Carbon Deposition Characteristics in Thermal Conversion of Methane for Sustainable Fuel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrothermal Synthesis of CoAl Spinel Oxides
- (1)
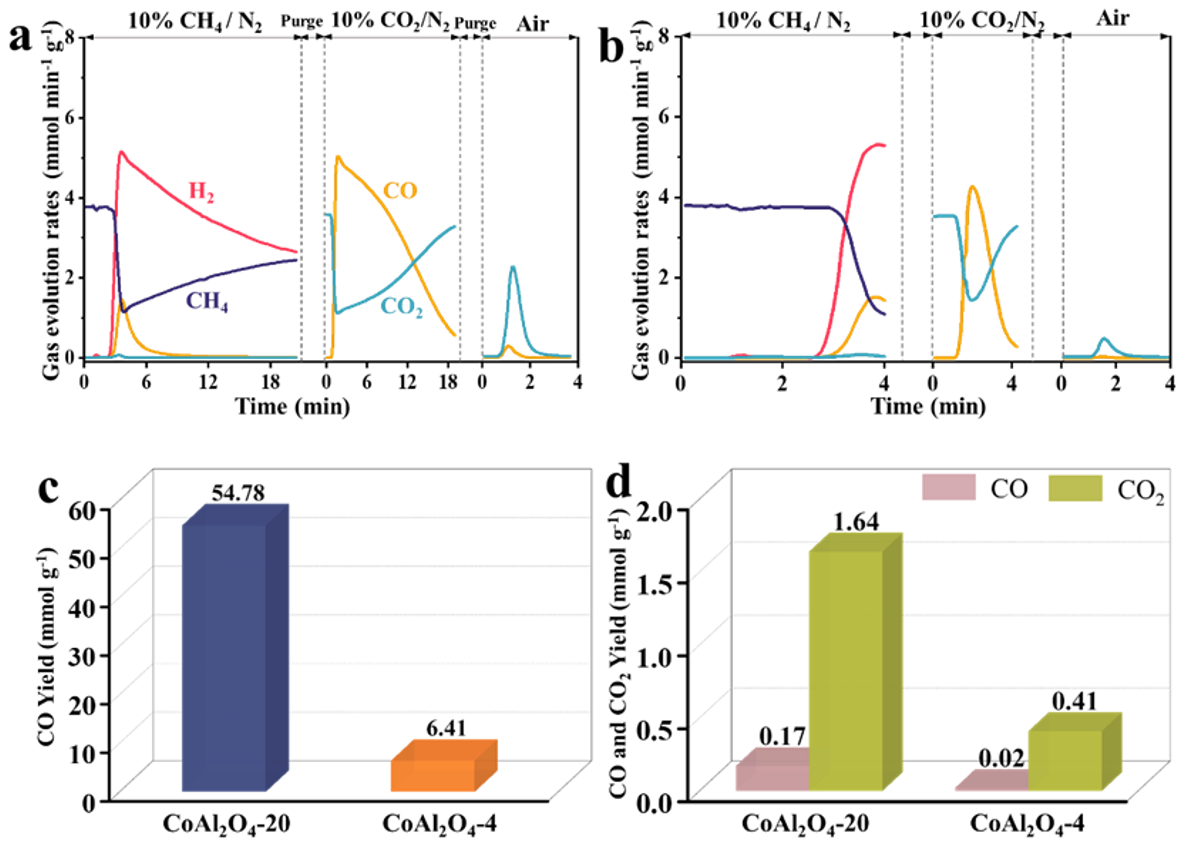
- Reaction time: Reaction durations of 20 and 4 min were selected, with the corresponding samples labeled as CoAl2O4−20 and CoAl2O4−4, respectively.
