Effect of Cell-to-Cell Internal Resistance Variations on the Thermal Performance of Lithium-Ion Batteries for Urban Air Mobility
Abstract
:1. Introduction
2. Methodology
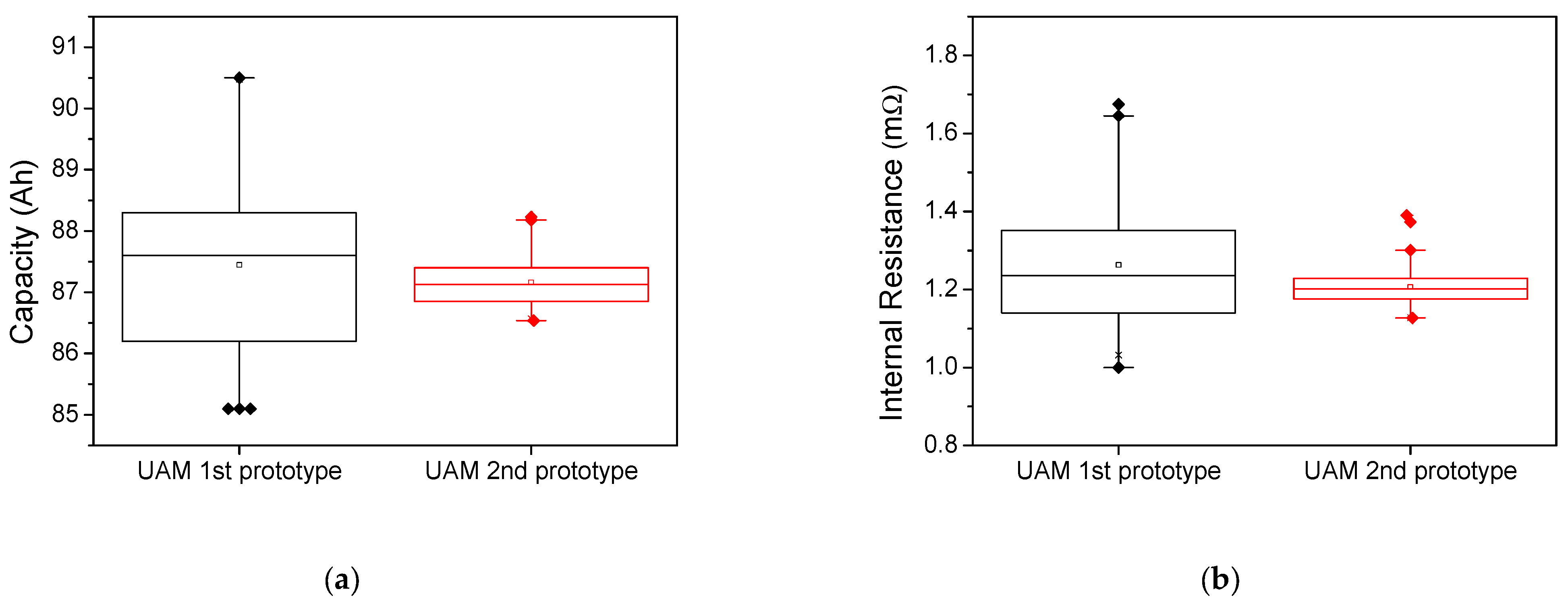
2.1. Lithium-Ion Battery Module
2.2. Governing Equation and Simulation Setup
3. Simulation Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garrow, L.A.; German, B.J.; Leonard, C.E. Urban air mobility: A Comprehensive Review and Comparative Analysis with Autonomous and Electric Ground Transportation for Informing Future Research. Transp. Res. Part C Emerg. Technol. 2021, 132, 103377. [Google Scholar] [CrossRef]
- Liu, T.; Yang, X.G.; Ge, S.; Leng, Y.; Wang, C.Y. Ultrafast Charging of Energy-Dense Lithium-ion Batteries for Urban Air Mobility. eTransportation 2021, 7, 100103. [Google Scholar] [CrossRef]
- Qiao, X.; Chen, G.; Lin, W.; Zhou, J. The Impact of Battery Performance on Urban Air Mobility Operations. Aerospace 2023, 10, 631. [Google Scholar] [CrossRef]
- Sripad, S.; Viswanathan, V. The Promise of Energy-efficient Battery-powered Urban Aircraft. Proc. Natl. Acad. Sci. USA 2021, 118, e2111164118. [Google Scholar] [CrossRef] [PubMed]
- Lee, G. Electro-thermal Analysis of a Pouch–type Lithium–ion Battery with a High Discharge Rate for Urban Air Mobility. Batteries 2023, 9, 476. [Google Scholar] [CrossRef]
- Paul, S.; Diegelmann, C.; Kabza, H.; Tillmetz, W. Analysis of Ageing Inhomogeneities in Lithium-ion Battery Systems. J. Power Sources 2013, 239, 642–650. [Google Scholar] [CrossRef]
- Chiu, K.C.; Lin, C.H.; Yeh, S.F.; Lin, Y.H.; Huang, C.S.; Chen, K.C. Cycle Life Analysis of Series Connected Lithium-ion Batteries with Temperature Difference. J. Power Sources 2014, 263, 75–84. [Google Scholar] [CrossRef]
- Offer, G.J.; Yufit, V.; Howey, D.A.; Wu, B.; Brandon, N.P. Module Design and Fault Diagnosis in Electric Vehicle Batteries. J. Power Sources 2012, 206, 383–392. [Google Scholar] [CrossRef]
