Phase Analysis of Alkali-Activated Slag Hybridized with Low-Calcium and High-Calcium Fly Ash
Abstract
:1. Introduction
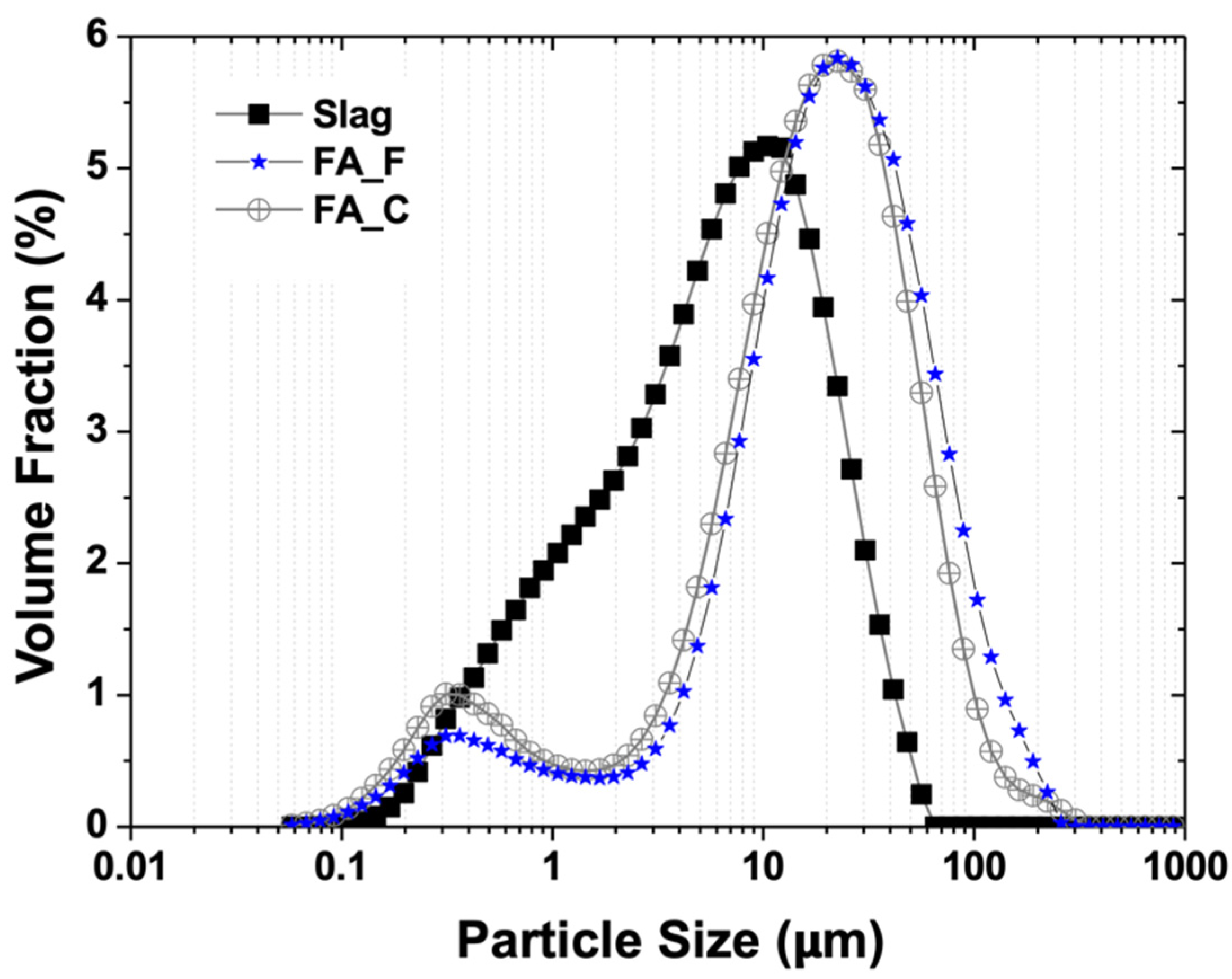
2. Materials and Methods
3. Results and Discussion
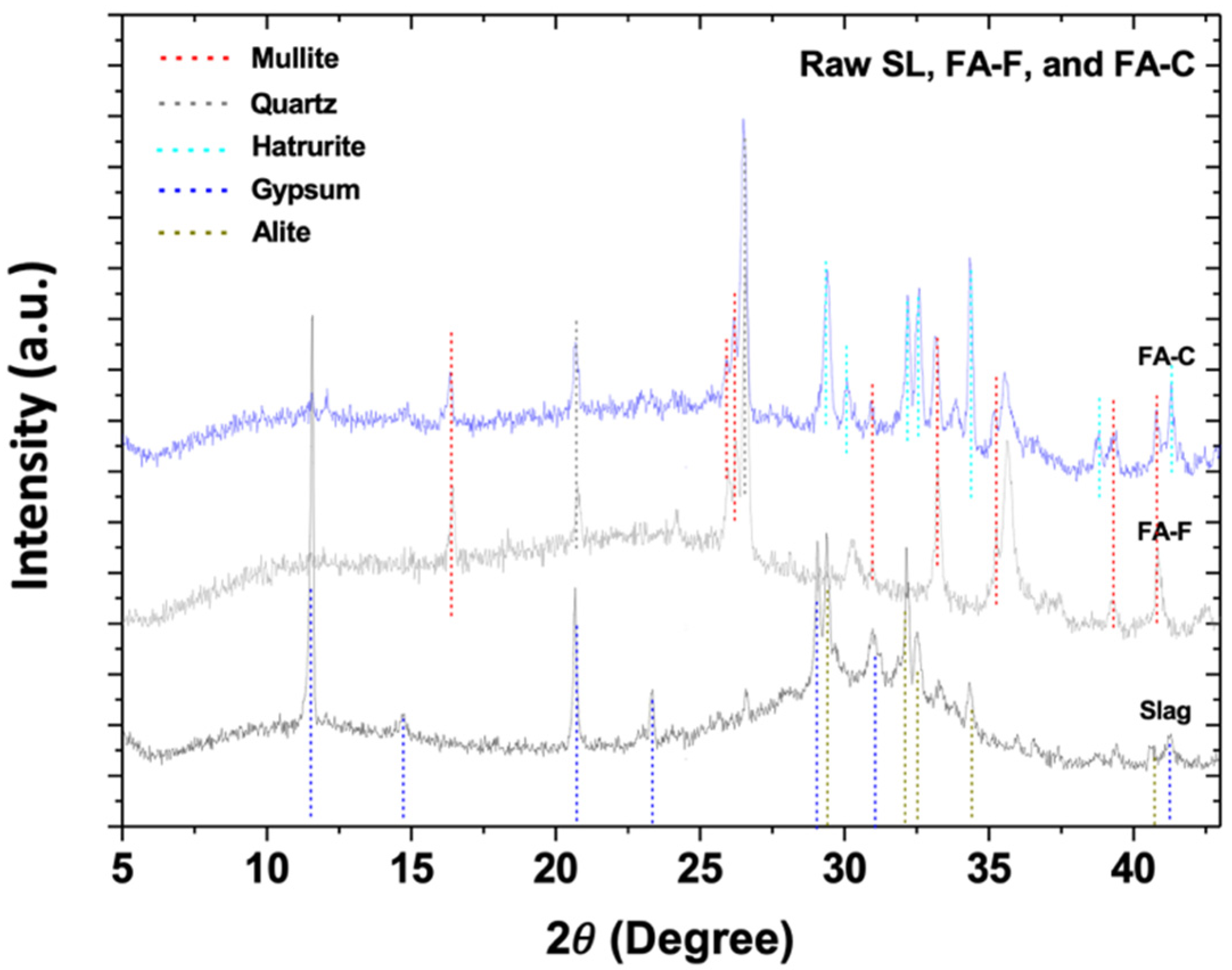
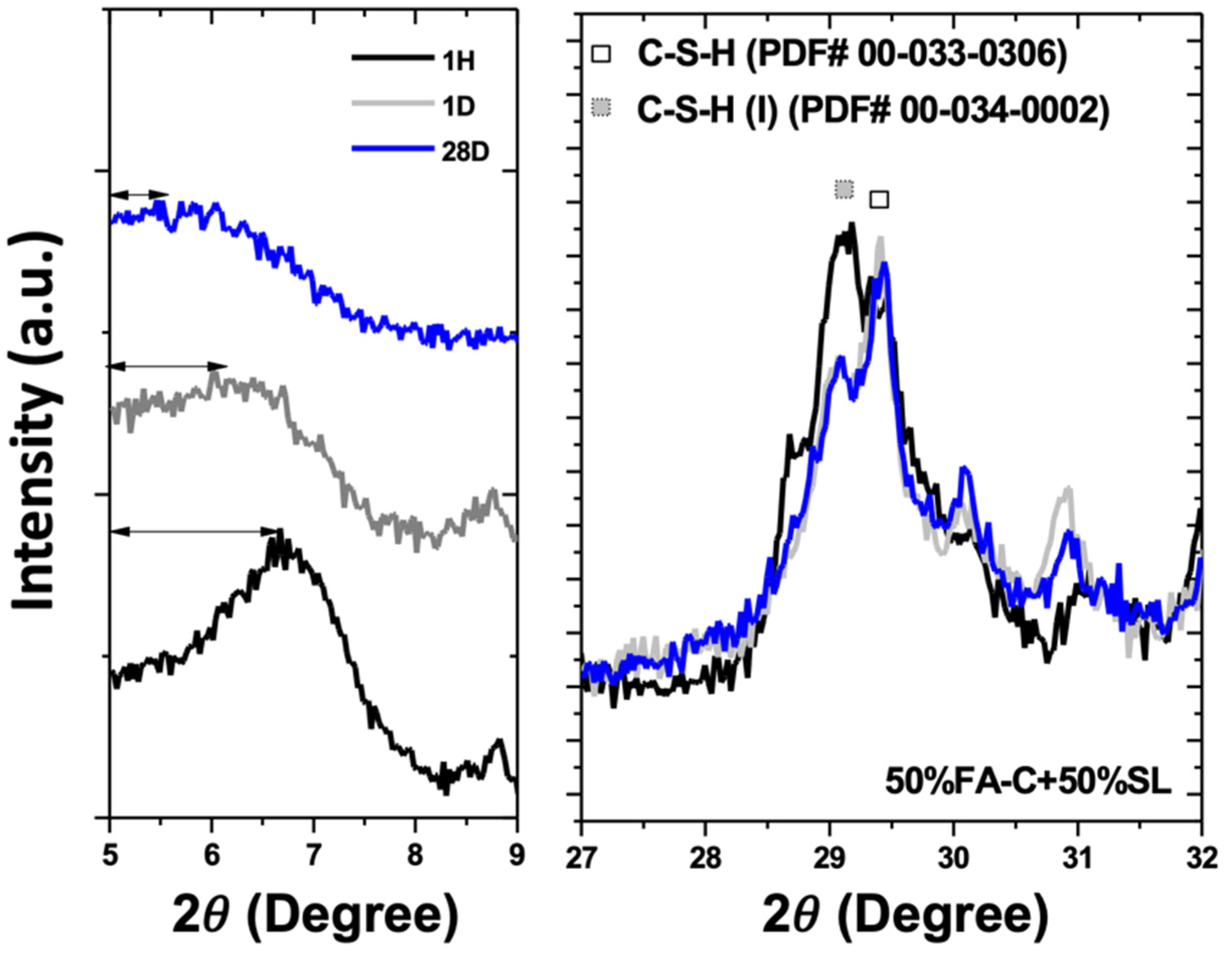
3.1. X-Ray Diffractometry
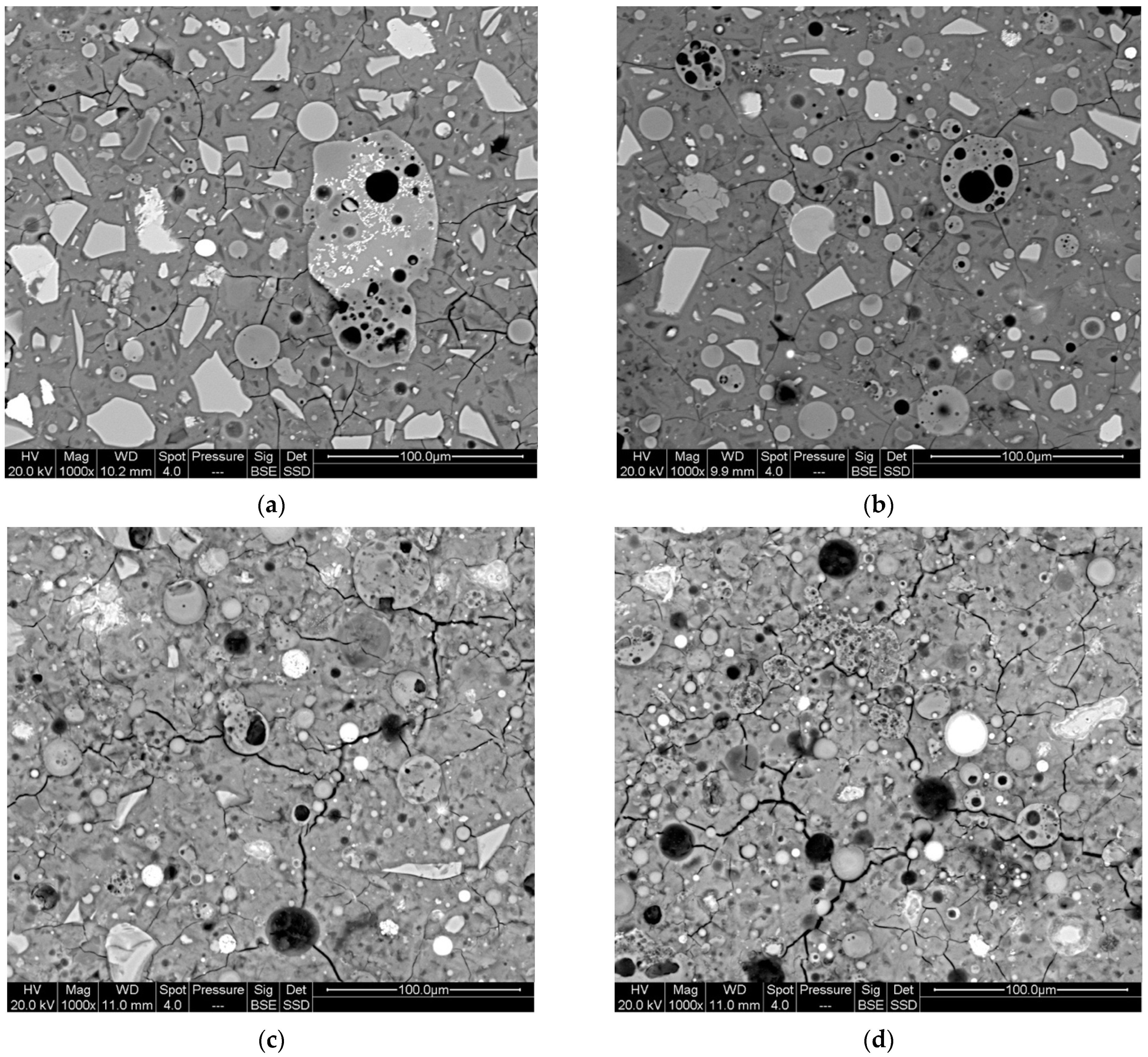
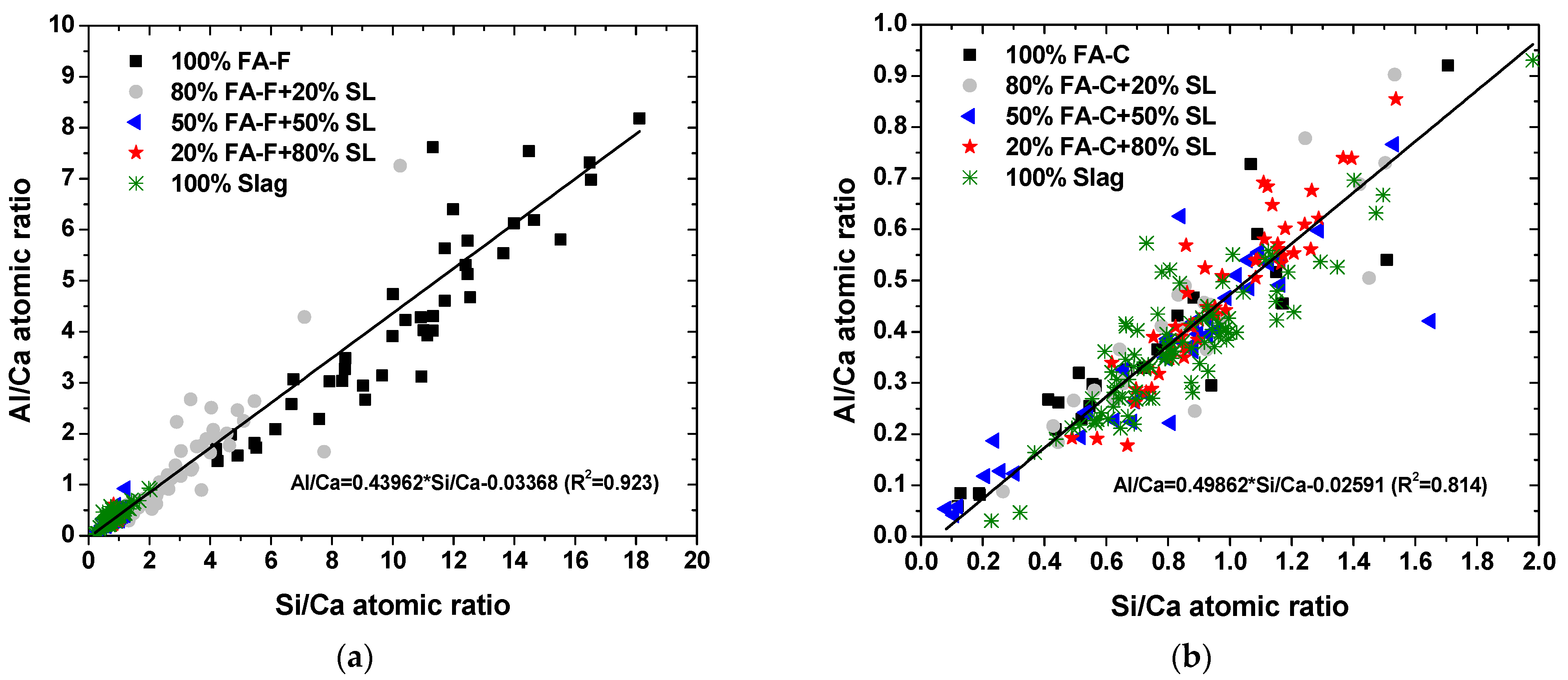
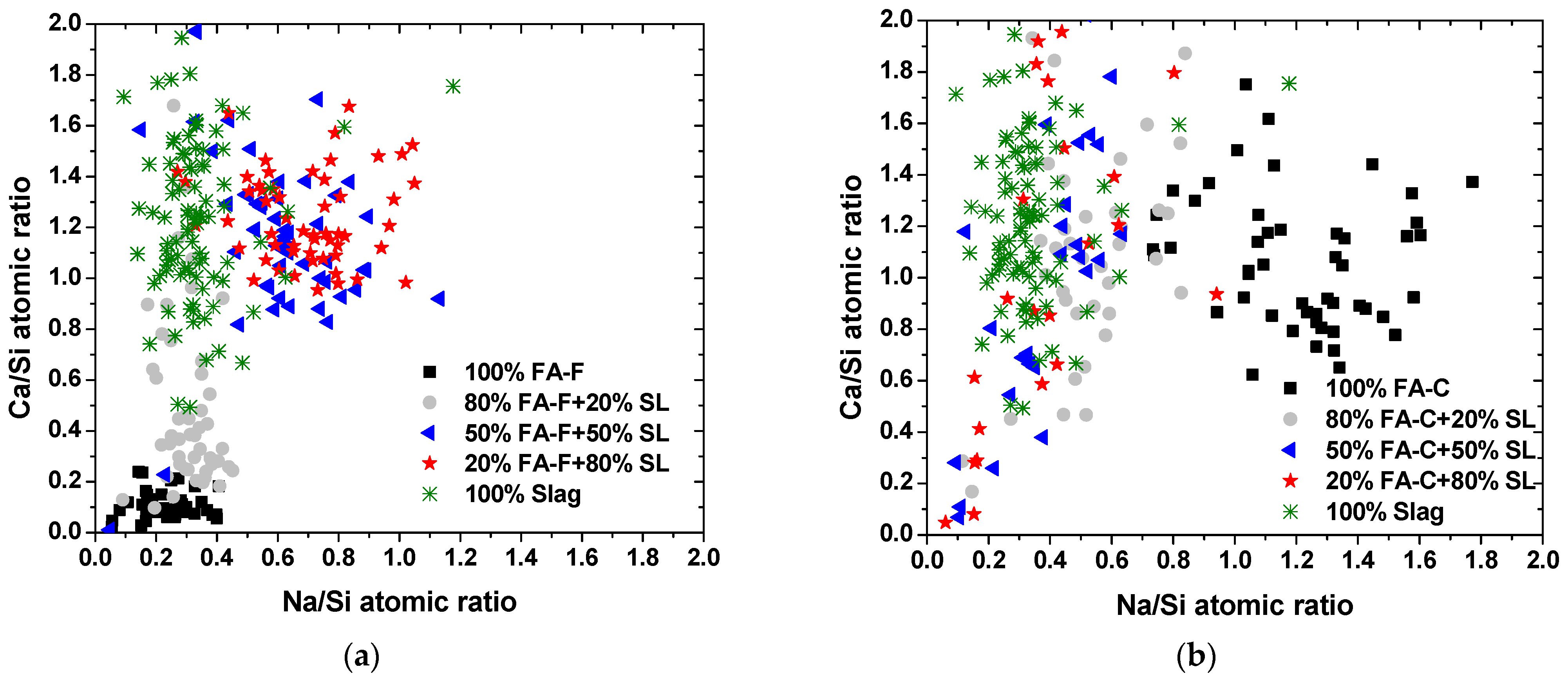
3.2. SEM-EDS Microanalysis
4. Conclusions
- (1)
- The type and composition of precipitated C-A-S-H, N-A-S-H, and N-C-A-S-H depend on the fly ash composition and slag-to-fly ash ratio.
- (2)
- There is a structural limitation on the incorporation of Al3+ for Si4+ in the tetrahedral silicate chain of C-A-S-H, N-A-S-H, and N-C-A-S-H, regardless of the starting material chemistry.
- (3)
- In an NaOH-activated hybrid slag–fly ash system, the framework structure of precipitated gels is an assemblage of aluminosilicate units of varying Ca and Na contents. The heterogeneity of composition in the microstructure could be a result of the heterogeneous spatial distribution of Ca and Na cations.
- (4)
- The C-S-H (I) formed in hydrated AAS seems to be reactive with the dissolved (calcium, alumina, silicate) species from fly ash, resulting in a gradual conversion to a distinct C-S-H with probably different compositions, nanostructures, and degree of crystallinity at an early age.
