Abstract
Temperature and moisture are essential factors in germination and seedling growth. The purpose of this research was to assess the germination and growth of wheat (Triticum aestivum L.) seeds under various abiotic stressors. It was conducted in the Agronomy Institute of the Hungarian University of Agriculture and Life Sciences, Gödöllő, Hungary. Six distinct temperature levels were used: 5, 10, 15, 20, 25, and 30 °C. Stresses of drought and waterlogging were quantified using 25 water levels based on single-milliliter intervals and as a percentage based on thousand kernel weight (TKW). Seedling density was also tested. Temperature significantly influenced germination duration and seedling development. 20 °C was ideal with optimal range of 15 °C to less than 25 °C. Germination occurred at water amount of 75% of the TKW, and its ideal range was lower and narrower than the range for seedling development. Seed size provided an objective basis for defining germination water requirements. The current study established an optimal water supply range for wheat seedling growth of 525–825 percent of the TKW. Fifteen seeds within a 9 cm Petri dish may be preferred to denser populations.
1. Introduction
Wheat is the predominant grain crop and a mainstay source of food for most of the world’s population [1]. This field crop is nutritionally essential globally, ranking second only after maize production [2]. It is involved in many bakery and pastry industries [3]. It is commonly utilized to produce flour, malt, and various food products, including bread, pasta, and morning cereals [2]. Wheat is a primary source of protein and starch, providing 20–30% of daily caloric intake in most societies [4]. Because it is widespread many different ecosystems, plants face various abiotic challenges, such as drought and rising temperature, due to global warming, which results in huge yield loss [5,6].
Germination is a physiological mechanism in which sequential biological and biochemical activities occur to initiate and develop a seedling [7,8]. It is a critical stage in the life of wheat plants, as it determines seedling establishment and the effective use of available nutrients and water resources [9,10]. It is regulated by the interaction of the surrounding environmental conditions, the seeds’ physiological state, and the germ [11]. Temperature, light, pH, water availability, and soil moisture most affect seed germination among abiotic factors [12]. Seeds have a specific range of environmental requirements necessary for optimal germination [13]. The success of propagation depends on the seeds’ physiological response towards overlapping various external abiotic factors. Therefore, seed germination reflects inhabitants’ size, abundance, and distribution [14,15,16].
Seed germination begins with water adsorption by the dormant dry seed and is accomplished by the commencement of radicles by lengthening the embryo’s axis [17]. A sequence of ordered morphogenetic and physiological mechanisms, including energy transfer, nutrient intake, and physiological and biochemical changes, are part of this process [14,16,18]. A seed’s water adsorption is a three-stage procedure, with phase I being a fast initial uptake, phase II being a plateau, and phase III being a rise in water adsorption, but only with the initiation of germination [19]. Germination phase I, called imbibition, the rapid water take-up, results in softening and swelling the seed coat close to or at an ideal temperature [20]. Seed coat rupturing permits the emergence of radicle and shoot. The seed’s physiology is then activated, and it begins to respire [18,19]. Primary germination indicators include re-establishing critical processes, including translation and DNA repairing, followed by cell expansion and division [16,20]. Physically germination has a two-sequent-stages mechanism: endosperm rupture, and the evolving radicle follows the breakdown of the micropylar endosperm [14,21,22]. Enzymatic activity to degrade carbohydrate and lipid for reserved materials mobilization is essential in germination and early seedling development [23,24,25]. The biological, bio-chemical enzymic activity in these stages is highly affected by temperature and water availability [26].
Seed germination necessitates the presence of water. It hydrates the crucial processes of protoplasm, supplies dissolved oxygen, softens the seed’s outer layer, and improves permeability [18,26]. Water stress harms germination percentage [5,27,28]. The water level has more complicated germination effects for wheat since it is the primary determinant for seed imbibition and germination. Water contributes to the subsequent germination metabolic phases [25,29]. It is vital for seed enzyme activation, breakdown, translocation, and endospermic stored materials [29,30]. Water stress declines enzymic activities that cause adverse effects on the metabolic process of carbohydrates, decrease the water potential and the amount of soluble potassium and calcium, and cause changes in seeds hormones [31,32].
The temperature has a major impact on specifying the germination time duration [32]. The temperature effect on germination can be described as a substantial temperature scale: minimal, optimal, and maximum temperatures to initiate [33]. The optimum temperature results in the most significant germination rate (%) in the shortest time. Each germination stage has its substantial temperature; due to the intricacy of the germination process, the temperature response may vary throughout the germination phases. The seeds’ response to temperature relies on variety, seed quality, time from harvest, and some other factors [34]. Many studies regarding the regulation of germination by temperature have been conducted. Riley (1981) demonstrated that the energy state of cells and the behavior of certain enzymes change as the temperature changes [35]. The ATP content and protein synthesis rate increase as the temperature reaches the optimum and decreases as it goes either way [19,35]. The intra-equilibrium of reactive oxygen species indispensable for seed germination is hindered by abnormal respiration [12,35,36]. The antioxidant system highly affected wheat seed germination [37,38].
Drought, thermal, and drought + thermal stresses significantly impact the yield and quality more than each separately. For example, wheat yield losses in literature were 57% after drought stress, 76% after drought + thermal stresses, and only 31% after thermal stresses [39].
This study was conducted to investigate the performance of wheat seed germination ability under different moisture conditions, temperatures, and seed and seedling densities per Petri dish. A fundamental understanding of germination is vital to the field of crop production. The objectives of this study were: the first was (1) to determine the optimal range of water amount for germination based on water volume of single-milliliter intervals and water quantity as percentages based on thousand kernels weight (TKW). This method was used to investigate the null hypothesis “The difference in seed weight and size has no role in germination when the amount of water is constant”. Therefore, part of this pre-experiment is to prove the alternative hypothesis’ validity, namely that “the water requirements of the seeds differ depending on their size and weight”. Theoretically, larger seeds need more water to germinate compared to smaller seeds. Therefore, if the alternative hypothesis is correct, it will be a more accurate method to find the optimal needed amount of water for germination, and the second was (2) to determine the effects of germination temperatures and germination time of wheat seeds. The third objective was (3) to investigate the effect number of seeds and seedling density in a Petri dish on germination percentage and open the Petri dish’s top cover. Opened Petri dish has various side effects of moisture losses and exposure to contamination. This part was designed to answer the question of “Do the number of seeds and the density of seedlings affect the germination percentage and viability of seedlings under the same water quantity?”. This research was conducted following the standard of the NO. 48 of 2004 (IV.21.) of the Ministry of Agriculture and Rural Development concerning the production and marketing of seed of agriculture crop species, Hungary, which following the International Seed Test Association (ISTA), the circumstances was changed according to our research questions.
This study yields critical information on germination demands that can evaluate tolerance to ranges of drought and temperature stresses.
