Thermal and Oxidative Stability of Biocrude Oil Derived from the Continuous Hydrothermal Liquefaction of Spirulina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Continuous Biocrude Oil Production
2.3. Storage Environment of Biocrude Oil
2.4. Analysis
3. Results and Discussion
3.1. Physical Properties
3.2. Element Distribution and Calorific Value
3.3. Boiling Point Distribution
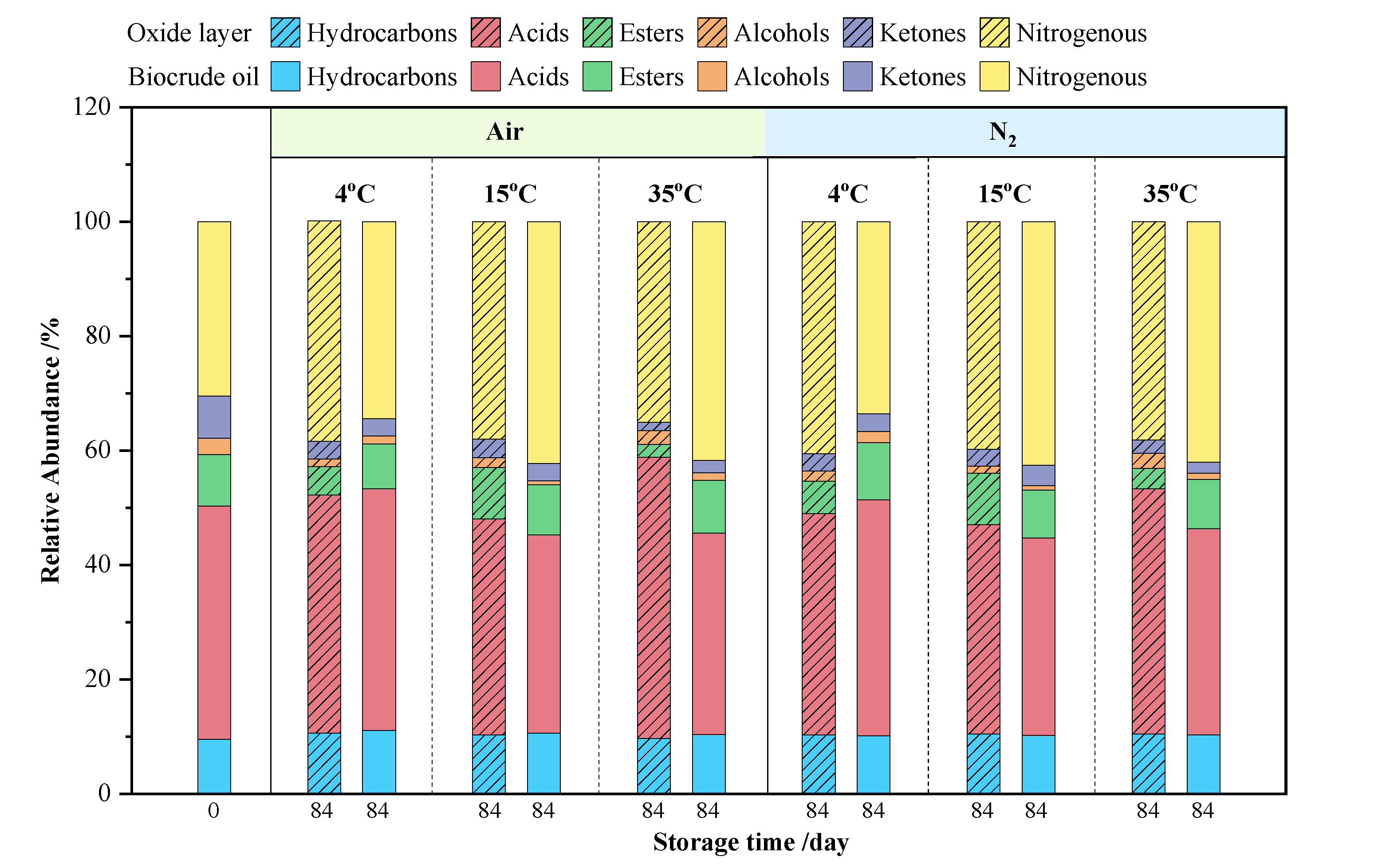
3.4. GC-MS Analysis
3.5. FT-IR Analysis
3.6. Relationship between Storage Conditions and Characteristics of Biocrude Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, S.; Shao, Y.; Bao, T.; Zhu, J. Review on Nitrogen Transformation during Microalgae Thermochemical Liquefaction: Recent Advances and Future Perspectives. Energy Fuels 2023, 37, 1525–1544. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Chen, D.; Yin, L.; Duan, P. Hydro-upgrading of algal bio-oil in tetralin for the production of high-quality liquid fuel: Process intensification. Fuel Process. Technol. 2021, 224, 107034. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Y.; Yang, W.; Li, Y.; Yang, H.; Chen, Y.; Wang, X.; Chen, H. Valorization of the microalgae fixing CO2 from flue gas by co-hydrothermal liquefaction with high-protein microalgae: Denitrogenation of bio-oil by ash and high energy recovery. Fuel 2023, 340, 127566. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Chen, W.; Lin, B.; Huang, M.; Chang, J. Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour. Technol. 2015, 184, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lee, T.H.; Li, Y.; Chen, W.; Zhang, Y. Spray and combustion characteristics of pure hydrothermal liquefaction biofuel and mixture blends with diesel. Fuel 2021, 294, 120498. [Google Scholar] [CrossRef]
- Eboibi, B.E.; Eboibi, O.; Amabogha, B.; Okan, O.L.; Agarry, S.E. Influence of Seawater and Reaction Temperature on Biocrude Yield and Composition During Hydrothermal Liquefaction of Spirulina sp. Microalgal Biomass. Waste Biomass Valoriz. 2023, 15, 3055–3076. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Z.; Lu, J.; Watson, J.; Kong, D.; Wang, K.; Zhang, Y.; Liu, Z. Establishment and performance of a plug-flow continuous hydrothermal reactor for biocrude oil production. Fuel 2020, 280, 118605. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, Z. Effect of Aging in Nitrogen and Air on the Properties of Biocrude Produced by Hydrothermal Liquefaction of Spirulina. Energy Fuels 2019, 33, 9870–9878. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, W.; Liu, H. Aging and emulsification analyses of hydrothermal liquefaction bio-oil derived from sewage sludge and swine leather residue. J. Clean. Prod. 2020, 266, 122050. [Google Scholar] [CrossRef]