- (5)
- The low cementitious capability of alkali-activated FA-C may be attributed to the unstable N-C-A-S-H gel structure with concurrent high Na and Ca contents.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qian, J.; Shi, C.; Wang, Z. Activation of blended cements containing fly ash. Cem. Concr. Res. 2001, 31, 1121–1127. [Google Scholar] [CrossRef]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Thomas, R.J.; Ye, H.; Radlińska, A.; Peethamparan, S. Alkali-activated slag concrete: A closer look at sustainable alternatives to portland cement. Concr. Int. 2016, 38, 33–38. [Google Scholar]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vazquez, T. Alkali-activated fly ash/slag cements: Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Lee, N.; Lee, H. Reactivity and reaction products of alkali-activated, fly ash/slag paste. Constr. Build. Mater. 2015, 81, 303–312. [Google Scholar] [CrossRef]
- Juenger, M.; Winnefeld, F.; Provis, J.; Ideker, J. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Puertas, F.; Fernández-Jiménez, A. Mineralogical and microstructural characterisation of alkali-activated fly ash/slag pastes. Cem. Concr. Compos. 2003, 25, 287–292. [Google Scholar] [CrossRef]
- Liu, J.-C.; Chen, Z.; Cai, R.; Ye, H. Quantitative effects of mixture parameters on alkali-activated binder-based ultra-high strength concrete at ambient and elevated temperatures. J. Adv. Concr. Technol. 2022, 20, 1–17. [Google Scholar] [CrossRef]
- Wetzel, A.; Middendorf, B. Influence of silica fume on properties of fresh and hardened ultra-high performance concrete based on alkali-activated slag. Cem. Concr. Compos. 2019, 100, 53–59. [Google Scholar] [CrossRef]
- Cai, R.; Ye, H. Clinkerless ultra-high strength concrete based on alkali-activated slag at high temperatures. Cem. Concr. Res. 2021, 145, 106465. [Google Scholar] [CrossRef]
- Huang, L.; Liu, J.-C.; Cai, R.; Ye, H. Mechanical degradation of ultra-high strength alkali-activated concrete subjected to repeated loading and elevated temperatures. Cem. Concr. Compos. 2021, 121, 104083. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, H. Sequestration and release of nitrite and nitrate in alkali-activated slag: A route toward smart corrosion control. Cem. Concr. Res. 2021, 143, 106398. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, H. Influence of metakaolin and limestone on chloride binding of slag activated by mixed magnesium oxide and sodium hydroxide. Cem. Concr. Compos. 2022, 127, 104397. [Google Scholar] [CrossRef]