- (2)
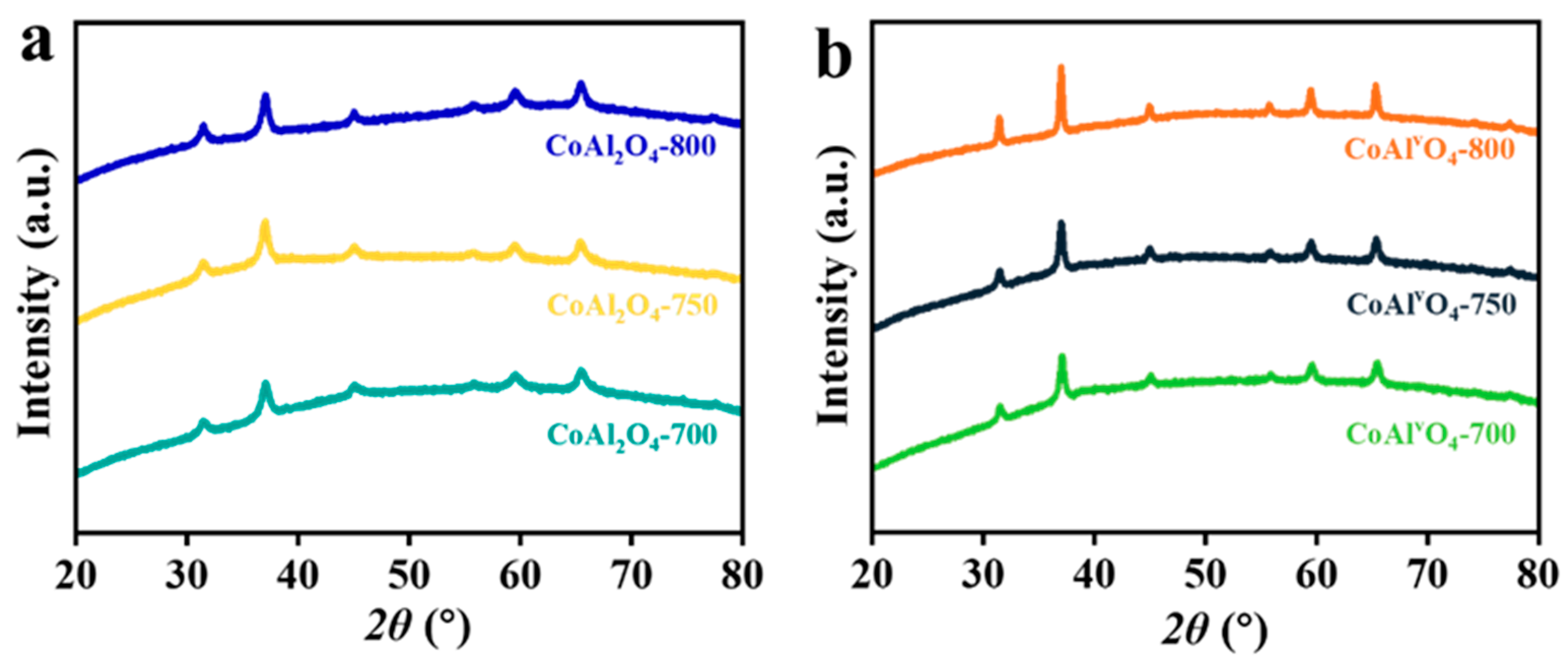
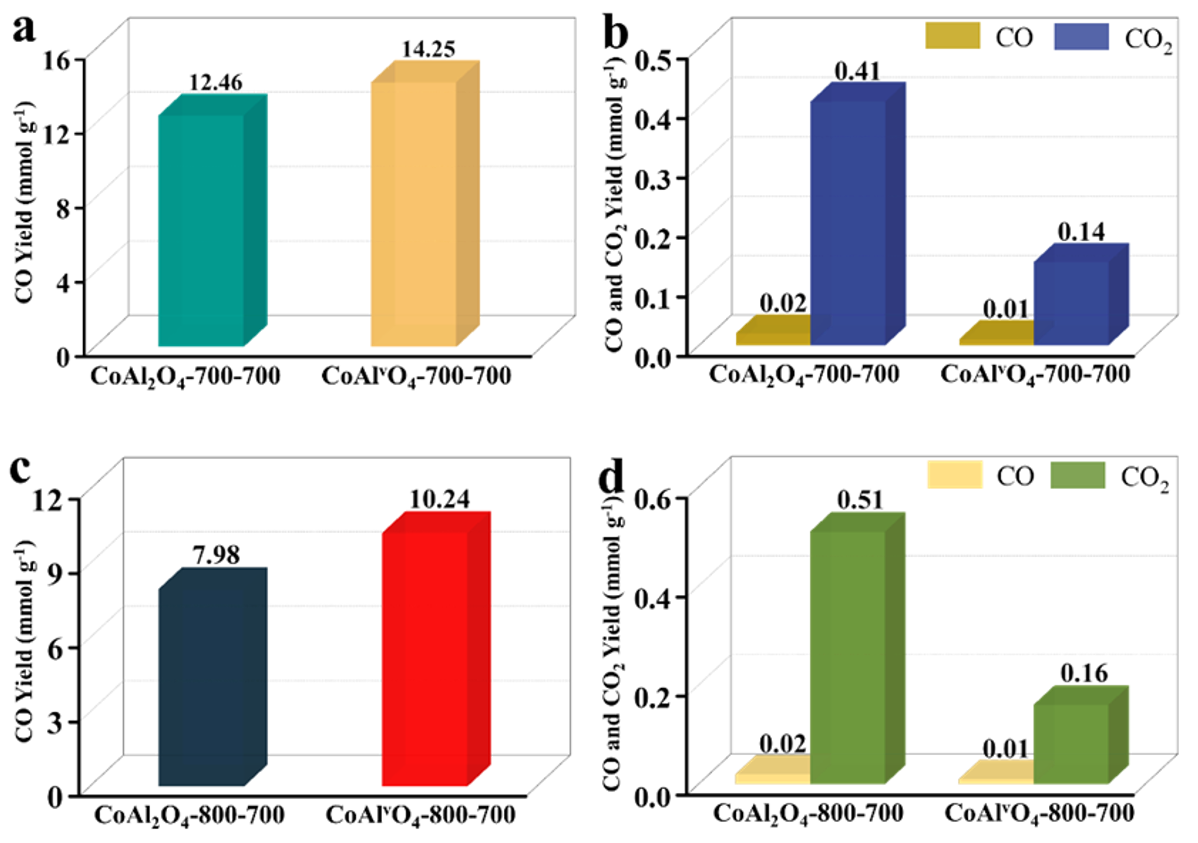
- Calcination temperature of metal oxide: Temperatures of 700 and 800 °C were selected, and the corresponding samples were denoted as CoAl2O4−700−700, CoAlvO4−700−700 and CoAl2O4−800−700, CoAlvO4−800−700, respectively.