2. Materials and Methods
The present study was conducted to determine the temperature and drought effect on germination vigor and seedling growth. The seeds of a regionally commonly grown variety of Hungarian wheat named Alfold 90 were obtained from a grower and used. It is a high winter tolerance wheat variety with 75–95 cm plant height, with awed spikes. Alfold 90 is sensitive to powdery mildew disease and total tolerance to stem rust disease. It is an early Hungarian variety and cultivates in dry regions. It was conducted in the Agronomy Institute/the Hungarian University of Agriculture and Life Sciences, 2100 Gödöllő, Hungary. Highly sensitive and highly accurate incubators made by Memmert, Schwabach, Germany, with natural convection or forced air circulation and double doors (interior glass, exterior stainless steel) for a clear view without a drop in temperature were used. The germination capacity can be 100%, and it declines for several factors, i.e., storage time and conditions, but it was over 98% for the used stock. These seed characterizations are: TKW is 39–44 g, test weight 78–82 kg, protein content 14–17%, wet gluten content 34–40%, and the farinograph value is 80–90%, which fall into the A2 category. Alfold 90 is described as a drought-tolerant wheat variety. The study was subdivided into three experiments conducted in the following subsections.
2.1. Temperature Experiment
This study compares wheat seed germination under six distinct temperatures, 5, 10, 15, 20, 25, and 30 °C. After Petri dishes were labeled, 20 seeds of wheat were placed in each and exposed to an identical amount of distilled water, 5 mL. The electrical conductivity of distilled water was measured 1.5 mhos/cm. Every day, four Petri dishes of each temperature level were subjected to physical measurements. The measured parameters were the number of not-germinated seeds and radicles and the first proper leaf lengths, termed a shoot in this research. The germination stander measurement is that 80% of the seedlings in Petri dishes reach the length of 1 cm.
2.2. Water Quantity Experiment
Wheat seeds were treated to a total of 25 distilled water levels, 12 levels based on milliliter intervals, and 13 levels based on TKW in sterile Petri dishes of 9 cm diameter. The Petri dishes were lined with sterile filter paper, Table 1. TKW was determined using a seed counter machine, which was 42.76 g.
TKW × Seed n/100,000 = 1% of the propose quantity of water
42.76 × 20 = 855.2, 855.2/100,000 = 0.008552
Table 1.
Treatments of water levels experiment based on the two water basses, a single-milliliter interval and TKW.
Table 1’s outcome represents 1% water quantity of TKW [40]. This number was multiplied by the proposed percentage for each water treatment, as presented in Table 1. Twenty seeds were placed in each labeled Petri dish with five replications. This experiment was implied under a constant incubation chamber temperature level of 20 °C. All seedlings’ radicle and shoot lengths were physically measured after ten days. After labeling, these radicles and shoots for each Petri dish were then exposed to 65 °C for 48 h in an oven. Dry weights were acquired by weighing the radicles and the shoots of the 20 seedlings of each treatment replicate.
2.3. Seed Density Experiment
This part was designed to study the seed’s number effect on the germination performance under the same quantity of water. Three sets of seeds, 15, 20, and 25 per P.D, were placed in Petri dishes. First, they were treated with 6 mL of distilled water. Ten replications were implied to reduce the experimental error of the experiment and boost the accuracy. Then, they were incubated at a constant temperature of 20 °C in a controlled germination chamber. The measured traits were categorized into four groups: number of seeds that were not germinated, number of germinated seeds with the only radicle, seedling with short shoots (less than 4 cm), and normal seedling. The normal seedling term refers to the morphological stage compared to the other in the same condition: the seedling with taller shoots than 4 cm. These categories were made to achieve an aggregated value according to Equation (2) [40]:
where: NOT-G = not-germinated seeds, RO = the number of germinated seeds with only a radicle, S.P. = the number of germinated seeds with short shoots, and NP = the number of germinated seeds with the normal shoot.
Aggregated value = (NOT-G × 0) + (RO × 0.33) + (SP × 0.67) + (NP × 1)
2.4. Statistical Analysis
The statistics reported were presented as a mean value for each treatment of the experiment. For the water amount experiment, analysis of variance (ANOVA) and Fisher’s test of least significant differences was utilized to determine significant differences at a 5% probability level (GenStat 12th edition, PL20.1 m, the United Kingdom). In addition, To fit the obtained data and depict the best fit of the temperature levels, a sigmoid curve model was made (JMP Proc 13.2.1 of SAS, Canberra, the United States, and Microsoft Excel 365). Normality check was conducted using Kolmogorov–Smirnov and Shapiro–Wilk by SPSS version 27, IBM, New York, NY, USA.
3. Results
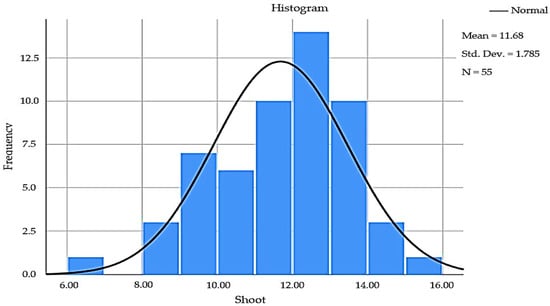
Based on the statistically computed data (Table 2), as the Kolmogorov–Smirnov and Shapiro–Wilk are greater than 0.05, it can be stated that the normality curve is symmetric [41,42], as presented in the histogram (Figure 1).
3.1. Temperature Experiment
3.1.1. Germination Duration
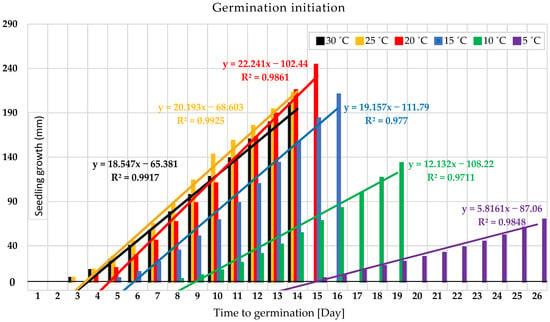
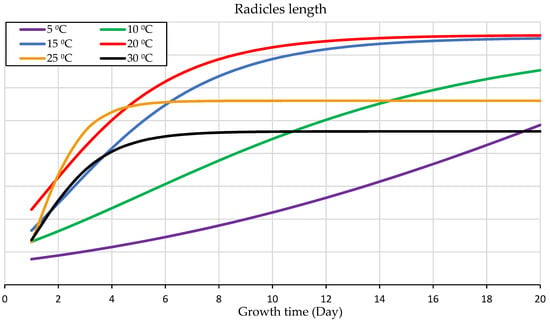
As determined in the temperature gradient experiment, 30 °C and 25 °C, which rapidly initiated germination, were faster to reach the standard measurement point in this study of 1 cm shoot length in 48 h, Figure 2. Under 20 °C, that took longer for its germinated shoots to reach 1 cm in the temperature gradient experiment, three days after subjecting the seed to the treatment. Figure 2 illustrates that wheat seeds germinated under a constant temperature of 15 °C, which needed five days to reach the shoot measurement stander, followed by a temperature of 10 °C that needed a more prolonged duration up to 8 days in the temperature gradient experiment. Seed germination under 5 °C took the most prolonged duration to initiate germination. Germinated shoot reached the length of 1 cm after 15 days of the planting date (Figure 2). The percentage differences within the germination range between the two limits were 152.941%. Regardless of 5 °C and 10 °C, the temperatures of 20 °C, 25 °C, and 30 °C have minor margin differences in germination duration, followed by 15 °C, which took slightly longer.
Figure 2.
Germination duration from planting (day 1) to germination initiation and seedling development under different temperatures.
