Analysis of Thiodiphenol in Rat Urine as a Biomarker of Exposure to Temephos
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Animal Treatment
2.4. Extraction of Temephos and Its Metabolites
2.5. Analysis of Temephos and Its Metabolites by Liquid Chromatography
2.6. Recovery of Temephos Metabolites
2.7. Toxicokinetic Parameters of Temephos Metabolites
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Temephos. In Pesticide Residues in Food. Joint FAO/WHO Meeting on Pesticide Residues; WHO: Geneva, Switzerland, 2006; pp. 403–427. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 5 July 2021).
- Verdín-Betancourt, F.A.; Figueroa, M.; Soto-Ramos, A.G.; de Lourdes López-González, M.; Castañeda-Hernández, G.; Ber-nal-Hernández, Y.Y.; Rojas-García, A.E.; Sierra-Santoyo, A. Toxicokinetics of temephos after oral administration to adult male rats. Arch. Toxicol. 2021, 95, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Flores, A.; Camacho-Hernández, I.; Sierra-Santoyo, A.; Solís-Heredia, M.J.; Verdín-Betancourt, F.A.; Parra-Forero, L.Y.; López-González, M.L.; Hernández-Ochoa, I.; Quintanilla-Vega, B. Temephos decreases sperm quality and fertilization rate and is metabolized in rat reproductive tissues at low-dose exposure. Toxicol. Sci. 2021, 184, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Vani, J.M.; de Carvalho Schweich, L.; de Oliveira, K.R.W.; Auharek, S.A.; Cunha-Laura, A.L.; Antoniolli-Silva, A.C.M.B.; Nazario, C.E.D.; Oliveira, R.J. Evaluation of the effects of the larvicides temephos on reproductive performance, embryofetal development and DNA integrity of Swiss mice. Pest. Biochem. Physiol. 