- (3)
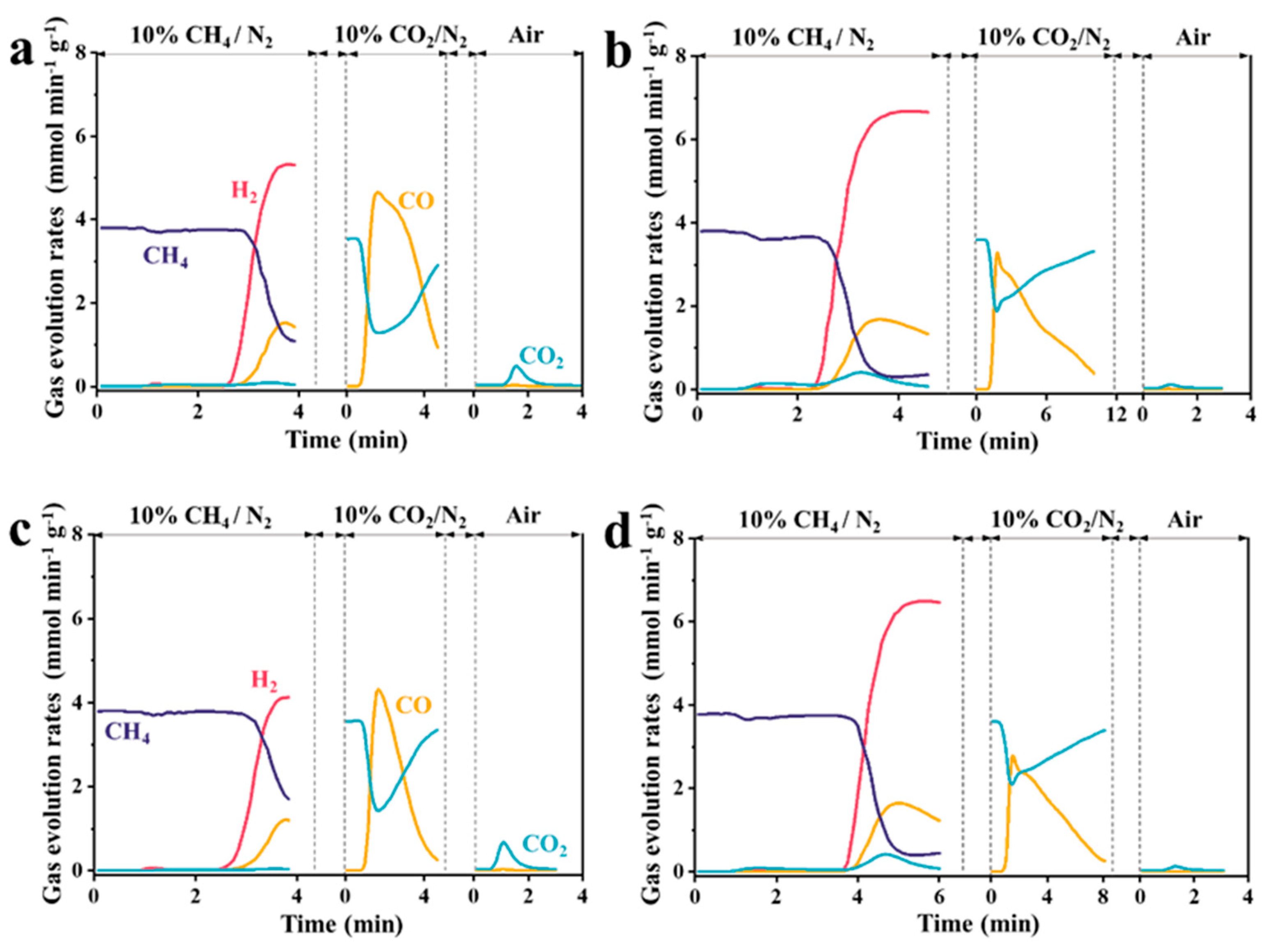
- Reaction temperature: Temperatures of 700 and 800 °C were selected, and the corresponding samples were denoted as CoAl2O4−750−700, CoAlvO4−750−700 and CoAl2O4−750−800, CoAlvO4−750−800, respectively.
2.2. Physical and Chemical Characterization