- Ye, H.; Chen, Z.; Huang, L. Mechanism of sulfate attack on alkali-activated slag: The role of activator composition. Cem. Concr. Res. 2019, 125, 105868. [Google Scholar] [CrossRef]
- Ye, H.; Yang, G.; Fu, C. Effect of dolomite filler on the sulfuric acid resistance of alkali-activated slag binders. J. Mater. Civil. Eng. 2021, 33, 04021219. [Google Scholar] [CrossRef]
- Cai, R.; Wu, T.; Fu, C.; Ye, H. Thermal degradation of potassium-activated ternary slag-fly ash-silica fume binders. Constr. Build. Mater. 2022, 320, 126304. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Sulphuric acid resistance of plain, polymer modified, and fly ash cement concretes. Constr. Build. Mater. 2009, 23, 3485–3491. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.; Lee, H. Setting and mechanical properties of alkali-activated fly ash/slag concrete manufactured at room temperature. Constr. Build. Mater. 2013, 47, 1201–1209. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Quantitative analysis of phase assemblage and chemical shrinkage of alkali-activated slag. J. Adv. Concr. Technol. 2016, 14, 245–260. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.; Jun, S.S.; Choi, S.; Clark, S.M. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Dick, W.A. Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cem. Concr. Compos. 2010, 32, 142–147. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.; Van Deventer, J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D. Compatibility studies between NASH and CASH gels. Study in the ternary diagram Na 2 O–CaO–Al 2 O 3–SiO 2–H 2 O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A.; Macphee, D.E. Effect of calcium additions on N–A–S–H cementitious gels. J. Am. Ceram. Soc. 2010, 93, 1934–1940. [Google Scholar] [CrossRef]
- Provis, J.L.; Myers, R.J.; White, C.E.; Rose, V.; van Deventer, J.S. X-ray microtomography shows pore structure and tortuosity in alkali-activated binders. Cem. Concr. Res. 2012, 42, 855–864. [Google Scholar] [CrossRef]
- Xiao, R.; Jiang, X.; Zhang, M.; Polaczyk, P.; Huang, B. Analytical investigation of phase assemblages of alkali-activated materials in CaO-SiO2-Al2O3 systems: The management of reaction products and designing of precursors. Mater. Des. 2020, 194, 108975. [Google Scholar] [CrossRef]
- Winnefeld, F.; Leemann, A.; Lucuk, M.; Svoboda, P.; Neuroth, M. Assessment of phase formation in alkali activated low and high calcium fly ashes in building materials. Constr. Build. Mater. 2010, 24, 1086–1093. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Fly ash-slag interaction during alkaline activation: Influence of activators on phase assemblage and microstructure formation. Constr. Build. Mater. 2016, 122, 594–606. [Google Scholar] [CrossRef] [Green Version]
