Zooplankton Structure and Ecological Niche Differentiation of Dominant Species in Tahe Bay, Lushun, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Survey Sites
2.2. Sample Collection and Processing
2.3. Measurement of Physical and Chemical Indicators of Water Quality
2.4. Data Analysis and Processing
2.4.1. Degree of Dominance Index
2.4.2. Biodiversity Indicators
2.4.3. Ecological Niche Width Index
2.4.4. Pianka Ecological Niche Overlap Index
2.4.5. Mathematical Statistics
3. Results and Analyses
3.1. Analysis of the Zooplankton Structure
3.1.1. Zooplankton Species Composition and Species Diversity
3.1.2. Analysis of Dominant Species
3.2. Ecological Niche Width (Bi) and Overlap (Qik) of Dominant Species
3.3. Zooplankton Community Structure in Relation to Environmental Factors
3.4. Factors Influencing Ecological Niche Differentiation of Dominant Zooplankton Species
4. Discussion
4.1. Plankton Species Composition and Seasonal Variation
4.2. Ecological Niche Width Analysis
4.3. Ecological Niche Overlap Analysis
4.4. Analysis of Ecological Niche Differentiation of Dominant Zooplankton Species in Relation to Environmental Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, J.S.; Yin, Z.Q.; Li, Y.; Xu, M.; Yang, M.; Niu, L.H. Bibliometrics and visualization analysis of research in the field of sustainable development of the blue economy (2006–2021). Front. Mar. Sci. 2022, 15, 936612. [Google Scholar] [CrossRef]
- Xia, P.; Zhang, Z.D.; Dai, X.F.; Wang, H.X.; Wang, P.X. Distribution characteristics of phytoplankton in the offshore waters of Zhejiang Province in April 2009 and the relationship with environmental factors. J. Mar. Sci. 2011, 29, 125–135. [Google Scholar]
- Jiang, H.C.; Liu, N.; Gao, J.Q.; Su, B.; Li, J.H.; He, J.L.; Liu, A.Y. Characteristics of zooplankton community and relationship with environmental factors in Forty Mile Bay, Yantai. Acta Ecol. Sin. 2017, 37, 1318–1327. [Google Scholar]
- Yildiz, I.; Feyzioglu, A.M. Distribution of mesozooplankton along to Anatolian coast in Black Sea over autumn period. Indian J. Geo-Mar. Sci. 2016, 45, 269–276. [Google Scholar]
- Chen, J.Y.; Geng, Z.M.; Zhang, Q.Y.; Shao, Y.; Zheng, X. Analysis of plankton community structure and key environmental factors in Baiyangdian. J. Qingdao Agric. Univ. (Nat. Sci. Ed.) 2024, 41, 50–61. [Google Scholar]
