Methylene Blue-Loaded Dissolving Microneedles: Potential Use in Photodynamic Antimicrobial Chemotherapy of Infected Wounds
Abstract
:1. Introduction

2. Materials and Methods
2.1. Chemicals
2.2. Microorganisms
2.3. Preparation of Dissolving MNs Containing Methylene Blue
2.4. MN Compressibility Testing
2.5. Parafilm™ Insertion
2.6. MN Permeation
2.7. Analysis of Methylene Blue Permeation
2.8. Microorganism Susceptibility Testing and Photodynamic Therapy
2.9. Statistical Analysis
3. Results and Discussion
3.1. Compressibility Testing

3.2. Parafilm™ Insertion

3.3. In Vitro Permeation of Methylene Blue
| MN insertion time | Methylene blue permeated into the Franz cell (mg/mL) by MNs prepared from blends containing | |
|---|---|---|
| 0.05% methylene blue | 5% methylene blue | |
| 5 min | 0.01 ± 0.01 | 0.05 ± 0.02 |
| 30 min | 0.03 ± 0.02 | 2.36 ± 0.97 |
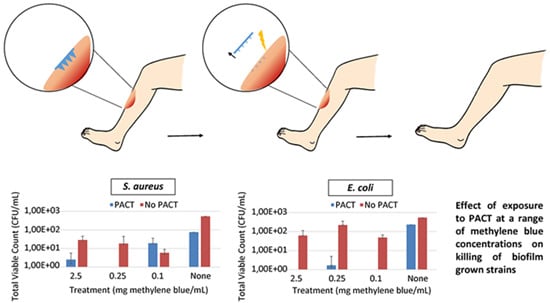
3.4. Microorganism Susceptibility

| Treatment | Methylene blue concentration (mg/mL) | |||
|---|---|---|---|---|
| 2.5 | 0.25 | 0.1 | None | |
| PACT | 99.5 ± 0.6 | 100.0 ± 0 | 96.4 ± 3.0 | 89.4 ± 8.9 |
| No PACT | 94.7 ± 3.2 | 96.6 ± 4.9 | 98.9 ± 0.6 | 0 ± 0 |
| Treatment | Methylene blue concentration (mg/mL) | |||
|---|---|---|---|---|
| 2.5 | 0.25 | 0.1 | None | |
| PACT | 100.0 ± 0 | 99.7 ± 0.6 | 100.0 ± 0 | 56.6 ± 14.0 |
| No PACT | 88.4 ± 9.8 | 59.1 ± 24.9 | 90.9 ± 3.4 | 0 ± 0 |
| Treatment | Methylene blue concentration (mg/mL) | |||
|---|---|---|---|---|
| 2.5 | 0.25 | 0.1 | None | |
| PACT | 99.9 ± 0.1 | 99.8 ± 0.2 | 99.9 ± 0 | 86.2 ± 9.9 |
| No PACT | 66.3 ± 9.2 | 69.1 ± 19.0 | 57.3 ± 34.9 | 0 ± 0 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Howell-Jones, R.S.; Wilson, M.J.; Hill, K.E.; Howard, A.J.; Price, P.E.; Thomas, D.W. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J. Antimicrob. Chemother. 2005, 55, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.D.; Wolcott, R.D.; Percival, S.L. Biofilms in wounds: Management strategies. J. Wound Care 2008, 17, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Noel, S.P.; Courtney, H.S.; Bumgardner, J.D.; Haggard, W.O. Chitosan sponges to locally deliver amikacin and vancomycin: A pilot in vitro evaluation. Clin. Orthop. Relat. Res. 2010, 468, 2074–2080. [Google Scholar] [CrossRef] [PubMed]
- Huiras, P.; Logan, J.; Papadopoulos, S.; Whitney, D. Local antimicrobial administration for prophylaxis of surgical site infections. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2012, 32, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Cassidy, C.M.; Loughlin, R.G.; Brown, A.; Tunney, M.M.; Jenkins, M.G.; McCarron, P.A. Delivery of methylene blue and meso-tetra (N-methyl-4-pyridyl) porphine tetra tosylate from cross-linked poly(vinyl alcohol) hydrogels: A potential means of photodynamic therapy of infected wounds. J. Photochem. Photobiol. B 2009, 96, 223–231. [Google Scholar] [CrossRef]
- Bowler, P.G.; Welsby, S.; Towers, V.; Booth, R.; Hogarth, A.; Rowlands, V.; Joseph, A.; Jones, S.A. Multidrug-resistant organisms, wounds and topical antimicrobial protection. Int. Wound J. 2012, 9, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Cookson, B. A review: Clinical significance of emergence of bacterial antimicrobial resistance in the hospital environment. J. Appl. Microbiol. 2005, 99, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Zeina, B.; Greenman, J.; Purcell, W.M.; Das, B. Killing of cutaneous microbial species by photodynamic therapy. Br. J. Dermatol. 