- Bruen, T.; Macro, J. Modelling and Experimental Evaluation of Parallel Connected Lithium Ion Cells for an Electric Vehicle Battery System. J. Power Sources 2016, 310, 91–101. [Google Scholar] [CrossRef]
- Fernández, C.P.; Bruen, T.; Widanage, W.D.; Gama-Valdez, M.A.; Marco, J. A Study of Cell-to-Cell Interactions and Degradation in Parallel Strings: Implications for the Battery Management System. J. Power Sources 2016, 329, 574–585. [Google Scholar] [CrossRef]
- Shi, W.; Hu, X.; Jin, C.; Jiang, J.; Zhang, Y.; Yip, T. Effects of Imbalanced Currents on Large-format LiFePO4/graphite Batteries Systems Connected in Parallel. J. Power Sources 2016, 313, 198–204. [Google Scholar] [CrossRef]
- Gong, X.; Xiong, R.; Mi, C.C. Study of the Characteristics of Battery Packs in Electric Vehicles with Parallel-Connected Lithium-Ion Battery Cells. IEEE Trans. Ind. Appl. 2015, 51, 1872–1879. [Google Scholar] [CrossRef]
- Liu, X.; Ai, W.; Marlow, M.N.; Patel, Y.; Wu, B. The Effect of Cell-to-cell Variations and Thermal Gradients on the Performance and Degradation of Lithium-ion Battery Packs. Appl. Energy 2019, 248, 489–499. [Google Scholar] [CrossRef]
- Song, Z.; Yang, X.G.; Yang, N.; Delgado, F.P.; Hofmann, H.; Sun, J. A Study of Cell-to-cell Variation of Capacity in Parallel-Connected Lithium-ion Battery Cells. eTransportation 2021, 7, 100091. [Google Scholar] [CrossRef]
- Traub, L.W. Optimal Battery Weight Fraction for Maximum Aircraft Range and Endurance. J. Aircr. 2016, 53, 1177–1179. [Google Scholar] [CrossRef]
- Shi, C.; Wang, T.; Liao, X.; Qie, B.; Yang, P.; Chen, M.; Wang, X.; Srinivasan, A.; Cheng, Q.; Ye, Q.; et al. Accordion-like stretchable Li-ion batteries with high energy density. Energy Storage Mater. 2019, 17, 136–142. [Google Scholar] [CrossRef]
- AVL. FIRE M User Manual 2020R 2. Available online: https://www.avl.com/cruise-m (accessed on 11 August 2022).
