.; Wu, H.; Hu, H.; Dong, Z.; Dang, Y.; Qi, Q.; Wang, Y.; Du, S.; Lu, Y. Zein nanoparticles as nontoxic delivery system for maytansine in the treatment of non-small cell lung cancer. Drug Deliv. 2020, 27, 100–109. [Google Scholar] [CrossRef]
- Shinde, P.; Agraval, H.; Singh, A.; Yadav, U.C.; Kumar, U. Synthesis of Luteolin Loaded Zein Nanoparticles for Targeted Cancer Therapy Improving Bioavailability and Efficacy. J. Drug Deliv. Sci. Technol. 2019, 52, 369–378. [Google Scholar] [CrossRef]
- El Sharkawi, F.Z.; Ewais, S.M.; Fahmy, R.H.; Rashed, L.A. PTEN and TRAIL Genes Loaded Zein Nanoparticles as Potential Therapy for Hepatocellular Carcinoma. J. Drug Target. 2017, 25, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, L.; Chen, Y.; Zhang, H.; Zhong, J.; Sun, Y.; Kong, W. Zein-Based Nanofibers for Drug Delivery: Classes and Current Applications. Curr. Pharm. Des. 2015, 21, 3199–3207. [Google Scholar] [CrossRef]
- Wongsasulak, S.; Pathumban, S.; Yoovidhya, T. Effect of Entrapped α-Tocopherol on Mucoadhesivity and Evaluation of the Release, Degradation, and Swelling Characteristics of Zein–Chitosan Composite Electrospun Fibers. J. Food Eng. 2014, 120, 110–117. [Google Scholar] [CrossRef]
- El Fawal, G.; Omar, A.M.; Abu-Serie, M.M. Nanofibers Based on Zein Protein Loaded with Tungsten Oxide for Cancer Therapy: Fabrication, Characterization and In Vitro Evaluation. Sci. Rep. 2023, 13, 22216. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.G.; Dodero, V.I. Gliadin proteolytical resistant peptides: The interplay between structure and self-assembly in gluten-related disorders. Biophys. Rev. 2021, 13, 1147–1154. [Google Scholar] [CrossRef]
- Voci, S.; Gagliardi, A.; Ambrosio, N.; Salvatici, M.C.; Fresta, M.; Cosco, D. Gliadin Nanoparticles Containing Doxorubicin Hydrochloride: Characterization and Cytotoxicity. Pharmaceutics 2023, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Farzan, M.; Varshosaz, J.; Mirian, M.; Minaiyan, M.; Pezeshki, A. Hyaluronic Acid-Conjugated Gliadin Nanoparticles for Targeted Delivery of Usnic Acid in Breast Cancer: An In Vitro/In Vivo Translational Study. J. Drug Deliv. Sci. Technol. 2023, 84, 104459. [Google Scholar] [CrossRef]
- Huang, X.; Liu, B.; Ma, J.; Wei, S.; Wang, L.; Yin, S.; Yang, X. Development of Gliadin@AgNPs hybrid nanoparticles as building blocks for constructing antimicrobial protein-based porous materials. Chem. Eng. J. 2024, 482, 148924. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, X.; Meng, F.; Guo, H.; Liu, Z.; Wang, H.; Xu, J.; Jin, H.; Jiang, L. Wheat gliadin hydrolysates based nano-micelles for hydrophobic naringin: Structure characterization, interaction, and in vivo digestion. Food Chem. X 2024, 21, 101136. [Google Scholar] [CrossRef]
- Marcano, R.G.V.; Khalil, N.M.; de Lurdes Felsner, M.; Mainardes, R.M. Mitigating amphotericin B cytotoxicity through gliadin-casein nanoparticles: Insights into synthesis, optimization, characterization, in vitro release and cytotoxicity evaluation. Int. J. Biol. Macromol. 2024, 260, 129471. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Obeso, C.; Hartzell, E.J.; Scheel, R.A.; Kaplan, D.L. Delivering on the promise of recombinant silk-inspired proteins for drug delivery. Adv. Drug Deliv. Rev. 2023, 192, 114622. [Google Scholar] [CrossRef]