2001, 144, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Sun, I.F.; Lee, S.S.; Chiu, C.C.; Lin, S.D.; Lai, C.S. Hyperbaric oxygen therapy with topical negative pressure: an alternative treatment for the refractory sternal wound infection. J. Card. Surg. 2008, 3, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, C.M.; Tunney, M.M.; McCarron, P.A.; Donnelly, R.F. Drug delivery strategies for photodynamic antimicrobial chemotherapy: From benchtop to clinical practice. J. Photochem. Photobiol. B 2009, 95, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M. Antifungal photodynamic therapy. Microbiol. Res. 2008, 163, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M.; Woolfson, D.A. Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. J. Photochem. Photobiol. B 2007, 86, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCarron, P.A.; Lightowler, J.M.; Woolfson, D.A. Bioadhesive patch-based delivery of 5-aminolevulinic acid to the nail for photodynamic therapy of onychomycosis. J. Control. Release 2005, 103, 381–392. [Google Scholar] [CrossRef]
- Kearney, M.-C.; Brown, S.; McCrudden, M.T.C.; Brady, A.J.; Donnelly, R.F. Potential of microneedles in enhancing delivery of photosensitising agents for photodynamic therapy. Photodiagn. Photodyn. Ther. 2014, 11, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, H.U.; Lee, Y.C.; Kim, G.H.; Park, E.C.; Han, S.H.; Lee, J.G.; Choi, S.; Heo, N.S.; Kim, D.L.; et al. Wound healing potential of antibacterial microneedles loaded with green tea extracts. Mater. Sci. Eng. C 2014, 42, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; McAllister, D.; Allen, M.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, J.H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Tuan-Mahmood, T.M.; McCrudden, M.T.C.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Garland, M.J.; Singh, T.R.; Donnelly, R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Morrow, D.I.J.; McCrudden, M.T.C.; Alkilani, A.Z.; Vicente-Pérez, E.M.; O’Mahony, C.; González-Vázquez, P.; McCarron, P.A.; Woolfson, A.D. Hydrogel-forming and dissolving microneedles for enhanced delivery of photosensitizers and precursors. Photochem. Photobiol. 2014, 90, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, J.P.; Del Giglio, A.; De Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fátima Turchiello, R.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagn. Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Majithiya, R.; Singh, T.R.R.; Morrow, D.I.J.; Garland, M.J.; Demir, Y.K.; Migalska, K.; Ryan, E.; Gillen, D.; Scott, C.J.; et al. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm. Res. 2011, 28, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Chee, H.Y. In vitro antifungal activity of equol against Candida albicans. Mycobiology 2010, 38, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, C.M.; Donnelly, R.F.; Elborn, J.S.; Magee, N.D.; Tunney, M.M. Photodynamic Antimicrobial Chemotherapy (PACT) in combination with antibiotics for treatment of Burkholderia cepacia complex infection. J. Photochem. Photobiol. B 2012, 106, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.C.; Alkilani, A.Z.; McCrudden, C.M.; McAlister, E.; McCarthy, H.O.; Woolfson, D.A.; Donnelly, R.F. Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs. J. Control. Release 2014, 180, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Demir, Y.K.; Akan, Z.; Kerimoglu, O. Characterization of polymeric microneedle arrays for transdermal drug delivery. PLoS ONE 2013, 8, e77289. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Moffatt, K.; Alkilani, A.Z.; Vicente-Pérez, E.M.; Barry, J.; McCrudden, M.T.; Woolfson, A.D. Hydrogel-forming microneedle arrays can be effectively inserted in skin by self-application: A pilot study centred on pharmacist intervention and a patient information leaflet. Pharm. Res. 2014, 31, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.J.; Migalska, K.; Tuan-Mahmood, T.M.; Raghu Raj Singh, T.; Majithija, R.; Caffarel-Salvador, E.; McCrudden, C.M.; McCarthy, H.O.; Woolfson, A.D.; Donnelly, R.F. Influence of skin model on in vitro performance of drug-loaded soluble microneedle arrays. Int. J. Pharm. 