- Pals, C.R.; Newman, J. Thermal Modeling of the Lithium/polymer Battery I. Discharge Behavior of a Single Cell. J. Electrochem. Soc. 1995, 142, 3274–3281. [Google Scholar] [CrossRef]
- Bernardi, D.; Pawlikowski, E.; Newman, J. A General Energy Balance for Battery Systems. J. Electrochem. Soc. 1985, 132, 5. [Google Scholar] [CrossRef]
- Jiaqiang, E.; Yue, M.; Chen, J.; Zhu, H.; Deng, Y.; Zhu, Y.; Zhang, F.; Wen, M.; Zhang, B.; Kang, S. Effects of the Different Air Cooling Strategies on Cooling Performance of a Lithium-ion Battery Module with Baffle. Appl. Therm. Eng. 2018, 144, 231–241. [Google Scholar]

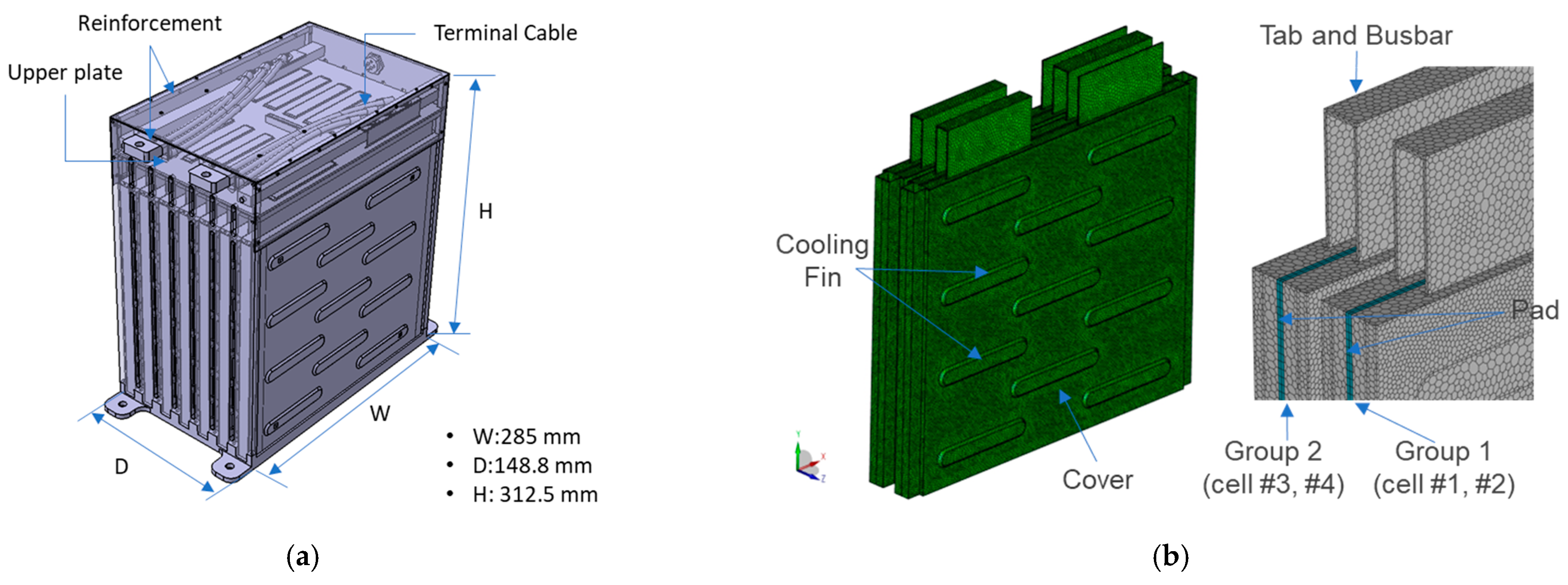
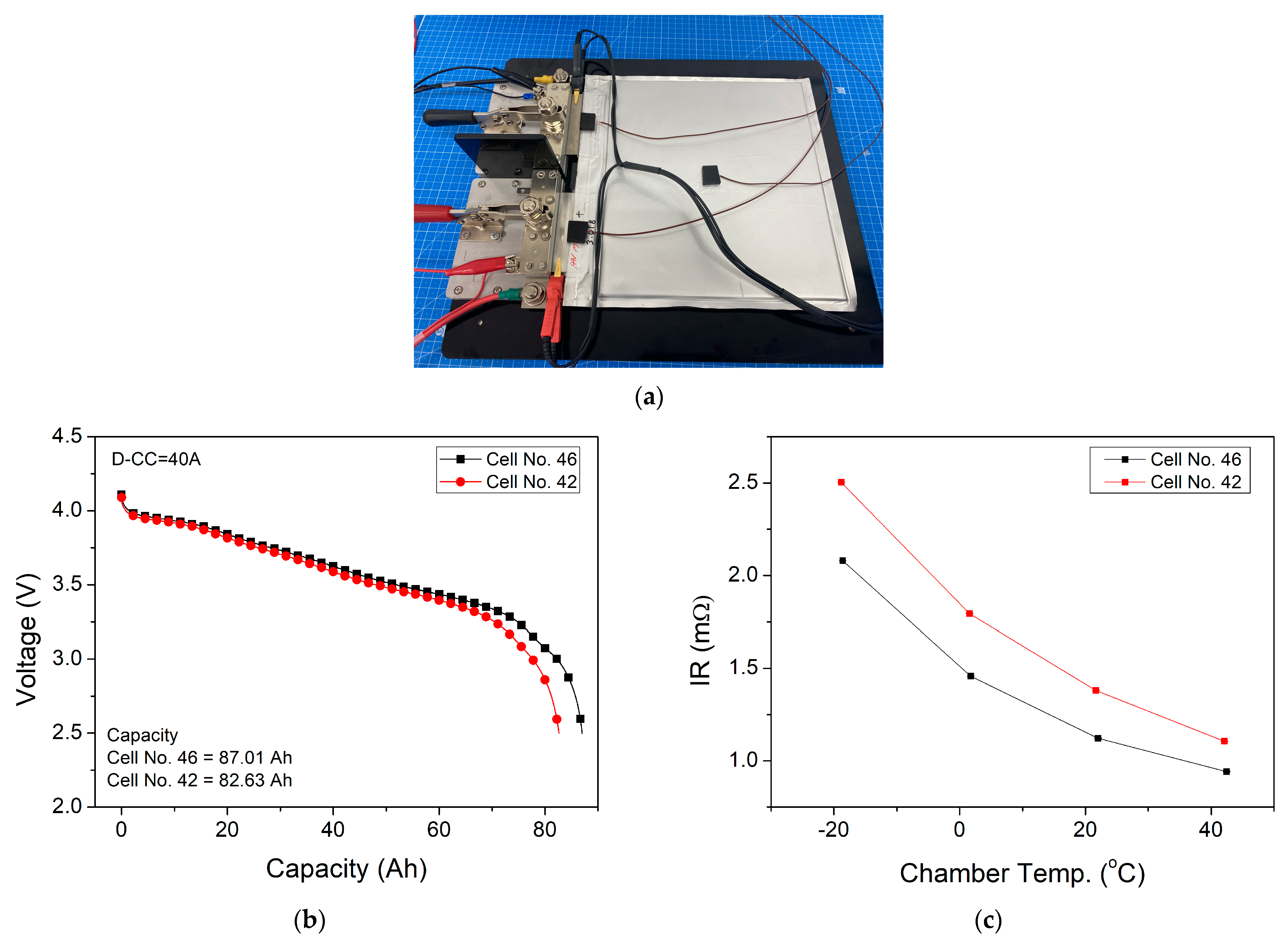