- Sobko, E.I.; Klimov, S.I.; Neverova, N.V.; Moreva, O.Y. The Structure of the Zooplankton Communities in Sukhoe More Bay, Southeastern White Sea. Russ. J. Mar. Biol. 2023, 49, 488–497. [Google Scholar] [CrossRef]
- Cataldo, D.H.; Boltovskoy, D. Yearly reproductive activity of Limnoperna fortunei (Bivalvia) as inferred from the occurrence of its larvae in the plankton of the lower Paraná river and the Río de la Plata estuary (Argentina). Aquat. Ecol. 2000, 34, 307–317. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Shang, H.X.; Ma, L.; Xu, Y. Zooplankton community structure and its relationship with environmental factors in typical aquaculture waters of Lushunkou, Dalian. J. Aquac. 2022, 43, 1–6. [Google Scholar]
- Zhao, W.; Yin, X.W.; Li, B.T.; Huo, Y.Z.; Liu, Q. Community structure and spatial and temporal pattern of zooplankton in Heishi Reef, Dalian. J. Dalian Fish. Univ. 2005, 4, 270–277. [Google Scholar]
- Fu, F.Y.; Han, P.W.; Fang, Z.; Liu, B.L. Relationship between the horizontal distribution of zooplankton dominant species in spring and winter in the Northwest Pacific Ocean and the factors affecting them. J. Dalian Ocean. Univ. 2022, 37, 489–496. [Google Scholar]
- Hou, Z.W.; Sun, X.Y.; Liu, Y.L.; Zhang, C.; Zhang, W.J. Spatial ecological position of dominant zooplankton species off Yantai. Acta Ecol. Sin. 2020, 40, 5822–5833. [Google Scholar]
- Yang, J.Q.; Chen, L.; Yang, Y.Y.; Zhou, J.; Shi, Y.R. Spatial and temporal ecological position analysis of zooplankton dominant species in spring and summer in the Yangtze River estuary and adjacent waters. J. Fish. Sci. China 2022, 29, 1625–1635. [Google Scholar]
- Li, G.X.; Yin, Z.Q.; Wang, D.; Zhou, Y.Y.; Zhang, D.W. Ecological niches of dominant zooplankton species and their differentiation in the waters of Beranzi. J. Shanghai Ocean. Univ. 2024, 33, 1199–1210. [Google Scholar]
- Wang, D.; Yin, Z.Q.; Zhang, D.W.; Wang, Y.; Chen, L.; Yang, J. Evaluation of the Environmental Impact of Artificial Habitat on Seawater Quality in Tahe Bay, Lushun. OAJRC Environ. Sci. 2023, 4, 1–12. [Google Scholar] [CrossRef]
- Habib, O.A.; Tippett, R.; Murphy, K.J. Seasonal changes in phytoplankton community structure in relation to physic-chemical factors in Loch Lomond. Hydrobiologia 1997, 350, 63–79. [Google Scholar] [CrossRef]