Temperature is critical in determining the duration of germinating [43,44,45]. The findings indicate that seeds accumulate the heat units from the thermal energy they absorbed, and once they reach the necessary level to initiate metabolic activity, germination begins at a pace dependent on the ambient temperature condition.
3.1.2. Seedling Development
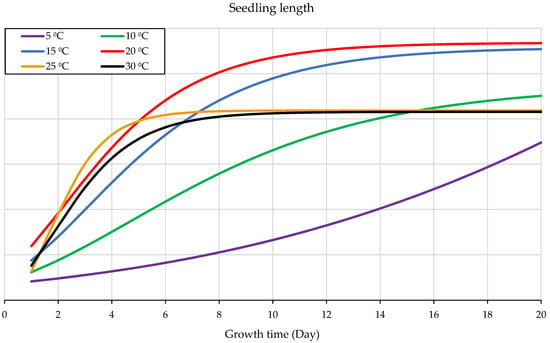
The experiment of the temperature gradient of 6 distinct constant temperatures, 5 °C, 10 °C, 15 °C, 20 °C, 25 °C, and 30 °C, presented that seedling growth under 20 °C gave the most outstanding performance and the most salient rate of growth (Figure 3). It was the most favorable growth temperature for seedling growth with a y-value: 22.241x − 102.44 (Figure 2). Similarly, with slightly lower development performance under 15 °C, y-value: 19.157x − 111.79. The temperature of 10 °C shows the same pattern, but with much slower development, y-value: 12.132x − 108.22. The experiment under 5 °C showed the most negligible seedling growth and required a longer time for development, y-value: 5.8161x − 87.06 (Figure 2). Slightly identical performance was shown under 25 °C and a higher temperature level when they grow quicker in the very early stage of seedling development, followed by slowing the rate of the development (Figure 3). It indicates that each stage of wheat seedling development needs a different temperature. This figure illustrates that the upper germination range of 25 °C and 30 °C gives quicker germination and early seedling growth within less than five days, but not for the subsequent seedling growth phase. Thus, the optimum range for seedling growth is around 20 °C with a greater growth rate followed by 15 °C (Figure 3).
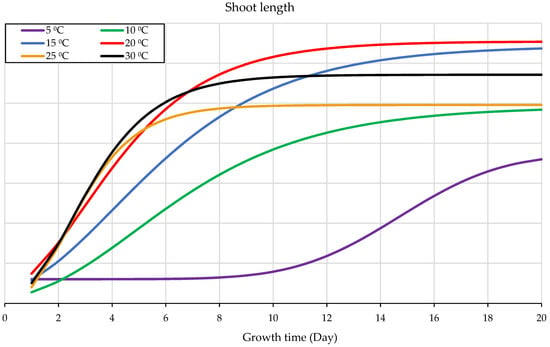
The seedling’s shoots and radicles showed the same development performance at and close to the optimal range of temperature; however, their growth pattern differs when the indicator arrow of the temperature crosses the optimum limits of growth range tails (Figure 4 and Figure 5). The shoot formed and developed better, especially in the very early growth stage, than the radicle under a temperature scope higher than the optimum range (Figure 4). However, the radicle has a distinct growth pattern and need for temperature compared to the shoot because it grows better at temperatures lower than the optimum range, particularly in the later stage than the shoot (Figure 5).
The highest growth and development of the shoots occurred at a temperature of 20 °C, and the least occurred at 5 °C. Therefore, the optimal temperature for the shoot and radicle development is at 20 °C, followed by 15 °C. When the temperature changes, either the shoots or the radicles will be more impacted than the other. The radicle is more resistant to cold than the shoot, and vice versa; the shoot is more resistant to temperatures over the ideal threshold than the radicle.
3.2. Water Quantity Experiment
Wheat seed quality parameters such as stress tolerance, uniformity, and germination rate can be determined using water absorption and temperature analysis. Availability of moisture reflects severe limitations on seed germination of Triticum astivum L. [39,40]. Therefore, seeds of the crop with stringent requirements for germination can be more effectively felicitously established than crop seeds with fewer constraints [46].
The water quantity experiment was carried out on two bases: single-milliliter intervals 0–12 mL and as percentages of TKW to develop a technique for comparing various wheat varieties with varied TKW in a future experiment. Furthermore, the two bases of water quantity application were compared and investigated simultaneously in parallel, which has a better reflectance representing water demands. Table 3 reveals significant variations among the water quantity potentials 0–12 mL for all the examined parameters: the number of non-germinated seeds, length of the radicle, length of shoot, seedling length in total, dry weight of the seedlings, seedlings’ corrected dry weight that was accomplished by subtracting the ungerminated seeds, dry weight of the radicles, and dry weight of shoots.
Table 3.
Parameters relating to germination and seedling characteristics of Triticum aestivum L. seeds respond to the application base’s water potential of single-milliliter water quantity intervals.
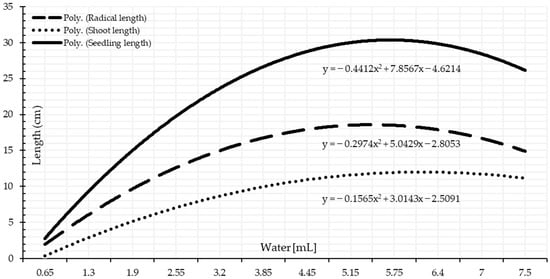
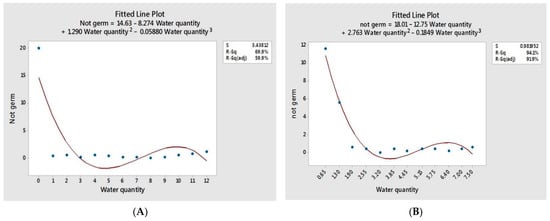
Germination percentage increased significantly as water quantity increased up until the potential water quantity, followed by a slight decrease as the water quantity level raised due to waterlogging, as presented in Table 3, Figure 6 and Figure A1. A similar pattern was seen when water was applied according to the TKW with statistically significant differences, Table 4 and Figure 7. Seeds of the wheat crop can be germinated under a minimal water quantity, 0.65 mL, Table 4, which represents 75% of TKW, Table 1. As water is essential for germination, seeds can germinate moistures near the wilting threshold point to activate and stimulate germination metabolic processes. The wheat seeds require internal 40% moisture to germinate [46]. If the interior moisture content were less than the critical moisture content limit, wheat seeds would not germinate. Under 0.65 mL, over half of the seeds germinated. Thus, water application based on the TKW enables better knowledge of the limitations and optimal water requirements since seed size is critical for attaining the 40% internal seed moisture level. These findings corroborate research conducted by [47], stating that “seed size has importance in predicting germination under stress conditions”. The optimal range for germination is 4.45–7.00 mL, Figure 7, representing 525–825% of the TKW.
Table 4.
Parameters relating to germination and seedling characteristics of Triticum aestivum L. seeds respond to the water potential of the TKW base.
