New Insights into the Role of SGLT-2 Inhibitors in the Prevention of Dementia
Abstract
1. Introduction
1.1. Global Prevalence of Diabetes Mellitus and Its Complications
1.2. Discovery and Development of SGLT-2 Inhibitors
1.3. Protective Effects of SGLT-2 Inhibitors on the Heart and Kidneys
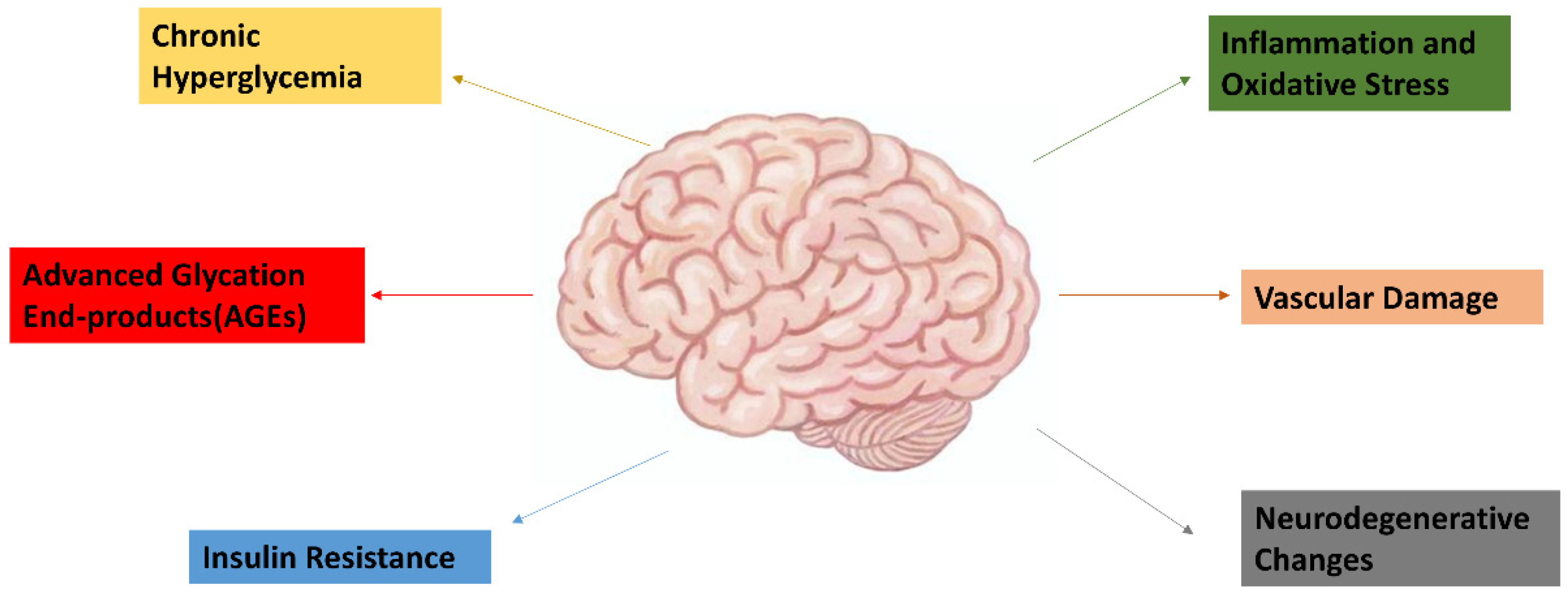
1.4. Pathophysiology of Dementia and Its Link to Diabetes
1.5. Existing Treatments for Dementia and Their Limitations
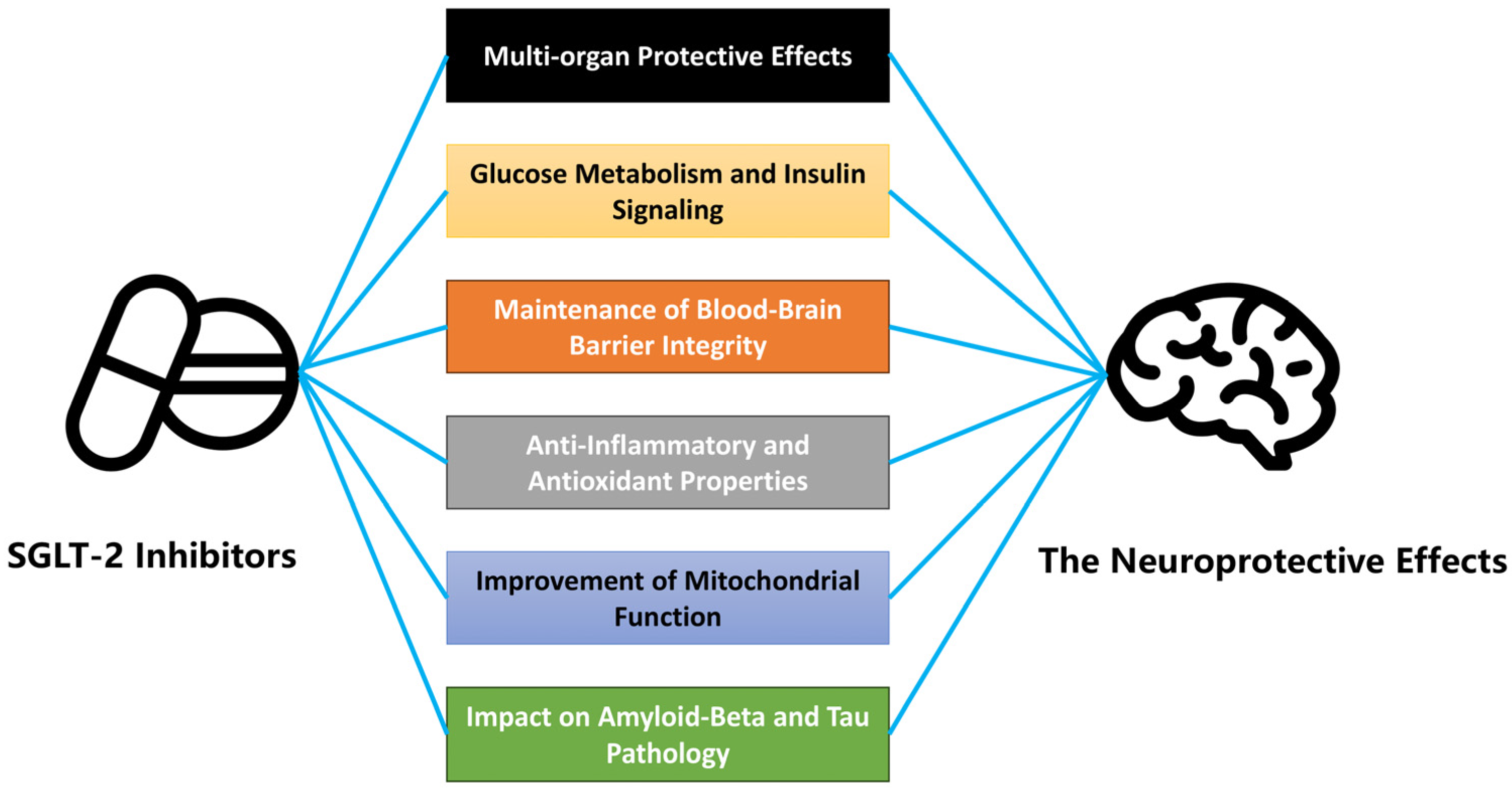
2. The Neuroprotective Potential of SGLT-2 Inhibitors
2.1. Early Animal Studies on the Neuroprotective Effects of SGLT-2 Inhibitors
| Study | SGLT-2 Type | Main Mechanisms | Key Findings |
|---|---|---|---|
| Yaribeygi et al. (2024) [50] | Empagliflozin, Dapagliflozin |
|
|
| Khamieset et al. (2024) [51] | Empagliflozin |
|
|
| Chmiel et al. (2023) [52] | Dapagliflozin |
|
|
2.2. The Neuroprotective Effects of SGLT-2 Inhibitors in Human Studies
3. Mechanisms of Action
3.1. Molecular Mechanisms of SGLT-2 Inhibitors
3.2. Effects on Glucose Metabolism and Insulin Signaling
3.3. Blood–Brain Barrier Integrity and Neuroprotection
3.4. Anti-Inflammatory and Antioxidant Properties
3.5. Mitochondrial Function and Neuroprotection
3.6. Impact on Amyloid-Beta and Tau Pathology
4. Clinical Evidence
| Study | Population | Sample Size | Intervention | Outcome | Results |
|---|---|---|---|---|---|
| Wu et al. (2023) [53] | Patients aged ≥ 66 years with diabetes in Canada | 106,903 | SGLT2 inhibitors vs. DPP-4 inhibitors | Time to incident dementia | SGLT2 inhibitors associated with a lower risk of dementia compared to DPP-4 |
| Siao et al. (2022) [57] | Patients with type 2 diabetes in Taiwan | 206,494 | SGLT2 inhibitors vs. non-SGLT2 inhibitors | Incident dementia | SGLT2 inhibitor group had a lower risk of incident dementia |
| Chen et al. (2024) [68] | Diabetic patients with AF in Taiwan | 2430 | SGLT2 inhibitors vs. non-SGLT2 inhibitors | Incident dementia | SGLT2 inhibitors associated with reduced risks of incident dementia |
| Perna et al. (2018) [69] | Elderly (>65 years of age) men and women with T2DM in Italy | 39 | SGLT2 inhibitors vs. incretins | Cognitive status change | Cognitive status did not change significantly during the 12 months of treatment in either of the groups |
| Mui et al. (2021) [70] | Type 2 diabetes mellitus patients in Hong Kong | 39,828 | SGLT2 inhibitors vs. DPP-4 inhibitors | New-onset dementia | The use of SGLT2 inhibitors is associated with a significantly lower risk of dementia |
| Shin et al. (2024) [71] | Type 2 diabetes mellitus patients aged 40–69 in Korea | 221,770 | SGLT2 inhibitors vs. DPP-4 inhibitors | New-onset dementia | The use of SGLT2 inhibitors is associated with a significantly lower risk of dementia hazard ratios of 0.65 (95% CI 0.58–0.73) |
5. Discussion
5.1. Future Directions