- Akin, S.; Winemiller, K.; Gelwick, F. Seasonal and spatial variations in fish and macrocrustacean assemblage structure in the Mad Island Marsh estuary, Texas. Estuar. Coast. Shelf Sci. 2003, 57, 269–282. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, H.H.; Ji, Y.L.; Su, K. Characteristics of zooplankton community structure in spring and autumn in the waters adjacent to Dongjiakou Harbour, Qingdao. J. Appl. Oceanogr. 2023, 42, 16–27. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Izaguirre, I.; O’farrell, I.; Unrein, F.; Sinistro, R.; Afonso, M.D.; Tell, G. Algal Assemblages Across a Wetland, from a Shallow Lake to Relictual Oxbow Lakes (Lower Paraná River, South America). Hydrobiologia 2004, 511, 25–36. [Google Scholar] [CrossRef]
- Haiyuan, C.; Kui, W.; Sijun, H.; Nianzhi, J.; Feng, C. Distinct patterns of picocyanobacterial communities in winter and summer in the Chesapeake Bay. Appl. Environ. Microbiol. 2010, 76, 2955–2960. [Google Scholar]
- Resende, P.; Azeiteiro, M.U.; Gonçalves, F.; Pereira, J.M. Distribution and ecological preferences of diatoms and dinoflagellates in the west Iberian Coastal zone (North Portugal). Acta Oecologica 2007, 32, 224–235. [Google Scholar] [CrossRef]
- Tian, Y.X.; Han, J.X.; Chen, B.C.; Xie, W.D.; Pan, L.R.; Meng, X. Species diversity and interspecific ecological niche characteristics of Acacia spp. in Northwest Guangxi. Guangxi For. Sci. 2022, 51, 466–474. [Google Scholar]
- Pianka, E.R. The structure of lizard communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Zhang, J.; Wang, X.L.; Xu, Y.; Li, X.; He, L. Changes of zooplankton community and its relationship with environmental factors in Bohai Bay. J. Mar. Sci. 2018, 36, 93–101. [Google Scholar]
- Li, Z.L.; Zhang, H.Y.; Zhao, L.; Xu, F. Spatial and temporal distribution of phytoplankton community structure in the Bohai Sea and its influencing factors. Mar. Sci. 2021, 45, 10–20. [Google Scholar]
- Wang, Z.L. Distribution of planktonic Copepoda in Dalian Bay. Adv. Mar. Sci. 1995, 1, 47–54. [Google Scholar]
- Zhou, X.S.; Li, N.; Sun, B.W.; Gao, X.P. Seasonal changes of plankton community structure in Baiyangdian and its relationship with environmental factors. Water Resour. Hydropower Eng. 2021, 52, 110–119. [Google Scholar]
- Tang, C.H.; Yi, Y.J.; Yang, Z.F.; Yang, Z.; Zerizghi, T.; Wang, X.; Cui, X.L.; Duan, P.Y. Planktonic indicators of trophic states for a shallow lake (Baiyangdian Lake, China). Limnologica 2019, 78, e125712. [Google Scholar] [CrossRef]