- Lozano-Pérez, A.A.; Rivero, H.C.; Hernández, M.D.C.P.; Pagán, A.; Montalbán, M.G.; Víllora, G.; Cénis, J.L. Silk Fibroin Nanoparticles: Efficient Vehicles for the Natural Antioxidant Quercetin. Int. J. Pharm. 2017, 518, 11–19. [Google Scholar] [CrossRef]
- Gupta, V.; Aseh, A.; Ríos, C.N.; Aggarwal, B.B.; Mathur, A.B. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int. J. Nanomed. 2009, 4, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Opálková Šišková, A.; Kozma, E.; Opálek, A.; Kroneková, Z.; Kleinová, A.; Nagy, Š.; Kronek, J.; Rydz, J.; Eckstein Andicsová, A. Diclofenac embedded in silk fibroin fibers as a drug delivery system. Materials 2020, 13, 3580. [Google Scholar] [CrossRef] [PubMed]
- Tallian, C.; Herrero-Rollett, A.; Stadler, K.; Vielnascher, R.; Wieland, K.; Weihs, A.M.; Pellis, A.; Teuschl, A.H.; Lendl, B.; Amenitsch, H. Structural insights into pH-responsive drug release of self-assembling human serum albumin-silk fibroin nanocapsules. Eur. J. Pharm. Biopharm. 2018, 133, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Feldt, M.; Bjarnadottir, O.; Kimbung, S.; Jirström, K.; Bendahl, P.-O.; Veerla, S.; Grabau, D.; Hedenfalk, I.; Borgquist, S. Statin-induced anti-proliferative effects via cyclin D1 and p27 in a window-of-opportunity breast cancer trial. J. Transl. Med. 2015, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-M.; Liu, Y.-L.; Lin, Y.-C.; Shun, C.-T.; Wu, M.-S.; Chen, C.-C. Inhibition of autophagy enhances anticancer effects of atorvastatin in digestive malignancies. Cancer Res. 2010, 70, 7699–7709. [Google Scholar] [CrossRef]
- Tamburrino, D.; Crippa, S.; Partelli, S.; Archibugi, L.; Arcidiacono, P.G.; Falconi, M.; Capurso, G. Statin use improves survival in patients with pancreatic ductal adenocarcinoma: A meta-analysis. Dig. Liver Dis. 2020, 52, 392–399. [Google Scholar] [CrossRef]
- Kanoujia, J.; Singh, M.; Singh, P.; Saraf, S.A. Novel genipin crosslinked atorvastatin loaded sericin nanoparticles for their enhanced antihyperlipidemic activity. Mater. Sci. Eng. C 2016, 69, 967–976. [Google Scholar] [CrossRef]
- Suktham, K.; Koobkokkruad, T.; Wutikhun, T.; Surassmo, S. Efficiency of resveratrol-loaded sericin nanoparticles: Promising bionanocarriers for drug delivery. Int. J. Pharm. 2018, 537, 48–56. [Google Scholar] [CrossRef]
- Parisi, O.I.; Fiorillo, M.; Scrivano, L.; Sinicropi, M.S.; Dolce, V.; Iacopetta, D.; Puoci, F.; Cappello, A.R. Sericin/poly (ethylcyanoacrylate) nanospheres by interfacial polymerization for enhanced bioefficacy of fenofibrate: In vitro and in vivo studies. Biomacromolecules 2015, 16, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Scrivano, L.; Iacopetta, D.; Sinicropi, M.S.; Saturnino, C.; Longo, P.; Parisi, O.I.; Puoci, F. Synthesis of sericin-based conjugates by click chemistry: Enhancement of sunitinib bioavailability and cell membrane permeation. Drug Deliv. 2017, 24, 482–490. [Google Scholar] [CrossRef][Green Version]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural polymer-based hydrogels: From polymer to biomedical applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef] [PubMed]
- Singhal, P.; Vashisht, H.; Nisar, S.; Mehra, S.; Rattan, S. Stimulus Responsive Soy-Protein Based Hydrogels through Grafting HEMA for Biomedical Applications. Ind. Crop. Prod. 2022, 178, 114621. [Google Scholar] [CrossRef]
- Gutschmidt, D.; Hazra, R.S.; Zhou, X.; Xu, X.; Sabzi, M.; Jiang, L. Electrospun, Sepiolite-Loaded Poly(Vinyl Alcohol)/Soy Protein Isolate Nanofibers: Preparation, Characterization, and Their Drug Release Behavior. Int. J. Pharm. 2021, 594, 120172. [Google Scholar] [CrossRef] [PubMed]
