Permeable Reactive Barrier Remediation Technique Using Carbonized Food Waste in Ground Contaminated with Combined Cu and Pb
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Soil Sample
2.1.2. Carbonized Food Waste (CFW)
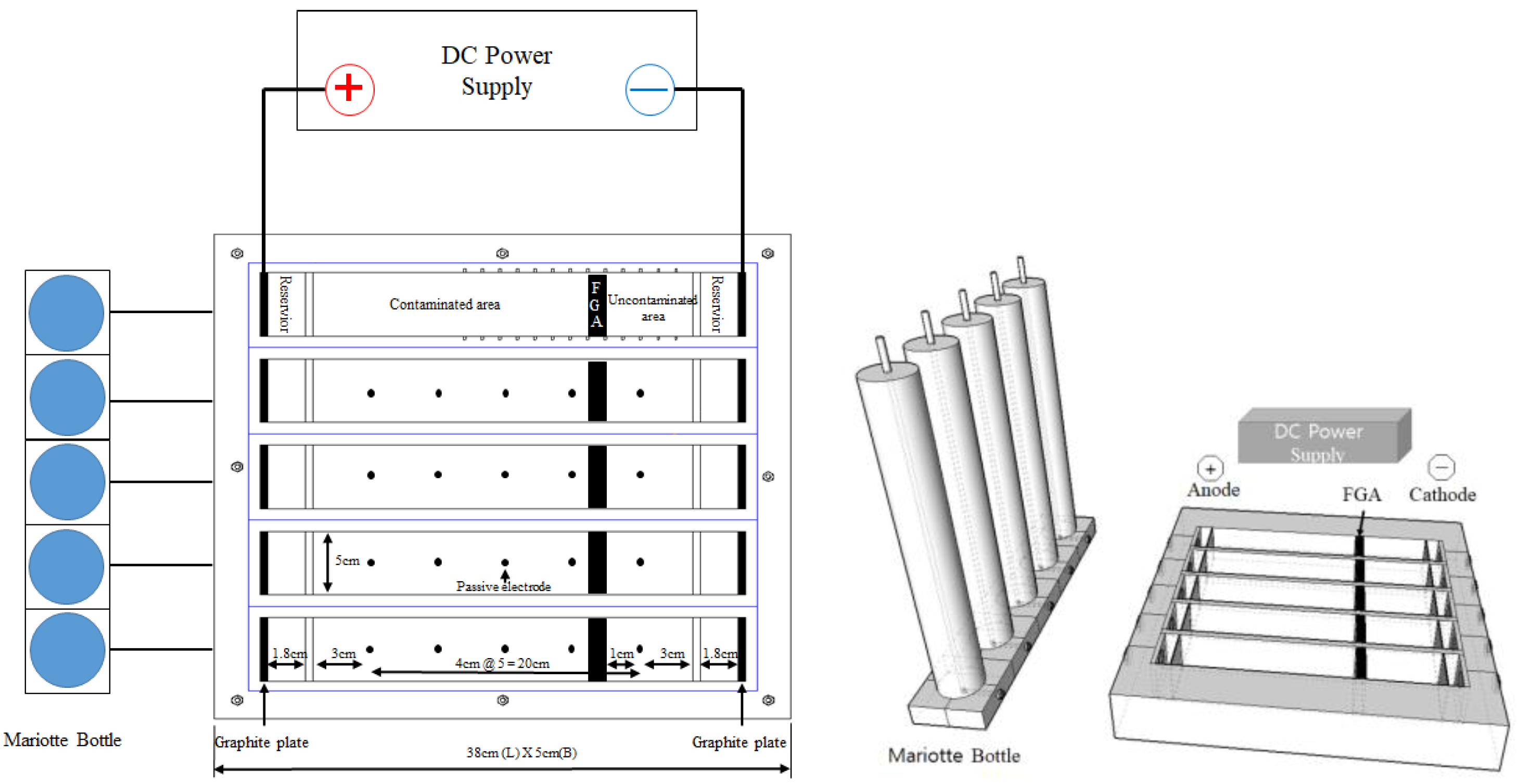
2.2. Experiments
3. Results
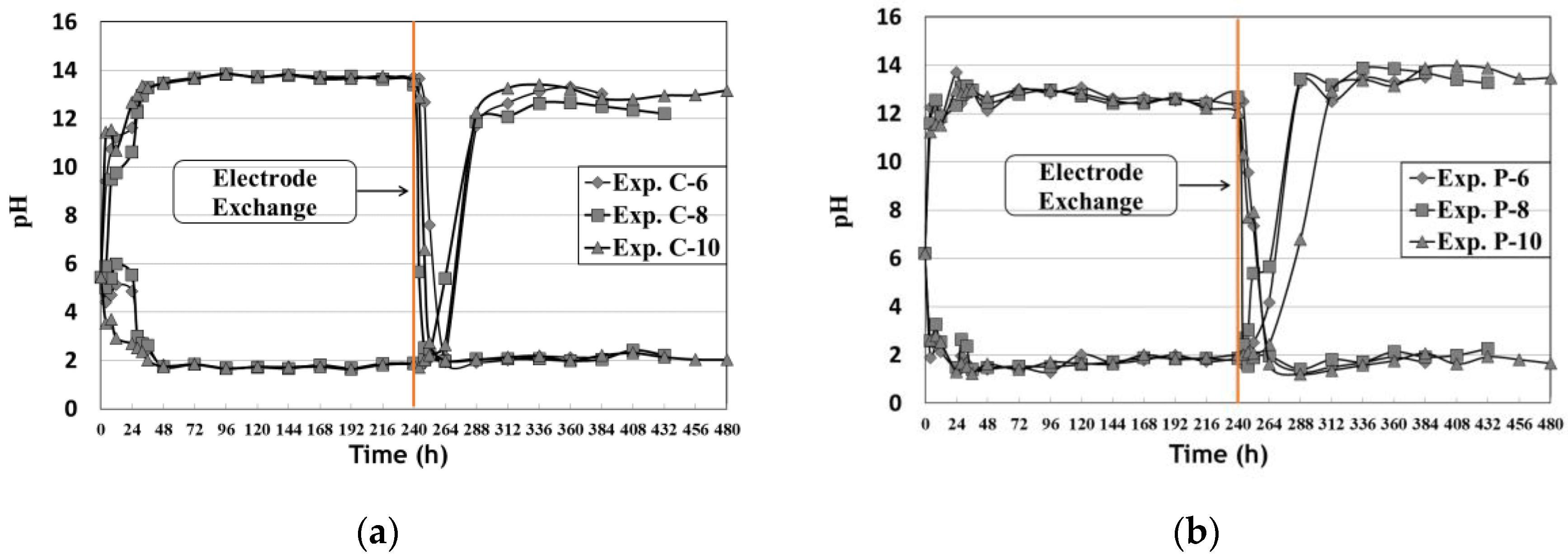
3.1. pH Change
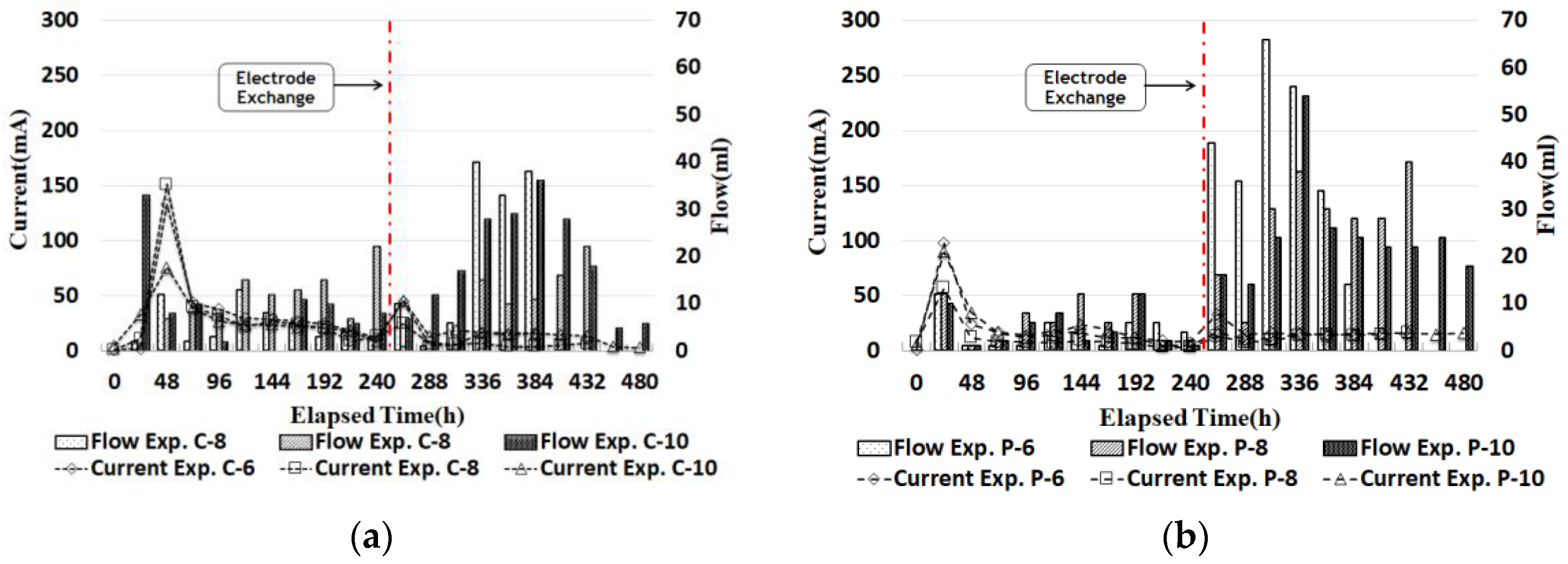
3.2. Current Density
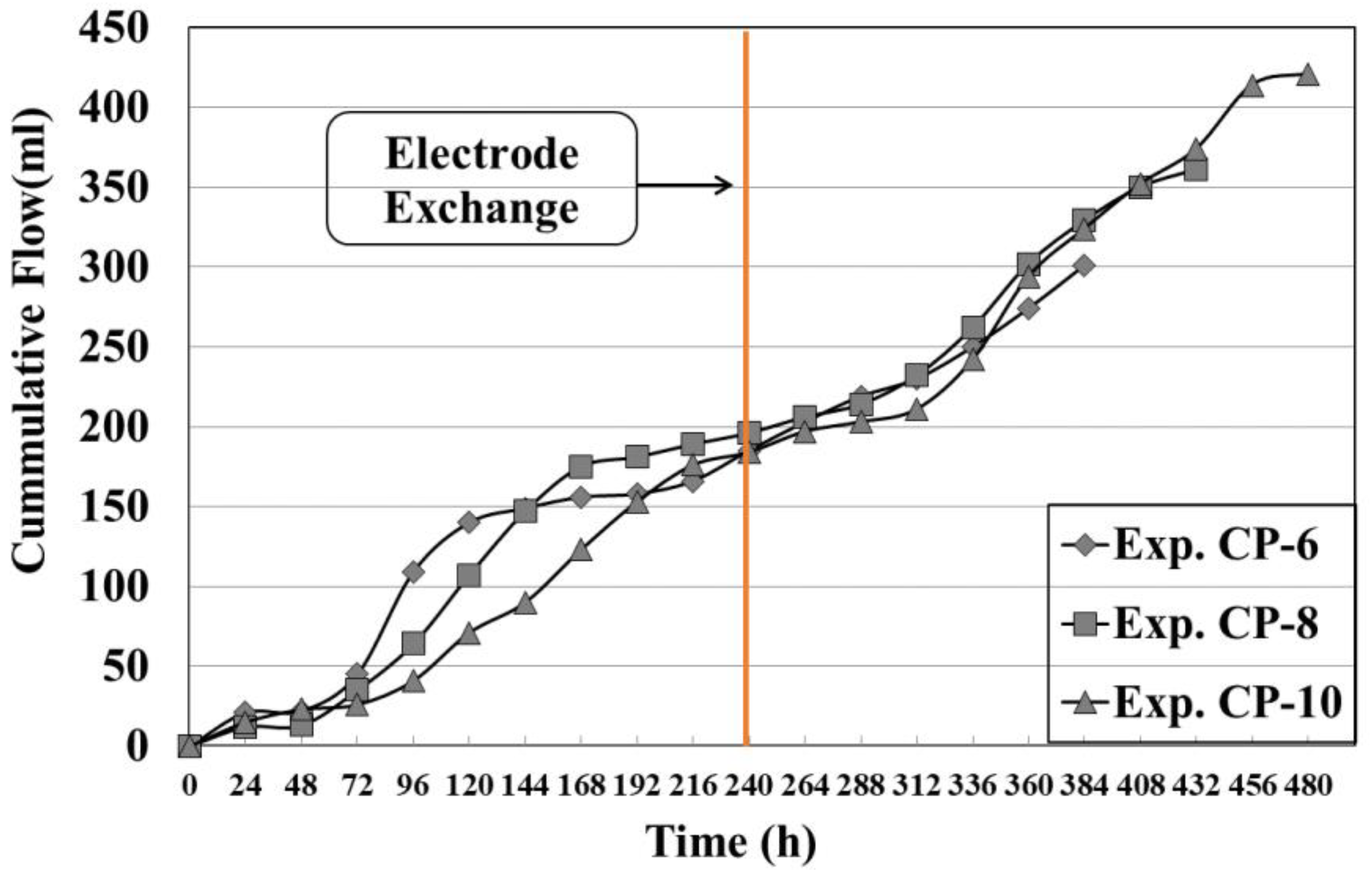
3.3. Electroosmosis Flow
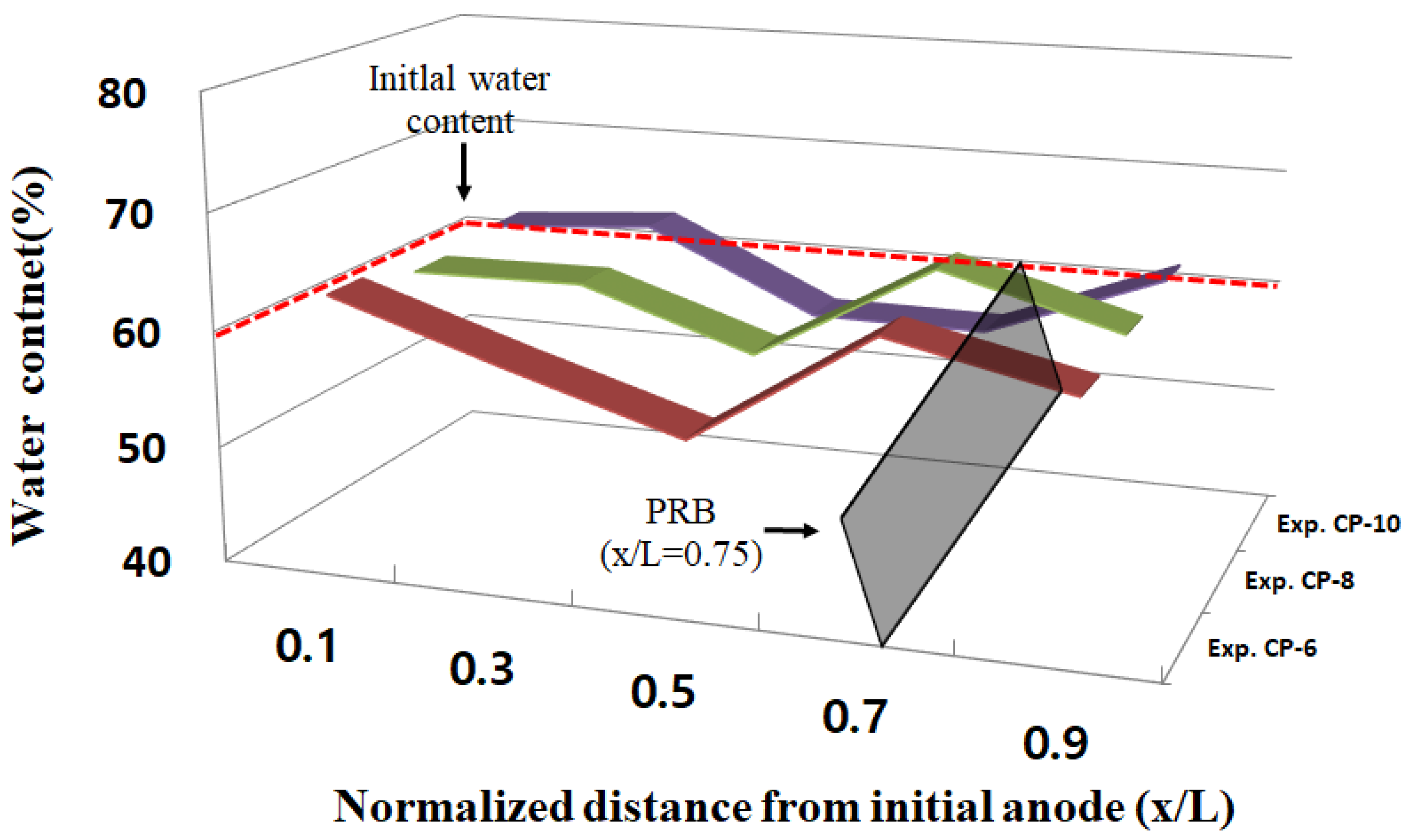
3.4. Water Content
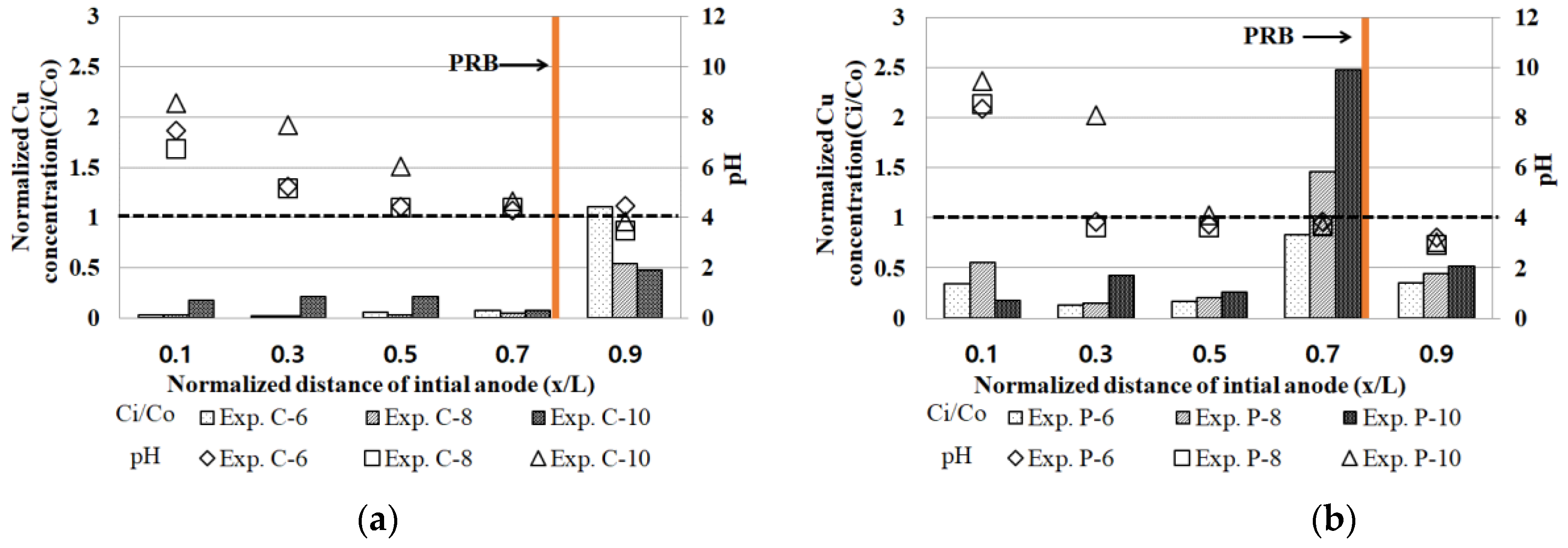
3.5. Residual Heavy Metals and pH
3.6. Currnet-Effluent Realtionship
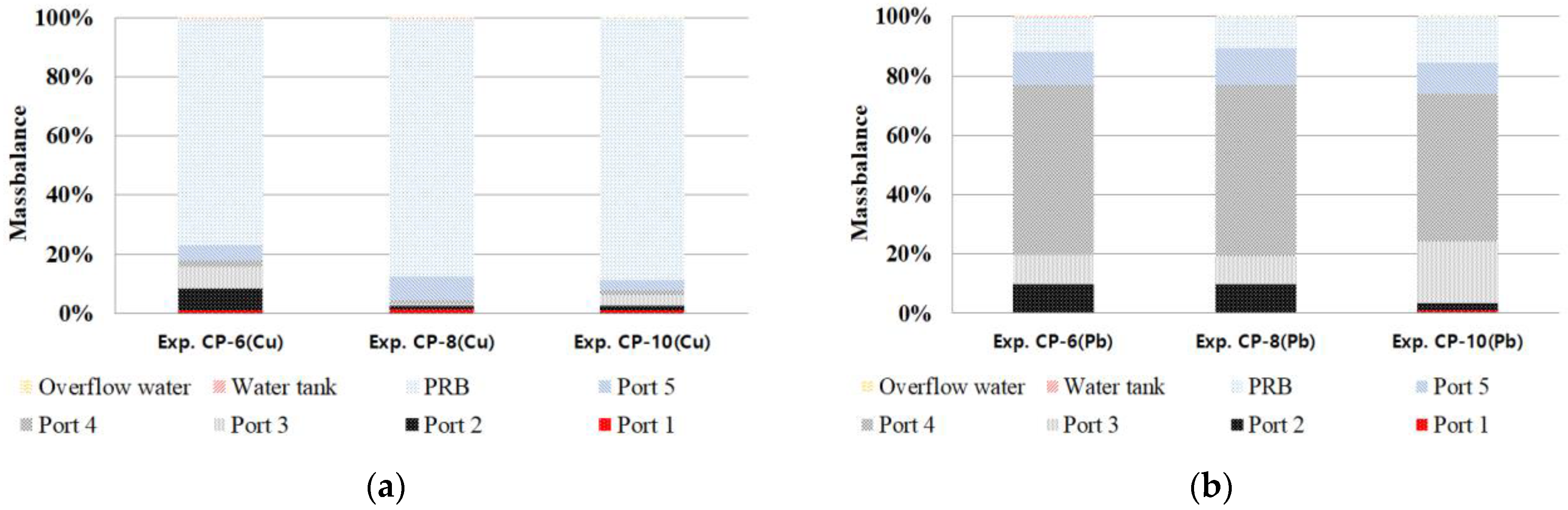
3.7. Mass Balance
4. Results and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bo, S.; Luo, J.; An, Q.; Xiao, Z.; Wang, H.; Cai, W.; Zhai, S.; Li, Z. Efficiently