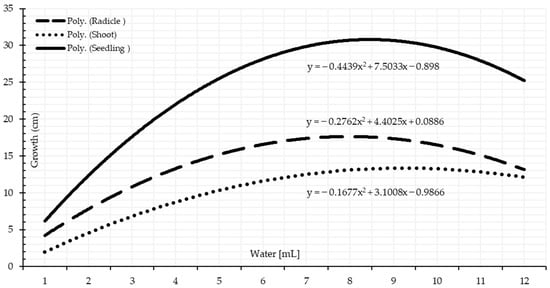
The germination test, nearly always coupled with vigor tests, such as a seedling growth test, is one of the most remarkable seed quality and seedling performance tests [48,49]. There were significant differences among the seedling performance depending on supplied moisture percentages. Under a low water quantity, the seedling length presents the least mean values, and their performance statistically significantly rose as the quantity of water increased Table 3 and Table 4. The optimal range of moisture amount starts from 3.85 mL, representing 450% of TKW, Table 4. Parallel to this, significant seed length performance started under 5 mL according to water quantity applied based on single-milliliter intervals. It implies that moisture percentages calculated using TKW are more precise, consistent, and reliable. Figure 6 and Figure 7 show that the optimal water amount range is approximately 4 to 9 mL on both water supplement bases. As a result, water stress significantly lowers seedlings’ vigor; however, seedling length augmented significantly at the launching point of the optimum range.
The statistical analysis of variance revealed significant variations among the applied water quantities on each radicle length and shoot length. The radicles length progressively significantly increases as the applied water quantity increases, Table 3 and Table 4. There is an optimum range for wheat radicle development, which gradually decreases as the water quantity increases than the upper limit. Shoot growth followed a different pattern than the radicle. As a result, it has the sense to measure the whole seedling, Figure 6 and Figure 7. Water stresses of drought and waterlogging possess a greater impact on the radicles than the shoots.
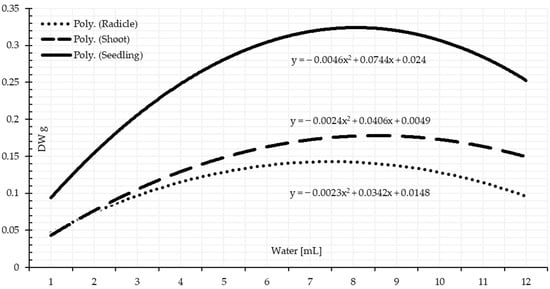
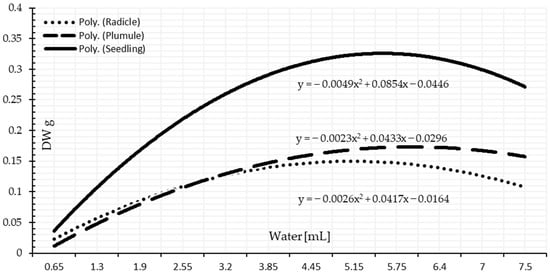
The dry weight test is another laboratory test to determine the vigor of the seeds and drought tolerance of the seedlings. Significant differences among the amount of accumulated dry matter of the seedlings based on the availability of water (Table 3 and Table 4). The dry matter increased gradually as the quantity of supplied water increased than the lower limit of the optimal range. Under drought stress, the creation of the dry matter is expressed on a chronological basis by accumulating the required amount to make a unite of dry matter. More water quantity had no beneficial influence on increasing the dry matter accumulation (Figure 8 and Figure 9). Seedlings under more water supplements over the threshold range are marginally adversely impacted by surplus water compared to the required quantity. This extra water reduces the rate of dry matter accumulation in the seedlings of wheat. As the data demonstrates, a shoot-accumulated dry matter unit demands more water than a unit of radicle-accumulated dry matter. This finding corroborates the one published by [47]. Water supply had a significant effect on the proportion of seeds germinating, the rate of seedling development, and the accumulation of dry matter: The greater the degree of hydro stress, the greater the decline in these parameters’ levels. Hydrological constraints and potential ranges exist at each germination and seedling development stage, which is in line with a study by [49].
Figure 8.
Seedling growth based on dry matter in response to the water amount based on 1 mL intervals.
3.3. Seed Number Experiment
The ANOVA revealed no significant differences among germination percentages of the seedling densities corresponding to the aggregated values of 15, 20, and 25 seeds of wheat in a Petri dish, Table 5. In addition, the subsets of the aggregated values presented no significant variations: seedlings with normal radicles, seedlings with short shoots, seedlings with only radicles, and the portion non-germinated seeds. Therefore, because increasing seedling density beyond the optimal has an opposite impact by the opening of Petri dishes lids, Figure 10, and there were no significant variations among used densities, 15 seeds per Petri dish are more appropriate than higher densities for wheat germination experiments in vitro.
Table 5.
Parameters relating to germination and seedling characteristics of Triticum aestivum L. seeds respond to the number of seeds per Petri dish.
4. Discussion
4.1. Temperature Experiment
4.1.1. Germination Duration
Temperature is critical in regulating the length of germination duration [50,51]. The findings suggest that the crop seeds accumulate heat units from the thermal energy they absorb [52]. Once they reach the required level to commence the intracellular metabolic activity, the germination process activates at a pave depending on the ambient temperature condition [53,54]. Following the temperature gradient sub-experiment, the temperature range of 25 to 30 °C commenced germination initiation with no significant variation in the time necessary to achieve the same germination threshold and statistically followed by 20 °C, Figure 2. Seeds subjected to temperature level of 15 °C needed more time to initiate germination. However, moderate temperature of roughly 15–25 °C is demanded to commence germination of wheat seed. Changes in the state of the cell’s energy supply and enzyme activity occurred, and protein synthesis was severely curtailed when the temperature rose over this threshold [35,55]. At 10 °C, germination needed a more extended time, nine days from the commencement of treatment, as validated by other investigations [56,57]. To achieve the standard measurement point of germination at 5 °C, the wheat seeds required an extended time of up to 15 days, Figure 2. According to the literature, the minimum limit of germination temperature for wheat seeds is 4 °C [56]. According to the same research, wheat seeds could not initiate germination over 37 °C. Seed can tolerate fluctuating temperature at or near the upper limit, but under constant temperature, lower temperature, suggesting 30 °C by this current research, is necessary to commence germination. Temperatures of the ideal range of germination 15° to 25 °C resulted in minor changes in germination time and internal biological activity. Although 30 °C can initiate germination rapidly, it has a reverse effect on metabolic activities in the following germination process.
4.1.2. Seedling Development
The most salient performance values and the most outstanding seedling development rate appeared at 20 °C compared to the other tested constant temperatures (Figure 2 and Figure 3). Sigmoid curves, Figure 3, demonstrate that seedling development under 15 °C followed a similar pattern to that at 20 °C, but with a slightly lower growth rate. However, the seedling development under more than 25 °C had a reverse effect on seedling development, which is in line with other studies [57,58]. There are discrepancies with other research that reported a broader optimal range owing to day–night temperature fluctuations. However, this present research presents the accumulation of the temperature in vitro at a constant temperature, which explains the narrower. For the same reason, seedling development needed a longer time with a lower growth rate. Although nearly identical development pattern to that of the 10 °C was presented at 25 °C and 30 °C, nonetheless, it was far faster (Figure 3); it is because of the reverse impact of the high temperature on enzymes activity, protein synthesis, and biochemical energy content increased and ROS formation, not because of the temperature accumulation [59]. The slightly analogous of seedling development under 25 °C and 30 °C presents that their growth rate was rapid in early stages but slowed in later stages (Figure 3). It indicates that each stage of wheat seedling development needs a different temperature. This figure illustrates that the upper germination range of 25 °C and 30 °C gives quicker germination and early seedling growth within less than five days, but not for the subsequent seedling growth phase. In brief, the best fitting starting point for seedling growth is 20 °C with a high development rate (Figure 3).
