Lower-Carbon Hydrogen Production from Wastewater: A Comprehensive Review
Abstract
:1. Introduction
2. Wastewater Analysis
3. Hydrogen Production Technologies and Simultaneous Wastewater Treatment
- Research on treating wastewater by producing hydrogen;
- Research assessing the effectiveness of wastewater treatment;
- Studies assessing the production of hydrogen by wastewater treatment.
3.1. Biological Treatment
3.1.1. Bio Photolysis
3.1.2. Dark Fermentation
3.1.3. Photofermentation
3.1.4. Photoelectrochemical Methods
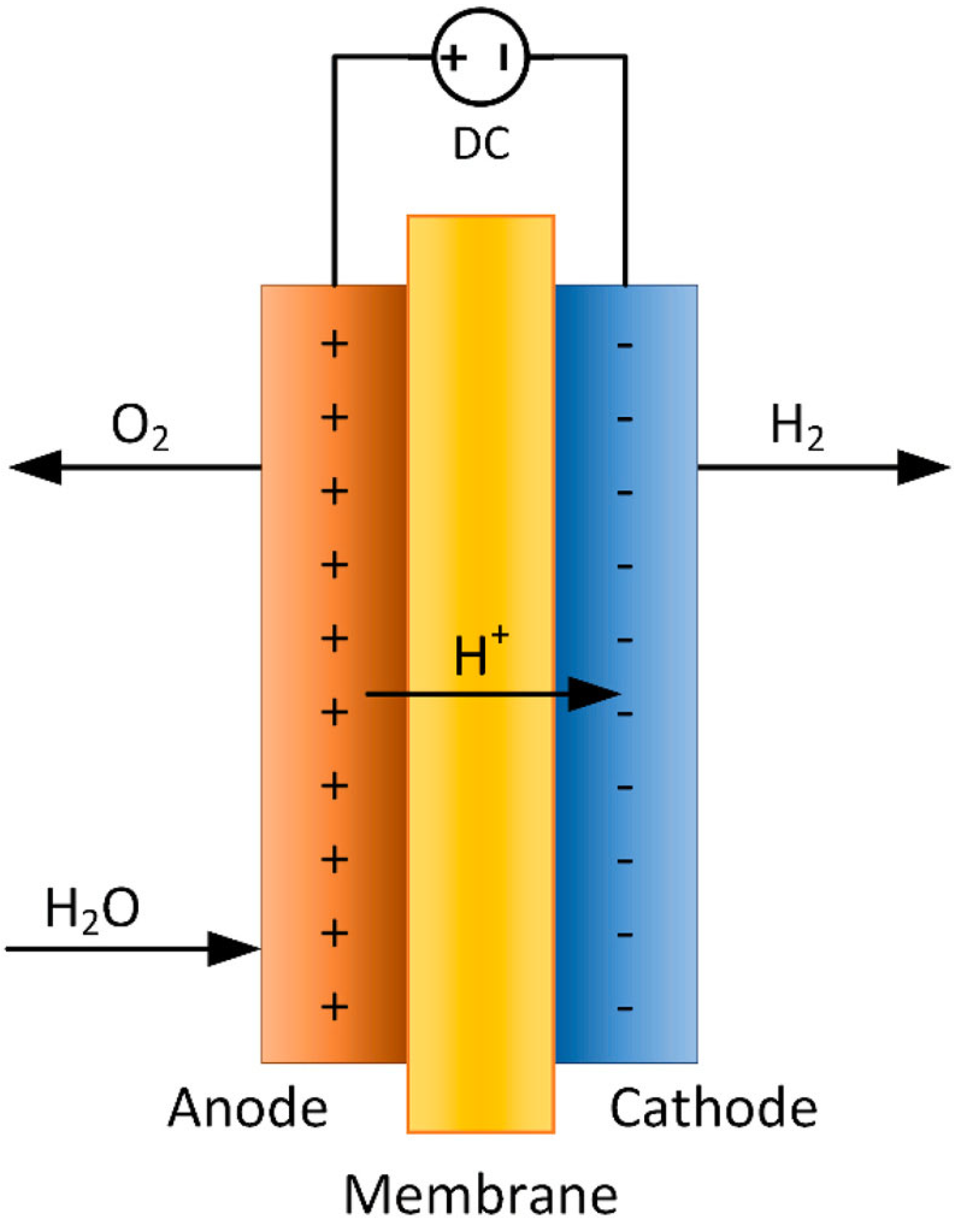
3.1.5. Electrolysis
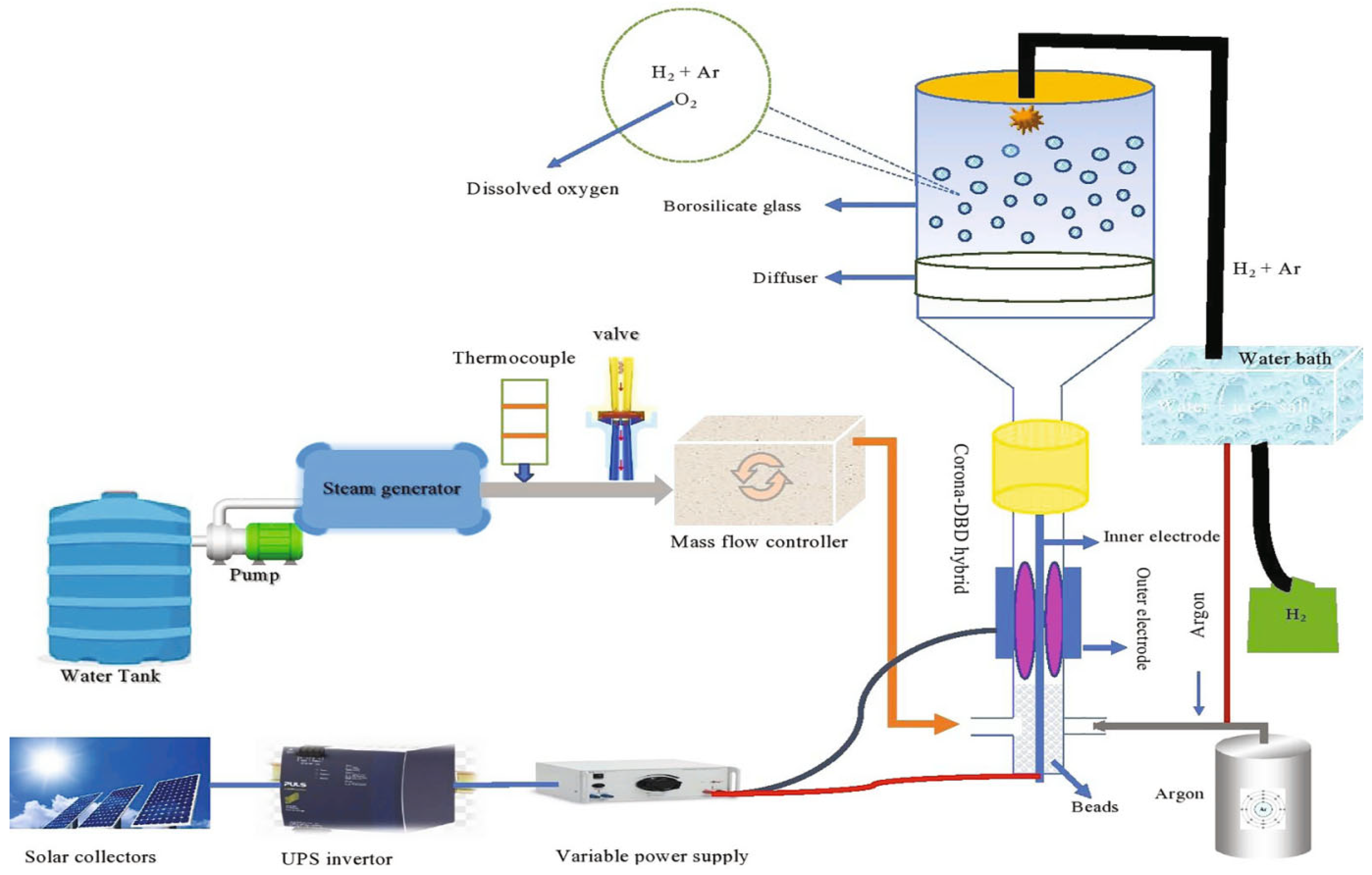
3.1.6. Plasmolysis