- Jakobsen, H.H.; Blanda, E.; Staehr, P.A.; Højgård, J.K.; Rayner, T.A.; Pedersen, M.F.; Jepsen, P.M.; Hansen, B.W. Development of phytoplankton communities: Implications of nutrient injections on phytoplankton composition, pH and ecosystem production. J. Exp. Mar. Biol. Ecol. 2015, 473, 81–89. [Google Scholar] [CrossRef]
- Ismail, H.A.; Lim, C.C.; Omar WM, W. Evaluation of spatial and temporal variations in zooplankton community structure with reference to water quality in Teluk Bahang Reservoir, Malaysia. Trop. Ecol. 2019, 60, 186–198. [Google Scholar] [CrossRef]
- Chen, X.C.; Zhu, L.Y.; Huang, Y.; Gong, W.J.; Hao, Y. Research on zooplankton community structure in the South Yellow Sea. Mar. Sci. 2017, 41, 41–49. [Google Scholar]
- Feng, Y.C.; Zheng, X.Y.; Wang, Z.N.; Liu, B.; Lan, S.R. Characteristics of ecological niche of understorey herb layer populations in pure and mixed fir forests. J. Ecol. Rural. Environ. 2019, 35, 217–224. [Google Scholar]
- Fan, L.J.; Hu, X.D.; Chen, W.M.; Wang, C.M. Zooplankton ecological niche and its ecological differentiation factors in Lake Baima. J. Hydroecology 2022, 43, 59–66. [Google Scholar]
- Luo, H.; Wu, Q.; Chen, Q.; Chen, W.L.; Chen, H.X. Characterisation of zooplankton dominant species succession and ecological niche analysis in Jiuquwan Reservoir after ecological restoration. J. Ecol. Environ. 2021, 30, 320–330. [Google Scholar]
- Duan, Y.; Wang, A.Y.; Wang, X.L.; Liu, X.Z.; Chai, Y. Ecological characteristics and spatial and temporal distribution of zooplankton in spring and summer in the northern Yellow Sea. Fish. Sci. 2017, 36, 456–461. [Google Scholar]
- Zhao, Q.; Liu, H.; Zhang, X.W.; Qi, Z.L.; Li, C. Zooplankton community structure and ecological niche in Tangshan Sea Ranch. Hebei Fish. 2021, 10, 17–24+30. [Google Scholar]
- Wathne, J.A.; Haug, T.; Lydersen, C. Prey preference and niche overlap of ringed seals Phoca hispida and harp seals P. groenlandica in the Barents Sea. Mar. Ecol. Prog. Ser. 2000, 194, 233–239. [Google Scholar] [CrossRef]
- Liu, K.; Yu, C.G.; Xu, Y.J.; Jiang, X.Q.; Zheng, J. Spatial ecological niche analysis of major crustacean species in spring and autumn in the eastern waters of Zhoushan Islands. J. Zhejiang Univ. (Sci. Ed.) 2021, 48, 450–460+480. [Google Scholar]