2.3. Reactivity Tests
3. Results and Discussion
3.1. Effect of Different Factors on the Production of Carbon Deposition
3.1.1. Reaction Time
3.1.2. Calcination Temperature of Metal Oxide
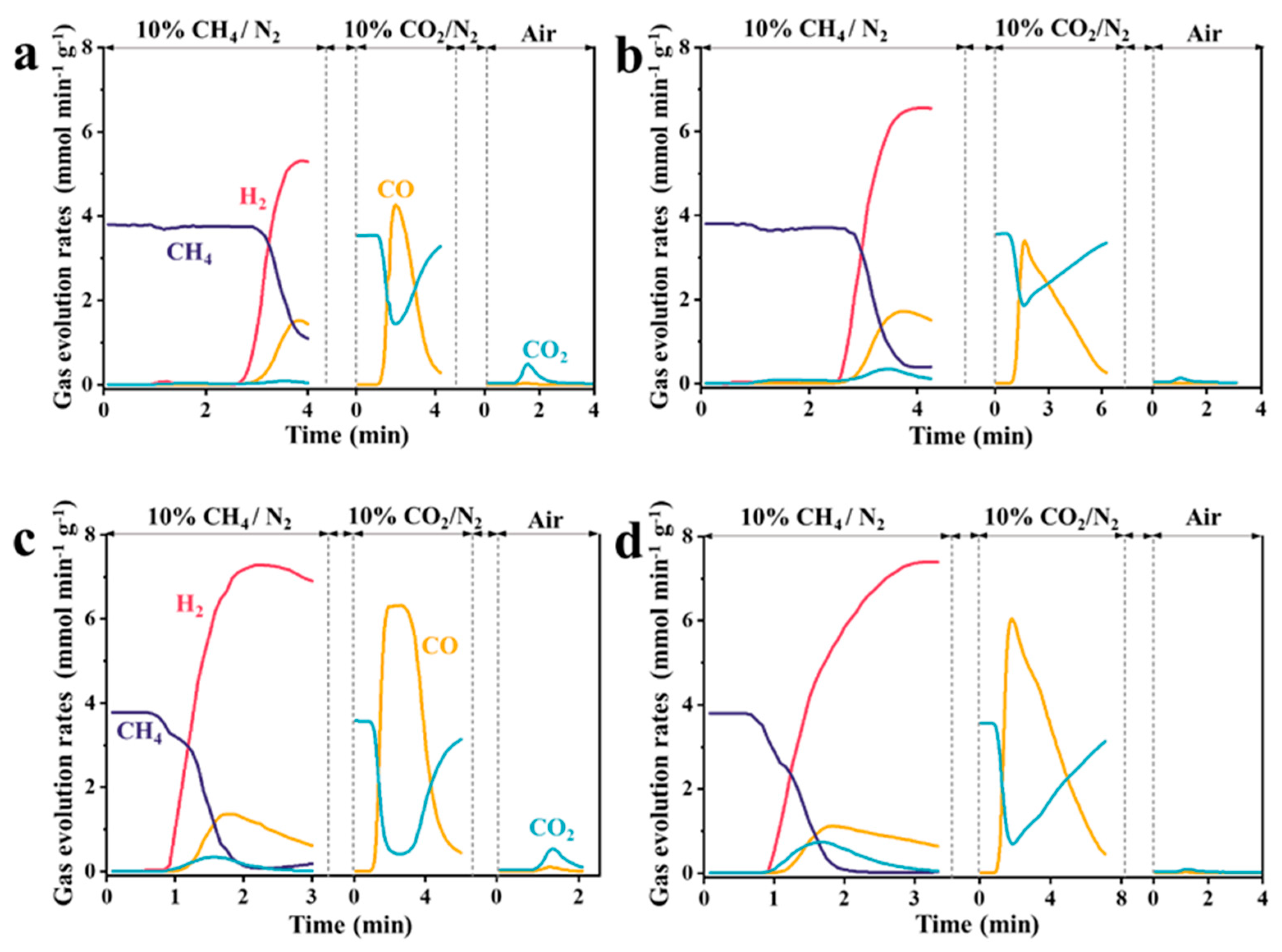
3.1.3. Reaction Temperature