- Haha, M.B.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Escalante-García, J.I.; Fuentes, A.F.; Gorokhovsky, A.; Fraire-Luna, P.E.; Mendoza-Suarez, G. Hydration products and reactivity of blast-furnace slag activated by various alkalis. J. Am. Ceram. Soc. 2003, 86, 2148–2153. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage mechanisms of alkali-activated slag. Cem. Concr. Res. 2016, 88, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Taylor, H.F. Cement Chemistry; Thomas Telford: London, UK, 1997. [Google Scholar]
- Renaudin, G.; Russias, J.; Leroux, F.; Cau-dit-Coumes, C.; Frizon, F. Structural characterization of C–S–H and C–A–S–H samples—Part II: Local environment investigated by spectroscopic analyses. J. Solid State Chem. 2009, 182, 3320–3329. [Google Scholar] [CrossRef]
- Fu, C.; Ye, H.; Zhu, K.; Fang, D.; Zhou, J. Alkali cation effects on chloride binding of alkali-activated fly ash and metakaolin geopolymers. Cem. Concr. Compos. 2020, 114, 103721. [Google Scholar] [CrossRef]
- Garbev, K.; Beuchle, G.; Bornefeld, M.; Black, L.; Stemmermann, P. Cell dimensions and composition of nanocrystalline calcium silicate hydrate solid solutions. part 1: Synchrotron-based x-ray diffraction. J. Am. Ceram. Soc. 2008, 91, 3005–3014. [Google Scholar] [CrossRef]