The shoot and radicle have comparable development patterns within and around the ideal temperature range, but their growth behavior differs when the temperature rises or lowers from this range (Figure 4 and Figure 5). The shoot developed better than the radicle when the temperature is somewhat higher than the ideal range, particularly on the early growth seedling stages (Figure 4). Radicle development has a specific pattern in response to temperature. It grows more rapidly than the shoot at temperatures slightly below the ideal range for seedling development, especially in the later stages. Temperature deviations from the ideal range influence either the radicles or the shoots more than the other. The radicle is more tolerant of cold than the shoot, and vice versa; the shoot is more tolerant of temperatures over the ideal threshold than the radicle, which results agrees with other researchers [40,60,61].
4.2. Water Amount Experiment
Germination is one of the most eminent seed quality and performance tests linked to vigor tests, such as a seedling growth test [62,63,64]. Water absorption and temperature studies may offer information about wheat seeds’ stress tolerance, uniformity, and germination rate. The availability of moisture may significantly impact seed germination [65,66]. Several cereal crops demand similar germination requirements as wheat [64]. To a greater extent, crop seeds with complex germination criteria will succeed in germinating than those with fewer limitations [67,68]. The water quantity experiment was carried out on two bases: single-milliliter intervals 0–12 mL and as percentages of thousand kernel weight (TKW) to develop a technique for comparing various wheat varieties with varied TKW in a future experiment. These bases of water quantity application were compared and investigated simultaneously in parallel, which has a better reflectance representing water demands. As demonstrated in the result chapter, Table 3 and Table 4 reveal significant variations among water quantities potential according to both water application bases for all the examined parameters: the number of non-germinated seeds, length of the radicle, length of shoot, seedling length in total, dry weight of the seedlings, seedlings’ corrected dry weight that accomplished by subtracting the ungerminated seeds, dry weight of the radicles, and dry weight of shoots. These different effects of water quantity on germination optimization agree with a study by [66].
As demonstrated by the results, germination percentage rose dramatically as water volume rose to the optimal water level, followed by a reduction somewhat as the water level increased owing to waterlogging. This is due to the demanded water limit necessary to initiate metabolic and physiological processes for germination and oxygen availability in the higher portion of water. Oxygen is critical for germination initiation [20,67]. When water levels are increased over the ideal range, oxygen availability for the seeds reduces [68]. As a result, the more water application, the less accessible oxygen availability. The wheat seeds require internal 40% moisture of the seed size to initiate germination [55]. In line with this current research, wheat seeds can initiate germination at 75% water amount of the TKW. This amount of water is sufficient to activate the metabolic processes of germination and enzymes. If the interior moisture content were less than the critical moisture content limit, wheat seeds would not germinate. Under 0.65 mL, over half of the seeds germinated. Thus, water application based on the TKW provides a better understanding of the constraints and optimal water requirements since seed size is critical for attaining the 40% internal seed moisture level. These findings corroborate research conducted by [40,57], stating that “seed size has importance in predicting germination under stress conditions”. As water increases more than the upper limit of the optimal range for germination, a reverse effect of waterlogging will reduce the germination percentage because of its negative effect on the intracellular process. Therefore, this study stated that the optimal range for germination is 4.45–7.00 mL, Figure 7, representing 525–825% of the TKW, and the TKW is an objective and more accurate base for water application.
Significant differences among the seedling performance depending on supplied moisture percentages were observed. As the water was applied at the lowest quantity necessary, the seedling length had the lowest mean values, and as water quantity increased, seedling development significantly increased, as presented in Table 3 and Table 4. The ideal moisture content range begins at 3.85 mL, or 450% of TKW, as shown in Table 4. Parallel to this, significant seedling length performance started under 5 mL according to water quantity applied based on single-milliliter intervals. In the same pattern, significant seedling performance started under 5 mL according to water quantity applied based on single-milliliter intervals. Water stresses of drought or waterlogging significantly reversely affect seedlings’ vigor, seedling length values rises dramatically at the starting point of the ideal range of water application, and moisture percentage based on the TKW is more accurate and reliable. Water stress reduces enzymatic activity, which has a deleterious effect on glucose metabolism, decreases water potential and soluble calcium and potassium, and alters seed hormones [69,70,71]. Since all of these intracellular mechanisms are affected by the water stress, the seedling growth rate finally deviates from the average growth and reduces.
The radicle growth values of wheat seedlings progressively descended as the quantity of water rose over the optimal range. Shoot growth followed a distinct pattern compared to the radicle. This is in line with the finding of other research [72,73]. As a result, measuring the whole seedling is realistic; the radicle and plumule statistically remained near the ideal range as the water level rose. Either way of water stresses greatly affected the radicles more than the shoots. It can be explained in terms of the physiological aspect of radicle development; with adequate moisture availability, the seedling exhibited a slower radicle development rate and accelerated shoot development.
Significant variation among the accumulated dry matter of the seedlings is based on moisture availability, as presented in Table 3 and Table 4. The dry matter values increased progressively as supplied water increased than the lower limit of the optimal range. Under drought stress, the creation of the dry matter is expressed on a chronological basis by accumulating the required amount to make a unite of dry matter. Seedlings under more water supplements over the threshold range are marginally adversely impacted by surplus water compared to the required quantity. The shoot-accumulated dry matter unit demanded a larger water quantity than a unit of radicle dry matter. This finding is in line with the result [47,74], where the researchers evaluated the effect of water potential levels 0.0 and −0.2 MPa on the germination stages of Pinus yunnanensis seeds. Moisture availability significantly impacted the germination rate, seedling development rate, and the accumulation of built dry matter on the radicle and the shoot: the more acute the moisture stress, the greater the reduction in dry matter accumulated values. There are water limitations and potential ranges for each germination phase and seedling development, supported by other research [47,75].
Seeds of wheat subjected to 0.65 mL of water were germinated. As a result, water application based on the TKW enables better knowledge of the limitations and optimal water requirements, as seed size is critical in achieving the required internal seed moisture. These findings corroborate research conducted by other researchers [47], stating that “seed size has importance in predicting germination under stress conditions”.
4.3. Seed Number Experiment
The data demonstrate that seed numbers are a critical factor in seedling growth bio-assays in vitro. Increased water volumes or denser wheat seedlings increase the amount of phytotoxin contained in each seed, hence increasing inhibition [51,52]. Greater than optimal seeding density has a detrimental effect on seedling development in Petri dishes and the opening of Petri dish lids. They are exposed to water loss, which has a detrimental effect on seedling development. Results presented no significant differences among different seeds and seedling densities of 15, 20, and 25 in a Petri dish for the measured traits, Table 5. Since high seedling density has an opposite impact by the opening of Petri dishes lids and there were no significant variations among used densities, 15 seeds per Petri dish are more appropriate than higher densities for wheat germination experiments in vitro. In plant breeding initiatives (identical breed seeds are sometimes scarce), it is critical to determine the ideal number of seeds to use in a Petri dish experiment. It is beneficial in resources optimization, particularly for plant breeders.