2018, 148, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Chaparro, A.; Verdín-Betancourt, F.A.; Sierra-Santoyo, A. Human biotransformation pathway of temephos using an in silico approach. Chem. Res. Toxicol. 2020, 33, 2765–2774. [Google Scholar] [CrossRef] [PubMed]
- Blinn, R.C. Metabolic Fate of Abate Insecticide in the Rat. J. Agric. Food Chem. 1969, 17, 118–122. [Google Scholar] [CrossRef]
- Shafik, M.T. The determination of 4,4′-thiodiphenol in human and rat urine as an indication of exposure to low levels of Abate. Bull. Environ. Contam. Toxicol. 1970, 5, 311–316. [Google Scholar] [CrossRef]
- Aiub, C.A.F.; Coelho, E.C.A.; Sodré, E.; Pinto, L.F.R.; Felzenszwalb, I. Genotoxic evaluation of the organophosphorous pesticide temephos. Genet. Mol. Res. 2002, 1, 159–166. [Google Scholar]
- Bezerra de Mélo, M.E.; da Costa Merlo, K.; de Carvalho Fernandes, R.R.; Feitosa Luna, C.; Nunes Diniz, G.T.; Jansem de Almeida Catanho, M.T.; Regis, L. Mutagenic effects of the organophosphate insecticide temephos on mice bone marrow cells. Rev. Inst. Adolfo Lutz 2008, 67, 96–201. [Google Scholar]
- Ba-Omar, T.A.; Al-Kharusi, I.; Victor, R. Effects of pesticide temephos on the liver of Aphanius dispar (Rüppell 1828) (Pisces: Cyprinodontidae): A microscopic study. Sultan Qaboos Univ. J. Sci. [SQUJS] 2013, 18, 11. [Google Scholar] [CrossRef]