3.2. Characterization of Carbon Deposition
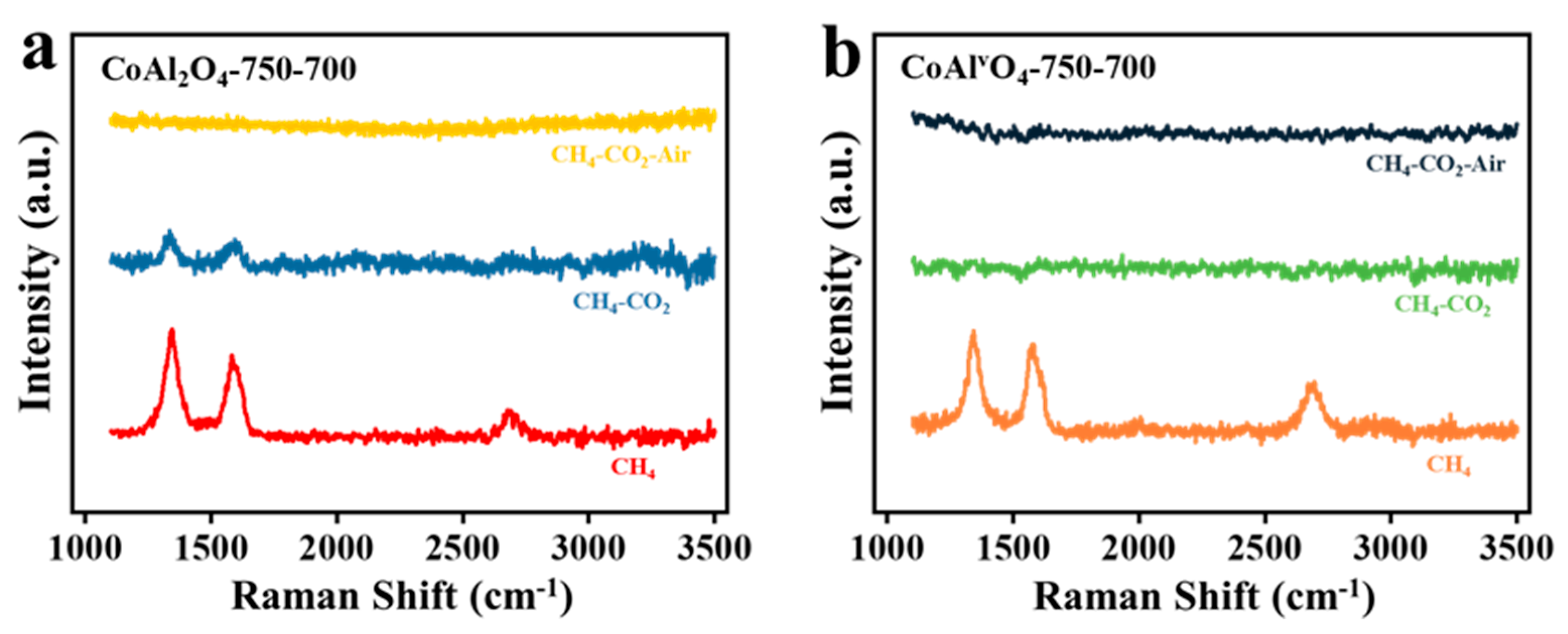
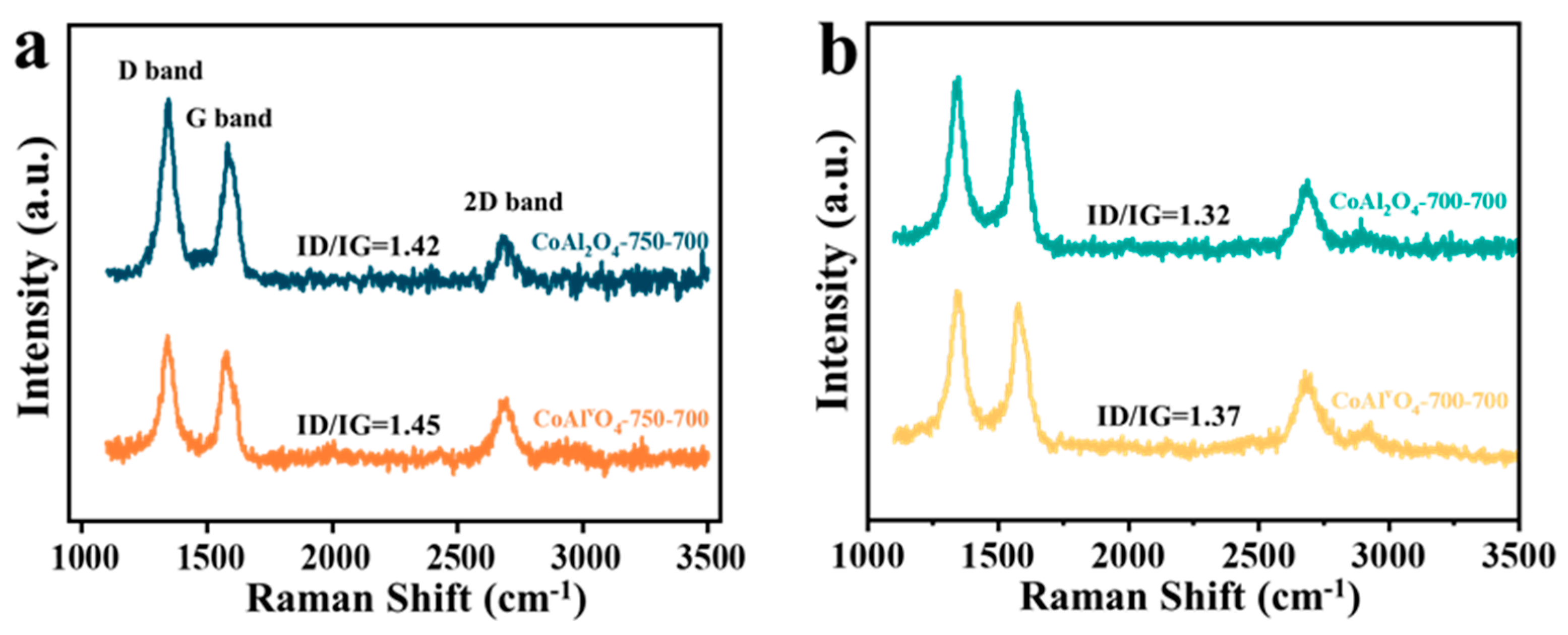
3.2.1. Raman Analysis
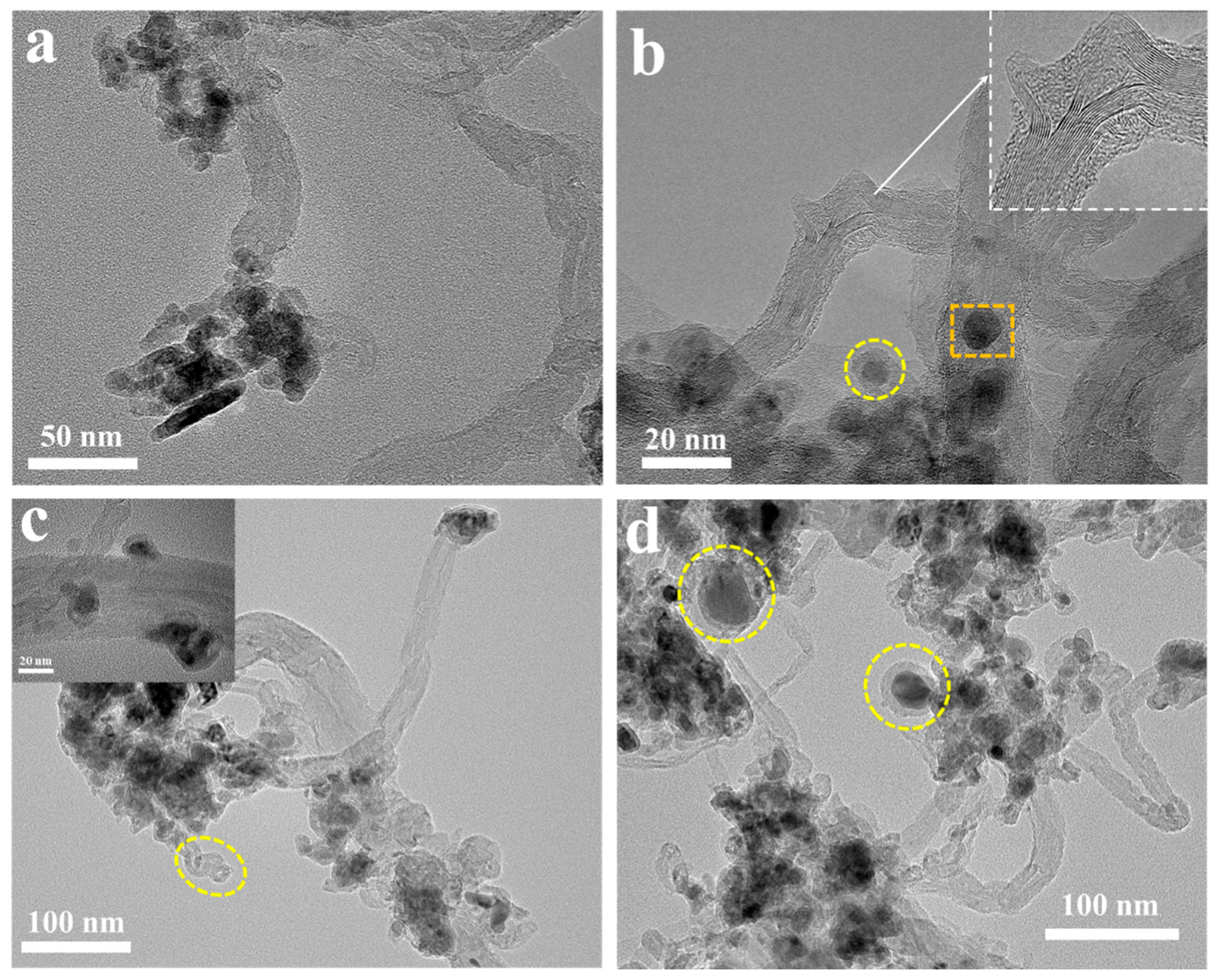
3.2.2. Microstructure Characterization
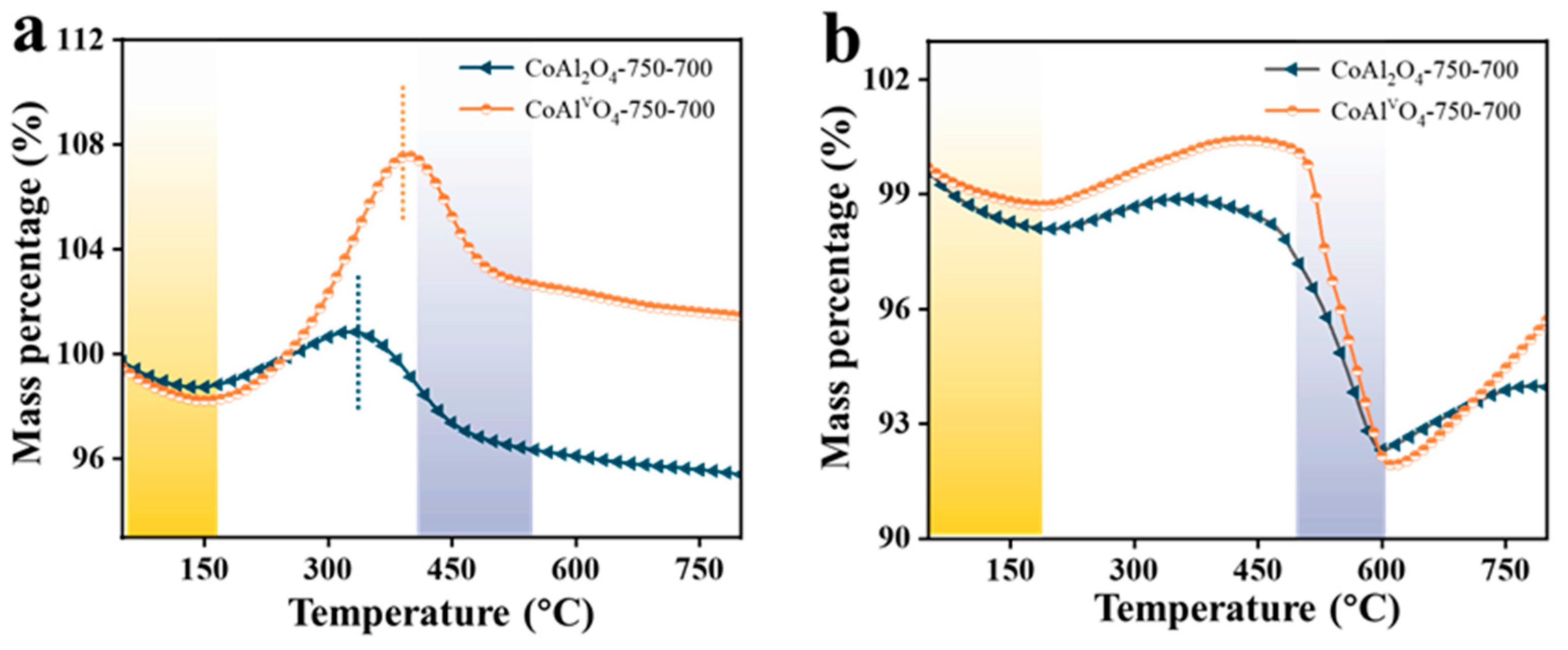
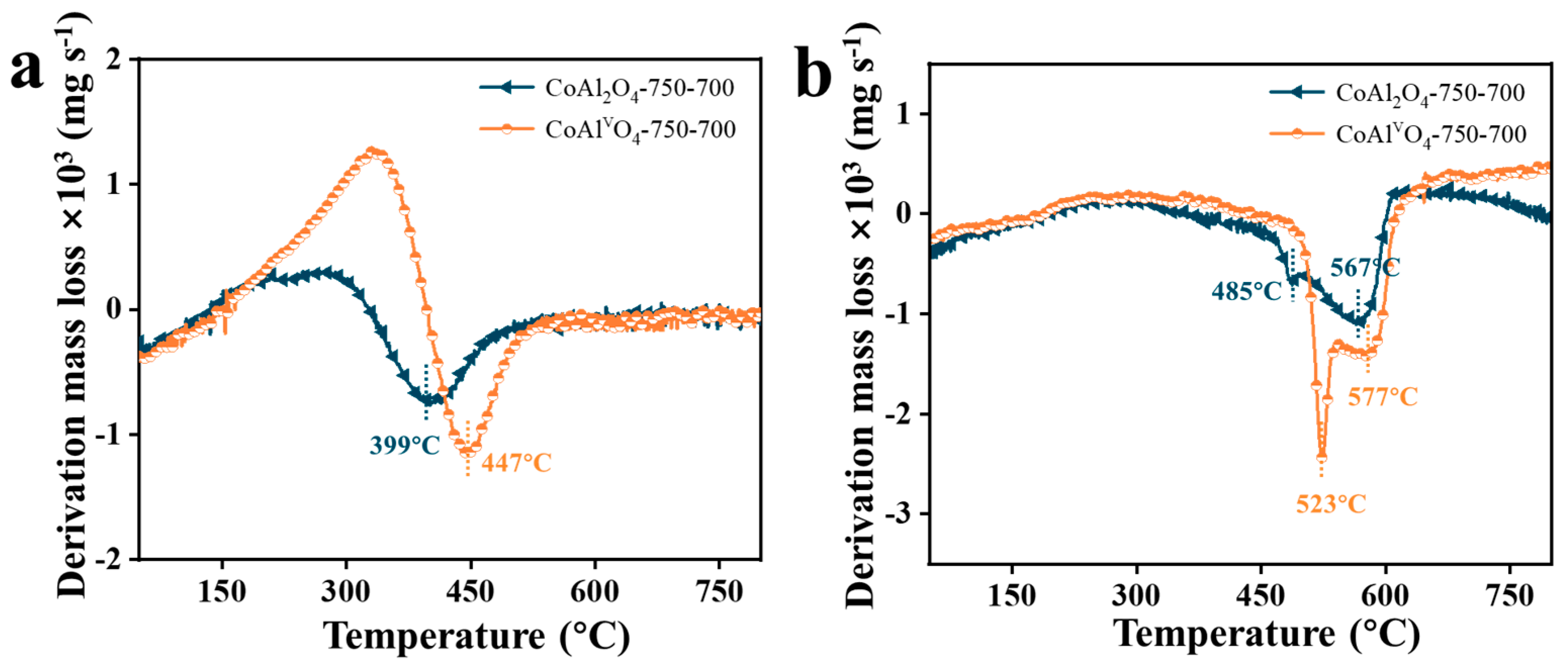
3.2.3. Thermogravimetric (TG) Analysis
4. Conclusions
- (1)
- The composition of carbon deposition over CoAl2O4 and CoAlvO4 depends on the material itself and reaction time; calcination temperature and reaction temperature do not affect the compositions of carbon deposition.
