Astodrimer Sodium Nasal Spray versus Placebo in Non-Hospitalised Patients with COVID-19: A Randomised, Double-Blinded, Placebo-Controlled Trial
Abstract
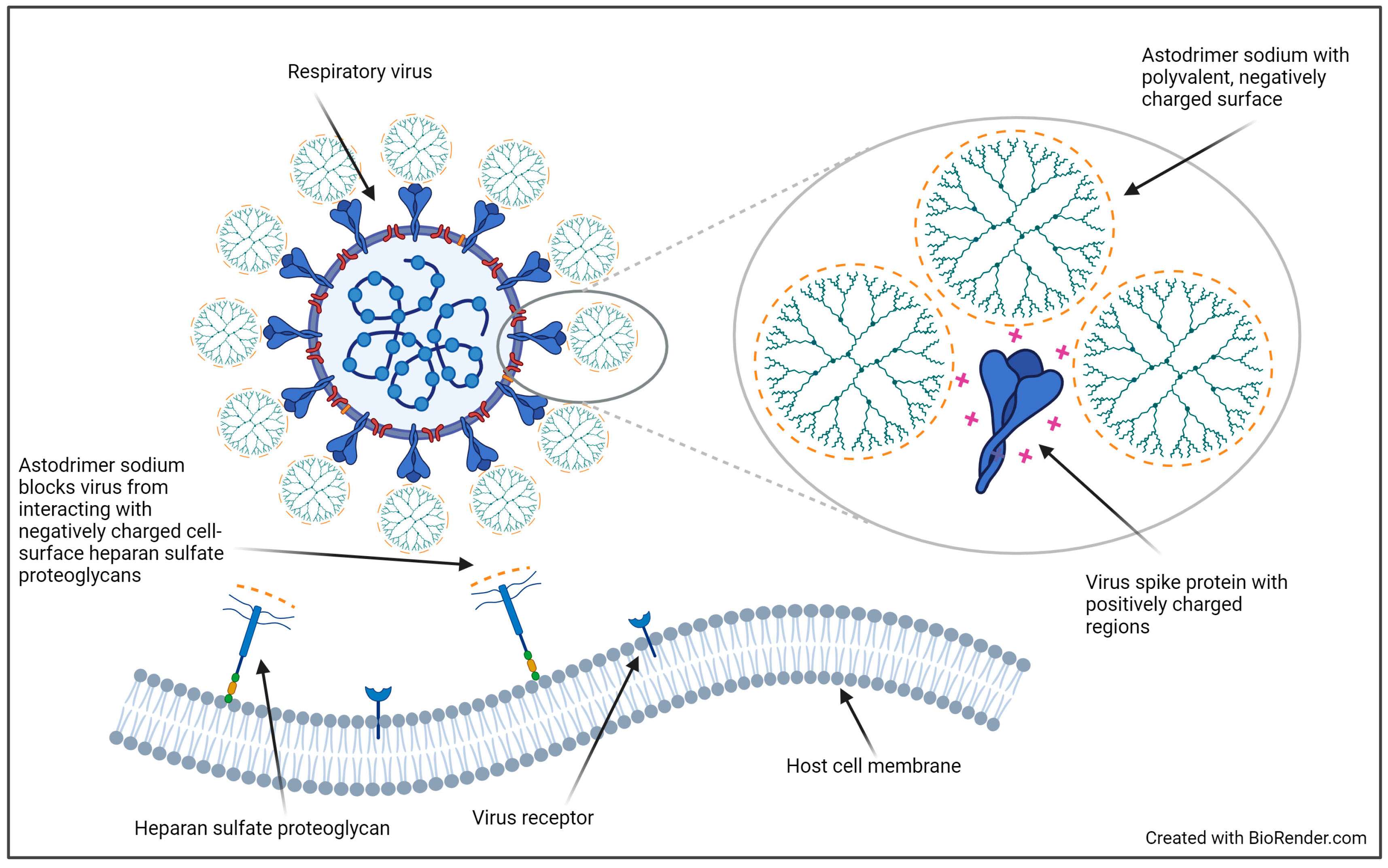
:1. Introduction
2. Materials and Methods
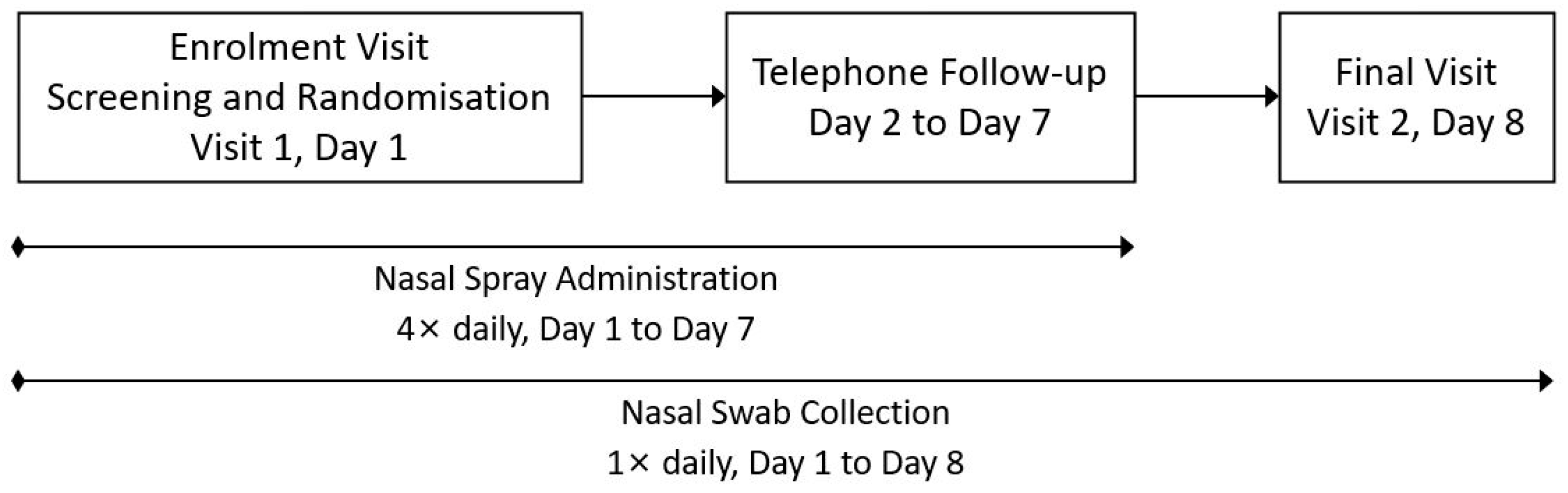
2.1. Study Design
2.2. Participants
2.3. Randomisation and Masking
2.4. Procedures
2.5. Outcomes
2.5.1. Primary Performance Outcome
2.5.2. Secondary Performance Outcomes
2.5.3. Safety Outcomes
2.6. Statistical Analysis
2.6.1. Analysis Populations
2.6.2. Primary Efficacy Analysis
2.6.3. Secondary and Exploratory Efficacy Analyses
2.6.4. Safety Analyses
2.6.5. Sample Size Determination
3. Results
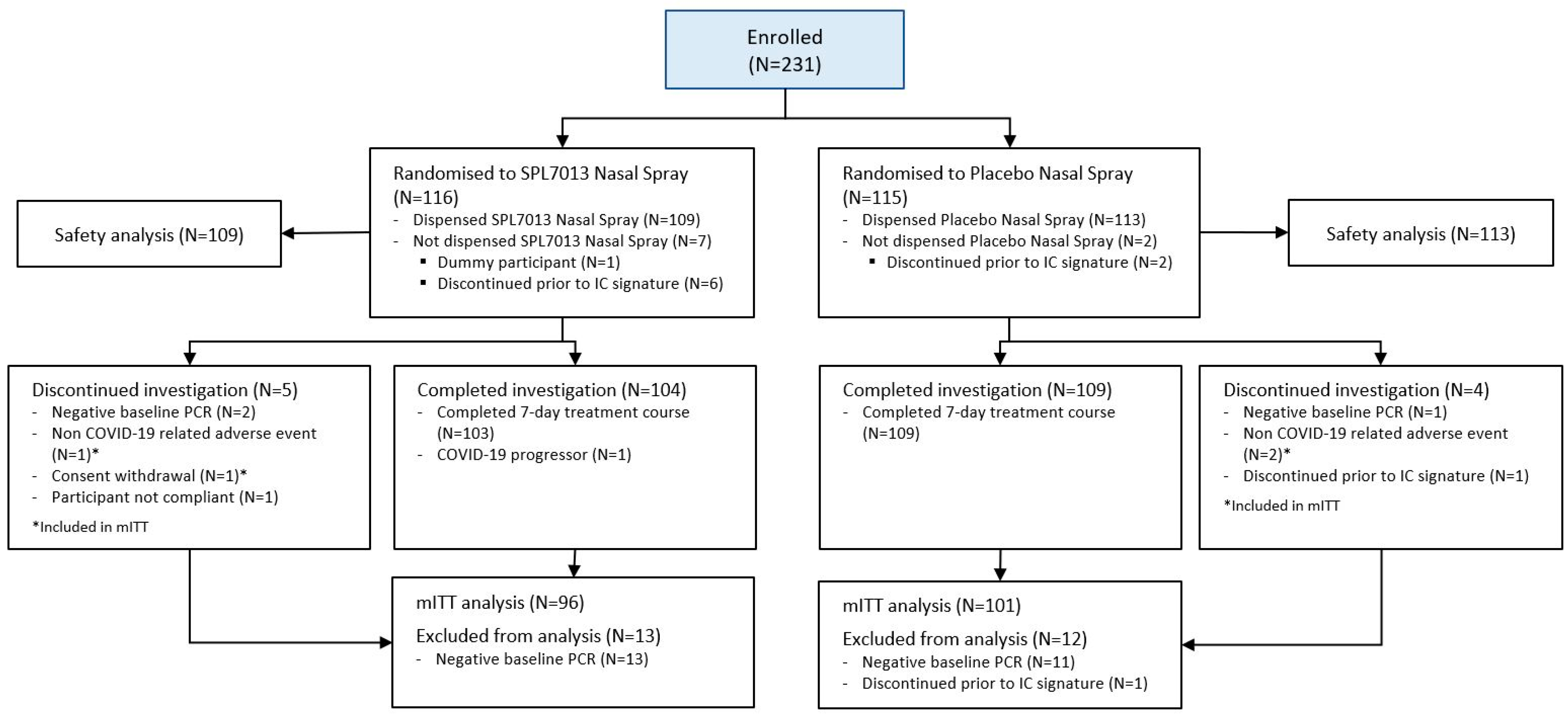
3.1. Populations and Baseline Data
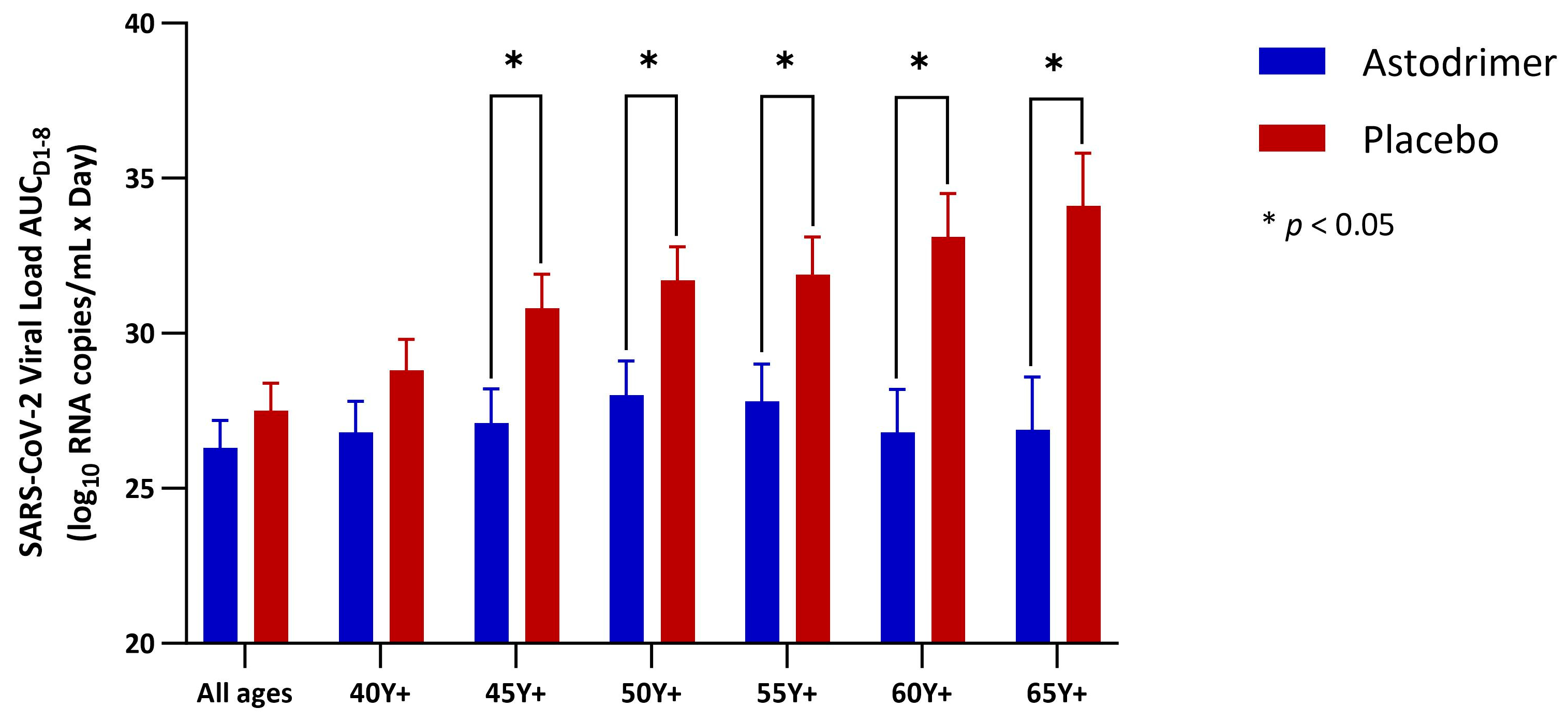
3.2. Viral Load
3.3. Symptoms
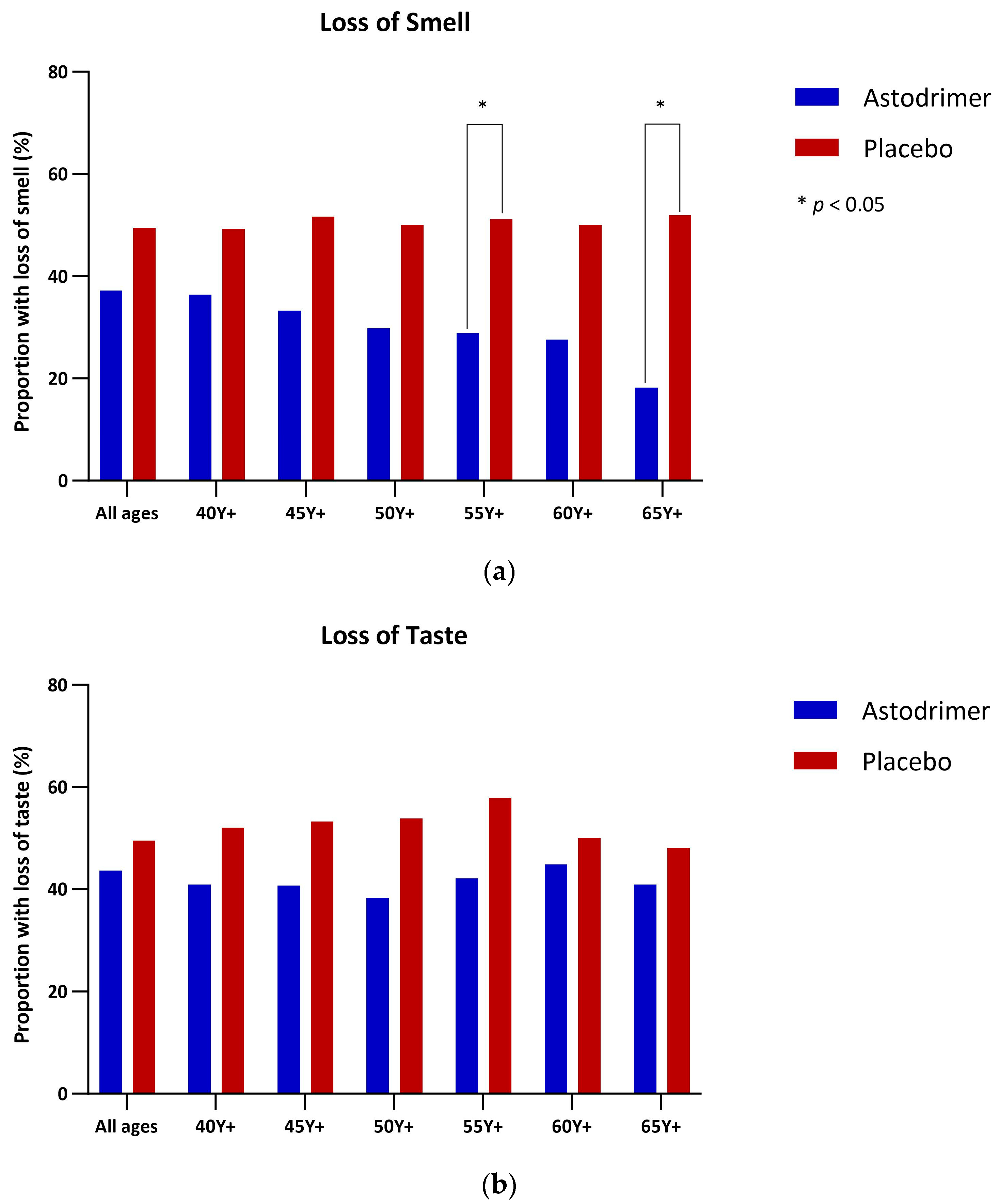
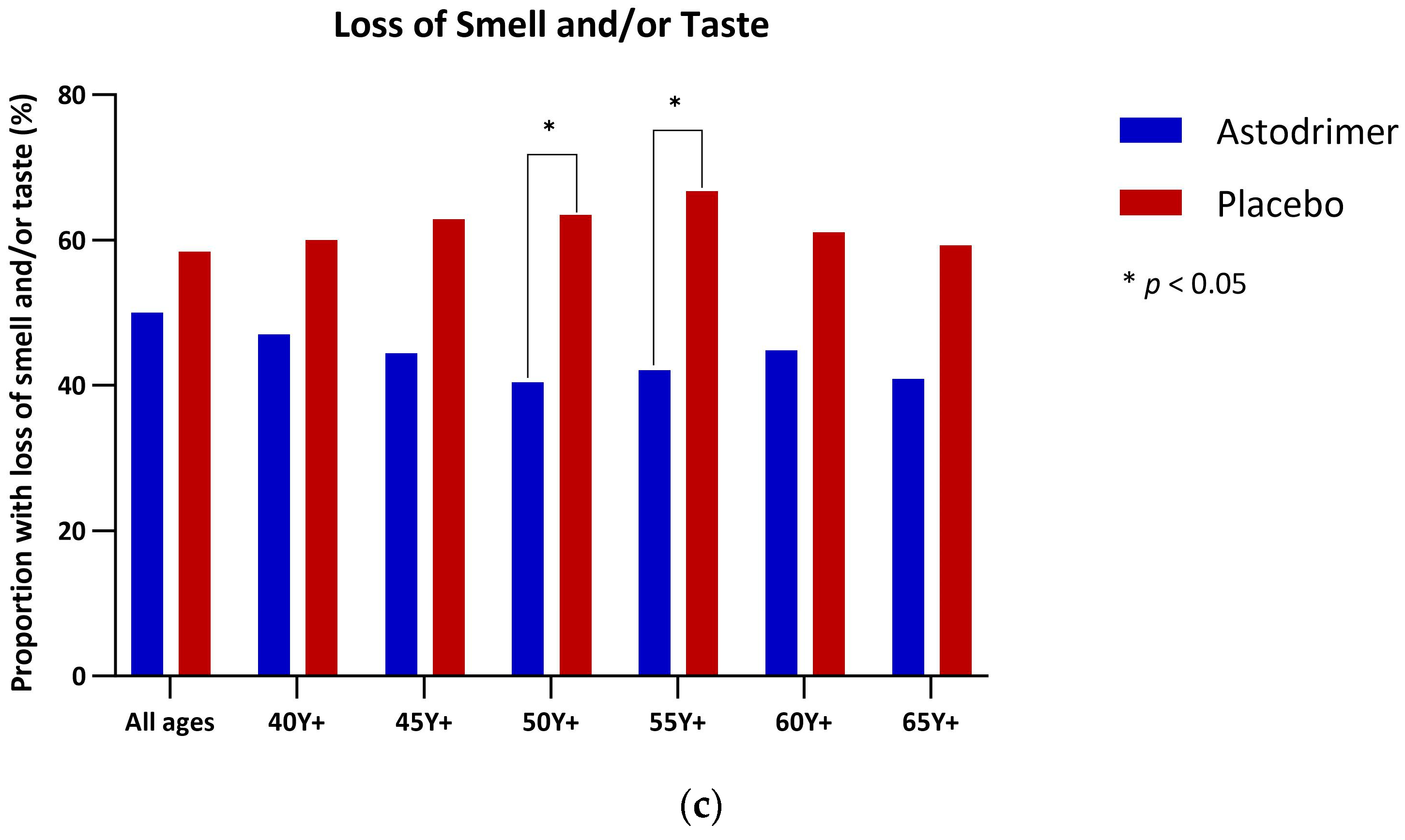
3.4. Anosmia and Ageusia
3.5. Return to Usual Activity
3.6. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Little, P.; Read, R.C.; Amlôt, R.; Chadborn, T.; Rice, C.; Bostock, J.; Yardley, L. Reducing Risks from Coronavirus Transmission in the Home-the Role of Viral Load. BMJ 2020, 369, m1728. [Google Scholar] [CrossRef]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian Hamsters as a Small Animal Model for SARS-CoV-2 Infection and Countermeasure Development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef] [PubMed]
- Dabisch, P.A.; Biryukov, J.; Beck, K.; Boydston, J.A.; Sanjak, J.S.; Herzog, A.; Green, B.; Williams, G.; Yeager, J.; Bohannon, J.K.; et al. Seroconversion and Fever Are Dose-Dependent in a Nonhuman Primate Model of Inhalational COVID-19. PLoS Pathog. 2021, 17, e1009865. [Google Scholar] [CrossRef] [PubMed]
