Feasibility Study of Bio-Sludge Hydrochar as Blast Furnace Injectant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Raw Materials
2.2. Experimental Method
3. Results
3.1. Basic Performance of Fuel
3.2. Hazardous Element Analysis of Fuel
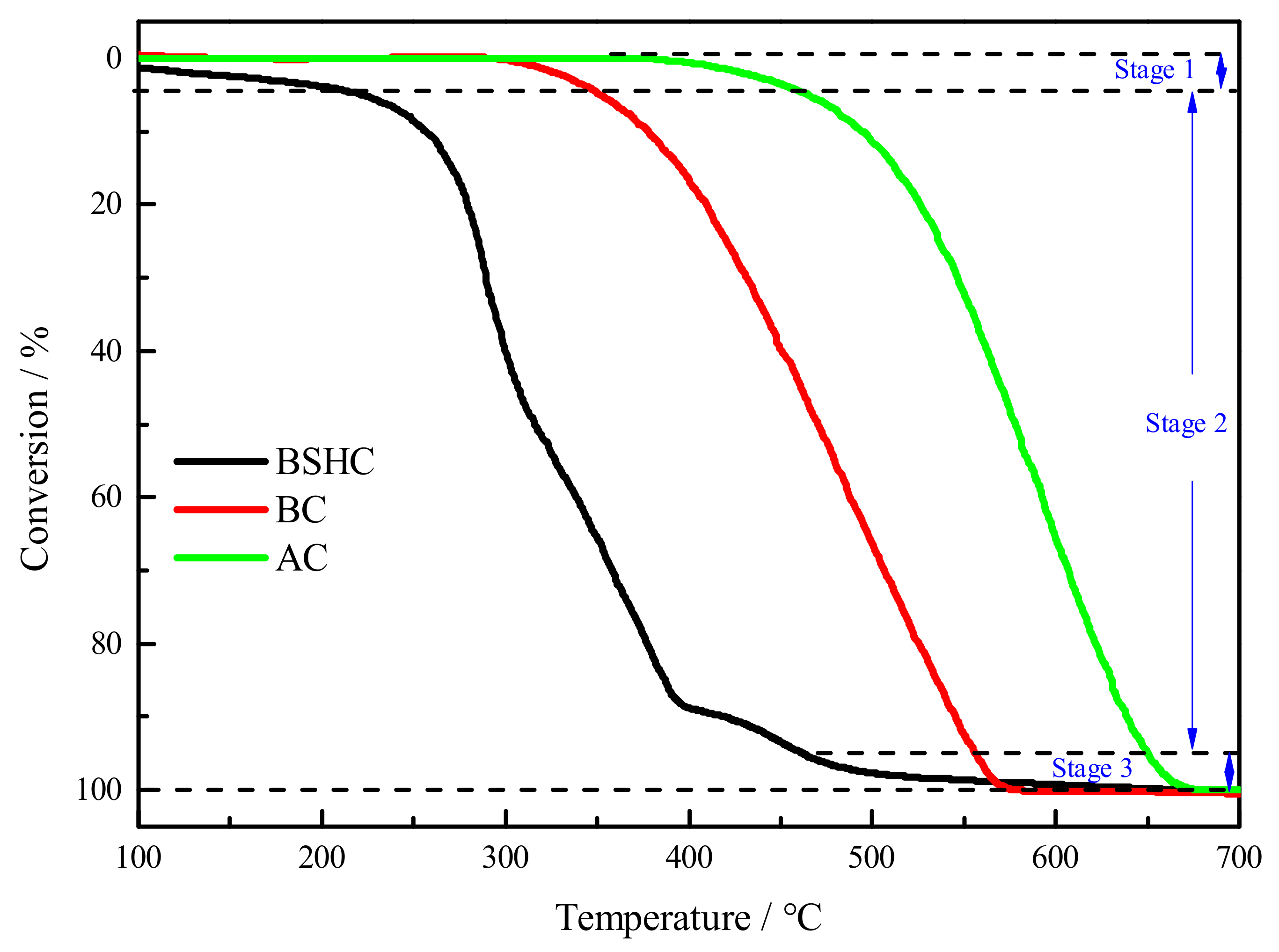
3.3. Combustion Performance of Fuel
3.4. Safety Performance of Fuel
3.5. Study on the Properties of Mixtures
3.5.1. Determination of Mixture Ratio
3.5.2. The Combustion Performance of Mixed Samples
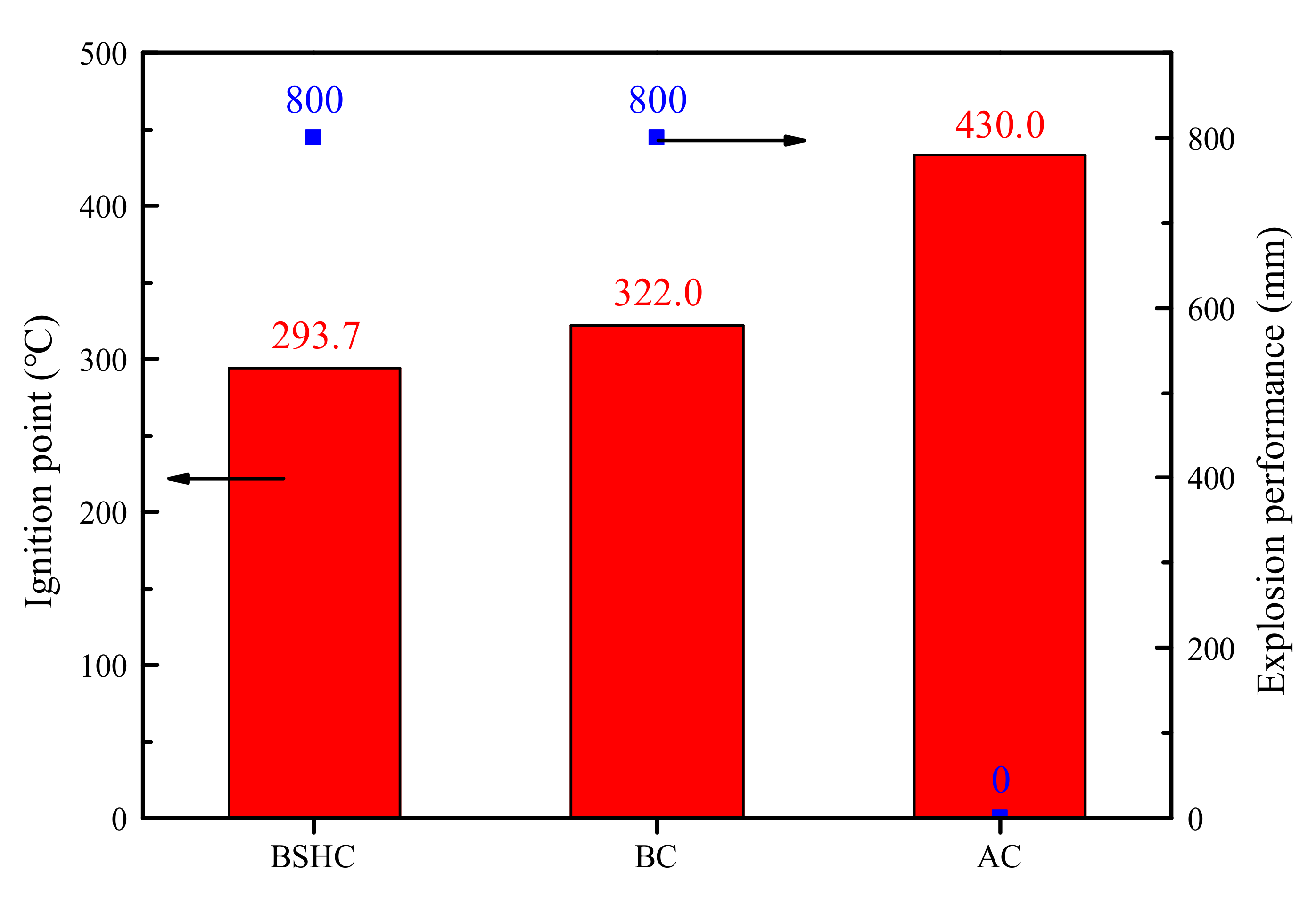
3.5.3. The Safety Performance of Mixed Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, W.; Wang, G.; Jiao, K.; Ning, X.; Zhang, J.; Guo, X.; Li, J.; Wang, C. Conversion mechanism and gasification kinetics of biomass char during hydrothermal carbonization. Renew. Energy 2021, 173, 318–328. [Google Scholar] [CrossRef]
- Song, M.; Wang, J.; Zhao, J. Coal endowment, resource curse, and high coal-consuming industries location: Analysis based on large-scale data. Resour. Conserv. Recycl. 2018, 129, 333–344. [Google Scholar] [CrossRef]
- Ren, Y.-S.; Apergis, N.; Ma, C.; Baltas, K.; Jiang, Y.; Liu, J.-L. FDI, economic growth, and carbon emissions of the Chinese steel industry: New evidence from a 3SLS model. Environ. Sci. Pollut. Res. 2021, 28, 52547–52564. [Google Scholar] [CrossRef] [PubMed]
- Krenek, A. How to implement a WTO-compatible full border carbon adjustment as an important part of the European Green Deal. Österreichische Gesellschaft für Europapolitik (ÖGfE) Policy Brief 2020. Available online: https://www.oegfe.at/wp-content/uploads/2020/01/OEGfE_Policy_Brief-2020.02-2.pdf (accessed on 3 May 2022).