- (2)
- In the CO2 oxidation, similar trends were observed on both CoAl2O4 and CoAlvO4 for C→CO. However, a certain concentration of CO2 was detected on CoAl2O4 in the air oxidation process, whereas CoAlvO4 exhibits an extremely low CO2 concentration, suggesting that carbon deposition on CoAlvO4 is almost completely removed during the CO2 oxidation process. This trend is also confirmed by Raman spectra.
- (3)
- Raman spectroscopy showed that ID/IG in CoAlvO4 is higher than that of CoAl2O4, indicating the decrease in the degree of graphitization of the carbon deposition on CoAlvO4. Thermogravimetric analysis showed that the strong alkali etching in CoAl2O4 only affects contents in different types of carbon deposition. It is mainly attributed to the cation vacancies produced by alkali etching, which efficiently modulates the surface electronic structure of CoAl2O4, thus resulting in differences in carbon deposition on CoAlvO4 surface compared to CoAl2O4.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serbin, S.; Radchenko, M.; Pavlenko, A.; Burunsuz, K.; Radchenko, A.; Chen, D. Improving Ecological Efficiency of Gas Turbine Power System by Combusting Hydrogen and Hydrogen-Natural Gas Mixtures. Energies 2023, 16, 3618. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.H.A.; Fennell, P.S.; Shen, L.H.; Fan, L.S. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef]
- Lee, C.T.; Hashim, H.; Ho, C.S.; Fan, Y.V.; Klemeš, J.J. Sustaining the low-carbon emission development in Asia and beyond: Sustainable energy, water, transportation and low-carbon emission technology. J. Clean. Prod. 2017, 146, 1–13. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Nagaraja, S.S.; Singh, E.; Cenker, E.; Amer, A. Review of life cycle assessments (LCA) for mobility powertrains. Transp. Eng. 2022, 10, 100148. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, A.; Xiao, H.; Jano-Ito, M.; Alnaeli, M.; Alnajideen, M.; Mashruk, S.; Valera-Medina, A. Emission reduction and cost-benefit analysis of the use of ammonia and green hydrogen as fuel for marine applications. Green Energy Resour. 2023, 1, 100046. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Catalyst-assisted chemical looping for CO2 conversion to CO. Appl. Catal. B Environ. 2015, 164, 184–191. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.; Zhao, Z.; He, F.; Huang, Z.; Wei, G.; Xia, C. Reaction schemes of barium ferrite in biomass chemical looping gasification for hydrogen-enriched syngas generation via an outer-inner looping redox reaction mechanism. Energy Convers. Manag. 2019, 189, 81–90. [Google Scholar] [CrossRef]
- Omoniyi, O.A.; Dupont, V. Chemical looping steam reforming of acetic acid in a packed bed reactor. Appl. Catal. B Environ. 2018, 226, 258–268. [Google Scholar] [CrossRef]
- Jiao, F.; Li, J.; Pan, X.; Xiao, J.; Li, H.; Ma, H.; Wei, M.; Pan, Y.; Zhou, Z.; Li, M.; et al. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef]
- Shen, Q.; Huang, F.; Tian, M.; Zhu, Y.; Li, L.; Wang, J.; Wang, X. Effect of Regeneration Period on the Selectivity of Synthesis Gas of Ba-Hexaaluminates in Chemical Looping Partial Oxidation of Methane. ACS Catal. 2019, 9, 722–731. [Google Scholar] [CrossRef]
- Ashok, J.; Kathiraser, Y.; Ang, M.L.; Kawi, S. Bi-functional hydrotalcite-derived NiO–CaO–Al2O3 catalysts for steam reforming of biomass and/or tar model compound at low steam-to-carbon conditions. Appl. Catal. B Environ. 2015, 172–173, 116–128. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Zhang, J.; Zhang, T.; Ye, M.; Liu, Z. Regeneration of catalysts deactivated by coke deposition: A review. Chin. J. Catal. 2020, 41, 1048–1061. [Google Scholar] [CrossRef]
- Guisnet, M.; Magnoux, P. Organic chemistry of coke formation. Appl. Catal. A Gen. 2001, 212, 83–96. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, S.; Ma, S.; Xiang, W. Carbon formation on iron-based oxygen carriers during CH4 reduction period in Chemical Looping Hydrogen Generation process. Chem. Eng. J. 2017, 325, 322–331. [Google Scholar] [CrossRef]
- Huang, J.; Yan, Y.; Saqline, S.; Liu, W.; Liu, B. High performance Ni catalysts prepared by freeze drying for efficient dry reforming of methane. Appl. Catal. B Environ. 2020, 275, 119109. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, C.; Li, X.; Wang, C.; Wang, X.; Cao, H.; Zhao, M.; Wu, G.; Su, W.; Ma, T.; et al. N-doping activated defective Co3O4 as an efficient catalyst for low-temperature methane oxidation. Appl. Catal. B Environ. 2020, 269, 118757. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.S.; Kang, D.; Kim, M.; Lee, J.W. Enhanced redox performance of LaFeO3 perovskite through in-situ exsolution of iridium nanoparticles for chemical looping steam methane reforming. Chem. Eng. J. 2023, 468, 143662. [Google Scholar] [CrossRef]