- Memoli, M.J.; Czajkowski, L.; Reed, S.; Athota, R.; Bristol, T.; Proudfoot, K.; Fargis, S.; Stein, M.; Dunfee, R.L.; Shaw, P.A.; et al. Validation of the Wild-Type Influenza A Human Challenge Model H1N1pdMIST: An A(H1N1)Pdm09 Dose-Finding Investigational New Drug Study. Clin. Infect. Dis. 2015, 60, 693–702. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.-M.; Wan, L.; Xiang, T.-X.; Le, A.; Liu, J.-M.; Peiris, M.; Poon, L.L.M.; Zhang, W. Viral Dynamics in Mild and Severe Cases of COVID-19. Lancet Infect. Dis. 2020, 20, 656–657. [Google Scholar] [CrossRef]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 Viral Load Is Associated with Increased Disease Severity and Mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Raftopoulos, V.; Vorou, R.; Papadima, K.; Mellou, K.; Spanakis, N.; Kossyvakis, A.; Gioula, G.; Exindari, M.; Froukala, E.; et al. Association Between Upper Respiratory Tract Viral Load, Comorbidities, Disease Severity, and Outcome of Patients with SARS-CoV-2 Infection. J. Infect. Dis. 2021, 223, 1132–1138. [Google Scholar] [CrossRef]
- da Silva, S.J.R.; de Lima, S.C.; da Silva, R.C.; Kohl, A.; Pena, L. Viral Load in COVID-19 Patients: Implications for Prognosis and Vaccine Efficacy in the Context of Emerging SARS-CoV-2 Variants. Front. Med. 2021, 8, 836826. [Google Scholar] [CrossRef]
- Kawasuji, H.; Takegoshi, Y.; Kaneda, M.; Ueno, A.; Miyajima, Y.; Kawago, K.; Fukui, Y.; Yoshida, Y.; Kimura, M.; Yamada, H.; et al. Transmissibility of COVID-19 Depends on the Viral Load around Onset in Adult and Symptomatic Patients. PLoS ONE 2020, 15, e0243597. [Google Scholar] [CrossRef]
- Meyerowitz, E.A.; Scott, J.; Richterman, A.; Male, V.; Cevik, M. Clinical Course and Management of COVID-19 in the Era of Widespread Population Immunity. Nat. Rev. Microbiol. 2024, 22, 75–88. [Google Scholar] [CrossRef]