- Benitez-Trinidad, A.B.; Herrera-Moreno, J.F.; Vázquez-Estrada, G.; Verdín-Betancourt, F.A.; Sordo, M.; Ostrosky-Wegman, P.; Bernal-Hernández, Y.Y.; Medina-Díaz, I.M.; Barrón-Vivanco, B.S.; Robledo-Marenco, M.L.; et al. Cytostatic and genotoxic effect of temephos in human lymphocytes and HepG2 cells. Toxicol. In Vitro 2015, 29, 779–786. [Google Scholar] [CrossRef]
- Kim, S.H.; Bae, J.W.; Kim, D.H.; Jeong, D.J.; Ha, J.J.; Yi, J.K.; Kwon, W.S. Detrimental effects of temephos on male fertility: An in vitro study on a mouse model. Reprod. Toxicol. 2020, 96, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Laurentino, A.O.M.; Durante de Medeiros, F.; de Oliveira, J.; da Rosa, N.; Mateus Gomes, T.; de Medeiros Peretti, E.; Somariva Prophiro, J.; Fortunato, J.J. Effects of prenatal exposure to temephos on behavior and social interaction. Neuropsychiatr. Dis. Treat. 2019, 15, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Laws, E.R., Jr.; Morales, F.R.; Hayes, W.J., Jr.; Joseph, C.R. Toxicology of Abate in volunteers. Arch. Environ. Health 1967, 14, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Verdín-Betancourt, F.A.; Figueroa, M.; López-González, M.L.; Gómez, E.; Bernal-Hernández, Y.Y.; Rojas-García, A.E.; Sierra-Santoyo, A. In vitro inhibition of human red blood cell acetylcholinesterase (AChE) by temephos-oxidized products. Sci. Rep. 2019, 9, 14758. [Google Scholar] [CrossRef]
- Cruz-Hurtado, M.C.; López-González, M.d.L.; Escobar-Wilches, D.C.; Sierra-Santoyo, A. Analysis of 3′,5′-dichloro-2,3,4-trihydroxy-2-methylbutylanilide (DTMBA) as a new potential biomarker of exposure to vinclozolin in urine. Toxicol. Appl. Pharmacol. 2018, 346, 1–8. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- CENAPRECE Productos Recomendados Por el CENAPRECE Para el Combate de Insectos Vectores de Enfermedades a Partir del 2024. 2024. Available online: https://www.gob.mx/cms/uploads/attachment/file/906167/Productos_Recomendados_por_CENAPRECE_para_el_Combate_de_Insectos_Vectores_2024.PDF (accessed on 26 July 2024).
- Steimer, J.L.; Plusquellec, Y.; Guillaume, A.; Boisvieux, J.F. A time-lag model for pharmacokinetics of drugs subject to enterohepatic circulation. J. Pharm. Sci. 1982, 71, 297–302. [Google Scholar] [CrossRef]
- Herrera-Moreno, J.F.; Medina-Díaz, I.M.; Bernal-Hernández, Y.Y.; Barrón-Vivanco, B.S.; González-Arias, C.A.; Mo-reno-Godínez, M.E.; Verdín-Betancourt, F.A.; Sierra-Santoyo, A.; Rojas-García, A.E. Organophosphorus pesticide exposure biomarkers in a Mexican popula-tion. Environ. Sci. Pollut. Res. 2021, 28, 50825–50834. [Google Scholar] [CrossRef]
- Buratti, F.; Volpe, M.; Meneguz, A.; Vittozzi, L.; Testaia, E. CYP-specific bioactivation of four organophosphorothioate pesticides by human liver microsomes. Toxicol. Appl. Pharmacol. 2003, 186, 143–154. [Google Scholar] [CrossRef]
- Foxemberg, R.J.; McGarrigle, B.P.; Knaak, J.B.; Kostyniak, P.J.; Olson, J.R. Human hepatic cytochrome P450-specific metabolism of parathion and chlorpyrifos. Drug Metab. Dispos. 2007, 35, 189–193. [Google Scholar] [CrossRef]
- Buratti, F.M.; Leoni, C.; Testai, E. The Human Metabolism of Organophosphorothionate Pesticides: Consequences for Toxicological Risk Assessment. J. Fur Verbraucherschutz Leb. 2007, 2, 37–44. [Google Scholar] [CrossRef]