5.2. Limitation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mittal, A.; Babu, D.; Mittal, A. Herbal Medicines for Diabetes Management and its Secondary Complications. Curr. Diabetes Rev. 2021, 17, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Damanik, J.; Yunir, E. Type 2 Diabetes Mellitus and Cognitive Impairment. Acta Med. Indones. 2021, 53, 213–220. [Google Scholar] [PubMed]
- Tinajero, M.G.; Malik, V.S. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol. Metab. Clin. N. Am. 2021, 50, 337–355. [Google Scholar] [CrossRef]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hébert, J.R.; Hodge, A.; de Courten, B. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2022, 23, e13349. [Google Scholar] [CrossRef]
- Amanat, S.; Ghahri, S.; Dianatinasab, A.; Fararouei, M.; Dianatinasab, M. Exercise and Type 2 Diabetes. Adv. Exp. Med. Biol. 2020, 1228, 91–105. [Google Scholar] [CrossRef]
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 110–116. [Google Scholar] [CrossRef]
- Thipsawat, S. Early detection of diabetic nephropathy in patient with type 2 diabetes mellitus: A review of the literature. Diabetes Vasc. Dis. Res. 2021, 18, 14791641211058856. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef]
- Chow, E.; Clement, S.; Garg, R. Euglycemic diabetic ketoacidosis in the era of SGLT-2 inhibitors. BMJ Open Diabetes Res. Care 2023, 11, e003666. [Google Scholar] [CrossRef] [PubMed]
- Bendotti, G.; Montefusco, L.; Pastore, I.; Lazzaroni, E.; Lunati, M.E.; Fiorina, P. The anti-inflammatory and immunological properties of SGLT-2 inhibitors. J. Endocrinol. Investig. 2023, 46, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.A.; Wright, E.M.; Vallon, V. Probing SGLT2 as a therapeutic target for diabetes: Basic physiology and consequences. Diabetes Vasc. Dis. Res. 2015, 12, 78–89. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Davidson, J.A.; Del Prato, S. The role of the kidneys in glucose homeostasis: A new path towards normalizing glycaemia. Diabetes Obes. Metab. 2012, 14, 5–14. [Google Scholar] [CrossRef]
- Babu, A. Canagliflozin for the treatment of type 2 diabetes. Drugs Today 2013, 49, 363–376. [Google Scholar] [CrossRef]
- Frąk, W.; Hajdys, J.; Radzioch, E.; Szlagor, M.; Młynarska, E.; Rysz, J.; Franczyk, B. Cardiovascular Diseases: Therapeutic Potential of SGLT-2 Inhibitors. Biomedicines 2023, 11, 2085. [Google Scholar] [CrossRef]
- Caruso, I.; Giorgino, F. SGLT-2 inhibitors as cardio-renal protective agents. Metab. Clin. Exp. 2022, 127, 154937. [Google Scholar] [CrossRef]