- Lewis, D. Is the Coronavirus Airborne? Experts Can’t Agree. Nature 2020, 580, 175. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Mao, Y.; Jones, R.M.; Tan, Q.; Ji, J.S.; Li, N.; Shen, J.; Lv, Y.; Pan, L.; Ding, P.; et al. Aerosol Transmission of SARS-CoV-2? Evidence, Prevention and Control. Environ. Int. 2020, 144, 106039. [Google Scholar] [CrossRef]
- Yan, J.; Grantham, M.; Pantelic, J.; Bueno de Mesquita, P.J.; Albert, B.; Liu, F.; Ehrman, S.; Milton, D.K. EMIT Consortium Infectious Virus in Exhaled Breath of Symptomatic Seasonal Influenza Cases from a College Community. Proc. Natl. Acad. Sci. USA 2018, 115, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Paull, J.R.A.; Heery, G.P.; Bobardt, M.D.; Castellarnau, A.; Luscombe, C.A.; Fairley, J.K.; Gallay, P.A. Virucidal and Antiviral Activity of Astodrimer Sodium against SARS-CoV-2 in Vitro. Antiviral Res. 2021, 191, 105089. [Google Scholar] [CrossRef] [PubMed]
- Gallay, P.A.; Luscombe, C.A.; Stauffer, W.; Bobardt, M.D.; Heery, G.P.; Castellarnau, A.; Seta, A.; Paull, J.R.A. Effects of Astodrimer Sodium against SARS-CoV-2 Variants (α, β, γ, δ, κ) in Vitro [CROI Abstract 478]. Special Issue: Abstracts from the Virtual 2022 Conference on Retroviruses and Opportunistic Infections. Top. Antivir. Med. 2022, 30, 182. [Google Scholar] [PubMed]
- Paull, J.R.A.; Luscombe, C.A.; Castellarnau, A.; Heery, G.P.; Bobardt, M.D.; Gallay, P.A. Protective Effects of Astodrimer Sodium 1% Nasal Spray Formulation against SARS-CoV-2 Nasal Challenge in K18-hACE2 Mice. Viruses 2021, 13, 1656. [Google Scholar] [CrossRef] [PubMed]