selective adsorption of Pb(II) with functionalized alginate-based adsorbent inbatch/column systems: Mechanism and application simulation. J. Clean. Prod. 2020, 250, 119585. [Google Scholar] [CrossRef]
- García-Carmona, M.; Romero-Freire, A.; Aragon, M.S.; Garzon, F.J.M.; Peinado, F.J.M. Evaluation of remediation techniques in soils affected by residual contamination with heavy metals and arsenic. J. Environ. Manag. 2017, 191, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Hou, D.; Ye, J.; Zhang, Y.; Ok, Y.S.; Song, Y.; Coulon, F.; Peng, T.; Tian, L. Lead-based paint remains a major public health concern: A critical review of global production, trade, use, exposure, health risk, and implications. Environ. Int. 2018, 121, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Manjón, l.; Ramírez-Andreotta, M.D.; Sáez, A.E.; Root, R.A.; Hild, J.; Janes, M.K.; Alexander-Ozinskas, A. Ingestion and inhalation of metal(loid)s through preschool gardening: An exposure and risk assessment in legacy mining communities. Sci. Total Environ. 2020, 718, 134639. [Google Scholar] [CrossRef]
- Han, J.G.; Kim, D.C.; Hong, G. The effects of pH on Microfluidics Flow Characteristics of Heavy Metals. J. Kor. Geosyn. Soc. 2015, 14, 23–32. [Google Scholar] [CrossRef]
- Lee, J.Y. The Remediation of Heavy Metal in the Contaminated Soil by Permeable Reactive Barrier with Carbonized Foods Waste (CFW). Ph.D. Thesis, Chung-Ang University, Seoul, Republic of Korea, 2010. [Google Scholar]
- Bhagat, S.K.; Tung, T.M.; Yaseen, Z.M. Development of artificial intelligence formodeling wastewater heavy metal removal: State of the art, applicationassessment and possible future research. J. Clean. Prod. 2020, 250, 11947. [Google Scholar] [CrossRef]