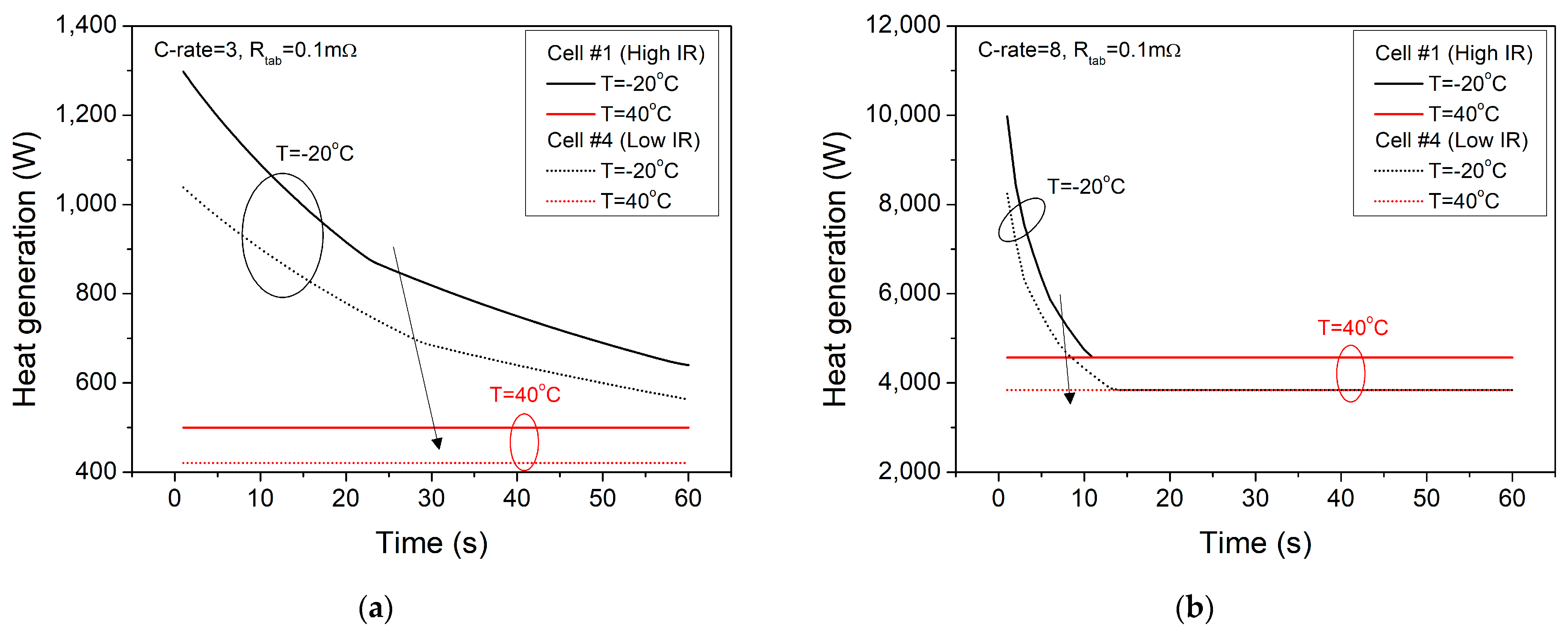
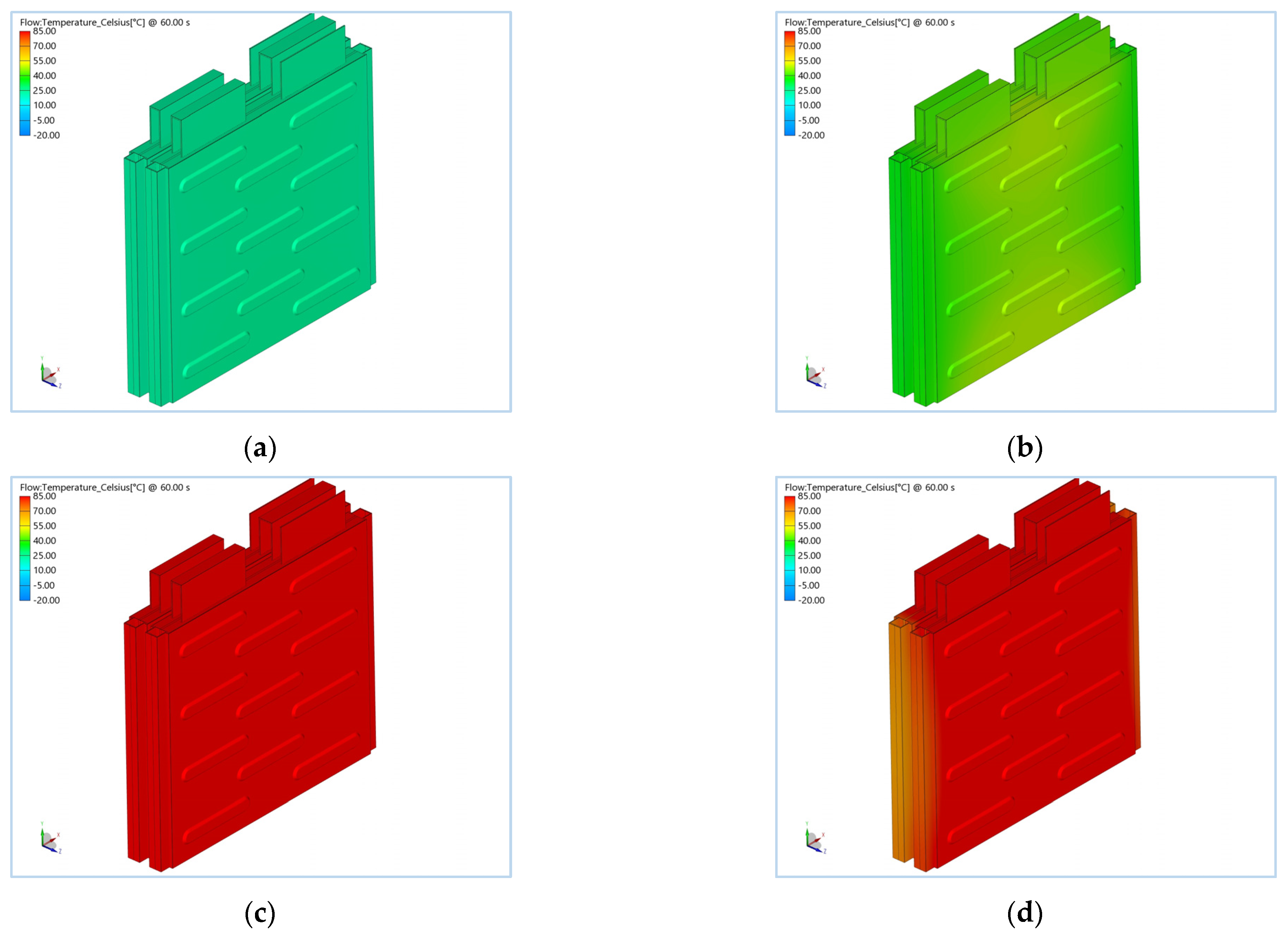

| Items | Specification |
|---|---|
| Nominal capacity | 90 Ah |
| Nominal voltage | 3.7 V |
| Energy density | 300 Wh/kg |
| Max. charge voltage | 4.25 V |
| Cut-off voltage | 2.75 V |
| Material system | Cathode: NCM–811 Anode: Graphite Electrolyte: Carbonate-based |
| Max. continuous discharge current | 8 C |
| Material | Air | Cover (Aluminum) | Cell (LIBs) | Thermal Insulation Pad |
|---|---|---|---|---|
| Density (kg/m3) | 1.1841 | 2702 | 2100 | 1060 |
| Specific heat capacity (J/(kg·K)) | 1003.6 | 903.0 | 1100 | 796 |
| Thermal conductivity (W/(m·K)) | 0.026 | 237 | 1/30/30 | 0.202 |