2012, 434, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.J.; Caffarel-Salvador, E.; Migalska, K.; Woolfson, A.D.; Donnelly, R.F. Dissolving polymeric microneedle arrays for electrically assisted transdermal drug delivery. J. Control. Release 2012, 159, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Vilela, S.F.; Junqueira, J.C.; Barbosa, J.O.; Majewski, M.; Munin, E.; Jorge, A.O. Photodynamic inactivation of Staphylococcus aureus and Escherichia coli biofilms by malachite green and phenothiazine dyes: An in vitro study. Arch. Oral Biol. 2012, 57, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Szeimies, R.M.; Jori, G.; Abels, C.H. Antibacterial photodynamic therapy in dermatology. Photochem. Photobiol. Sci. 2004, 3, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, S.A.G.; Demidova, T.N.; Aalders, M.C.G.; Hasan, T.; Hamblin, M.R. Photodynamic therapy for Staphylococcus aureus infected burn wounds in mice. Photochem. Photobiol. Sci. 2005, 4, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Payne, W.G.; Naidu, D.K.; Wheeler, C.K.; Barkoe, D.; Mentis, M.; Salas, R.E.; Smith, D.J.; Robson, M.C. Wound Healing in Patients With Cancer. Eplasty 2008, 8, e9. [Google Scholar] [PubMed]
- Zolfaghari, P.S.; Packer, S.; Singer, M.; Nair, S.P.; Bennett, J.; Street, C.; Wilson, M. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Usacheva, M.N.; Teichert, M.C.; Biel, M.A. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg. Med. 2001, 29, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Khanal, A.; Bui, M-P.N.; Seo, S.S. Microgel-encapsulated methylene blue for the treatment of breast cancer cells by photodynamic therapy. J. Breast Cancer 2014, 17, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Monfrecola, G.; Procacci, E.M.; Bevilacqua, M.; Manco, A.; Calabro, G.; Santoianni, P. In vitro effects of 5-aminolaevulinic acid plus visible light on Candida albicans. Photochem. Photobiol. Sci. 2004, 3, 419–422. [Google Scholar] [CrossRef]
- Bliss, J.M.; Bigelow, B.E.; Foster, T.H.; Haidaris, C.G. Susceptibility of Candida species to photodynamic effects of Photofrin. Antimicrob. Agents Chemother. 2004, 48, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious films. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Boehm, R.D.; Miller, P.R.; Singh, R.; Shah, A.; Stafslien, S.; Daniels, J.; Narayan, R.J. Indirect rapid prototyping of antibacterial acid anhydride copolymer microneedles. Biofabrication. 2012, 4, 011002. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caffarel-Salvador, E.; Kearney, M.-C.; Mairs, R.; Gallo, L.; Stewart, S.A.; Brady, A.J.; Donnelly, R.F. Methylene Blue-Loaded Dissolving Microneedles: Potential Use in Photodynamic Antimicrobial Chemotherapy of Infected Wounds. Pharmaceutics 2015, 7, 397-412. https://doi.org/10.3390/pharmaceutics7040397
Caffarel-Salvador E, Kearney M-C, Mairs R, Gallo L, Stewart SA, Brady AJ, Donnelly RF. Methylene Blue-Loaded Dissolving Microneedles: Potential Use in Photodynamic Antimicrobial Chemotherapy of Infected Wounds. Pharmaceutics. 2015; 7(4):397-412. https://doi.org/10.3390/pharmaceutics7040397
Chicago/Turabian StyleCaffarel-Salvador, Ester, Mary-Carmel Kearney, Rachel Mairs, Luigi Gallo, Sarah A. Stewart, Aaron J. Brady, and Ryan F. Donnelly. 2015. "Methylene Blue-Loaded Dissolving Microneedles: Potential Use in Photodynamic Antimicrobial Chemotherapy of Infected Wounds" Pharmaceutics 7, no. 4: 397-412. https://doi.org/10.3390/pharmaceutics7040397
APA StyleCaffarel-Salvador, E., Kearney, M.-C., Mairs, R., Gallo, L., Stewart, S. A., Brady, A. J., & Donnelly, R. F. (2015). Methylene Blue-Loaded Dissolving Microneedles: Potential Use in Photodynamic Antimicrobial Chemotherapy of Infected Wounds. Pharmaceutics, 7(4), 397-412. https://doi.org/10.3390/pharmaceutics7040397