- De Freitas, E.D.; de Almeida, H.J.; de Almeida Neto, A.F.; Vieira, M.G.A. Continuous adsorption of silver and copper by Verde-lodo bentonite in afixedbedflow-through column. J. Clean. Prod. 2018, 171, 613621. [Google Scholar] [CrossRef]
- Nfor, B.; Fai, P.B.A.; Tamungang, S.A.; Fobil, J.N.; Basu, N. Soil contamination and bioaccumulation of heavy metals by a tropical earthworm species (Alma nilotica) at informale-waste recycling sites in Douala, Cameroon. Environ. Toxicol. Chem. 2022, 41, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.P.; Melo, L.C.A.; de Abreu, C.A.; Coscione, A.R.; Paz-Ferreiro, J. Leaching and fractionation of heavy metals in mining soils amended with biochar. Soil Tillage Res. 2016, 164, 25–33. [Google Scholar] [CrossRef]
- Min, X.; Wang, Y.; Chai, L.; Yang, Z.; Liao, Q. High-resolution analyses reveal structural diversity patterns of microbial communities in chromite ore processing residue (COPR) contaminated soils. Chemosphere 2017, 183, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Wang, W.; Shrivastava, A.K. Cadmium-mediated morphological, biochemical and physiological tuning in three different anabaena species. Aquat. Toxicol. 2018, 202, 36–45. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Cho, W.R.; Chang, J.M.; Lee, J.Y. Evaluation of the potential stabilization of lead-contaminated soil using coal gasification slag and flue gas desulfurization gypsum. J. Kor. Soc. Waste Manag. 2021, 38, 257–265. [Google Scholar] [CrossRef]
- White Paper of Environmental, Ministry of Environment in Korea, Feb. 2021. Available online: https://www.me.go.kr/home/web/policy_data/read.do?menuId=10260&seq=7856 (accessed on 26 March 2024).