- Kluger, A.Y.; Tecson, K.M.; Barbin, C.M.; Lee, A.Y.; Lerma, E.V.; Rosol, Z.P.; Rangaswami, J.; Lepor, N.E.; Cobble, M.E.; McCullough, P.A. Cardiorenal Outcomes in the CANVAS, DECLARE-TIMI 58, and EMPA-REG OUTCOME Trials: A Systematic Review. Rev. Cardiovasc. Med. 2018, 19, 41–49. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J.V. The Serendipitous Story of SGLT2 Inhibitors in Heart Failure. Circulation 2019, 139, 2537–2541. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Gale, S.A.; Acar, D.; Daffner, K.R. Dementia. Am. J. Med. 2018, 131, 1161–1169. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y. Tau and neuroinflammation in Alzheimer’s disease: Interplay mechanisms and clinical translation. J. Neuroinflammat. 2023, 20, 165. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Lu, L.Y.; Wu, M.Y.; Kao, Y.S.; Hung, C.H. Non-alcoholic fatty liver disease and the risk of dementia: A meta-analysis of cohort studies. Clin. Mol. Hepatol. 2022, 28, 931–932. [Google Scholar] [CrossRef]
- Falsetti, L. Molecular Research on Alzheimer’s Disease. Biomedicines 2023, 11, 1883. [Google Scholar] [CrossRef]
- Chung, J.Y.; Kim, H.S.; Song, J. Iron metabolism in diabetes-induced Alzheimer’s disease: A focus on insulin resistance in the brain. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2018, 31, 705–714. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [PubMed]
- Kostev, K.; Tanislav, C. Diabetes and Co-Existing Coronary Artery Disease are Associated with an Increased Risk of Dementia. Eur. J. Prev. Cardiol. 2024, zwae196. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Xu, W.; Ou, Y.N.; Cao, X.P.; Tan, M.S.; Tan, L.; Yu, J.T. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res. Rev. 2019, 55, 100944. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.C.; Hsu, J.L.; Tung, H.Y.; Chou, C.C.; Bai, C.H. Increased dementia risk predominantly in diabetes mellitus rather than in hypertension or hyperlipidemia: A population-based cohort study. Alzheimer’s Res. Ther. 2017, 9, 7. [Google Scholar] [CrossRef]
- Folch, J.; Ettcheto, M.; Busquets, O.; Sánchez-López, E.; Castro-Torres, R.D.; Verdaguer, E.; Manzine, P.R.; Poor, S.R.; García, M.L.; Olloquequi, J.; et al. The Implication of the Brain Insulin Receptor in Late Onset Alzheimer’s Disease Dementia. Pharmaceuticals 2018, 11, 11. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Balázs, N.; Bereczki, D.; Kovács, T. Cholinesterase inhibitors and memantine for the treatment of Alzheimer and non-Alzheimer dementias. Ideggyogy. Szle. 2021, 74, 379–387. [Google Scholar] [CrossRef]
- Annweiler, C.; Beauchet, O. Possibility of a new anti-alzheimer’s disease pharmaceutical composition combining memantine and vitamin D. Drugs Aging 2012, 29, 81–91. [Google Scholar] [CrossRef]
- Boggio, P.S.; Valasek, C.A.; Campanhã, C.; Giglio, A.C.; Baptista, N.I.; Lapenta, O.M.; Fregni, F. Non-invasive brain stimulation to assess and modulate neuroplasticity in Alzheimer’s disease. Neuropsychol. Rehabil. 2011, 21, 703–716. [Google Scholar] [CrossRef]