5. Conclusions
- Sigmoid curves provide an excellent fit for the analyzed experimental data and present increased germination temperature, generally increased germination, and seedling development rate. Overall, 20 °C was ideal for seedling development. The germination has a broader range of 20 °C to 30 °C.
- Seed size plays a role in the required amount of water for germination. Therefore, it provides a more precise impression for the application water quantity. Germination of various percentages can occur in a broad range of water quantities commencing at 0.65 mL, which represents 75% of TKW, but the optimal range for germination is 4.45–7.00 mL, representing 525–825% of the TKW.
- Dry weight can be a good indicator of seedling growth since the accumulation of dry matter is consistent and is in line with the other measurement of seedling growth, such as shoot and radicle lengths.
- No significant differences among seed densities were observed; therefore, lower density is recommended for lab experiments, 15 wheat seeds per 9 cm Petri dish. In a nutshell,
This research suggests conducting wheat seeds germination experiments under 20 °C, and water quantity as a percentage based on the TKW for optimizing the suitability of water quantity and a maximum of 15 seeds per Petri dish; more seeds have no advantage. This is common practice when seed number is limited and in breeding projects.
Author Contributions
Methodology, writing, original paper, H.K.; methodology, Z.K.; funding acquisition, I.B.; acquisition, C.G.; acquisition, A.E.; conceptualization, reviewing, Á.T. All authors have read and agreed to the published version of the manuscript.
Funding
The Hungarian University of Agriculture and Life Sciences (MATE) supported this study with financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This manuscript’s data, tables, and figures are original.
Acknowledgments
The authors would like to convey their heartfelt appreciation to Zsófia Kovács, a researcher from the genetic laboratory, for her outstanding notes and work. We want to thank Katalin Dobine, the lab technician, for aiding us with the experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of polystyrene nanoplastics (psnps) on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yin, Y.; Liu, C.; Zhao, Z.; Guo, M. Effect of germination time on the compositional, functional and antioxidant properties of whole wheat malt and its end-use evaluation in cookie-making. Food Chem. 2021, 349, 129125. [Google Scholar] [CrossRef] [PubMed]
- Beta, T.; Qiu, Y.; Liu, Q.; Borgen, A.; Apea-Bah, F.B. Purple wheat (Triticum sp.) seeds. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 103–125. [Google Scholar] [CrossRef]
- Lemmens, E.; De Brier, N.; Goos, P.; Smolders, E.; Delcour, J.A. Steeping and germination of wheat (Triticum aestivum L.). i. unlocking the impact of phytate and cell wall hydrolysis on bio-accessibility of iron and zinc elements. J. Cereal Sci. 2019, 90, 102847. [Google Scholar] [CrossRef]
- Yan, M.; Xue, C.; Xiong, Y.; Meng, X.; Li, B.; Shen, R.; Lan, P. Proteomic dissection of the similar and different responses of wheat to drought, salinity and submergence during seed germination. J. Proteom. 2020, 220, 103756. [Google Scholar] [CrossRef]
- Herman, J.J.; Sultan, S.E.; Horgan-Kobelski, T.; Riggs, C. Adaptive transgenerational plasticity in an annual plant: Grandparental and parental drought stress enhance performance of seedlings in dry soil. Integr. Comp. Biol. 2012, 52, 77–88. [Google Scholar] [CrossRef]
- Poudel, R.; Finnie, S.; Rose, D.J. Effects of wheat kernel germination time and drying temperature on compositional and end-use properties of the resulting whole wheat flour. J. Cereal Sci. 2019, 86, 33–40. [Google Scholar] [CrossRef]
- Khalid, N.; Tarnawa, Á.; Kende, Z.; Kassai, K.M.; Jolánkai, M. Viability of maize (Zea mays L.) seeds influenced by water, temperature, and salinity stress. Acta Hydrol. Slovaca 2021, 22, 113–117. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Khan, H.; Munsif, F.; Nie, L. Ascorbic acid priming enhances seed germination and seedling growth of winter wheat under low temperature due to late sowing in pakistan. Agronomy 2019, 9, 757. [Google Scholar] [CrossRef]
- Kende, Z.; Sallai, A.; Kassai, K.; Mikó, P.; Percze, A.; Birkás, M. The effects of tillage-induced soil disturbance on weed infestation of winter wheat. Pol. J. Environ. Stud. 2017, 26, 1131–1138. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef]
- Rizzardi, M.A.; Luiz, A.R.; Roman, E.S.; Vargas, L. Temperatura cardeal e potencial hídrico na germinação de sementes de corda-de-viola (Ipomoea triloba). Planta Daninha 2009, 27, 13–21. [Google Scholar] [CrossRef]
- Kildisheva, O.A.; Dixon, K.W.; Silveira, F.A.O.; Chapman, T.; Di Sacco, A.; Mondoni, A.; Turner, S.R.; Cross, A.T. Dormancy and germination: Making every seed count in restoration. Restor. Ecol. 2020, 28, S256–S265. [Google Scholar] [CrossRef]
- McCormick, M.K.; Taylor, D.L.; Whigham, D.F.; Burnett, R.K. Germination patterns in three terrestrial orchids relate to abundance of mycorrhizal fungi. J. Ecol. 2016, 104, 744–754. [Google Scholar] [CrossRef]
- Cone, J.W.; Spruit, C.J.P. Imbibition conditions and seed dormancy of Arabidopsis thaliana. Physiol. Plant. 1983, 59, 416–420. [Google Scholar] [CrossRef]
- Bentsink, L.; Koornneef, M. Seed Dormancy and Germination; The Arabidopsis Book/American Society of Plant Biologists: Rockville, MD, USA, 2008. [Google Scholar] [CrossRef]
- Fu, F.F.; Peng, Y.S.; Wang, G.B.; El-Kassaby, Y.A.; Cao, F.L. Integrative analysis of the metabolome and transcriptome reveals seed germination mechanism in Punica granatum L. J. Integr. Agric. 2021, 20, 132–146. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1994; p. 421. [Google Scholar]
- Manz, B.; Mü, K.; Kucera, B.; Volke, F.; Leubner-Metzger, G. Water uptake and distribution in germinating tobacco seeds investigated in vivo by nuclear magnetic resonance imaging 1. Plant Physiol. 2005, 138, 1538–1551. [Google Scholar] [CrossRef]
- Xue, X.; Du, S.; Jiao, F.; Xi, M.; Wang, A.; Xu, H.; Jiao, Q.; Zhang, X.; Jiang, H.; Chen, J.; et al. The regulatory network behind maize seed germination: Effects of temperature, water, phytohormones, and nutrients. Crop J. 2021, 9, 718–724. [Google Scholar] [CrossRef]
- Gallardo, K.; Job, C.; Groot, S.P.C.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomic analysis of arabidopsis seed germination and priming. Plant Physiol. 2001, 126, 835–848. [Google Scholar] [CrossRef]