- Hong, S.C. Remediation Characteristics of Diesel Contaminated Soil by Electrokinetic. Master’s Thesis, Chung-Ang University, Seoul, Republic of Korea, 2014. [Google Scholar]
- Park, C.O.; Kim, J.S.; Seo, S.W.; Lee, Y.J.; Lee, J.Y.; Park, M.J.; Kong, S.H. Enhanced separation technique of heavy metal (Pb, Zn) in contaminated agricultural soils near abandoned metal mine. J. Kor. Soc. Soil Ground Environ. 2013, 18, 41–53. [Google Scholar]
- Kim, J.H.; Kim, M.G.; Kim, W.R.; Han, O.H. Study on as removal from mine tailing using MGS gravity separator. J. Kor. Inst. Res. Recycl. 2014, 23, 36–43. [Google Scholar]
- Lin, H.; Wang, Z.; Liu, C.; Dong, Y. Technologies for removing heavy metal from contaminated soils on farmland: A review. Chemosphere. 2022, 305, 135457. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.S.; Dinh, V.C.; Nguyen, T.A.H.; Kim, K.W. Soil contamination and health risk assessment from heavy metals exposure near mining area in Bac Kan province, Vietnam. Environ. Geochem. Health. 2022, 44, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Seol, M.S. A study of the remediation of lead contaminated soil in a clay shooting range with soil washing. J. Kor. Soc. Soil Groundwater Environ. 2010, 15, 23–31. [Google Scholar]
- Park, C.O.; Kim, J.W.; Park, J.H.; Lee, Y.J.; Yang, I.J.; Lee, J.Y. Application of a soil separation system for the remediation of arsenic contaminated soil in a metal mining area. J. Kor. Soc. Soil Groundwater Environ. 2013, 8, 56–64. [Google Scholar] [CrossRef]
- Acar, Y.B.; Alshawabkeh, A.N. Principles of electrokinetic remediation. Environ. Sci. Technol. 1993, 7, 2638–2647. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Sandia National Laboratories In-Situ Electrokinetic Extraction Technology: Innovative Technology Evaluation Report; EPA/540/R-97/509; EPA: Washington, DC, USA, 1999.
- Alshawabek, A.N.; Yeung, A.T.; Bricka, M.R. Practical aspects of in situ electrokinetic extraction. J. Environ. Eng. 1999, 125, 27–35. [Google Scholar] [CrossRef]
- Acar, Y.B.; Alshawabkeh, A.N.; Gale, R.J. Fundamentals of extracting species from soils by electrokinetics. Waste Manag. 1993, 3, 141–151. [Google Scholar] [CrossRef]
- Acar, Y.B.; Gale, R.J.; Alshabkeh, A.N.; Marks, R.E.; Puppala, S.; Bricka, M.; Parker, R. Electrokinetic remediation: Basics and technology status. J. Hazard. Mater. 1995, 40, 117–137. [Google Scholar] [CrossRef]
- Probstein, R.F.; Hicks, R.E. Removal of contaminants from soils by electric fields. Science 1993, 260, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shady, A.; Ismail, S.; Yossif, T.M.H.; Yassin, S.A.; Ali, M.E.A.; Habib, A.A.M.; Khalil, A.K.A.; Tag-Elden, M.A.; Emam, T.M.; Mahmoud, A.A.; et al. Comprehensive review of progress made in soil electrokinetic research during 1993–2020, Part I: Process design modifications with brief summaries of main output. S. Afr. J. Chem. Eng. 2023, 44, 156–256. [Google Scholar] [CrossRef]
- Peng, G.; Tian, G. Using electrode electrolytes to enhance electrokinetic removal of heavy metals from electroplating sludge. Chem. Eng. J. 2010, 165, 388–394. [Google Scholar] [CrossRef]
- Kim, B.K.; Baek, K.; Ko, S.H.; Yang, J.W. Research and field experiences on electrokinetic remediation in South Korea. Sep. Purif. Technol. 2011, 19, 116–123. [Google Scholar] [CrossRef]
- Ren, L.; Lu, H.; He, L.; Zhang, Y. Enhanced electrokinetic technologies with oxidization–reduction for organically-contaminated soil remediation. Chem. Eng. J. 2014, 247, 111–124. [Google Scholar] [CrossRef]
- Dizon, A.; Orazem, M.E. Advances and challenges of electrokinetic dewatering of clays and soils. Curr. Opin. Electrochem. 2020, 22, 17–24. [Google Scholar] [CrossRef]