- Song, C.; Meng, Z.; Wang, X.B.; Han, Q.X. Ecological niches of dominant zooplankton species and their ecological differentiation in Zhoushan waters during the summer of 2019–2020. Acta Oceanol. Sin. 2022, 44, 127–139. [Google Scholar]
- Peng, S.Y.; Li, X.Z.; Wang, H.F.; Zhang, B.L. Ecological niche of dominant macrobenthic species in the South Yellow Sea in spring. Acta Ecol. Sin. 2015, 35, 1917–1928. [Google Scholar]
- Zhu, Z.; Sun, X.; Yang, X.R.; Tang, X.F.; Hu, Y.Q.; Zhang, M. Characteristics of seasonal dynamics of phytoplankton community in Chaohu Lake and its influencing factors. Environ. Monit. China 2024, 40, 129–142. [Google Scholar]
- Tao, M.; Xiong, Y.; Li, B.; Wang, Z.J.; Huang, J. Spatial and temporal distribution of phytoplankton communities in the Sichuan section of the Jialing River and their environmental impact factors. Resour. Environ. Yangtze Basin 2021, 30, 1680–1694. [Google Scholar]
- Devreker, D.S.S.; Seuront, L. Effects of chlorophyll concentration and temperature variation on the reproduction and survival of Temora longicornis (Copepoda, Calanoida) in the Eastern English Channel. J. Exp. Mar. Biol. Ecol. 2004, 318, 145–162. [Google Scholar] [CrossRef]
- Lenz, P.H.; Herma, E.; Hartline, D.K. Temperature compensation in the escape response of a marine copepod, Calanus finmarchicus (Crustacea). Biol. Bull. 2005, 209, 75–85. [Google Scholar] [CrossRef]
- Wang, X.X.; Huo, J.; Yang, Y.Y.; Liu, X.B.; Zhang, X.M. Zooplankton community structure and seasonal changes in the nearshore waters of northern Shandong Peninsula. Ecol. Sci. 2024, 43, 163–170. [Google Scholar]
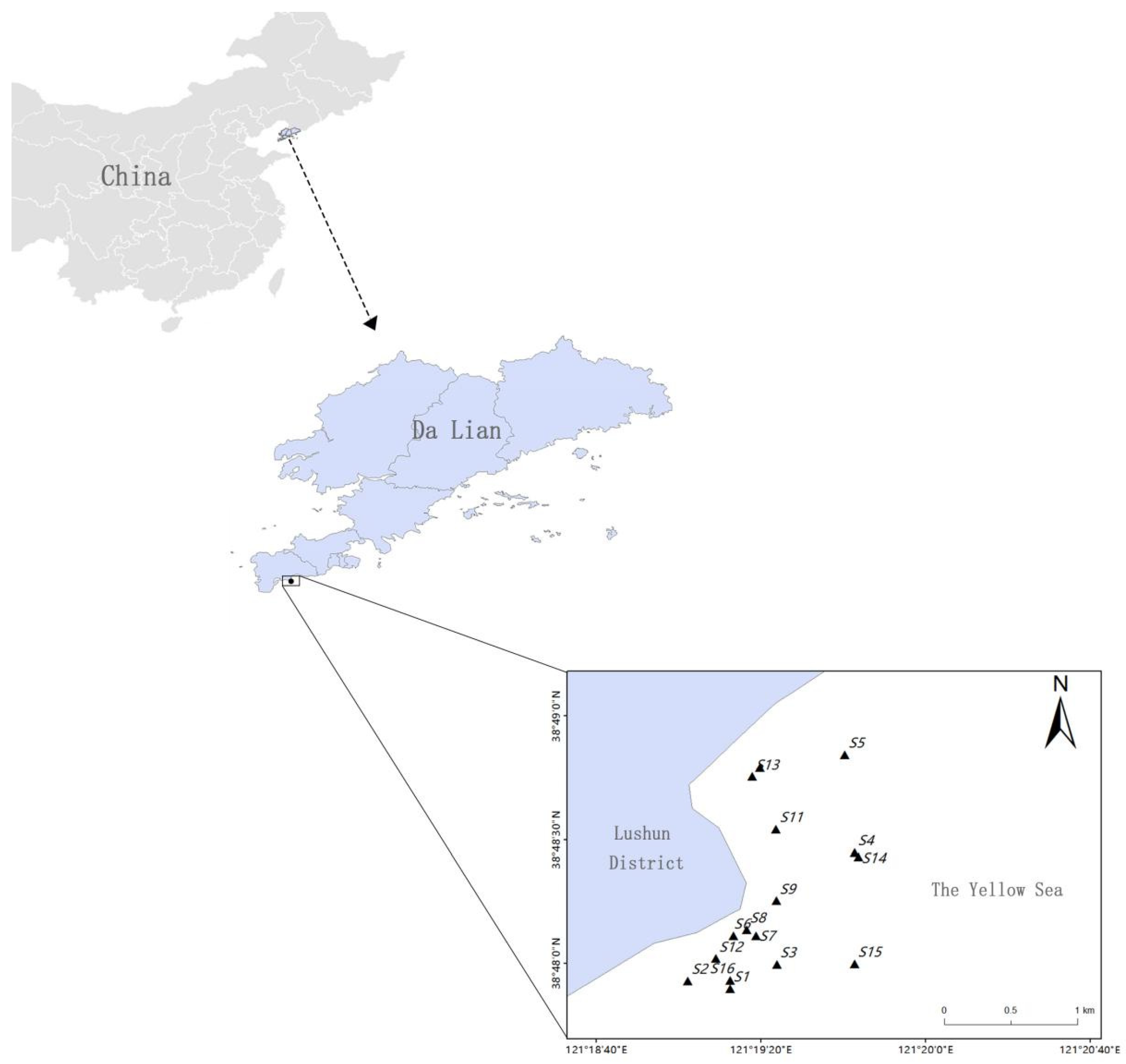




| Station | Latitude | Longitude |
|---|---|---|
| S1 | 38°47′53.84″ N | 121°19′12.46″ E |
| S2 | 38°47′59.54″ N | 121°19′2.25″ E |
| S3 | 38°47′59.78″ N | 121°19′23.92″ E |
| S4 | 38°48′26.92″ N | 121°19′42.74″ E |
| S5 | 38°48′50.63″ N | 121°19′40.40″ E |
| S6 | 38°48′25.86″ N | 121°19′43.69″ E |
| S7 | 38°47′59.85″ N | 121°19′23.90″ E |
| S8 | 38°47′55.86″ N | 121°19′2.25″ E |
| S9 | 38°48′6.73″ N | 121°19′13.31″ E |
| S10 | 38°48′6.62″ N | 121°19′18.79″ E |
| S11 | 38°48′8.16″ N | 121°19′16.48″ E |
| S12 | 38°48′15.21″ N | 121°19′23.76″ E |
| S13 | 38°48′47.46″ N | 121°19′19.75″ E |
| S14 | 38°48′32.54″ N | 121°19′23.68″ E |