- Piekarz, J.; Picheta, N.; Burdan, O.; Kurek, M.; Chrościńska-Krawczyk, M. Phytotherapy in Alzheimer’s Disease-A Narrative Review. Biomedicines 2024, 12, 1812. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, D.; Kawahara, M.; Konoha-Mizuno, K.; Hama, R.; Ogawara, T. The Role of Zinc in the Development of Vascular Dementia and Parkinson’s Disease and the Potential of Carnosine as Their Therapeutic Agent. Biomedicines 2024, 12, 1296. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Brellenthin, A.G.; Lee, D.C.; Lefferts, E.C.; Lefferts, W.K.; Dougherty, R.J.; Kim, Y. Physical Activity Intensity and Risk of Dementia. Am. J. Prev. Med. 2024, 66, 948–956. [Google Scholar] [CrossRef]
- Nordestgaard, L.T.; Christoffersen, M.; Frikke-Schmidt, R. Shared Risk Factors between Dementia and Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 9777. [Google Scholar] [CrossRef]
- Reuben, D.B.; Kremen, S.; Maust, D.T. Dementia Prevention and Treatment: A Narrative Review. JAMA Intern. Med. 2024, 184, 563–572. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Saleh, F.M.; Ali, F.E.M.; Rashwan, E.K.; Atwa, A.M.; Abd El-Ghafar, O.A.M. Neuroprotective effect of canagliflozin against cisplatin-induced cerebral cortex injury is mediated by regulation of HO-1/PPAR-γ, SIRT1/FOXO-3, JNK/AP-1, TLR4/iNOS, and Ang II/Ang 1-7 signals. Immunopharmacol. Immunotoxicol. 2023, 45, 304–316. [Google Scholar] [CrossRef]
- Heimke, M.; Lenz, F.; Rickert, U.; Lucius, R.; Cossais, F. Anti-Inflammatory Properties of the SGLT2 Inhibitor Empagliflozin in Activated Primary Microglia. Cells 2022, 11, 3107. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Hemmati, M.A.; Nasimi, F.; Pakdel, R.; Jamialahmadi, T.; Sahebkar, A. Empagliflozin alleviates diabetes-induced cognitive impairments by lowering nicotinamide adenine dinucleotide phosphate oxidase-4 expression and potentiating the antioxidant defense system in brain tissue of diabetic rats. Behav. Brain Res. 2024, 460, 114830. [Google Scholar] [CrossRef]
- Khamies, S.M.; El-Yamany, M.F.; Ibrahim, S.M. Canagliflozin Mitigated Cognitive Impairment in Streptozotocin-Induced Sporadic Alzheimer’s Disease in Mice: Role of AMPK/SIRT-1 Signaling Pathway in Modulating Neuroinflammation. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2024, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Piątkowska-Chmiel, I.; Herbet, M.; Gawrońska-Grzywacz, M.; Pawłowski, K.; Ostrowska-Leśko, M.; Dudka, J. Molecular and neural roles of sodium-glucose cotransporter 2 inhibitors in alleviating neurocognitive impairment in diabetic mice. Psychopharmacology 2023, 240, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Iskander, C.; Wang, C.; Xiong, L.Y.; Shah, B.R.; Edwards, J.D.; Kapral, M.K.; Herrmann, N.; Lanctôt, K.L.; Masellis, M.; et al. Association of Sodium-Glucose Cotransporter 2 Inhibitors With Time to Dementia: A Population-Based Cohort Study. Diabetes Care 2023, 46, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Hierro-Bujalance, C.; Infante-Garcia, C.; Del Marco, A.; Herrera, M.; Carranza-Naval, M.J.; Suarez, J.; Alves-Martinez, P.; Lubian-Lopez, S.; Garcia-Alloza, M. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Alzheimer’s Res. Ther. 2020, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.J.; Kim, S.; Jeong, H.J.; Ah, Y.M.; Yu, Y.M. Sodium-glucose cotransporter-2 inhibitors and their potential role in dementia onset and cognitive function in patients with diabetes mellitus: A systematic review and meta-analysis. Front. Neuroendocrinol. 2024, 73, 101131. [Google Scholar] [CrossRef]
- Tang, H.; Shao, H.; Shaaban, C.E.; Yang, K.; Brown, J.; Anton, S.; Wu, Y.; Bress, A.; Donahoo, W.T.; DeKosky, S.T.; et al. Newer glucose-lowering drugs and risk of dementia: A systematic review and meta-analysis of observational studies. J. Am. Geriatr. Soc. 2023, 71, 2096–2106. [Google Scholar] [CrossRef]
- Siao, W.Z.; Lin, T.K.; Huang, J.Y.; Tsai, C.F.; Jong, G.P. The association between sodium-glucose cotransporter 2 inhibitors and incident dementia: A nationwide population-based longitudinal cohort study. Diabetes Vasc. Dis. Res. 2022, 19, 14791641221098168. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, P.; Li, Y.; Chen, Z.; Shi, A. Mechanistic evaluation of the inhibitory effect of four SGLT-2 inhibitors on SGLT 1 and SGLT 2 using physiologically based pharmacokinetic (PBPK) modeling approaches. Front. Pharmacol. 2023, 14, 1142003. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Norton, L.; Defronzo, R.A. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr. Rev. 2011, 32, 515–531. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, W.; Jin, S.; Wang, Y.; Pang, Y.; Wang, B.; Zhao, N.; Song, Y.; Qi, J. Canagliflozin protects against hyperglycemia-induced cerebrovascular injury by preventing blood-brain barrier (BBB) disruption via AMPK/Sp1/adenosine A2A receptor. Eur. J. Pharmacol. 2024, 968, 176381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, H.; Liu, X.; Guo, X. Hypoglycemic medicines in the treatment of Alzheimer’s disease: Pathophysiological links between AD and glucose metabolism. Front. Pharmacol. 2023, 14, 1138499. [Google Scholar] [CrossRef] [PubMed]
- La Grotta, R.; de Candia, P.; Olivieri, F.; Matacchione, G.; Giuliani, A.; Rippo, M.R.; Tagliabue, E.; Mancino, M.; Rispoli, F.; Ferroni, S.; et al. Anti-inflammatory effect of SGLT-2 inhibitors via uric acid and insulin. Cell. Mol. Life Sci. CMLS 2022, 79, 273. [Google Scholar] [CrossRef] [PubMed]
- Maltese, G.; Koufakis, T.; Kotsa, K.; Karalliedde, J. Can sodium-glucose cotransporter 2 inhibitors ‘spin the thread of life’? Trends Endocrinol. Metab. TEM 2023, 34, 1–4. [Google Scholar] [CrossRef]
- Złotek, M.; Kurowska, A.; Herbet, M.; Piątkowska-Chmiel, I. GLP-1 Analogs, SGLT-2, and DPP-4 Inhibitors: A Triad of Hope for Alzheimer’s Disease Therapy. Biomedicines 2023, 11, 3035. [Google Scholar] [CrossRef]