- Huyen Nguyen Lyckeskog, C.M.L.Å.; Sven-Ingvar Andersson, L.V.A.H.; Adhikari, S. Storage Stability of Bio-oils Derived from the Catalytic Conversion of Softwood Kraft Lignin in Subcritical Water. Energy Fuels 2016, 30, 3097–3106. [Google Scholar] [CrossRef]
- Kosinkova, J.; Ramirez, J.A.; Ristovski, Z.D.; Brown, R.; Rainey, T.J. Physical and Chemical Stability of Bagasse Biocrude from Liquefaction Stored in Real Conditions. Energy Fuels 2016, 30, 10499–10504. [Google Scholar] [CrossRef]
- Liu, G.; Du, H.; Sailikebuli, X.; Meng, Y.; Liu, Y.; Wang, H.; Zhang, J.; Wang, B.; Saad, M.G.; Li, J.; et al. Evaluation of Storage Stability for Biocrude Derived from Hydrothermal Liquefaction of Microalgae. Energy Fuels 2021, 35, 10623–10629. [Google Scholar] [CrossRef]
- Palomino, A.; Godoy-Silva, R.D.; Raikova, S.; Chuck, C.J. The storage stability of biocrude obtained by the hydrothermal liquefaction of microalgae. Renew. Energy 2020, 145, 1720–1729. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Yoshikawa, K.; Li, H.; Liu, Z. Effect of biomass origins and composition on stability of hydrothermal biocrude oil. Fuel 2021, 302, 121138. [Google Scholar] [CrossRef]
- Sluiter, A.; Hyman BH, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; NREL/TP-510-42621; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- ASTM E1755-01; Standard Test Method for Ash in Biomass. ASTM International Publishing: West Conshohocken, PA, USA, 2002.
- NB/T 34057.9-2020; Determination of Chemical Composition of Lignocellulosic Biomass Feedstocks—Part 9: Determination of Lipids. National Energy Administration Publishing: Beijing, China, 2020.
- GB/T 6432-2018; Determination of Crude Protein in Feeds-Kjeldahl Method. State Administration for Market Regulation Publishing: Beijing, China, 2018.
- NY/T 3494-2019; Agricultural Biomass Raw Materials—Determination of Cellulose, Hemicellulose, and Lignin. Ministry of Agriculture and Rural Affairs of the People’s Republic of China Publishing: Beijing, China, 2019.
- ASTM D445; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Lipids. ASTM International Publishing: West Conshohocken, PA, USA, 2000.