- Zare-Zardini, H.; Soltaninejad, H.; Ghorani-Azam, A.; Nafisi-Moghadam, R.; Haddadzadegan, N.; Ansari, M.; Saeed-Banadaki, S.H.; Sobhan, M.R.; Mozafari, S.; Zahedi, M. Slow release curcumin-containing soy protein nanoparticles as anticancer agents for osteosarcoma: Synthesis and characterization. Prog. Biomater. 2022, 11, 311–320. [Google Scholar] [CrossRef]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Fabrication of vitamin E-enriched nanoemulsions: Factors affecting particle size using spontaneous emulsification. J. Colloid Interface Sci. 2013, 391, 95–102. [Google Scholar] [CrossRef]
- Wan, J.; Li, D.; Song, R.; Shah, B.R.; Li, B.; Li, Y. Enhancement of physical stability and bioaccessibility of tangeretin by soy protein isolate addition. Food Chem. 2017, 221, 760–770. [Google Scholar] [CrossRef]
- Qian, X.; Ge, L.; Yuan, K.; Li, C.; Zhen, X.; Cai, W.; Cheng, R.; Jiang, X. Targeting and microenvironment-improving of phenylboronic acid-decorated soy protein nanoparticles with different sizes to tumor. Theranostics 2019, 9, 7417. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Y.; Borgard, H.; Jijiwa, M.; Nasu, M.; He, M.; Deng, Y. The function and mechanism of lipid molecules and their roles in the diagnosis and prognosis of breast cancer. Molecules 2020, 25, 4864. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Sabjan, K.B.; Munawar, S.M.; Rajendiran, D.; Vinoji, S.K.; Kasinathan, K. Nanoemulsion as oral drug delivery—A review. Curr. Drug Res. Rev. Former. Curr. Drug Abus. Rev. 2020, 12, 4–15. [Google Scholar] [CrossRef]
- Munir, M.; Zaman, M.; Waqar, M.A.; Khan, M.A.; Alvi, M.N. Solid lipid nanoparticles: A versatile approach for controlled release and targeted drug delivery. J. Liposome Res. 2024, 34, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.; Uthappa, U.; Altalhi, T.; Jung, H.-Y.; Han, S.S.; Kurkuri, M.D. Alginate based polymeric systems for drug delivery, antibacterial/microbial, and wound dressing applications. Mater. Today Commun. 2022, 33, 104813. [Google Scholar] [CrossRef]
- Moon, R.J.; Schueneman, G.T.; Simonsen, J. Overview of cellulose nanomaterials, their capabilities and applications. Jom 2016, 68, 2383–2394. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Kandi, V.; Vadakedath, S. Clinical trials and clinical research: A comprehensive review. Cureus 2023, 15, e35077. [Google Scholar] [CrossRef]
- Jager, W. The Importance of Clinical Trials in Advancing Medical Research. Ann. Clin. Trials Vaccines Res. 2023, 13, 93–98. [Google Scholar] [CrossRef]
- Hackshaw, A. A Concise Guide to Clinical Trials, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2024. [Google Scholar]
- Toyama, T.; Nitta, N.; Ohta, S.; Tanaka, T.; Nagatani, Y.; Takahashi, M.; Murata, K.; Shiomi, H.; Naka, S.; Kurumi, Y. Clinical trial of cisplatin-conjugated gelatin microspheres for patients with hepatocellular carcinoma. Jpn. J. Radiol. 2012, 30, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Livney, Y. Bioavailability of Vitamin D Encapsulated in Casein Micelles, Compared with Its Bioavailability in a Synthetic Emulsifier Currently Used for Supplementation and Enrichment. Clin. Trial 2013, ClinicalTrials.gov ID: NCT01807845. Available online: https://cdek.pharmacy.purdue.edu/trial/NCT01807845/ (accessed on 30 August 2024).