- Gallo, M.A.; Lawryk, N.J. Organic phosphorus pesticides. In Handbook of Pesticide Toxicology: Classes of Pesticides; Hayes, W.J., Jr., Laws, E.R., Jr., Eds.; Academic Press: San Diego, CA, USA, 1991; Volume 2, pp. 917–1123. [Google Scholar]
- Ellison, C.A.; Tian, Y.; Knaak, J.B.; Kostyniak, P.J.; Olson, J.R. Human cytochrome P450-Specific Metabolism of the Or-ganophosphorus Pesticides Methyl Parathion and Diazinon. Drug Metab. Dispos. 2012, 40, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jokanovic, M. Biotransformation of organophosphorus compounds. Toxicology 2001, 166, 139–160. [Google Scholar] [CrossRef]
- Sultatos, L.G. Mammalian toxicology of organophosphorus pesticides. J. Toxicol. Environ. Health 1995, 43, 271–289. [Google Scholar] [CrossRef]
- Ferguson, P.W.; Medon, P.J.; Nasri, E. Temephos (Abate) metabolism and toxicity in rats. Arch. Environ. Contam. Toxicol. 1985, 14, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, E.; Rose, R. Organophosphorus Chemicals: Potent inhibitors of the human metabolism of steroid hormones and xenobiotics. Drug Metab. Rev. 2006, 38, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Usmani, K.; Cho, T.; Rose, R.; Hodgson, E. Inhibition of the human liver microsomal and human cytochrome P450 1A2 and 3A4 metabolism of estradiol by deployment-related and other chemicals. Drug Metab. Dispos. 2006, 34, 1606–1614. [Google Scholar] [CrossRef]
- Murphy, S.D.; Sheever, K.L. Carboxylesterase and cholinesterase inhibition in rats. Arch. Environ. Health 1972, 24, 107–114. [Google Scholar] [CrossRef]