- Ahmed, S.; El-Sayed, M.M.; Kandeil, M.A.; Khalaf, M.M. Empagliflozin attenuates neurodegeneration through antioxidant, anti-inflammatory, and modulation of α-synuclein and Parkin levels in rotenone-induced Parkinson’s disease in rats. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2022, 30, 863–873. [Google Scholar] [CrossRef]
- Sim, A.Y.; Choi, D.H.; Kim, J.Y.; Kim, E.R.; Goh, A.R.; Lee, Y.H.; Lee, J.E. SGLT2 and DPP4 inhibitors improve Alzheimer’s disease-like pathology and cognitive function through distinct mechanisms in a T2D-AD mouse model. Biomed. Pharmacother. 2023, 168, 115755. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chang, H.C.; Lin, Y.J.; Chien, K.L.; Hsieh, Y.C.; Chung, F.P.; Lin, C.H.; Lip, G.Y.H.; Chen, S.A. The impact of sodium-glucose co-transporter-2 inhibitors on dementia and cardiovascular events in diabetic patients with atrial fibrillation. Diabetes/Metab. Res. Rev. 2024, 40, e3775. [Google Scholar] [CrossRef]
- Perna, S.; Mainardi, M.; Astrone, P.; Gozzer, C.; Biava, A.; Bacchio, R.; Spadaccini, D.; Solerte, S.B.; Rondanelli, M. 12-month effects of incretins versus SGLT2-Inhibitors on cognitive performance and metabolic profile. A randomized clinical trial in the elderly with Type-2 diabetes mellitus. Clin. Pharmacol. Adv. Appl. 2018, 10, 141–151. [Google Scholar] [CrossRef]
- Mui, J.V.; Zhou, J.; Lee, S.; Leung, K.S.K.; Lee, T.T.L.; Chou, O.H.I.; Tsang, S.L.; Wai, A.K.C.; Liu, T.; Wong, W.T.; et al. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors vs. Dipeptidyl Peptidase-4 (DPP4) Inhibitors for New-Onset Dementia: A Propensity Score-Matched Population-Based Study With Competing Risk Analysis. Front. Cardiovasc. Med. 2021, 8, 747620. [Google Scholar] [CrossRef]
- Shin, A.; Koo, B.K.; Lee, J.Y.; Kang, E.H. Risk of dementia after initiation of sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors in adults aged 40-69 years with type 2 diabetes: Population based cohort study. BMJ (Clin. Res. Ed.) 2024, 386, e079475. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Osadnik, T.; Goławski, M.; Lewandowski, P.; Pawlas, N. “Obesity and Insulin Resistance” Is the Component of the Metabolic Syndrome Most Strongly Associated with Oxidative Stress. Antioxidants 2022, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Kohan, D.E.; Fioretto, P.; Tang, W.; List, J.F. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014, 85, 962–971. [Google Scholar] [CrossRef]
- Erondu, N.; Desai, M.; Ways, K.; Meininger, G. Diabetic Ketoacidosis and Related Events in the Canagliflozin Type 2 Diabetes Clinical Program. Diabetes Care 2015, 38, 1680–1686. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A. Risks Associated with SGLT2 Inhibitors: An Overview. Curr. Drug Saf. 2018, 13, 84–91. [Google Scholar] [CrossRef]