- Castellarnau, A.; Heery, G.P.; Seta, A.; Luscombe, C.A.; Kinghorn, G.R.; Button, P.; McCloud, P.; Paull, J.R.A. Astodrimer Sodium Antiviral Nasal Spray for Reducing Respiratory Infections Is Safe and Well Tolerated in a Randomized Controlled Trial. Sci. Rep. 2022, 12, 10210. [Google Scholar] [CrossRef]
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A Minimal Common Outcome Measure Set for COVID-19 Clinical Research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Powers, J.H.; Guerrero, M.L.; Leidy, N.K.; Fairchok, M.P.; Rosenberg, A.; Hernández, A.; Stringer, S.; Schofield, C.; Rodríguez-Zulueta, P.; Kim, K.; et al. Development of the Flu-PRO: A Patient-Reported Outcome (PRO) Instrument to Evaluate Symptoms of Influenza. BMC Infect. Dis. 2016, 16, 1. [Google Scholar] [CrossRef]
- Powers, J.H.; Bacci, E.D.; Guerrero, M.L.; Leidy, N.K.; Stringer, S.; Kim, K.; Memoli, M.J.; Han, A.; Fairchok, M.P.; Chen, W.-J.; et al. Reliability, Validity, and Responsiveness of InFLUenza Patient-Reported Outcome (FLU-PRO©) Scores in Influenza-Positive Patients. Value Health 2018, 21, 210–218. [Google Scholar] [CrossRef]
- Dadras, O.; Afsahi, A.M.; Pashaei, Z.; Mojdeganlou, H.; Karimi, A.; Habibi, P.; Barzegary, A.; Fakhfouri, A.; Mirzapour, P.; Janfaza, N.; et al. The Relationship between COVID-19 Viral Load and Disease Severity: A Systematic Review. Immun. Inflamm. Dis. 2022, 10, e580. [Google Scholar] [CrossRef]
- Winchester, S.; John, S.; Jabbar, K.; John, I. Clinical Efficacy of Nitric Oxide Nasal Spray (NONS) for the Treatment of Mild COVID-19 Infection. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef]
- Aydin, S.; Benk, I.G.; Geckil, A.A. May Viral Load Detected in Saliva in the Early Stages of Infection Be a Prognostic Indicator in COVID-19 Patients? J. Virol. Methods 2021, 294, 114198. [Google Scholar] [CrossRef] [PubMed]