- Yang, Z.; Hollebone, B.P.; Wang, Z.; Yang, C.; Landriault, M. Effect of storage period on the dominant weathering processes of biodiesel and its blends with diesel in ambient conditions. Fuel 2013, 104, 342–350. [Google Scholar] [CrossRef]
- Pedraza, H.; Wang, H.; Han, X.; Zeng, Y.; Liu, J. Corrosion and aging risk assessment of an injection system for FCCBio-oil co-feed. Biomass Bioenergy 2023, 175, 106875. [Google Scholar] [CrossRef]
- Meng, J.; Moore, A.; Tilotta, D.; Kelley, S.; Park, S. Toward Understanding of Bio-Oil Aging: Accelerated Aging of Bio-Oil Fractions. ACS Sustain. Chem. Eng. 2014, 2, 2011–2018. [Google Scholar] [CrossRef]
- Oasmaa, A.; Korhonen, J.; Kuoppala, E. An Approach for Stability Measurement of Wood-Based Fast Pyrolysis Bio-Oils. Energy Fuels 2011, 25, 3307–3313. [Google Scholar] [CrossRef]
- Yu, Y.; Li, C.; Jiang, C.; Chang, J.; Shen, D. Aging Behaviors of Phenol-Formaldehyde Resin Modified by Bio-Oil under Five Aging Conditions. Polymers 2022, 14, 1352. [Google Scholar] [CrossRef]
- Vardon, D.R.; Sharma, B.K.; Scott, J.; Yu, G.; Wang, Z.; Schideman, L.; Zhang, Y.; Strathmann, T.J. Chemical properties of biocrude oil from the hydrothermal liquefaction of Spirulina algae, swine manure, and digested anaerobic sludge. Bioresour. Technol. 2011, 102, 8295–8303. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative stability of biodiesel: Causes, effects and prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Lee, T.H.; Wu, Z.; Si, B.; Lee, C.F.; Lin, A.; Sharma, B.K. Renewable diesel blendstocks produced by hydrothermal liquefaction of wet biowaste. Nat. Sustain. 2018, 1, 702–710. [Google Scholar] [CrossRef]
- Li, J.; Fang, X.; Bian, J.; Guo, Y.; Li, C. Microalgae hydrothermal liquefaction and derived biocrude upgrading with modified SBA-15 catalysts. Bioresour. Technol. 2018, 266, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yan, R.; Cai, C.; Chen, X.; Zhao, F.; Fan, L.; Xu, C.C.; Yang, W. Hydrothermal liquefaction of Cd-enriched Amaranthus hypochondriacus L. in ethanol–water co-solvent: Focus on low-N bio-oil and heavy metal/metal-like distribution. Fuel 2021, 303, 121235. [Google Scholar] [CrossRef]
- Fu, S.; Yang, J.; Shi, M.; Wei, D.; Yin, H.; Sun, J.; He, J. Re-express hydrothermal liquefaction bio-crude in petroleum way. Fuel 2017, 191, 164–169. [Google Scholar] [CrossRef]
- Valdez, P.J.; Tocco, V.J.; Savage, P.E. A general kinetic model for the hydrothermal liquefaction of microalgae. Bioresour. Technol. 2014, 163, 123–127. [Google Scholar] [CrossRef]
- Taghipour, A.; Hornung, U.; Ramirez, J.A.; Brown, R.J.; Rainey, T.J. Fractional distillation of algae based hydrothermal liquefaction biocrude for co-processing: Changes in the properties, storage stability, and miscibility with diesel. Energy Convers. Manag. 2021, 236, 114005. [Google Scholar] [CrossRef]
- Huyen, N.L.; Mattsson, C.; Olausson, L.; Andersson, S.; Vamling, L.; Theliander, H. Thermal stability of low and high Mw fractions of bio-oil derived from lignin conversion in subcritical water. Biomass Convers. Biorefin. 2017, 7, 401–414. [Google Scholar]
- Wang, C.; Ding, H.; Zhang, Y.; Zhu, X. Analysis of property variation and stability on the aging of bio-oil from fractional condensation. Renew. Energy 2020, 148, 720–728. [Google Scholar] [CrossRef]
- Ribeiro, L.A.B.; Martins, R.C.; Mesa-Pérez, J.M.; Bizzo, W.A. Study of bio-oil properties and ageing through fractionation and ternary mixtures with the heavy fraction as the main component. Energy 2019, 169, 344–355. [Google Scholar] [CrossRef]