- Masubelele, N.H.; Dewitte, W.; Menges, M.; Maughan, S.; Collins, C.; Huntley, R.; Nieuwland, J.; Scofield, S.; Murray, J.A.H. D-Type Cyclins Activate Division in the Root Apex to Promote Seed Germination in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 15694–15699. [Google Scholar] [CrossRef]
- Krock, B.; Schmidt, S.; Hertweck, C.; Baldwin, I.T. Vegetation-derived abscisic acid and four terpenes enforce dormancy in seeds of the post-fire annual, Nicotiana attenuata. Seed Sci. Res. 2002, 12, 239–252. [Google Scholar] [CrossRef]
- Petruzzelli, L.; Müller, K.; Hermann, K.; Leubner-Metzger, G. Distinct expression patterns of β-1,3-glucanases and chitinases during the germination of solanaceous seeds. Seed Sci. Res. 2003, 13, 139–153. [Google Scholar] [CrossRef]
- Bradford, K.J.; Nonogaki, H. Seed Development, Dormancy and Germination; Blackwell Publishing: Hoboken, NJ, USA, 2007; pp. 1–367. [Google Scholar] [CrossRef]
- Ozden, E.; Light, M.E.; Demir, I. Alternating temperatures increase germination and emergence in relation to endogenous hormones and enzyme activities in aubergine seeds. S. Afr. J. Bot. 2021, 139, 130–139. [Google Scholar] [CrossRef]
- Bradford, K.J. A water relations analysis of seed germination rates. Plant Physiol. 1990, 94, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Filho, J. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- Liu, Y.; Han, C.; Deng, X.; Liu, D.; Liu, N.; Yan, Y. Integrated physiology and proteome analysis of embryo and endosperm highlights complex metabolic networks involved in seed germination in wheat (Triticum aestivum L.). J. Plant Physiol. 2018, 229, 63–76. [Google Scholar] [CrossRef]
- Abido, W.A.E.; Zsombik, L. Effect of water stress on germination of some hungarian wheat landraces varieties. Shengtai Xuebao/Acta Ecol. Sin. 2020, 38, 422–428. [Google Scholar] [CrossRef]
- Aderounmu, A.F.; Nkemnkeng, F.J.; Anjah, G.M. Effects of seed provenance and growth media on the growth performance of Vitellaria paradoxa c.f. gaertn. Int. J. Biol. Chem. Sci. 2020, 14, 2659–2669. [Google Scholar] [CrossRef]
- Guan, B.; Zhou, D.; Zhang, H.; Tian, Y.; Japhet, W.; Wang, P. Germination responses of medicago ruthenica seeds to salinity, alkalinity, and temperature. J. Arid Environ. 2009, 73, 135–138. [Google Scholar] [CrossRef]
- Ostadian Bidgoly, R.; Balouchi, H.; Soltani, E.; Moradi, A. Effect of temperature and water potential on Carthamus tinctorius L. seed germination: Quantification of the cardinal temperatures and modeling using hydrothermal time. Ind. Crops Prod. 2018, 113, 121–127. [Google Scholar] [CrossRef]
- Dadach, M.; Mehdadi, Z.; Latreche, A. Effect of water stress on seed germination of Thymus serpyllum L. from tessala mount. J. Plant Sci. 2015, 10, 151–158. [Google Scholar] [CrossRef][Green Version]
- Riley, G.J.P. Effects of High Temperature on the Germination of Maize (Zea Mays L.). Planta 1981, 151, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Andronis, E.A.; Moschou, P.N.; Toumi, I.; Roubelakis-Angelakis, K.A. Peroxisomal polyamine oxidase and nadph-oxidase cross-talk for ros homeostasis which affects respiration rate in Arabidopsis thaliana. Front. Plant Sci. 2014, 5, 132. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. The signalling role of ros in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Kunos, V.; Cséplő, M.; Seress, D.; Eser, A.; Kende, Z.; Uhrin, A.; Bányai, J.; Bakonyi, J.; Pál, M.; Mészáros, K. The Stimulation of Superoxide Dismutase Enzyme Activity and Its Relation with the Pyrenophora teres f. teres Infection in Different Barley Genotypes. Sustainability 2022, 14, 2597. [Google Scholar] [CrossRef]
- Balla, K.; Rakszegi, M.; Li, Z.; Békés, F.; Bencze, S.; Veisz, O. Quality of winter wheat in relation to heat and drought shock after anthesis. Czech J. Food Sci. 2011, 29, 117–128. [Google Scholar] [CrossRef]
- Khaeim, H.; Kende, Z.; Jolánkai, M.; Kovács, G.P.; Gyuricza, C.; Tarnawa, Á. Impact of temperature and water on seed germination and seedling growth of maize (Zea mays L.). Agronomy 2022, 12, 397. [Google Scholar] [CrossRef]
- Drebee, H.A.; Razak, N.A.A. Measuring the efficiency of colleges at the university of al-qadisiyah-iraq: A data envelopment analysis approach. J. Ekon. Malays. 2018, 52, 163–179. [Google Scholar] [CrossRef]
- Bulmer, M.G.; Harrison, T.K. Principles of Statistics. J. R. Stat. Soc. Ser. A Stat. Soc. Ser. D. Stat. 1966, 16, 217. [Google Scholar] [CrossRef]
- Seefeldt, S.S.; Kidwell, K.K.; Waller, J.E. Base growth temperatures, germination rates and growth response of contemporary spring wheat (Triticum aestivum L.) cultivars from the us pacific northwest. Field Crops Res. 2002, 75, 47–52. [Google Scholar] [CrossRef]
- Sabouri, A.; Azizi, H.; Nonavar, M. Hydrotime model analysis of lemon balm (Melissa officinalis L.) using different distribution functions. S. Afr. J. Bot. 2020, 135, 158–163. [Google Scholar] [CrossRef]
- Barrero, J.M.; Jacobsen, J.V.; Talbot, M.J.; White, R.G.; Swain, S.M.; Garvin, D.F.; Gubler, F. Grain dormancy and light quality effects on germination in the model grass Brachypodium distachyon. New Phytol. 2012, 193, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M. Effect of water and temperature on seed germination and emergence as a seed hydrothermal time model. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 1686–1691. [Google Scholar]
- Gao, C.; Liu, F.; Zhang, C.; Feng, D.; Li, K.; Cui, K. Germination responses to water potential and temperature variation among provenances of Pinus yunnanensis. Flora Morphol. Distrib. Funct. Ecol. Plants 2021, 276–277, 151786. [Google Scholar] [CrossRef]
- da Silva, L.J.; de Medeiros, A.D.; Oliveira, A.M.S. SeedCalc, a new automated r software tool for germination and seedling length data processing. J. Seed Sci. 2019, 41, 250–257. [Google Scholar] [CrossRef]
- Eser, A.; Kassai, K.M.; Kato, H.; Kunos, V.; Tarnava, A.; Jolánkai, M. IMPACT of nitrogen topdressing on the quality parameters of winter wheat (Triticum aestivum L.) yield. Acta Aliment. 2020, 49, 244–253. [Google Scholar] [CrossRef]
- Zhang, K.; Ji, Y.; Fu, G.; Yao, L.; Liu, H.; Tao, J. Dormancy-breaking and germination requirements of Thalictrum squarrosum stephan ex willd. seeds with underdeveloped embryos. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100311. [Google Scholar] [CrossRef]