- Vegvari, C.; Hadjichrysanthou, C.; Cauët, E.; Lawrence, E.; Cori, A.; de Wolf, F.; Anderson, R.M. How Can Viral Dynamics Models Inform Endpoint Measures in Clinical Trials of Therapies for Acute Viral Infections? PLoS ONE 2016, 11, e0158237. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.G.; Andrew, M.K. COVID-19 Vaccination and Hybrid Immunity in Older Adults. Lancet Healthy Longev. 2023, 4, e364–e365. [Google Scholar] [CrossRef] [PubMed]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Khoury, J.; Amar, M.; Stein, N.; Goldstein, L.H.; Saliba, W. Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients. Clin Infect Dis 2023, 76, e342–e349. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory Transmucosal SARS-CoV-2 Invasion as a Port of Central Nervous System Entry in Individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Boldrini, M.; Canoll, P.D.; Klein, R.S. How COVID-19 Affects the Brain. JAMA Psychiatry 2021, 78, 682–683. [Google Scholar] [CrossRef]
- Anwar, M.M.; Badawi, A.M.; Eltablawy, N.A. Can the Coronavirus Infection Penetrates the Brain Resulting in Sudden Anosmia Followed by Severe Neurological Disorders? eNeurologicalSci 2020, 21, 100290. [Google Scholar] [CrossRef]
- Vilarello, B.J.; Jacobson, P.T.; Tervo, J.P.; Waring, N.A.; Gudis, D.A.; Goldberg, T.E.; Devanand, D.P.; Overdevest, J.B. Olfaction and Neurocognition after COVID-19: A Scoping Review. Front. Neurosci. 2023, 17, 1198267. [Google Scholar] [CrossRef]
| Astodrimer (n = 96) | Placebo (n = 101) | Total (n = 197) | |
|---|---|---|---|
| Age (Years) | 50 (16, 86) | 52 (19, 86) | 50 (16, 86) |
| ≥40 years (40 Y+) | 68 (70.8%) | 75 (74.3%) | 143 (72.6%) |
| ≥45 years (45 Y+) | 56 (58.3%) | 62 (61.4%) | 118 (59.9%) |