- Yoneshima, Y.; Morita, S.; Ando, M.; Nakamura, A.; Iwasawa, S.; Yoshioka, H.; Goto, Y.; Takeshita, M.; Harada, T.; Hirano, K. Phase 3 trial comparing nanoparticle albumin-bound paclitaxel with docetaxel for previously treated advanced NSCLC. J. Thorac. Oncol. 2021, 16, 1523–1532. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | Protein-Based Biopolymers | Synthetic Polymers |
|---|---|---|
| Water solubility | High | Varies, generally low |
| Biocompatibility | High | Varies, generally low |
| Biodegradability | High | Generally low |
| Toxicity | Low | Potentially high |
| Inertness | Inert | Potentially reactive |
| Availability | Easily available from natural sources | Depends on the synthesis process |
| Amphiphilic properties | Yes, facilitating interactions with solvents and drugs | Generally lacking |
| Conjugation abilities | Capable of forming covalent bonds with drugs and ligands | Good conjugation abilities but with potential side effects |
| Role in immune response | Less likely to cause immune response activation | Can cause inflammation and immune response activation |
| Use of organic solvents | Not required | Often required |
| Protein-Based Carrier | Drug | Application | Reference |
|---|---|---|---|
| Cassava starch acetate (CSA)—polyethylene glycol (PEG)—gelatin (G) nanocomposites | Cisplatin | Anticancer therapy | [33] |
| Eudragit-S100-coated gelatin nanoparticles | 5-amino salicylic acid (5-ASA) | Ulcerative colitis treatment | [34] |
| Graphene oxide nanocarriers covered by gelatin and polyvinylpyrrolidone (PVP) | Quercetin | Anticancer therapy | [35] |
| Carbon quantum dots complexed with gelatin and chitosan hydrogel | Curcumin | Anticancer therapy | [36] |
| Halloysite nanotubes complexed with gelatin microparticles | Carvedilol | Improved oral drug delivery system for hypertension and coronary artery pathologies | [37] |
| Gelatin nanoparticles | Methotrexate | Anticancer therapy | [39] |
| Insoluble gelatin type B/chitosan nanoparticles | Systems tested as good Pickering emulsifiers | Numerous different treatments | [42] |
| Gelatin/glucomannan)/tannic acid nanocomplexes | Systems tested as good Pickering emulsifiers | Numerous different treatments | [43] |
| A multilayer emulsion made up of gelatin, gum Arabic and tannic acid | Curcumin | Anticancer therapy | [44] |
| Aminated gelatin nanoparticles | Systems tested as good Pickering emulsifiers | Numerous different treatments | [46] |
| Gelatin nanoparticles | Tizanidine hydrochloride and Gatifloxacin | Muscle relaxation therapies and bacterial infection treatments, also anticancer therapy | [47] |
| Gelatin/folic acid nanoparticles | Irinotecan | Anticancer therapy | [48] |
| Gelatin nanoparticles of different sizes | Doxorubicin, iodixanol and cisplatin | Anticancer therapy | [49] |
| Protein-Based Carrier | Drug | Application | Reference |
|---|---|---|---|
| BSA/oxidized arabic gum nanoparticles | Piperine | Anticancer therapy | [51] |
| Folic acid–BSA grafted graphene oxide nanocomplexes | Doxorubicin | Anticancer therapy | [52] |
| Ethoniosomes coated with folic acid/BSA | Pterostilbene | Antidiabetic and anticancer therapies | [55] |
| Fe3+–BSA nanoparticles, grafted with folic acid and complexed with indocyanine green dye | Doxorubicin | Anticancer therapy | [56] |
| Protein-Based Carrier | Drug | Application | Reference |
|---|---|---|---|
| Collagen (poly 3-acrylamidophenylboronic acid, PAPBA) nanoparticles | Doxorubicin | Anticancer therapy | [57] |
| Type 1 collagen hydrogels | Luteolin | Wound-healing therapies | [58] |
| An innovative nanostructure made up of cellulose nanofibrils and collagen aerogels | 5-fluorouracil | Numerous different treatments | [59] |
| Porous microspheres made up of collagen and bacterial cellulose | BSA | Numerous different treatments | [60] |
| Collagen nanoparticles | Sylimarin | Brain disease therapies | [61] |
| Protein-Based Carrier | Drug | Application | Reference |
|---|---|---|---|
| Zein nanoparticles (phase separation method) | Doxorubicin | Anticancer therapy | [63] |
| Zein nanoparticles | Maytansine | Anticancer therapy | [64] |