- Wen, D.; Fu, R.; Li, Q. Removal of inorganic contaminants in soil by electrokinetic remediation technologies: A review. J. Hazard. Mater. 2021, 401, 123345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, A.; Cui, C. Remediation of heavy metal-contaminated soils by electrokinetic technology: Mechanisms and applicability. Chemosphere 2021, 165, 129071. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; He, J.; Liu, T.; Xin, X.; Hu, H. Removal of heavy metal from sludge by the combined application of a biodegradable biosurfactant and complexing agent in enhanced electrokinetic treatment. Chemosphere 2017, 189, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; He, J.; Xin, X.; Hu, H.; Liu, T. Biosurfactants enhanced heavy metals removal from sludge in the electrokinetic treatment. Chem. Eng. J. 2018, 334, 2579–2592. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, H.; Zhang, M.; Chen, R.; Chen, X.; Zheng, X.; Jin, Y. Cr(VI) recovery from chromite ore processing residual using an enhanced electrokinetic process by bipolar membranes. J. Membr. Sci. 2018, 566, 190–196. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Malarbì, D.; Vagliasindi, F.G.A. Removal of mercury from marine sediments by the combined application of a biodegradable non-ionic surfactant and complexing agent in enhanced-electrokinetic treatment. Electrochim. Acta 2016, 222, 1569–1577. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Malarbì, D.; Maddalena, R.; Greco, V.; Vagliasindi, F.G.A. Remediation of Hg-contaminated marine sediments by simultaneous application of enhancing agents and microwave heating (MWH). Chem. Eng. J. 2017, 321, 1–10. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Malarbì, D.; Greco, V.; Vagliasindi, F.G.A. Surfactant and MGDA enhanced-Electrokinetic treatment for the simultaneous removal of mercury and PAHs from marine sediments. Sep. Purif. Technol. 2017, 175, 330–339. [Google Scholar] [CrossRef]
- Qiu, M.; He, C. Efficient removal of heavy metal ions by forward osmosis membrane with a polydopamine modified zeolitic imidazolate framework incorporated selective layer. J. Hazard. Mater. 2019, 367, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, S.; Lopez-Vizcaino, R.; Rodrigo, M.A.; Canizares, P.; Navarro, V.; Roa, G.; Barrera, C.; Saez, C. Scale-up of electrokinetic permeable reactive barriers for the removal of organochlorine herbicide from spiked soils. J. Hazard. Mater. 2021, 417, 126078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Boparai, H.K.; Wang, J.; Sleep, B.E. Effect of low permeability zone location on remediation of Cr(VI)-contaminated media by electrokinetics combined with a modified-zeolite barrier. J. Hazard. Mater. 2022, 426, 127785. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shady, A.; Eissa, D.; Abdelmottaleb, O.; Hegab, R. New approaches to remediate heavy metals containing polluted soil via improved PCPSS. J. Environ. Chem. Eng. 2018, 6, 1322–1332. [Google Scholar] [CrossRef]
- Abou-Shady, A.; Peng, C. New process for ex situ electrokinetic pollutant removal. I:process evaluation. J. Ind. Eng. Chem. 2012, 18, 2162–2176. [Google Scholar] [CrossRef]
- Abou-Shady, A. Reclaiming salt-affected soils using electro-remediation technology: PCPSS evaluation. Electrochim. Acta 2016, 190, 511–520. [Google Scholar] [CrossRef]
- Abou-Shady, A.; El-Araby, H. Electro-agric, a novel environmental engineering perspective to overcome the global water crisis via marginal water reuse. Nat. Hazards Res. 2021, 1, 202–226. [Google Scholar] [CrossRef]
- Lockhart, N.C. Electro-osmotic dewatering of clay-Ⅲ. Influence of clay type, exchangeable cations and electrode materials. Colloid Surf. 1983, 6, 253–269. [Google Scholar] [CrossRef]
- Dalloway, B.J. Heavy Matals in Soils; John Wiley & Sons, Inc.: New York, NY, USA, 1990; Volume 339. [Google Scholar]
- Kim, W.S.; Kim, S.O.; Kim, K.W. Enhancement electrokinetic extraction of heavy metals from soils assisted by ion exchange membranes. J. Hazard. Mater. 2005, 118, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Apostolos, G.; Aris, N.; Despina, P.; Evangelos, G. Chelating agent-assisted electrokinetic removal of cadmium, lead and copper from contaminated soils. Environ. Pollut. 2009, 157, 3379–3386. [Google Scholar]
- Annamalai, S.; Santhanam, M.; Sundaram, M.; Curras, M.P. Electrokinetic remediation of inorganic and organic pollutants in textile effluent contaminated agricultural soil. Chemosphere 2014, 117, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Cappai, G.; Gioannis, G.D.; Muntoni, A.; Spiga, D.; Zijlstra, J. Combined use of a transformed red mud reactive barrier and electrokinetics for remediation of Cr/As contaminated soil. Chemosphere 2012, 86, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Kwon, M.J.; Choi, J.; Baek, K.; O’Loughlin, E.J. The transport behavior of As, Cu, Pb, and Zn during electrokinetic remediation of a contaminated soil using electrolyte conditioning. Chemosphere 2014, 117, 79–86. [Google Scholar] [CrossRef]