- Epping, R.; Kerkering, S.; Andersson, J.T. Influence of Different Compound Classes on the Formation of Sediments in Fossil Fuels During Aging. Energy Fuels 2014, 28, 5649–5656. [Google Scholar] [CrossRef]
- Bauserman, J.W.; George, W.; Mushrush, A.; Hardy, D.R. Organic Nitrogen Compounds and Fuel Instability in Middle Distillate Fuels. Ind. Eng. Chem. Res. 2008, 47, 2867–2875. [Google Scholar] [CrossRef]
- Abdul-Quadir, M.S.; Ferg, E.E.; Tshentu, Z.R.; Ogunlaja, A.S. Remarkable adsorptive removal of nitrogen containing compounds from hydrotreated fuel by molecularly imprinted poly-2-(1H-imidazol-2-yl)-4-phenol nanofibers†. R. Soc. Chem. 2018, 8, 8039–8050. [Google Scholar] [CrossRef]
- Ding, X.; Li, T.; Wang, J.; Wu, L.; Zheng, L.; Wang, Y. Catalytic co-liquefaction of microalgae + corn straw over K3PO4+γ-Al2O3 supported Fe and Ni mono-/bimetallic in-situ composite catalysts for the production of liquid biofuel. Chem. Eng. J. 2023, 471, 144668. [Google Scholar] [CrossRef]
- Cui, Z.; Cheng, F.; Jarvis, J.M.; Jena, U.; Brewer, C.E. Integrated Extraction and Catalytic Upgrading of Biocrude Oil from Co-hydrothermal Liquefaction of Crude Glycerol and Algae. Energy Fuels 2021, 35, 12165–12174. [Google Scholar] [CrossRef]
- Li, H.; Tian, C.; Lu, J.; He, C. Vacuum fractional distillation of biocrude oil and the immobilization of harmful metal. Fuel 2022, 326, 125013. [Google Scholar] [CrossRef]
- Si, B.; Watson, J.; Wang, Z.; Wang, T.; Acero Triana, J.S.; Zhang, Y. Storage stability of biocrude oil fractional distillates derived from the hydrothermal liquefaction of food waste. Renew. Energy 2024, 220, 119669. [Google Scholar] [CrossRef]





| Sample | Storage Temperature/°C | Atmosphere | |||
|---|---|---|---|---|---|
| 4 | 15 | 35 | Air | N2 | |
| Air-4 | √ | √ | |||
| N2-4 | √ | √ | |||
| Air-15 | √ | √ | |||
| N2-15 | √ | √ | |||
| Air-35 | √ | √ | |||
| N2-35 | √ | √ | |||
| ID | C (%) | H (%) | N (%) | O 1 (%) | HHV (MJ·kg−1) |
|---|---|---|---|---|---|
| Fresh biocrude | 69.26 ± 0.09 | 9.175 ± 0.15 | 7.475 ± 0.01 | 14.09 | 33.97 |
| Oxide layer | |||||
| Air-4UO | 67.41 ± 0.03 | 8.886 ± 0.04 | 7.047 ± 0.05 | 16.66 | 32.48 |
| Air-15UO | 68.93 ± 0.06 | 8.897 ± 0.01 | 7.202 ± 0.03 | 14.97 | 33.31 |
| Air-35UO | 69.88 ± 0.09 | 8.641 ± 0.01 | 7.177 ± 0.02 | 14.30 | 33.39 |
| N2-4UO | 65.26 ± 0.16 | 8.994 ± 0.07 | 6.817 ± 0.05 | 18.93 | 31.50 |
| N2-15UO | 66.54 ± 0.11 | 8.878 ± 0.04 | 6.936 ± 0.00 | 17.65 | 32.00 |
| N2-35UO | 67.11 ± 0.03 | 9.11 ± 0.01 | 7.230 ± 0.02 | 16.55 | 32.72 |
| Aged biocrude | |||||
| Air-4 | 65.63 ± 0.03 | 9.311 ± 0.06 | 6.855 ± 0.01 | 18.20 | 32.21 |
| Air-15 | 64.21 ± 0.01 | 9.522 ± 0.24 | 6.691 ± 0.02 | 19.58 | 31.78 |
| Air-35 | 64.49 ± 0.05 | 9.491 ± 0.05 | 6.718 ± 0.00 | 19.30 | 31.88 |
| N2-4 | 66.04 ± 0.01 | 9.364 ± 0.04 | 6.919 ± 0.02 | 17.68 | 32.51 |
| N2-15 | 64.41 ± 0.07 | 9.192 ± 0.10 | 6.688 ± 0.05 | 19.71 | 31.36 |
| N2-35 | 64.82 ± 0.02 | 9.226 ± 0.56 | 6.961 ± 0.02 | 18.99 | 31.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cao, M.; Lan, W.; Yin, D. Thermal and Oxidative Stability of Biocrude Oil Derived from the Continuous Hydrothermal Liquefaction of Spirulina. Sustainability 2024, 16, 4884. https://doi.org/10.3390/su16124884
Wang Y, Cao M, Lan W, Yin D. Thermal and Oxidative Stability of Biocrude Oil Derived from the Continuous Hydrothermal Liquefaction of Spirulina. Sustainability. 2024; 16(12):4884. https://doi.org/10.3390/su16124884
Chicago/Turabian StyleWang, Yingxian, Maojiong Cao, Weijuan Lan, and Dongxue Yin. 2024. "Thermal and Oxidative Stability of Biocrude Oil Derived from the Continuous Hydrothermal Liquefaction of Spirulina" Sustainability 16, no. 12: 4884. https://doi.org/10.3390/su16124884