- Chiaramonti, D.; Talluri, G.; Scarlat, N.; Prussi, M. The challenge of forecasting the role of biofuel in EU transport decarbonisation at 2050: A meta-analysis review of published scenarios. Renew. Sustain. Energy Rev. 2021, 139, 110715. [Google Scholar] [CrossRef]
- Holappa, L. A general vision for reduction of energy consumption and CO2 emissions from the steel industry. Metals 2020, 10, 1117. [Google Scholar] [CrossRef]
- Wu, R.; Lin, B. Does industrial agglomeration improve effective energy service: An empirical study of China’s iron and steel industry. Appl. Energy 2021, 295, 117066. [Google Scholar] [CrossRef]
- Mandova, H.; Leduc, S.; Wang, C.; Wetterlund, E.; Patrizio, P.; Gale, W.; Kraxner, F. Possibilities for CO2 emission reduction using biomass in European integrated steel plants. Biomass Bioenergy 2018, 115, 231–243. [Google Scholar] [CrossRef]
- Malico, I.; Pereira, R.N.; Gonçalves, A.C.; Sousa, A.M. Current status and future perspectives for energy production from solid biomass in the European industry. Renew. Sustain. Energy Rev. 2019, 112, 960–977. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, R.; Xiong, M.; Zhang, D.; Gu, H.; Chen, W. Conversion of cotton textile waste to clean solid fuel via surfactant-assisted hydrothermal carbonization: Mechanisms and combustion behaviors. Bioresour. Technol 2021, 321, 124450. [Google Scholar] [CrossRef]
- Alper, K.; Tekin, K.; Karagöz, S.; Ragauskas, A.J. Sustainable energy and fuels from biomass: A review focusing on hydrothermal biomass processing. Sustain. Energy Fuels 2020, 4, 4390–4414. [Google Scholar] [CrossRef]
- Guan, Y.; Tai, L.; Cheng, Z.; Chen, G.; Yan, B. Biomass molded fuel in China: Current status, policies and suggestions. Sci. Total Environ. 2020, 724, 138345. [Google Scholar] [CrossRef]
- Babich, A.; Senk, D.; Solar, J.; de Marco, I. Efficiency of biomass use for blast furnace injection. ISIJ Int. 2019, 59, 2212–2219. [Google Scholar] [CrossRef] [Green Version]
- Antonkiewicz, J.; Popławska, A.; Kołodziej, B.; Ciarkowska, K.; Gambuś, F.; Bryk, M.; Babula, J. Application of ash and municipal sewage sludge as macronutrient sources in sustainable plant biomass production. J. Environ. Manag. 2020, 264, 110450. [Google Scholar] [CrossRef]
- Wołejko, E.; Wydro, U.; Jabłońska-Trypuć, A.; Butarewicz, A.; Łoboda, T. The effect of sewage sludge fertilization on the concentration of PAHs in urban soils. Environ. Pollut. 2018, 232, 347–357. [Google Scholar] [CrossRef]
- Gerner, G.; Meyer, L.; Wanner, R.; Keller, T.; Krebs, R. Sewage sludge treatment by hydrothermal carbonization: Feasibility study for sustainable nutrient recovery and fuel production. Energies 2021, 14, 2697. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Y.; Liu, J.; Zhong, S.; Qian, Y.; Gao, P. An overlooked entry pathway of microplastics into agricultural soils from application of sludge-based fertilizers. Environ. Sci. Technol. 2020, 54, 4248–4255. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Li, A. Hydrothermal carbonization for energy-efficient processing of sewage sludge: A review. Renew. Sustain. Energy Rev. 2019, 108, 423–440. [Google Scholar] [CrossRef]
- Liu, T.; Lang, Q.; Xia, Y.; Chen, Z.; Li, D.; Ma, J.; Gai, C.; Liu, Z. Combination of hydrothermal carbonization and oxy-fuel combustion process for sewage sludge treatment: Combustion characteristics and kinetics analysis. Fuel 2019, 242, 265–276. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.-Y. Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Çebi, D.; Celiktas, M.S.; Sarptas, H. A Review on Sewage Sludge Valorization via Hydrothermal Carbonization and Applications for Circular Economy. Circ. Econ. Sustain. 2022, 1–23. [Google Scholar] [CrossRef]
- Nyktari, E.; Danso-Boateng, E.; Wheatley, A.D.; Holdich, R. Anaerobic digestion of liquid products following hydrothermal carbonisation of faecal sludge at different reaction conditions. Desalination Water Treat. 2017, 91, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Pels, J.; Cieplik, M.; Bleijendaal, L.; Nijman, M.; Zandvoort, M. Conversion of water plants to biomass fuel using torwash. 2014. Available online: https://repository.tudelft.nl/islandora/object/uuid:ea383ee8-2c42-4cbc-9654-099a78e4ddd2 (accessed on 3 May 2022).