- Yong, R.N.; Mohamed, A.M.O.; Warkentin, B.P. Principles of Contaminant Transport in Soils; Elsevier Science: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Wen, D.D.; Fu, R.B.; Zhang, W.; Gu, Y.Y. Enhanced electrokinetic remediation of heavy metals contaminated soils by stainless steel electrodes as well as the phenomenon and mechanism of electrode corrosion and crystallization. Huanjing Kexue 2017, 38, 1209–1217. [Google Scholar] [PubMed]
- Wang, J.; Huang, X.; Kao, J.; Stabnikova, O. Removal of heavy metals from kaolin using an upward electrokinetic soil remedial (UESR) technology. J. Hazard Mater. 2006, 136, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jin, C.; Sun, Z.; Huang, G.; She, Z. Assessment of acid enhancement schemes for electrokinetic remediation of Cd/Pb contaminated soil. Water Air Soil Pollut. 2016, 227, 1–12. [Google Scholar] [CrossRef]









| O | Ca | C | K | Cl | Na | P | Fe | Si | Mg | S | Al | Other | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 38.39 | 25.51 | 21.73 | 3.2 | 3.05 | 2.64 | 1.5 | 1.29 | 0.97 | 0.71 | 0.51 | 0.27 | 0.23 | 100 |
| Fixed factor | |||||||||||
| Electrode Gradient (V/cm) | Improver (M) | PRB Location (x/L) | Duration (Day) | ||||||||
| 1 | Acetic acid 0.05 | 0.75 | 10 | ||||||||
| Variable factor | |||||||||||
| Contaminant (ppm) | |||||||||||
| Copper | Lead | Complex pollution (Cu/Pb) | |||||||||
| 500 | 1000 | 500/1000 | |||||||||
| PRB thickness (cm) | |||||||||||
| 1 | 1 | 2 | |||||||||
| Duration after electrode exchange (Day) | |||||||||||
| 6 | 8 | 10 | 6 | 8 | 10 | 6 | 8 | 10 | |||
| Exp. C-6 | Exp. C-8 | Exp. C-10 | Exp. P-6 | Exp. P-8 | Exp. P-10 | Exp. CP-6 | Exp. CP-8 | Exp. CP-10 | |||
| Test | Initial Amount of Pollutant (mg) | Residual in the Soil (mg) | PRB (mg) | Amount Removed by EOF (mg) | Water Tanks (mg) | Mass Balance (%) | Removal (%) |
|---|---|---|---|---|---|---|---|
| Exp. C-6 | 180 | 26.24 | 72.84 | 0.09 | 1.57 | 99.99 | 85.42 |
| Exp. C-8 | 180 | 13.52 | 55.06 | 0.44 | 9.42 | 78.44 | 92.49 |
| Exp. C-10 | 180 | 22.24 | 72.10 | 0.38 | 2.15 | 96.87 | 87.65 |
| Test | Initial Amount of Pollutant (mg) | Residual in the Soil (mg) | PRB (mg) | Amount Removed by EOF (mg) | Water Tanks (mg) | Mass Balance (%) | Removal (%) |
|---|---|---|---|---|---|---|---|
| Exp. P-6 | 360 | 319.16 | 27.17 | 0.04 | 0.03 | 96.22 | 11.34 |
| Exp. P-8 | 360 | 238.28 | 65.35 | 0.07 | 0.05 | 84.37 | 33.81 |
| Exp. P-10 | 360 | 355.79 | 51.65 | 0.35 | 0.02 | 113.84 | 0.61 |
| Test | Initial Amount of Pollutant (mg) | Residual in the Soil (mg) | PRB (mg) | Amount Removed by EOF (mg) | Water Tanks (mg) | Mass Balance (%) | Removal (%) | |
|---|---|---|---|---|---|---|---|---|
| Cu | Exp. CP-6 | 180 | 43.62 | 143.50 | 0.26 | 0.72 | 104.50 | 75.77 |
| Exp. CP-8 | 180 | 18.69 | 131.42 | 0.35 | 0.30 | 83.75 | 89.62 | |
| Exp. CP-10 | 180 | 23.17 | 186.38 | 0.24 | 0.21 | 116.67 | 87.13 | |
| Pb | Exp. CP-6 | 360 | 282.57 | 37.22 | 37.22 | 0.78 | 89.12 | 21.51 |
| Exp. CP-8 | 360 | 308.38 | 36.37 | 36.37 | 0.00 | 95.84 | 14.34 | |
| Exp. CP-10 | 360 | 267.70 | 49.67 | 49.67 | 0.00 | 88.23 | 25.64 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-N.; Kim, J.-Y.; Lee, J.-Y.; Han, J.-G.; Kim, D.-C. Permeable Reactive Barrier Remediation Technique Using Carbonized Food Waste in Ground Contaminated with Combined Cu and Pb. Sustainability 2024, 16, 4794. https://doi.org/10.3390/su16114794
Kim D-N, Kim J-Y, Lee J-Y, Han J-G, Kim D-C. Permeable Reactive Barrier Remediation Technique Using Carbonized Food Waste in Ground Contaminated with Combined Cu and Pb. Sustainability. 2024; 16(11):4794. https://doi.org/10.3390/su16114794
Chicago/Turabian StyleKim, Dong-Nam, Ji-Yoon Kim, Jong-Young Lee, Jung-Geun Han, and Dong-Chan Kim. 2024. "Permeable Reactive Barrier Remediation Technique Using Carbonized Food Waste in Ground Contaminated with Combined Cu and Pb" Sustainability 16, no. 11: 4794. https://doi.org/10.3390/su16114794