- Duy, N.P.H.; Phuong, N.N.; Ngoc, L.T.B.; Tri, N.; Nguyen, H.-H.T.; Vo, D.-V.N.; Phuong, P.T.T. Deactivation and in-situ regeneration of Dy-doped Ni/SiO2 catalyst in CO2 reforming of methanol. Int. J. Hydrogen Energy 2023, 48, 21224–21239. [Google Scholar]
- Fan, Z.; Weng, W.; Zhou, J.; Gu, D.; Xiao, W. Catalytic decomposition of methane to produce hydrogen: A review. J. Energy Chem. 2021, 58, 415–430. [Google Scholar] [CrossRef]
- Lim, H.S.; Kang, D.; Lee, J.W. Phase transition of Fe2O3-NiO to NiFe2O4 in perovskite catalytic particles for enhanced methane chemical looping reforming-decomposition with CO2 conversion. Appl. Catal. B Environ. 2017, 202, 175–183. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, H.S.; Kim, H.S.; Lee, M.; Lee, J.W.; Kang, D. Carbon dioxide splitting and hydrogen production using a chemical looping concept: A review. J. CO2 Util. 2022, 63, 102139. [Google Scholar]
- Wang, L.; Qi, Y.; Yang, Z.; Wu, H.; Liu, J.; Tang, Y.; Wang, F. Pt nanoparticles supported LaCoO3 as highly efficient catalysts for photo-thermal catalytic CO2 methanation. Green Energy Resour. 2023, 1, 100036. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Song, Z.; Zhao, X.; Sun, J.; Mao, Y.; Wang, W. Co3O4-CeO2 for enhanced syngas by low-temperature methane conversion with CO2 utilization via a catalytic chemical looping process. Fuel Process. Technol. 2023, 245, 107741. [Google Scholar] [CrossRef]
- Zhu, M.; Song, Y.; Chen, S.; Li, M.; Zhang, L.; Xiang, W. Chemical looping dry reforming of methane with hydrogen generation on Fe2O3/Al2O3 oxygen carrier. Chem. Eng. J. 2019, 368, 812–823. [Google Scholar] [CrossRef]
- Kang, D.; Lee, J.W. Enhanced methane decomposition over nickel–carbon–B2O3 core–shell catalysts derived from carbon dioxide. Appl. Catal. B Environ. 2016, 186, 41–55. [Google Scholar] [CrossRef]
- Mo, W.; Ma, F.; Ma, Y.; Fan, X. The optimization of Ni–Al2O3 catalyst with the addition of La2O3 for CO2–CH4 reforming to produce syngas. Int. J. Hydrogen Energy 2019, 44, 24510–24524. [Google Scholar] [CrossRef]
- Liu, C.; Chen, D.; Cao, Y.; Zhang, T.; Mao, Y.; Wang, W.; Wang, Z.; Kawi, S. Catalytic steam reforming of in-situ tar from rice husk over MCM-41 supported LaNiO3 to produce hydrogen rich syngas. Renew. Energy 2020, 161, 408–418. [Google Scholar] [CrossRef]
- Sastre, D.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Chemical insights on the activity of La1-xSrxFeO3 perovskites for chemical looping reforming of methane coupled with CO2-splitting. J. CO2 Util. 2019, 31, 16–26. [Google Scholar]
- Gao, R.; Li, X.; Wang, Z.; Liu, Y.; Sun, J.; Zhu, Y.; Yao, S. Reaction regeneration cycle of Mo/HZSM-5 catalyst in methane dehydroaromatization with the addition of oxygen-containing components. Appl. Catal. A Gen. 2022, 647, 118916. [Google Scholar] [CrossRef]
- Qingli, X.; Zhengdong, Z.; Kai, H.; Shanzhi, X.; Chuang, M.; Chenge, C.; Huan, Y.; Yang, Y.; Yongjie, Y. Ni supported on MgO modified attapulgite as catalysts for hydrogen production from glycerol steam reforming. Int. J. Hydrogen Energy 2021, 46, 27380–27393. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Yang, Z.; Liang, T.; Liu, S.; Zhou, Z.; Li, X. Bimetallic Ni-M (M=Co, Cu and Zn) supported on attapulgite as catalysts for hydrogen production from glycerol steam reforming. Appl. Catal. A Gen. 2018, 550, 214–227. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, Y.; Mao, H.; Huang, H.; Hu, Y. Alkali Etching Induced CoAl-Layered Double Oxides with Regulatable Cation and Oxygen Vacancy Defects to Promote the Photothermal Degradation of Methanol. ChemNanoMat 2022, 8, e202200263. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, J.; Song, Z.; Pang, Y. Carbon Deposition Characteristics in Thermal Conversion of Methane for Sustainable Fuel. Sustainability 2024, 16, 5035. https://doi.org/10.3390/su16125035
Zhang X, Wang J, Song Z, Pang Y. Carbon Deposition Characteristics in Thermal Conversion of Methane for Sustainable Fuel. Sustainability. 2024; 16(12):5035. https://doi.org/10.3390/su16125035
Chicago/Turabian StyleZhang, Xiaorong, Jie Wang, Zhanlong Song, and Yingping Pang. 2024. "Carbon Deposition Characteristics in Thermal Conversion of Methane for Sustainable Fuel" Sustainability 16, no. 12: 5035. https://doi.org/10.3390/su16125035
APA StyleZhang, X., Wang, J., Song, Z., & Pang, Y. (2024). Carbon Deposition Characteristics in Thermal Conversion of Methane for Sustainable Fuel. Sustainability, 16(12), 5035. https://doi.org/10.3390/su16125035