- Song, T.; Zhang, J.; Wang, G.; Wang, H.; Xu, R. Influencing factors of the explosion characteristics of modified coal used for blast furnace injection. Powder Technol. 2019, 353, 171–177. [Google Scholar] [CrossRef]
- Aich, S.; Nandi, B.K.; Bhattacharya, S. Utilization of sal leaves and sal leaves char to improve the combustion performance of reject coal. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 2299–2312. [Google Scholar] [CrossRef]
- Dastidar, M.G.; Bhattacharyya, A.; Sarkar, B.K.; Dey, R.; Mitra, M.K.; Schenk, J. The effect of alkali on the reaction kinetics and strength of blast furnace coke. Fuel 2020, 268, 117388. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Li, Z.; Zhang, A.; Zhang, X.; Qing, S. Impact of slag composition activity on the behavior of phosphorus in the smelting reduction process of high-phosphorus iron ores. Int. J. Hydrogen Energy 2017, 42, 24487–24494. [Google Scholar] [CrossRef]
- Xie, J.; Zhou, P. Robust stochastic configuration network multi-output modeling of molten iron quality in blast furnace ironmaking. Neurocomputing 2020, 387, 139–149. [Google Scholar] [CrossRef]
- Wang, P.; Wang, G.; Zhang, J.; Lee, J.-Y.; Li, Y.; Wang, C. Co-combustion characteristics and kinetic study of anthracite coal and palm kernel shell char. Appl. Therm. Eng. 2018, 143, 736–745. [Google Scholar] [CrossRef]
- Takarada, T.; Tamai, Y.; Tomita, A. Reactivities of 34 coals under steam gasification. Fuel 1985, 64, 1438–1442. [Google Scholar] [CrossRef]
- Jin, L.-Z.; Niu, X.-M. Micromorphology and safety properties of meager and meager-lean coal for blast furnace injection. Int. J. Miner. Metall. Mater. 2021, 28, 774–781. [Google Scholar] [CrossRef]
- Rybak, W.; Moroń, W.; Ferens, W. Dust ignition characteristics of different coal ranks, biomass and solid waste. Fuel 2019, 237, 606–618. [Google Scholar] [CrossRef]
- Tong, W.; Liu, Q.; Ran, G.; Liu, L.; Ren, S.; Chen, L.; Jiang, L. Experiment and expectation: Co-combustion behavior of anthracite and biomass char. Bioresour. Technol 2019, 280, 412–420. [Google Scholar] [CrossRef]
- Guo, F.; Zhong, Z. Co-combustion of anthracite coal and wood pellets: Thermodynamic analysis, combustion efficiency, pollutant emissions and ash slagging. Environ. Pollut. 2018, 239, 21. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, J.; Xu, R.; Ning, X.; Zhang, N.; Wang, C.; Mao, X.; Li, J.; Wang, G.; Wang, C. Co-combustion kinetic analysis of biomass hydrochar and anthracite in blast furnace injection. Fuel 2022, 316, 123299. [Google Scholar] [CrossRef]
- Orre, J.; Ökvist, L.S.; Bodén, A.; Björkman, B. Understanding of Blast Furnace Performance with Biomass Introduction. Minerals 2021, 11, 157. [Google Scholar] [CrossRef]
- Sundqvist Ökvist, L.; Lundgren, M. Experiences of bio-coal applications in the blast furnace process—Opportunities and limitations. Minerals 2021, 11, 863. [Google Scholar] [CrossRef]
- Ng, K.W.; Giroux, L.; Todoschuk, T. Value-in-use of biocarbon fuel for direct injection in blast furnace ironmaking. Ironmak. Steelmak. 2018, 45, 406–411. [Google Scholar] [CrossRef]