- Wiciński, M.; Wódkiewicz, E.; Górski, K.; Walczak, M.; Malinowski, B. Perspective of SGLT2 Inhibition in Treatment of Conditions Connected to Neuronal Loss: Focus on Alzheimer’s Disease and Ischemia-Related Brain Injury. Pharmaceuticals 2020, 13, 379. [Google Scholar] [CrossRef]
- Little, K.; Llorián-Salvador, M.; Scullion, S.; Hernández, C.; Simó-Servat, O.; Del Marco, A.; Bosma, E.; Vargas-Soria, M.; Carranza-Naval, M.J.; Van Bergen, T.; et al. Common pathways in dementia and diabetic retinopathy: Understanding the mechanisms of diabetes-related cognitive decline. Trends Endocrinol. Metab. TEM 2022, 33, 50–71. [Google Scholar] [CrossRef]
- Reddy, V.P.; Aryal, P.; Soni, P. RAGE Inhibitors in Neurodegenerative Diseases. Biomedicines 2023, 11, 1131. [Google Scholar] [CrossRef]
- Dong, M.; Wen, S.; Zhou, L. The Relationship Between the Blood-Brain-Barrier and the Central Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter-2 Inhibitors. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 2583–2597. [Google Scholar] [CrossRef] [PubMed]
- Bode, D.; Semmler, L.; Wakula, P.; Hegemann, N.; Primessnig, U.; Beindorff, N.; Powell, D.; Dahmen, R.; Ruetten, H.; Oeing, C.; et al. Dual SGLT-1 and SGLT-2 inhibition improves left atrial dysfunction in HFpEF. Cardiovasc. Diabetol. 2021, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Shi, W.; Qiu, J.; Zhou, N.; Du, N.; Zhou, H.; Chen, X.; Ma, L. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion injury through improving mitochondrial homeostasis. Cardiovasc. Diabetol. 2022, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Taguchi, K.; Takagi, Y.; Hase, T.; Meguro, S.; Nakamura, Y. Mouse Models with SGLT2 Mutations: Toward Understanding the Role of SGLT2 beyond Glucose Reabsorption. Int. J. Mol. Sci. 2023, 24, 6278. [Google Scholar] [CrossRef]
- Ferrannini, E.; Mark, M.; Mayoux, E. CV Protection in the EMPA-REG OUTCOME Trial: A “Thrifty Substrate” Hypothesis. Diabetes Care 2016, 39, 1108–1114. [Google Scholar] [CrossRef]
- Peters, A.L.; Buschur, E.O.; Buse, J.B.; Cohan, P.; Diner, J.C.; Hirsch, I.B. Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care 2015, 38, 1687–1693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, C.-H.; Lu, L.-Y. New Insights into the Role of SGLT-2 Inhibitors in the Prevention of Dementia. Neurol. Int. 2024, 16, 1717-1730. https://doi.org/10.3390/neurolint16060124
Hung C-H, Lu L-Y. New Insights into the Role of SGLT-2 Inhibitors in the Prevention of Dementia. Neurology International. 2024; 16(6):1717-1730. https://doi.org/10.3390/neurolint16060124
Chicago/Turabian StyleHung, Cheng-Hsien, and Li-Yu Lu. 2024. "New Insights into the Role of SGLT-2 Inhibitors in the Prevention of Dementia" Neurology International 16, no. 6: 1717-1730. https://doi.org/10.3390/neurolint16060124
APA StyleHung, C.-H., & Lu, L.-Y. (2024). New Insights into the Role of SGLT-2 Inhibitors in the Prevention of Dementia. Neurology International, 16(6), 1717-1730. https://doi.org/10.3390/neurolint16060124