| ≥50 years (50 Y+) | 49 (51.0%) | 52 (51.5%) | 101 (51.3%) |
| ≥55 years (55 Y+) | 39 (40.6%) | 45 (44.6%) | 84 (42.6%) |
| ≥60 years (60 Y+) | 30 (31.3%) | 36 (35.6%) | 66 (33.5%) |
| ≥65 years (65 Y+) | 23 (24.0%) | 27 (26.7%) | 50 (25.4%) |
| Gender | |||
| Female | 60 (62.5%) | 57 (56.4%) | 117 (59.4%) |
| Male | 36 (37.5%) | 44 (43.6%) | 80 (40.6%) |
| Ethnicity | |||
| White | 81 (87.1%) | 79 (78.2%) | 160 (82.5%) |
| Asian (Indian, Pakistani or Bangladeshi) | 8 (8.6%) | 11 (10.9%) | 19 (9.8%) |
| Asian (Chinese) | 1 (1.1%) | 3 (3.0%) | 4 (2.1%) |
| Other | 1 (1.1%) | 6 (5.9%) | 7 (6.9%) |
| Height (cm) | 168.6 (9.1) | 168.5 (10.7) | 168.6 (9.9) |
| Weight (kg) | 82.0 (19.4) | 75.4 (16.5) | 78.6 (18.2) |
| BMI (kg/m2) | 28.8 (6.0) | 26.4 (4.7) | 27.6 (5.5) |
| Astodrimer | Placebo | Total | |
|---|---|---|---|
| COVID-19 vaccination status | (N = 96) | (N = 101) | (N = 197) |
| Participants not vaccinated | 4 (4.2%) | 4 (4.0%) | 8 (4.1%) |
| Participants with at least 1 vaccination | 90 (93.8%) | 96 (95.0%) | 186 (94.4%) |
| Missing | 2 | 1 | 3 |
| Vaccination history | (N = 68) | (N = 83) | (N = 151) |
| Number of vaccinations per participant | 3.5 (1.1) | 3.4 (0.8) | 3.5 (1.0) |
| Participants with 1 vaccination | 2 (2.9%) | 0 (0.0%) | 2 (1.3%) |
| Participants with 2 vaccinations | 7 (10.3%) | 11 (13.3%) | 18 (11.9%) |
| Participants with 3 vaccinations | 29 (42.6%) | 32 (38.6%) | 61 (40.4%) |
| Participants with 4 vaccinations | 18 (26.5%) | 32 (38.6%) | 50 (33.1%) |
| Participants with 5 vaccinations | 10 (14.7%) | 8 (9.6%) | 18 (11.9%) |
| Participants with 6 vaccinations | 1 (1.5%) | 0 (0.0%) | 1 (0.7%) |
| Participants with 7 vaccinations | 1 (1.5%) | 0 (0.0%) | 1 (0.7%) |
| Type of vaccine | |||