| Sample | Proximate Analysis (%) | Ultimate Analysis (%) | HHV (MJ/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FCd a | Vd | Ad | C | H | O | N | S | ||
| BS | 11.0 | 70.0 | 19.0 | 41.3 | 5.8 | 32.7 | 6.3 | 2.2 | 18.1 |
| Sample | Proximate Analysis (%) | Ultimate Analysis (%) | HHV (MJ/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FCd a | Vd | Ad | C | H | O a | N | S | ||
| BSHC | 15.87 | 61.09 | 23.04 | 46.50 | 6.04 | 19.45 | 4.22 | 0.74 | 23.45 |
| BC | 59.10 | 34.95 | 5.95 | 65.02 | 3.82 | 23.77 | 0.99 | 0.45 | 27.46 |
| AC | 82.49 | 8.85 | 8.66 | 85.26 | 3.40 | 0.40 | 1.08 | 1.09 | 30.65 |
| Sample | K | Na | P |
|---|---|---|---|
| BSHC | 0.077 | 0.110 | 2.489 |
| BC | ≤0.010 | 0.060 | 0.079 |
| AC | 0.155 | 0.790 | 0.006 |
| Sample | Ti (°C) | Tf (°C) | Tf − Ti (°C) | R0.5 × 10−4 (s−1) |
|---|---|---|---|---|
| BSHC | 221 | 462 | 241 | 5.27 |
| BC | 351 | 556 | 205 | 3.55 |
| AC | 465 | 650 | 185 | 2.88 |
| Sample | FCd a (%) | Vd (%) | Ad (%) | P (%) |
|---|---|---|---|---|
| 50%BSHC/50%AC | 49.18 | 34.97 | 15.86 | 1.25 |
| 40%BSHC/60%AC | 55.84 | 29.75 | 14.41 | 1.00 |
| 30%BSHC/70%AC | 62.50 | 24.52 | 12.98 | 0.75 |
| 20%BSHC/80%AC | 69.17 | 19.30 | 11.53 | 0.50 |
| 10%BSHC/90%AC | 75.83 | 14.07 | 10.10 | 0.25 |
| Sample | Ti (°C) | Tf (°C) | Tf − Ti (°C) | R0.5 × 10−4 (s−1) |
|---|---|---|---|---|
| BSHC | 221 | 462 | 241 | 5.27 |
| 50%BSHC/50%AC | 256 | 600 | 344 | 3.35 |
| 40%BSHC/60%AC | 260 | 610 | 350 | 3.16 |
| 30%BSHC/70%AC | 285 | 621 | 336 | 3.06 |
| 20%BSHC/80%AC | 307 | 625 | 318 | 2.98 |
| 10%BSHC/90%AC | 393 | 637 | 244 | 2.93 |
| AC | 465 | 650 | 185 | 2.88 |
| Sample | Ignition Point (°C) | Explosion Performance (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | Average Value | 1st | 2nd | 3rd | Max Value | |
| 50%BSHC/50%AC | 322 | 326 | 322 | 323.33 | 800 | 800 | 800 | 800 |
| 40%BSHC/60%AC | 341 | 320 | 327 | 329.33 | 200 | 210 | 200 | 210 |
| 30%BSHC/70%AC | 341 | 343 | 339 | 341.00 | 10 | 0 | 0 | 10 |
| 20%BSHC/80%AC | 380 | 400 | 388 | 389.33 | 0 | 0 | 0 | 0 |
| 10%BSHC/90%AC | 421 | 405 | 413 | 413.00 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Nanou, P.; Wray, H.; Zhang, J.; Lundstrom, I.; Lundqvist, S.; Wang, C. Feasibility Study of Bio-Sludge Hydrochar as Blast Furnace Injectant. Sustainability 2022, 14, 5510. https://doi.org/10.3390/su14095510
Liang W, Nanou P, Wray H, Zhang J, Lundstrom I, Lundqvist S, Wang C. Feasibility Study of Bio-Sludge Hydrochar as Blast Furnace Injectant. Sustainability. 2022; 14(9):5510. https://doi.org/10.3390/su14095510
Chicago/Turabian StyleLiang, Wang, Pavlina Nanou, Heather Wray, Jianliang Zhang, Ingemar Lundstrom, Stefan Lundqvist, and Chuan Wang. 2022. "Feasibility Study of Bio-Sludge Hydrochar as Blast Furnace Injectant" Sustainability 14, no. 9: 5510. https://doi.org/10.3390/su14095510
APA StyleLiang, W., Nanou, P., Wray, H., Zhang, J., Lundstrom, I., Lundqvist, S., & Wang, C. (2022). Feasibility Study of Bio-Sludge Hydrochar as Blast Furnace Injectant. Sustainability, 14(9), 5510. https://doi.org/10.3390/su14095510