| Ambient Temp. | Mean Temp. Diff. (°C) at 60 s | |||
|---|---|---|---|---|
| 1 C | 3 C | 5 C | 8 C | |
| −20 | 1.473 | 5.954 | 13.747 | 32.952 |
| 0 | 1.073 | 4.958 | 13.324 | 32.545 |
| 20 | 0.747 | 3.819 | 12.745 | 31.994 |
| 40 | 0.498 | 3.494 | 12.44 | 31.712 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the World Electric Vehicle Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, K.; Lee, G. Effect of Cell-to-Cell Internal Resistance Variations on the Thermal Performance of Lithium-Ion Batteries for Urban Air Mobility. World Electr. Veh. J. 2024, 15, 423. https://doi.org/10.3390/wevj15090423
Xin K, Lee G. Effect of Cell-to-Cell Internal Resistance Variations on the Thermal Performance of Lithium-Ion Batteries for Urban Air Mobility. World Electric Vehicle Journal. 2024; 15(9):423. https://doi.org/10.3390/wevj15090423
Chicago/Turabian StyleXin, Kuo, and Geesoo Lee. 2024. "Effect of Cell-to-Cell Internal Resistance Variations on the Thermal Performance of Lithium-Ion Batteries for Urban Air Mobility" World Electric Vehicle Journal 15, no. 9: 423. https://doi.org/10.3390/wevj15090423
APA StyleXin, K., & Lee, G. (2024). Effect of Cell-to-Cell Internal Resistance Variations on the Thermal Performance of Lithium-Ion Batteries for Urban Air Mobility. World Electric Vehicle Journal, 15(9), 423. https://doi.org/10.3390/wevj15090423