| Pfizer/BioNTech | 61 (89.7%) | 78 (94.0%) | 139 (92.1%) |
| Oxford/AstraZeneca | 33 (48.5%) | 41 (49.4%) | 74 (49.0%) |
| Moderna | 17 (25.0%) | 33 (39.8%) | 50 (33.1%) |
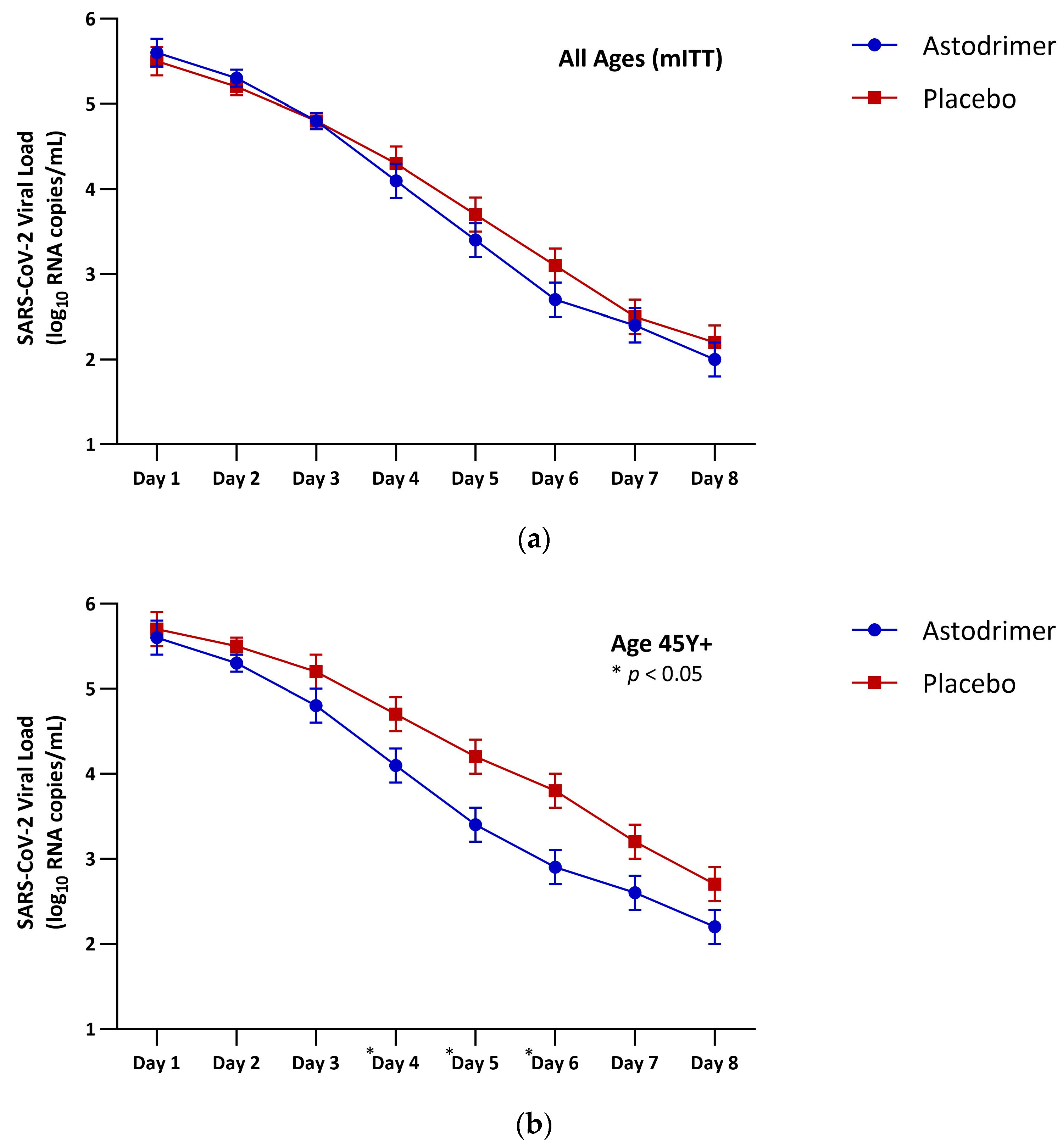
| Astodrimer | Placebo | Astodrimer Versus Placebo | |
|---|---|---|---|
| All ages (mITT) | |||
| N | 96 | 101 | 197 |
| LSM (SE) | 26.3 (0.9) | 27.5 (0.9) | −1.2 (1.2) |
| 95% CI | 24.6, 28.1 | 25.8, 29.2 | −3.6, 1.2 |
| p-value | 0.344 | ||
| Age 40 Y+ (mITT) | |||
| N | 68 | 75 | 143 |
| LSM (SE) | 26.8 (1.0) | 28.8 (1.0) | −2.0 (1.4) |
| 95% CI | 24.8, 28.9 | 26.9, 30.8 | −4.8, 0.8 |
| p-value | 0.159 | ||
| Age 45 Y+ (mITT) | |||
| N | 56 | 62 | 118 |
| LSM (SE) | 27.1 (1.1) | 30.8 (1.1) | −3.7 (1.5) |
| 95% CI | 24.9, 29.3 | 28.7, 32.9 | −6.8, −0.7 |
| p-value | 0.017 * | ||
| Age 50 Y+ (mITT) | |||
| N | 49 | 52 | 101 |
| LSM (SE) | 28.0 (1.1) | 31.7 (1.1) | −3.8 (1.6) |
| 95% CI | 25.7, 30.2 | 29.6, 33.9 | −6.9, −0.7 |
| p-value | 0.018 * | ||
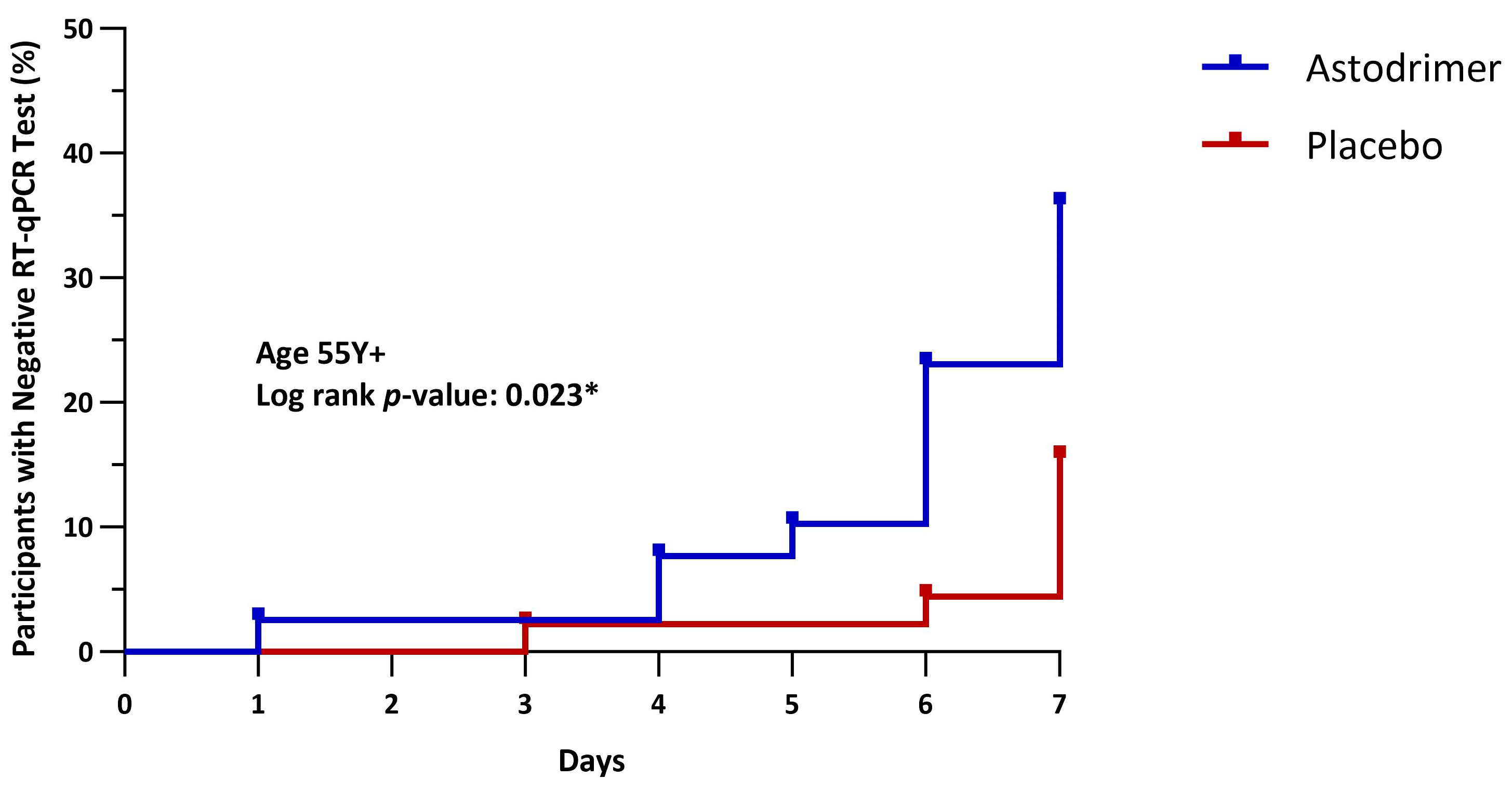
| Age 55 Y+ (mITT) | |||
| N | 39 | 45 | 84 |
| LSM (SE) | 27.8 (1.2) | 31.9 (1.2) | −4.2 (1.8) |
| 95% CI | 25.2, 30.4 | 29.5, 34.4 | −7.7, −0.6 |
| p-value | 0.024 * | ||
| Age 60 Y+ (mITT) | |||
| N | 30 | 36 | 66 |
| LSM (SE) | 26.8 (1.4) | 33.1 (1.4) | −6.2 (2.0) |
| 95% CI | 23.9, 29.8 | 30.4, 35.8 | −10.3, −2.2 |
| p-value | 0.003 * | ||
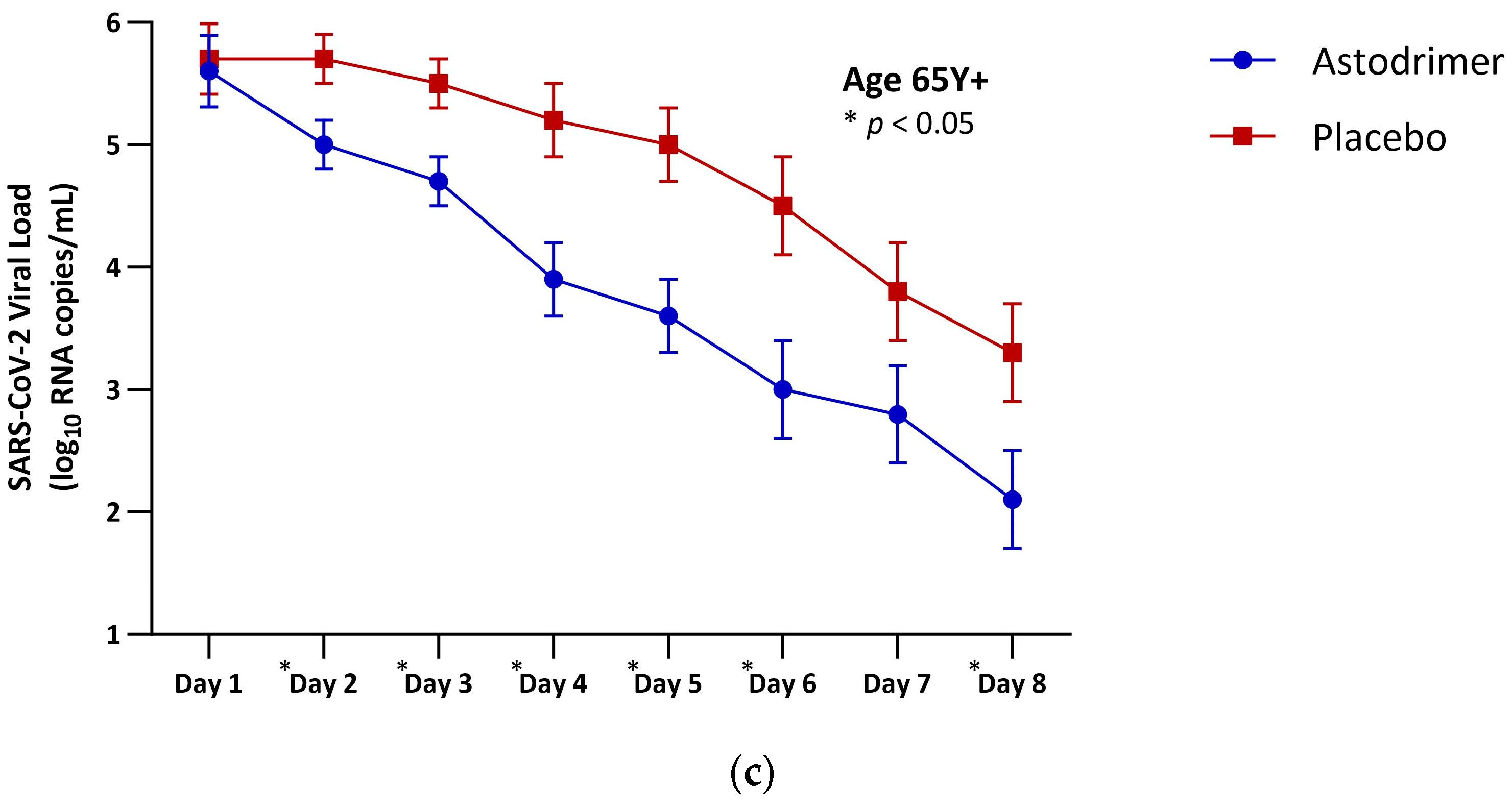
| Age 65 Y+ (mITT) | |||
| N | 23 | 27 | 50 |
| LSM (SE) | 26.9 (1.7) | 34.1 (1.7) | −7.3 (2.5) |
| 95% CI | 23.2, 30.5 | 30.8, 37.5 | −12.2, −2.3 |
| p-value | 0.005 * |
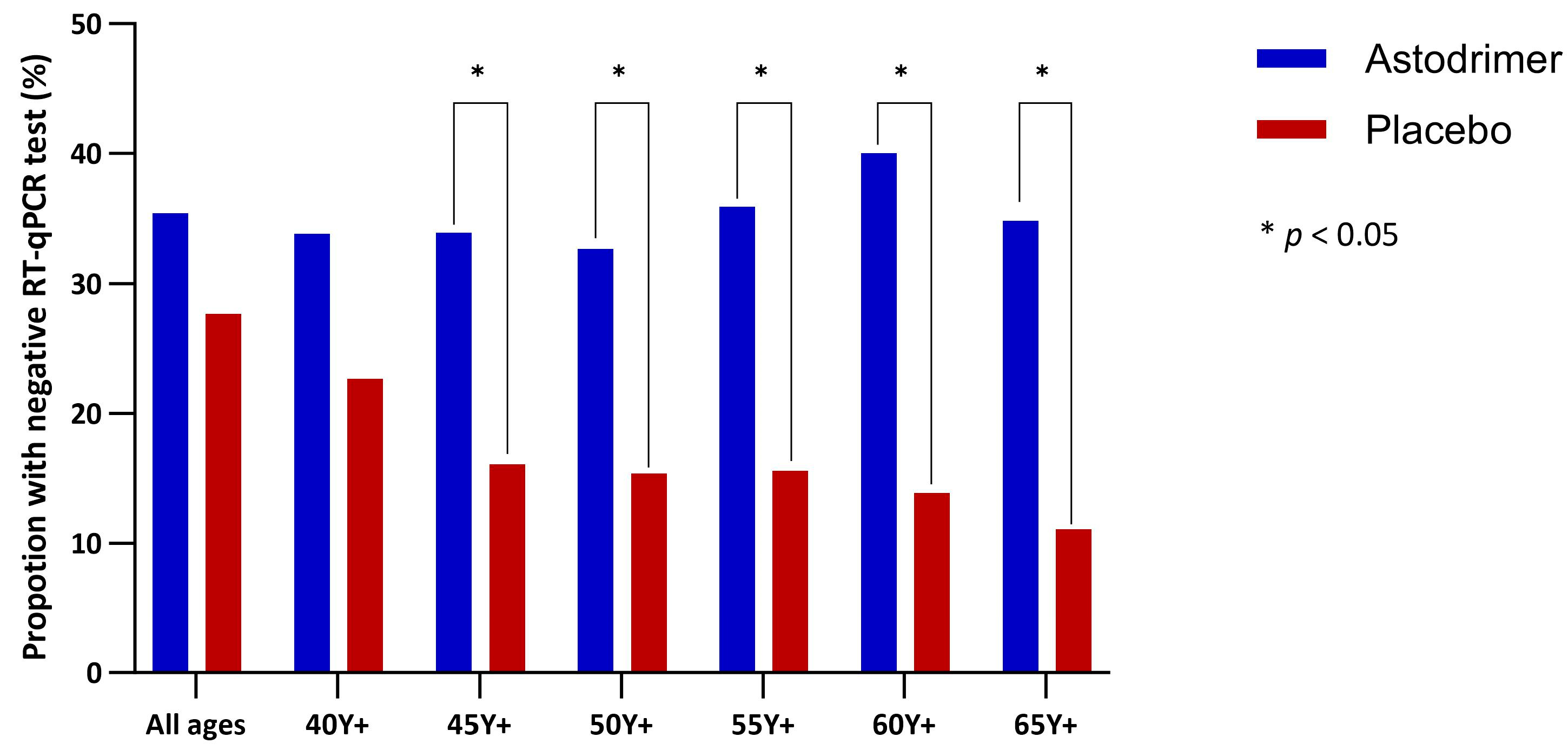
| Negative RT-qPCR at or by Day 8 | Fisher’s Exact Test | Hazard Ratio | ||
|---|---|---|---|---|
| Astodrimer | Placebo | |||