| Zein nanoparticles coated with sodium caseinate | Luteolin | Wound-healing therapies | [65] |
| Zein nanoparticles | PTEN (Phosphatase and Tensin homolog deleted from chromosome ten) and TRAIL (TNF- related apoptosis- inducing ligand) genes | Gene therapy and anticancer therapy | [66] |
| Zein nanofibers made up of chitosan and polyethylene oxide (PEO) | Alpha-tocopherol | Delivery of hydrophobic compounds to the gastrointestinal area | [68] |
| Zein nanofibers with the incorporation of tungsten oxide | Tested as innovative structures | Anticancer therapy | [69] |
| Protein-Based Carrier | Drug | Application | Reference |
|---|---|---|---|
| Gliadin nanoparticles coated by polyoxyethylene (2) oleyl ether | Doxorubicin hydrochloride | Anticancer therapy | [71] |
| Gliadin nanoparticles functionalised with hyaluronic acid | Usnic acid | Anticancer therapy | [72] |
| Hybrid gliadin/silver nanoparticles for the building of an innovative protein-based porous material | Tested as innovative structures | Antibacterial therapies | [73] |
| Nanomicelles made up of gliadin hydrolysates | Naringin | Anticancer therapy | [74] |
| Gliadin nanoparticles coated by caseins | Amphotericin B | Antifungal infections treatments | [75] |
| Protein-Based Carrier | Drug | Application | Reference |
|---|---|---|---|
| Silk fibroin nanoparticles | Quercetin | Anticancer and anti-inflammatory therapies | [77] |
| Silk fibroin–chitosan nanoparticles | Curcumin | Anticancer therapy | [78] |
| Silk fibroin/casein electrospun nanofibers | Diclofenac sodium salt | Anti-inflammatory therapies | [79] |
| Silk fibroin–human serum albumin nanocapsules | Methotrexate | Anti-inflammatory therapies | [80] |
| Silk sericin nanoparticles | Atorvastatin | Anticancer therapy | [84] |
| Silk sericin nanoparticles covered by pluronic F-68 | Resveratrol | Anticancer therapy | [85] |
| Silk sericin/poly(ethylcyanoacrylate) nanospheres | Fenoifibrate | Cholesterolemia therapies | [86] |
| Bioconjugate made of silk sericin with modifications | Sunitib | Anticancer therapy | [87] |
| Protein-Based Carrier | Drug | Application | Reference |
|---|---|---|---|
| 2-hydroxyethyl methacrylate—soy protein isolate (SPI) pH sensitive hydrogel | Paracetamol | Gatrointestinal disease therapies | [89] |
| PVA (Polyvinyl alcohol)/SPI nanofiber mats complexed with sepiolite nano needles | Ketoprofen | Anti-inflammatory therapies | [90] |
| Soybean protein-based nanoparticles | Curcumin | Anticancer therapy | [91] |
| Supersaturated nanoemulsions of SPI | Tangeretin | Numerous therapies involving hydrophobic pharmaceuticals | [93] |
| Soy protein nanoparticles coated by phenylboronic acid | Sialic acid | Anticancer therapy | [94] |
| Protein-Based Carrier | Drug | Application | Reference |
|---|---|---|---|
| Gelatin nanoparticles | Cisplatin | Clinical trial first stage—Advanced Hepatocellular Carcinoma | [86] |
| Casein micelles | Vitamin D | Clinical trial n° NCT01807845 | [87] |
| Albumin nanoparticles | Paclitaxel | Clinical trial n° NCT01620190 | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraro, C.; Dattilo, M.; Patitucci, F.; Prete, S.; Scopelliti, G.; Parisi, O.I.; Puoci, F. Exploring Protein-Based Carriers in Drug Delivery: A Review. Pharmaceutics 2024, 16, 1172. https://doi.org/10.3390/pharmaceutics16091172
Ferraro C, Dattilo M, Patitucci F, Prete S, Scopelliti G, Parisi OI, Puoci F. Exploring Protein-Based Carriers in Drug Delivery: A Review. Pharmaceutics. 2024; 16(9):1172. https://doi.org/10.3390/pharmaceutics16091172
Chicago/Turabian StyleFerraro, Claudia, Marco Dattilo, Francesco Patitucci, Sabrina Prete, Giuseppe Scopelliti, Ortensia Ilaria Parisi, and Francesco Puoci. 2024. "Exploring Protein-Based Carriers in Drug Delivery: A Review" Pharmaceutics 16, no. 9: 1172. https://doi.org/10.3390/pharmaceutics16091172
APA StyleFerraro, C., Dattilo, M., Patitucci, F., Prete, S., Scopelliti, G., Parisi, O. I., & Puoci, F. (2024). Exploring Protein-Based Carriers in Drug Delivery: A Review. Pharmaceutics, 16(9), 1172. https://doi.org/10.3390/pharmaceutics16091172