- Gao, C.; He, H.; Qiu, W.; Zheng, Y.; Chen, Y.; Hu, S.; Zhao, X. Oxidative stress, endocrine disturbance, and immune interference in humans showed relationships to serum bisphenol concentrations in a dense industrial area. Environ. Sci. Technol. 2021, 55, 1953–1963. [Google Scholar] [CrossRef]
- Lei, B.; Peng, W.; Xu, G.; Wu, M.; Wen, Y.; Xu, J.; Yu, Z.; Wang, Y. Activation of G protein-coupled receptor 30 by thi-odiphenol promotes proliferation of estrogen receptor a-positive breast cancer cells. Chemosphere 2017, 169, 204–211. [Google Scholar] [CrossRef]
- Martignoni, M.; Groothuis, G.M.M.; de Kanter, R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2006, 2, 875–894. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, D.; Liu, W.; Li, R.; Yan, S.; Jia, M.; Zhang, L.; Zhou, Z.; Zhu, W. Perinatal exposure to Bisphenol S (BPS) promotes obesity development by interfering with lipid and glucose metabolism in male mouse offspring. Environ. Res. 2019, 173, 189–198. [Google Scholar] [CrossRef] [PubMed]

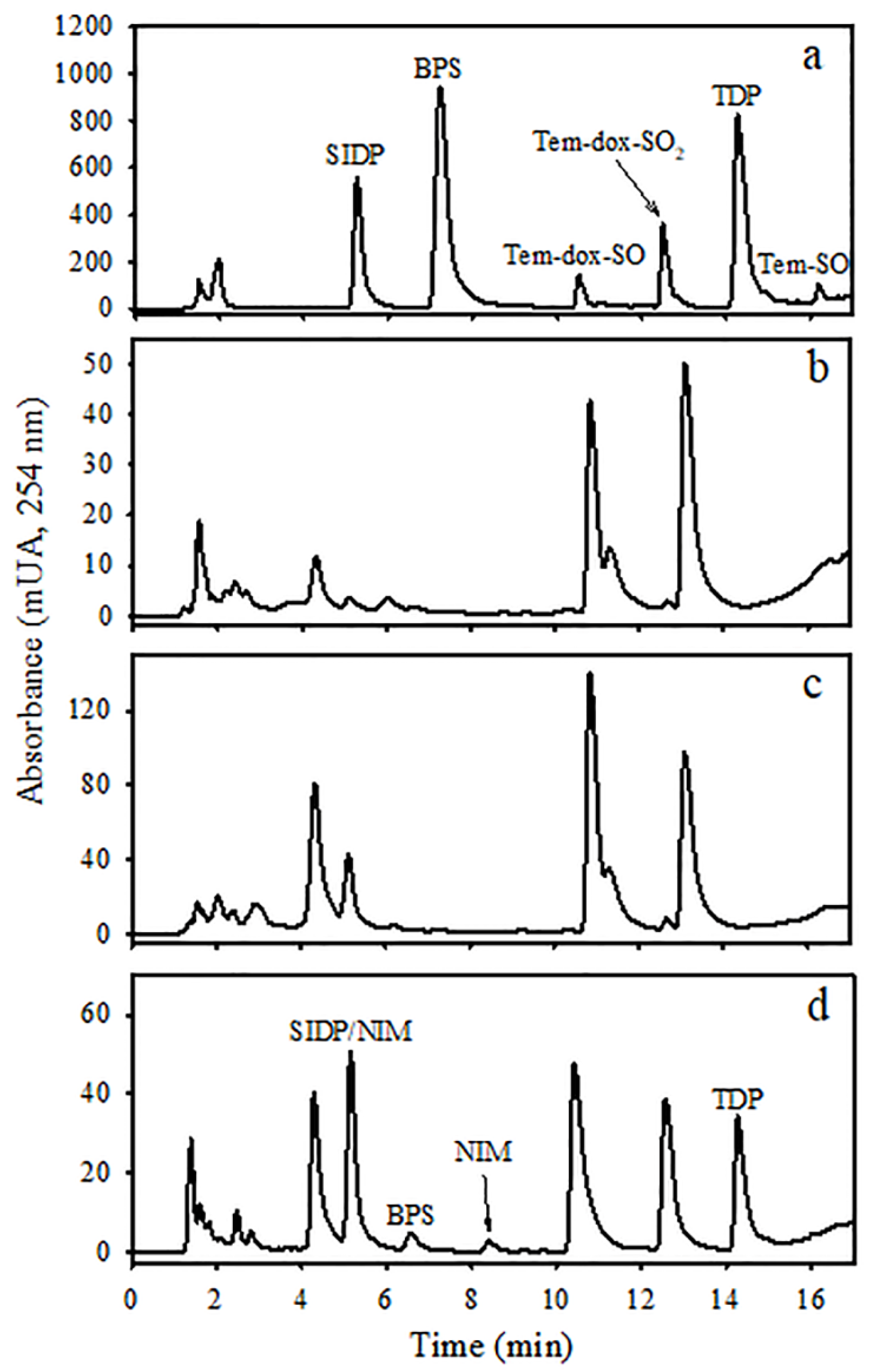
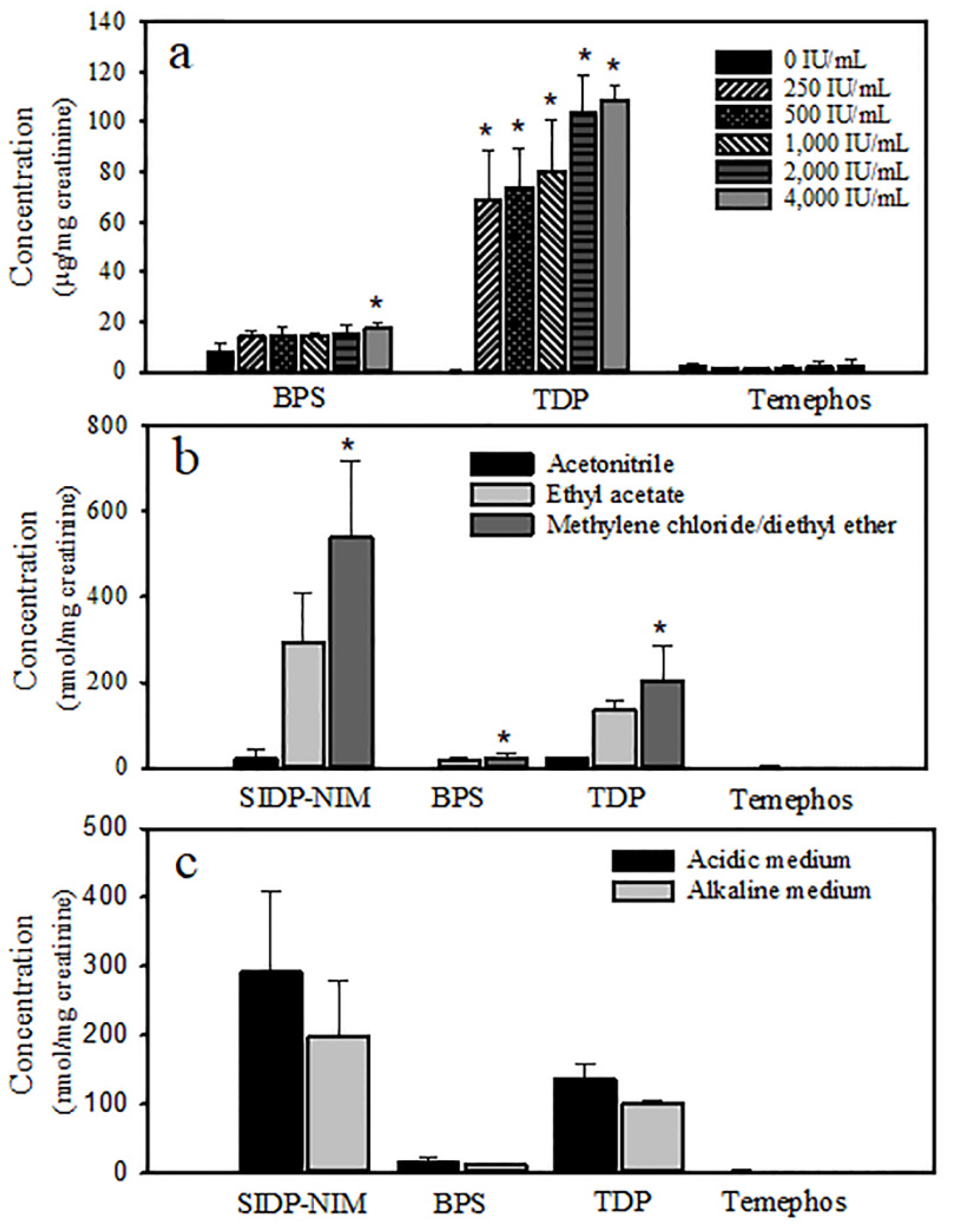
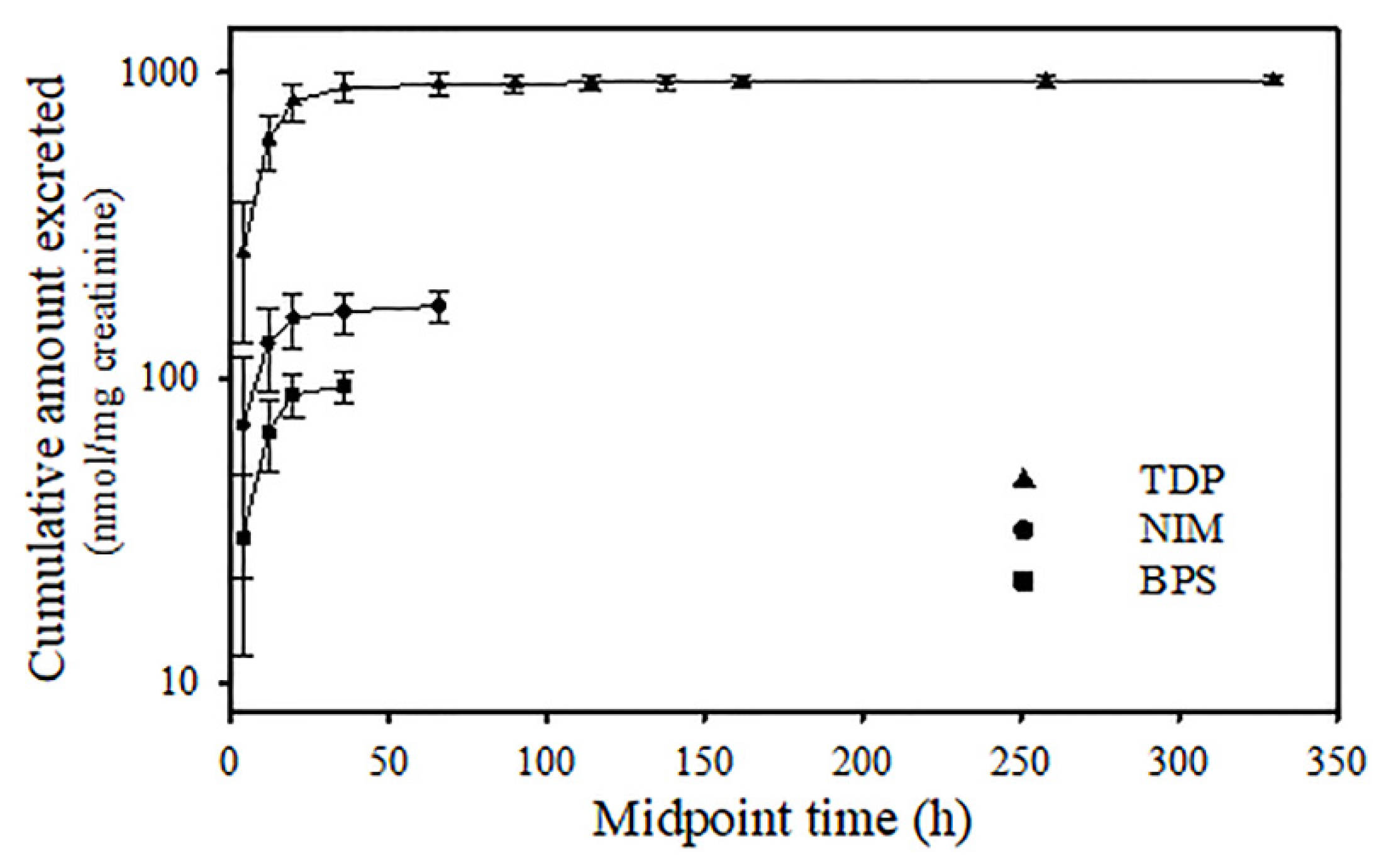
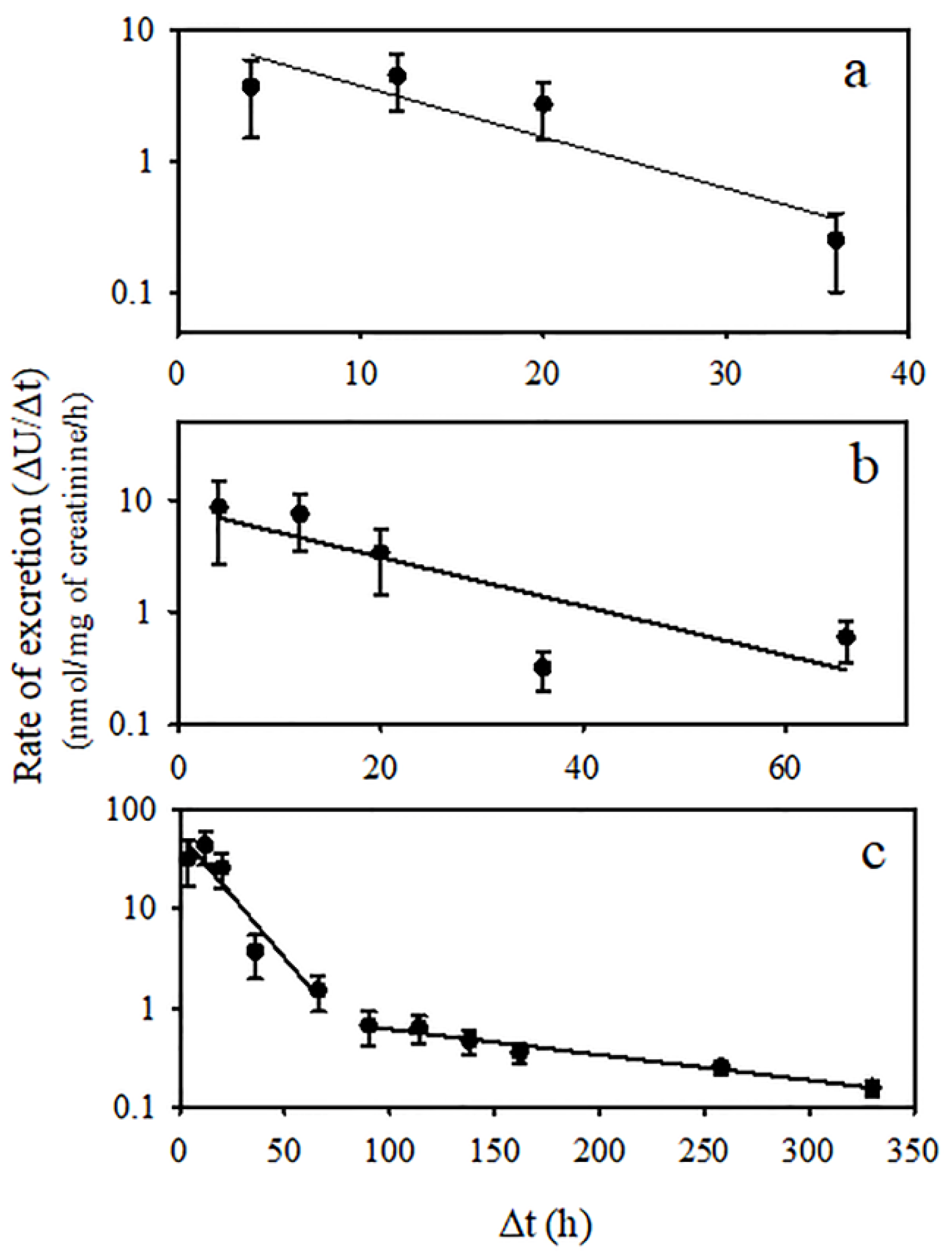
| Concentration (µg/mL) | ||||||
|---|---|---|---|---|---|---|
| Metabolite | 2 | 10 | 50 | |||
| Recovery (%) | Precision (%) | Recovery (%) | Precision (%) | Recovery (%) | Precision (%) | |
| BPS | 74.3 ± 12.9 | 3.3 | 100.6 ± 12.9 | 5.2 | 92.8 ± 10.3 | 4.9 |
| TDF | 90.5 ± 15.9 | 11.7 | 100.3 ± 19.8 | 3.5 | 87.4 ± 12.6 | 7.0 |
| Metabolite | KElim-U (h−1) | t1/2 Elim-U (h) | AUC0–∞ (nmol-h/mg Creatinine) | Cmax-U (nmol/mg Creatinine) | tmax-U (h) | ||