| All ages | 34/96 (35.4%) | 28/101 (27.7%) | p = 0.157 | 1.31 (0.79, 2.15), p = 0.297 |
| Age 40 Y+ | 23/68 (33.8%) | 17/75 (22.7%) | p = 0.097 | 1.54 (0.82, 2.87), p = 0.180 |
| Age 45 Y+ | 19/56 (33.9%) | 10/62 (16.1%) | p = 0.021 * | 2.24 (1.04, 4.81), p = 0.040 * |
| Age 50 Y+ | 16/49 (32.7%) | 8/52 (15.4%) | p = 0.035 * | 2.31 (0.99, 5.40), p = 0.053 |
| Age 55 Y+ | 14/39 (35.9%) | 7/45 (15.6%) | p = 0.029 * | 2.64 (1.06, 6.54), p = 0.036 * |
| Age 60 Y+ | 12/30 (40.0%) | 5/36 (13.9%) | p = 0.016 * | 3.27 (1.15, 9.29, p = 0.026 * |
| Age 65 Y+ | 8/23 (34.8%) | 3/27 (11.1%) | p = 0.047 * | 3.41 (0.90, 12.87), p = 0.070 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winchester, S.; Castellarnau, A.; Jabbar, K.; Nadir, M.; Ranasinghe, K.; Masramon, X.; Kinghorn, G.R.; John, I.; Paull, J.R.A. Astodrimer Sodium Nasal Spray versus Placebo in Non-Hospitalised Patients with COVID-19: A Randomised, Double-Blinded, Placebo-Controlled Trial. Pharmaceutics 2024, 16, 1173. https://doi.org/10.3390/pharmaceutics16091173
Winchester S, Castellarnau A, Jabbar K, Nadir M, Ranasinghe K, Masramon X, Kinghorn GR, John I, Paull JRA. Astodrimer Sodium Nasal Spray versus Placebo in Non-Hospitalised Patients with COVID-19: A Randomised, Double-Blinded, Placebo-Controlled Trial. Pharmaceutics. 2024; 16(9):1173. https://doi.org/10.3390/pharmaceutics16091173
Chicago/Turabian StyleWinchester, Stephen, Alex Castellarnau, Kashif Jabbar, Meera Nadir, Kapila Ranasinghe, Xavier Masramon, George R. Kinghorn, Isaac John, and Jeremy R. A. Paull. 2024. "Astodrimer Sodium Nasal Spray versus Placebo in Non-Hospitalised Patients with COVID-19: A Randomised, Double-Blinded, Placebo-Controlled Trial" Pharmaceutics 16, no. 9: 1173. https://doi.org/10.3390/pharmaceutics16091173