- Contreras-Negrete, G.; Pineda-García, F.; Nicasio-Arzeta, S.; De la Barrera, E.; González-Rodríguez, A. Differences in germination response to temperature, salinity, and water potential among prosopis laevigata populations are guided by the tolerance-exploitation trade-off. Flora 2021, 285, 151963. [Google Scholar] [CrossRef]
- Boyce, D.S. Heat and moisture transfer in ventilated grain. J. Agric. Eng. Res. 1966, 11, 255–265. [Google Scholar] [CrossRef]
- Hu, H.; Jing, N.; Peng, Y.; Liu, C.; Ma, H.; Ma, Y. 60Coγ-ray irradiation inhibits germination of fresh walnuts by modulating respiratory metabolism and reducing energy status during storage. Postharvest Biol. Technol. 2021, 182, 111694. [Google Scholar] [CrossRef]
- Sullivan, B.K.; Keough, M.; Laura, L.G. Copper sulphate treatment induces Heterozostera seed germination and improves seedling growth rates. Glob. Ecol. Conserv. 2022, 35, e02079. [Google Scholar] [CrossRef]
- Gong, M.; Chen, B.O.; Li, Z.G.; Guo, L.H. Heat-shock-induced cross adaptation to heat, chilling, drought and salt stress in maize seedlings and involvement of H2O2. J. Plant Physiol. 2001, 158, 1125–1130. [Google Scholar] [CrossRef]
- Schabo, D.C.; Martins, L.M.; Iamanaka, B.T.; Maciel, J.F.; Taniwaki, M.H.; Schaffner, D.W.; Magnani, M. Modeling aflatoxin b1 production by aspergillus flavus during wheat malting for craft beer as a function of grains steeping degree, temperature and time of germination. Int. J. Food Microbiol. 2020, 333, 108777. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Liu, H.; Wang, H.; Zou, D.; Qu, Z.; Wang, J.; Zheng, H.; Wang, J.; Yang, L.; Mei, Y.; et al. Effects of root characteristics on panicle formation in japonica rice under low temperature water stress at the reproductive stage. Field Crops Res. 2022, 277, 108395. [Google Scholar] [CrossRef]
- Wang, T.; Zang, Z.; Wang, S.; Liu, Y.; Wang, H.; Wang, W.; Hu, X.; Sun, J.; Tai, F.; He, R. Quaternary ammonium iminofullerenes promote root growth and osmotic-stress tolerance in maize via ros neutralization and improved energy status. Plant Physiol. Biochem. 2021, 164, 122–131. [Google Scholar] [CrossRef]
- Cao, K.; Xie, C.; Wang, M.; Wang, P.; Gu, Z.; Yang, R. Effects of soaking and germination on deoxynivalenol content, nutrition and functional quality of fusarium naturally contaminated wheat. LWT 2022, 160, 113324. [Google Scholar] [CrossRef]
- Bao, Y.; Pan, C.; Li, D.; Guo, A.; Dai, F. Stress response to oxytetracycline and microplastic-polyethylene in wheat (Triticum aestivum L.) during seed germination and seedling growth stages. Sci. Total Environ. 2022, 806, 150553. [Google Scholar] [CrossRef]
- Cheng, X.; Tian, B.; Gao, C.; Gao, W.; Yan, S.; Yao, H.; Wang, X.; Jiang, Y.; Hu, L.; Pan, X.; et al. Identification and expression analysis of candidate genes related to seed dormancy and germination in the wheat gata family. Plant Physiol. Biochem. 2021, 169, 343–359. [Google Scholar] [CrossRef]
- Thakur, P.S.; Chatterjee, A.; Rajput, L.S.; Rana, S.; Bhatia, V.; Prakash, S. Laser biospeckle technique for characterizing the impact of temperature and initial moisture content on seed germination. Opt. Lasers Eng. 2022, 153, 106999. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Q.; Sun, Y.; Zhang, J. Effects of oxygenated brackish water on germination and growth characteristics of wheat. Agric. Water Manag. 2021, 245, 106520. [Google Scholar] [CrossRef]
- Kim, Y.J.; Zhang, D.; Jung, K.H. Molecular basis of pollen germination in cereals. Trends Plant Sci. 2019, 24, 1126–1136. [Google Scholar] [CrossRef]
- Gabriele, M.; Pucci, L. Fermentation and germination as a way to improve cereals antioxidant and antiinflammatory properties. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress; Academic Press: Cambridge, MA, USA, 2022; pp. 477–497. [Google Scholar] [CrossRef]
- Campos, H.; Trejo, C.; Peña-Valdivia, C.B.; García-Nava, R.; Víctor Conde-Martínez, F.; Cruz-Ortega, R. Water availability effects on germination, membrane stability and initial root growth of Agave lechuguilla and A. salmiana. Flora 2020, 268, 151606. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rahimian, H.; Alizadeh, H.; Yousefi, A.R.; Gonzalez-Andujar, J.L.; Mac Sweeney, E.; Mastinu, A. Competitive ability effects of Datura stramonium L. and Xanthium strumarium L. on the development of maize (Zea mays L.) seeds. Plants 2021, 10, 1922. [Google Scholar] [CrossRef] [PubMed]
- Prerna, D.I.; Govindaraju, K.; Tamilselvan, S.; Kannan, M.; Vasantharaja, R.; Chaturvedi, S.; Shkolnik, D. Influence of nanoscale micro-nutrient α-fe2o3 on seed germination, seedling growth, translocation, physiological effects and yield of rice (Oryza sativa L.) and maize (Zea mays L.). Plant Physiol. Biochem. 2021, 162, 564–580. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hu, W.; Li, Y.; Zhu, H.; Zou, J.; Wang, Y.; Meng, Y.; Chen, B.; Zhao, W.; Wang, S.; et al. Prolonged drought affects the interaction of carbon and nitrogen metabolism in root and shoot of cotton. Environ. Exp. Bot. 2022, 197, 104839. [Google Scholar] [CrossRef]
- Thiébeau, P.; Beaudoin, N.; Justes, E.; Allirand, J.M.; Lemaire, G. Radiation use efficiency and shoot:root dry matter partitioning in seedling growths and regrowth crops of lucerne (Medicago sativa L.) after spring and autumn sowings. Eur. J. Agron. 2011, 35, 255–268. [Google Scholar] [CrossRef]
- Peng, X.; Li, J.; Sun, L.; Gao, Y.; Cao, M.; Luo, J. Impacts of water deficit and post-drought irrigation on transpiration rate, root activity, and biomass yield of festuca arundinacea during phytoextraction. Chemosphere 2022, 294, 133842. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, Z.; Wei, Z.; Hu, X. Effect of maternal environment on seed germination and seed yield components of Thlaspi arvense. Ind. Crops Prod. 2022, 181, 114790. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Morton, T.C.; Romeo, J.T. Solution volume and seed number: Often overlooked factors in allelopathic bioassays. J. Chem. Ecol. 1987, 13, 1481–1491. [Google Scholar] [CrossRef]
- Jolánkai, J.M.; Tarnawa, Á.; Kassai, M.K.; Eser, A.; Kende, Z. Water footprint of the protein formation of six of field crop species. Environ. Anal. Ecol. Stud. 2021, 8, 000686. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Lu, H.; Shu, Q.; Zhang, Y.; Chen, Q. New perspectives on physiological, biochemical and bioactive components during germination of edible seeds: A review. Trends Food Sci. Technol. 2022; in press. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).