| S15 | 38°48′01.16″ N | 121°19′08.97″ E |
| S16 | 38°48′45.30″ N | 121°19′17.80″ E |
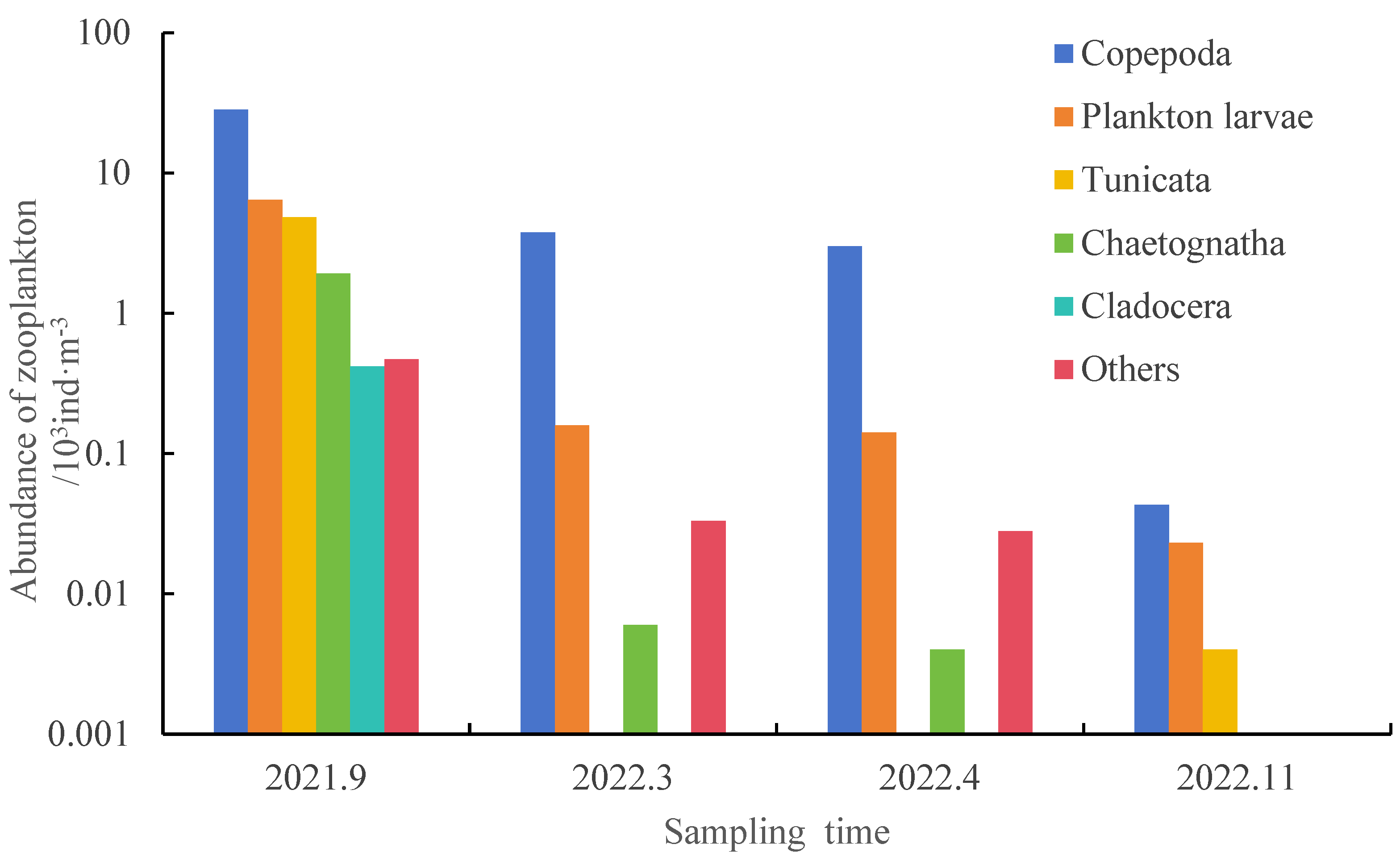
| Taxa | September 2021 (End of Summer) | March 2022 (End of Winter) | April 2022 (Spring) | November 2022 (Autumn) |
|---|---|---|---|---|
| Copepoda | 5 | 8 | 7 | 5 |
| Cladocera | 1 | 0 | 0 | 0 |
| Planktonic larvae | 2 | 6 | 6 | 3 |
| Tunicata | 1 | 0 | 0 | 1 |
| Chaetognatha | 1 | 1 | 1 | 1 |
| Others | 1 | 3 | 3 | 0 |
| Sampling Time | Zooplankton | ||
|---|---|---|---|
| Shannon–Wiener’s Diversity Index (H’) | Margalef’s Index (D) | Pielou Index (J) | |
| September 2021 | 2.05 | 0.94 | 0.70 |
| March 2022 | 0.86 | 1.93 | 0.14 |
| April 2022 | 2.22 | 1.81 | 0.34 |
| November 2022 | 2.81 | 4.99 | 0.29 |
| Median | 2.14 | 1.87 | 0.32 |
| Serial Number | Species Name | Dominance Index | |||
|---|---|---|---|---|---|
| September 2021 (End of Summer) | March 2022 (End of Winter) | April 2022 (Spring) | November 2022 (Autumn) | ||
| B01 | Oikopleura dioica | 0.114 | 0.027 | ||
| B02 | Parvocalanus crassirostris | 0.256 | |||
| B03 | Corycaeus affinis | 0.193 | |||
| B04 | Pluteus | 0.091 | |||
| B05 | Oithona setigera | 0.049 | |||
| B06 | Oithona similis | 0.051 | 0.035 | 0.044 | 0.227 |
| B07 | Sagitta crassa | 0.023 | |||
| B08 | Euterpina acutifrons | 0.820 | 0.819 | ||
| B09 | Copepoda nauplius | 0.030 | |||
| B010 | Harpacticidae | 0.118 | |||
| B011 | Paracalanus parvus | 0.245 | |||
| B012 | Polychaeta larvae | 0.082 | |||
| B013 | Copepoda larvae | 0.15 | |||
| Code | Bi | Qik | ||||||
|---|---|---|---|---|---|---|---|---|
| B01 | B02 | B03 | B04 | B05 | B06 | B07 | ||
| B01 | 0.71 | 1 | ||||||
| B02 | 0.94 | 0.78 | 1 | |||||
| B03 | 0.84 | 0.94 | 0.82 | 1 | ||||
| B04 | 0.94 | 0.44 | 0.89 | 0.60 | 1 | |||
| B05 | 0.96 | 0.75 | 0.50 | 0.86 | 0.29 | 1 | ||
| B06 | 1.00 | 0.34 | 0.84 | 0.40 | 0.93 | 0.00 | 1 | |
| B07 | 0.92 | 0.29 | 0.80 | 0.33 | 0.90 | 0.00 | 0.97 | 1 |
| Code | Bi | Qik | |
|---|---|---|---|
| B06 | B08 | ||
| B06 | 1.00 | 1 | |
| B08 | 0.34 | 0.01 | 1 |
| Code | Bi | Qik | ||
|---|---|---|---|---|
| B06 | B08 | B09 | ||
| B06 | 1.00 | 1 | ||
| B08 | 0.34 | 0.01 | 1 | |
| B09 | 0.96 | 0.91 | 0.42 | 1 |
| Code | Bi | Qik | |||||
|---|---|---|---|---|---|---|---|
| B01 | B010 | B011 | B06 | B012 | B013 | ||
| B01 | 0.90 | 1 | |||||
| B010 | 0.80 | 0.49 | 1 | ||||