|---|---|---|---|---|---|---|---|
| 1st Phase | 2nd Phase | 1st Phase | 2nd Phase | ||||
| BPS | - | 0.03912 | - | 17.7 | 2455 ± 974 | 41 ± 16 | 9 ± 6 |
| NIM | - | 0.02188 | - | 31.7 | 9541 ± 3843 | 88 ± 40 | 7 ± 4 |
| TDP | 0.02496 | 0.00255 | 27.8 | 272.1 | 297,540 ± 76,013 | 366 ± 115 | 10 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, M.-L.; López-González, M.d.L.; Uribe-Ramírez, M.; Rojas-García, A.E.; Verdín-Betancourt, F.A.; Sierra-Santoyo, A. Analysis of Thiodiphenol in Rat Urine as a Biomarker of Exposure to Temephos. J. Xenobiot. 2024, 14, 1889-1900. https://doi.org/10.3390/jox14040100
Shih M-L, López-González MdL, Uribe-Ramírez M, Rojas-García AE, Verdín-Betancourt FA, Sierra-Santoyo A. Analysis of Thiodiphenol in Rat Urine as a Biomarker of Exposure to Temephos. Journal of Xenobiotics. 2024; 14(4):1889-1900. https://doi.org/10.3390/jox14040100
Chicago/Turabian StyleShih, Miao-Ling, Ma. de Lourdes López-González, Marisela Uribe-Ramírez, Aurora Elizabeth Rojas-García, Francisco Alberto Verdín-Betancourt, and Adolfo Sierra-Santoyo. 2024. "Analysis of Thiodiphenol in Rat Urine as a Biomarker of Exposure to Temephos" Journal of Xenobiotics 14, no. 4: 1889-1900. https://doi.org/10.3390/jox14040100
APA StyleShih, M.-L., López-González, M. d. L., Uribe-Ramírez, M., Rojas-García, A. E., Verdín-Betancourt, F. A., & Sierra-Santoyo, A. (2024). Analysis of Thiodiphenol in Rat Urine as a Biomarker of Exposure to Temephos. Journal of Xenobiotics, 14(4), 1889-1900. https://doi.org/10.3390/jox14040100