- Richardson, I. Tobermorite/jennite-and tobermorite/calcium hydroxide-based models for the structure of CSH: Applicability to hardened pastes of tricalcium silicate, β-dicalcium silicate, Portland cement, and blends of Portland cement with blast-furnace slag, metakaolin, or silica fume. Cem. Concr. Res. 2004, 34, 1733–1777. [Google Scholar]
- Richardson, I. The nature of CSH in hardened cements. Cem. Concr. Res. 1999, 29, 1131–1147. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.t.; Alastuey, A.; Hernández, E.; Lopez-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]





| d-Spacing in This Study | Reacted Phase | Typical d-Spacing | Theoretical Value | (h k l) |
|---|---|---|---|---|
| 14.47–14.48 | Sodium Aluminosilicate Hydrate (Na2Al2Si2.5O9·6.2H2O) | - | 14.47 | (1 1 1) |
| 3.81 | - | 3.81 | (5 3 3) | |
| 12.83–13.18 | C-S-H (I) (CaO·SiO2·H2O) | 11.48–11.59 [35] a, 12.50–12.57 [24] b | 12.50 | (0 0 2) [37] |
| 3.06–3.07 | 3.07–2.97 [35] a | 3.07 | (1 1 *) [37] c | |
| 2.78–2.80 | 2.79–2.82 [24] b | 2.80 | (2 0 *) [37] c | |
| 1.83–1.84 | 1.83–1.84 [35] a | 1.83 | (0 2 *) [37] c | |
| 9.64–9.80 | Ettringite (Ca6Al2S3O50H64) | - | 9.72 | (1 0 0) |
| 5.61–5.62 | - | 5.61 | (1 1 0) | |
| 4.99–5.00 | - | 4.97 | (−1 −1 2) | |
| 4.70 | - | 4.70 | (1 0 4) | |