| B011 | 0.88 | 0.84 | 0.81 | 1 | |||
| B06 | 0.64 | 0.89 | 0.63 | 0.95 | 1 | ||
| B012 | 1.00 | 0.77 | 0.55 | 0.88 | 0.85 | 1 | |
| B013 | 0.68 | 0.82 | 0.73 | 0.89 | 0.91 | 0.60 | 1 |
| Zooplankton Community Indicator | Water Temperature (°C) | Salinity | pH | Dissolved Oxygen Concentration (mg/L) | Chemical Oxygen Demand (mg/L) | Ammonia–Nitrogen Content (μg/L) | Nitrate–Nitrogen Content (μg/L) | Nitrite–Nitrogen Content (μg/L) | Inorganic Phosphorus Content (μg/L) |
|---|---|---|---|---|---|---|---|---|---|
| H′ | 0.166 | −0.024 | −0.094 | −0.325 | −0.137 | 0.13 | 0.017 | 0.111 | −0.159 |
| J | 0.379 | −0.168 | −0.518 | −0.864 ** | −0.281 | 0.471 | 0.014 | 0.397 | −0.705 ** |
| D | −0.645 * | 0.677 ** | −0.256 | 0.343 | 0.244 | −0.658 * | −0.560 * | −0.608 * | 0.013 |
| Abundance of Copepoda | 0.935 ** | −0.920 ** | 0.391 | −0.546 * | −0.321 | 0.909 ** | 0.778 ** | 0.848 ** | 0.015 |
| Abundance of Tunicata | 0.780 ** | −0.727 ** | 0.211 | −0.582 * | −0.03 | 0.900 ** | 0.703 ** | 0.753 ** | −0.088 |
| Abundance of planktonic larvae | 0.927 ** | −0.837 ** | 0.286 | −0.616 * | −0.467 | 0.897 ** | 0.626 * | 0.881 ** | −0.124 |
| Abundance of other zooplankton | 0.408 | −0.414 | 0.118 | −0.327 | −0.016 | 0.231 | 0.486 | 0.145 | 0.087 |
| Abundance of Cladocera | 0.411 | −0.369 | 0.122 | −0.268 | −0.319 | 0.313 | 0.254 | 0.179 | −0.017 |
| Abundance of Chaetognatha | 0.587 * | −0.51 | 0.18 | −0.363 | −0.525 | 0.515 | 0.267 | 0.539 * | −0.115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yin, Z.; Wang, Y.; Li, G.; Zhang, D.; Yang, J.; Chen, L.; Gu, H.; Qin, Y.; Tian, T. Zooplankton Structure and Ecological Niche Differentiation of Dominant Species in Tahe Bay, Lushun, China. Sustainability 2024, 16, 8590. https://doi.org/10.3390/su16198590
Zhang Y, Yin Z, Wang Y, Li G, Zhang D, Yang J, Chen L, Gu H, Qin Y, Tian T. Zooplankton Structure and Ecological Niche Differentiation of Dominant Species in Tahe Bay, Lushun, China. Sustainability. 2024; 16(19):8590. https://doi.org/10.3390/su16198590
Chicago/Turabian StyleZhang, Yanrong, Zengqiang Yin, Yan Wang, Guoxing Li, Dawang Zhang, Jun Yang, Lei Chen, Haifeng Gu, Yuxue Qin, and Tao Tian. 2024. "Zooplankton Structure and Ecological Niche Differentiation of Dominant Species in Tahe Bay, Lushun, China" Sustainability 16, no. 19: 8590. https://doi.org/10.3390/su16198590