| 3.88 | - | 3.88 | (−1 −1 4) | |
| 3.24–3.25 | - | 3.24 | (3 0 0) | |
| 7.72–7.70 | Hydrotalcite-type Phase (Mg6Al2(CO3) (OH)16·4H2O) | 7.63–7.70 [35] | 7.84 | (0 0 6) |
| 3.86–3.87 | 3.88–3.82 [35] | 3.91 | (0 1 8) | |
| 2.60–2.64 | 2.59–2.56 [35] | 2.61 | (0 2 4) | |
| 2.29–2.31 | 2.28–2.31 [35] | 2.31 | (0 2 10) | |
| 3.03–3.04 | C-S-H (Ca1.5SiO3.5·xH2O) | - | 3.04 | - |
| 1.82 | - | 1.82 | - | |
| 7.13–7.14 | Sodium Aluminosilicate Hydrate (Na5Al5Si5O20·9H2O) | - | 7.11 | (1 1 0) |
| 6.67–6.78 | - | 6.68 | (0 0 1) | |
| 4.87–4.88 | - | 4.87 | (1 1 1) | |
| 3.14 | - | 3.14 | (2 2 1) | |
| 4.15–4.16 | Sodium Aluminum Silicate (NaAl(SiO4)) | - | 4.15 | (0 2 1) |
| 4.14 | - | 4.14 | (2 2 0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Jin, Y. Phase Analysis of Alkali-Activated Slag Hybridized with Low-Calcium and High-Calcium Fly Ash. Sustainability 2022, 14, 3767. https://doi.org/10.3390/su14073767
Jiang T, Jin Y. Phase Analysis of Alkali-Activated Slag Hybridized with Low-Calcium and High-Calcium Fly Ash. Sustainability. 2022; 14(7):3767. https://doi.org/10.3390/su14073767
Chicago/Turabian StyleJiang, Tao, and Ying Jin. 2022. "Phase Analysis of Alkali-Activated Slag Hybridized with Low-Calcium and High-Calcium Fly Ash" Sustainability 14, no. 7: 3767. https://doi.org/10.3390/su14073767