3.1.7. Photoreforming of Lignocellulose into Hydrogen
3.2. Non-Renewable Hydrogen Production Sources
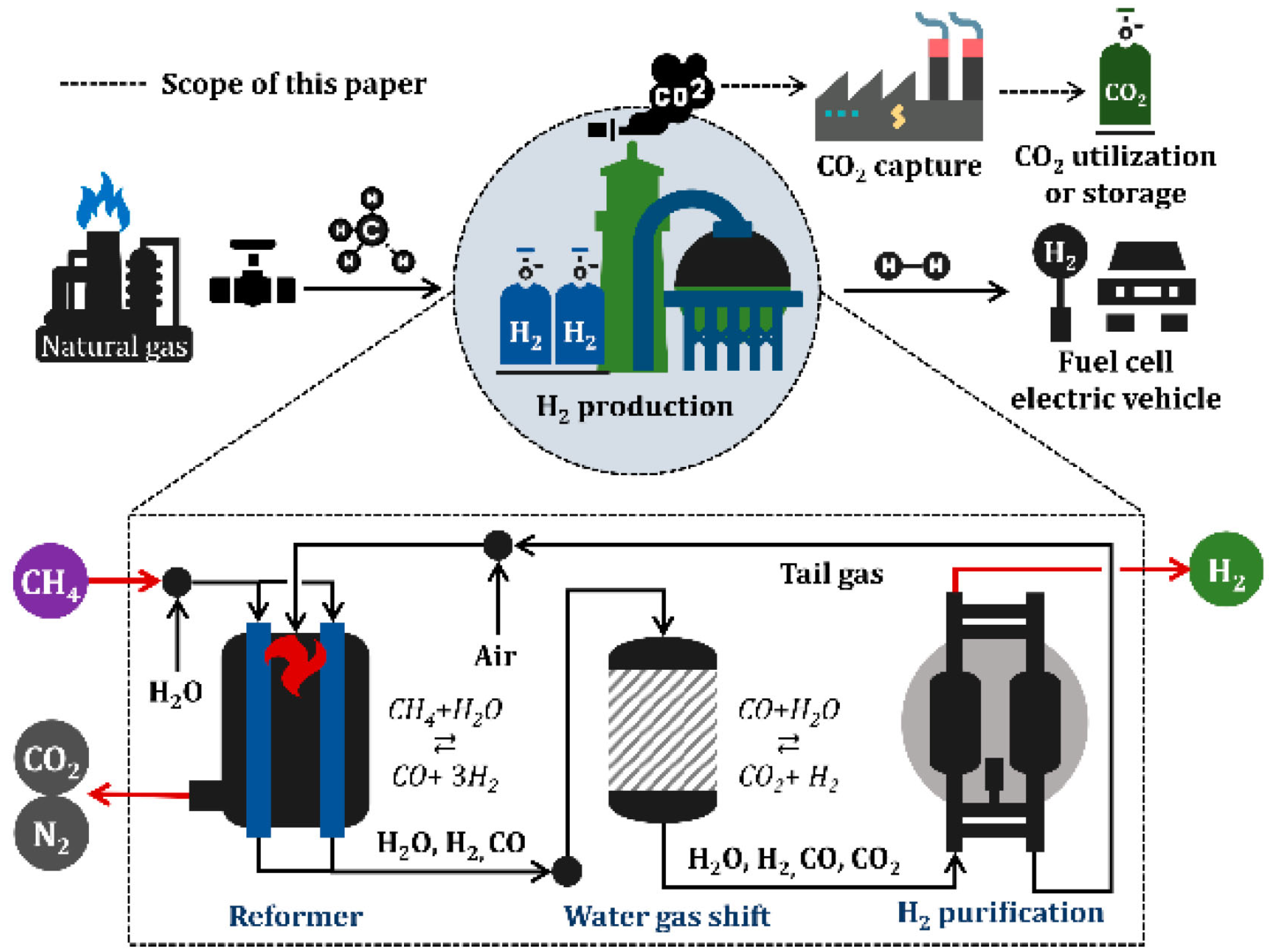
3.2.1. Steam Methane Reforming (SMR)
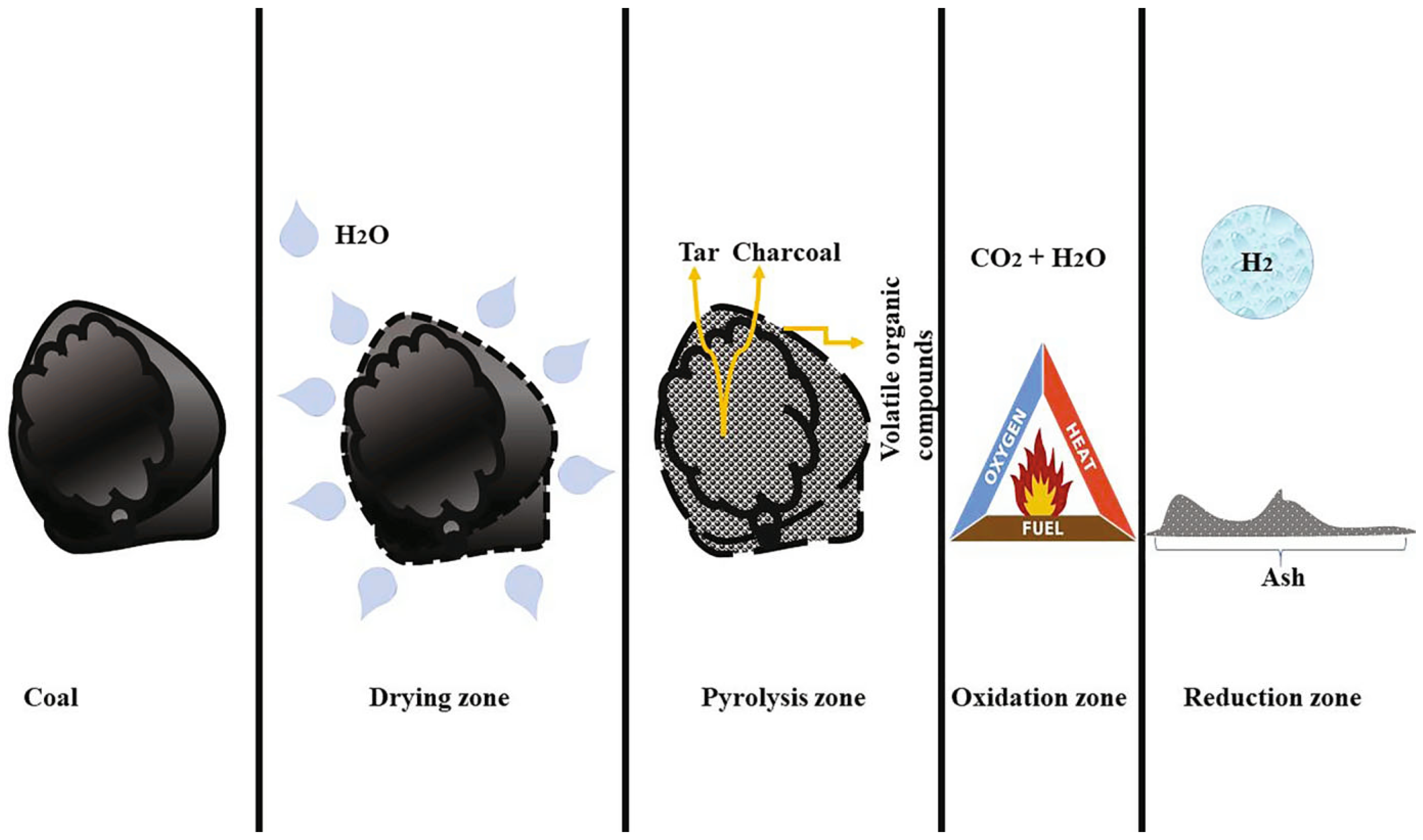
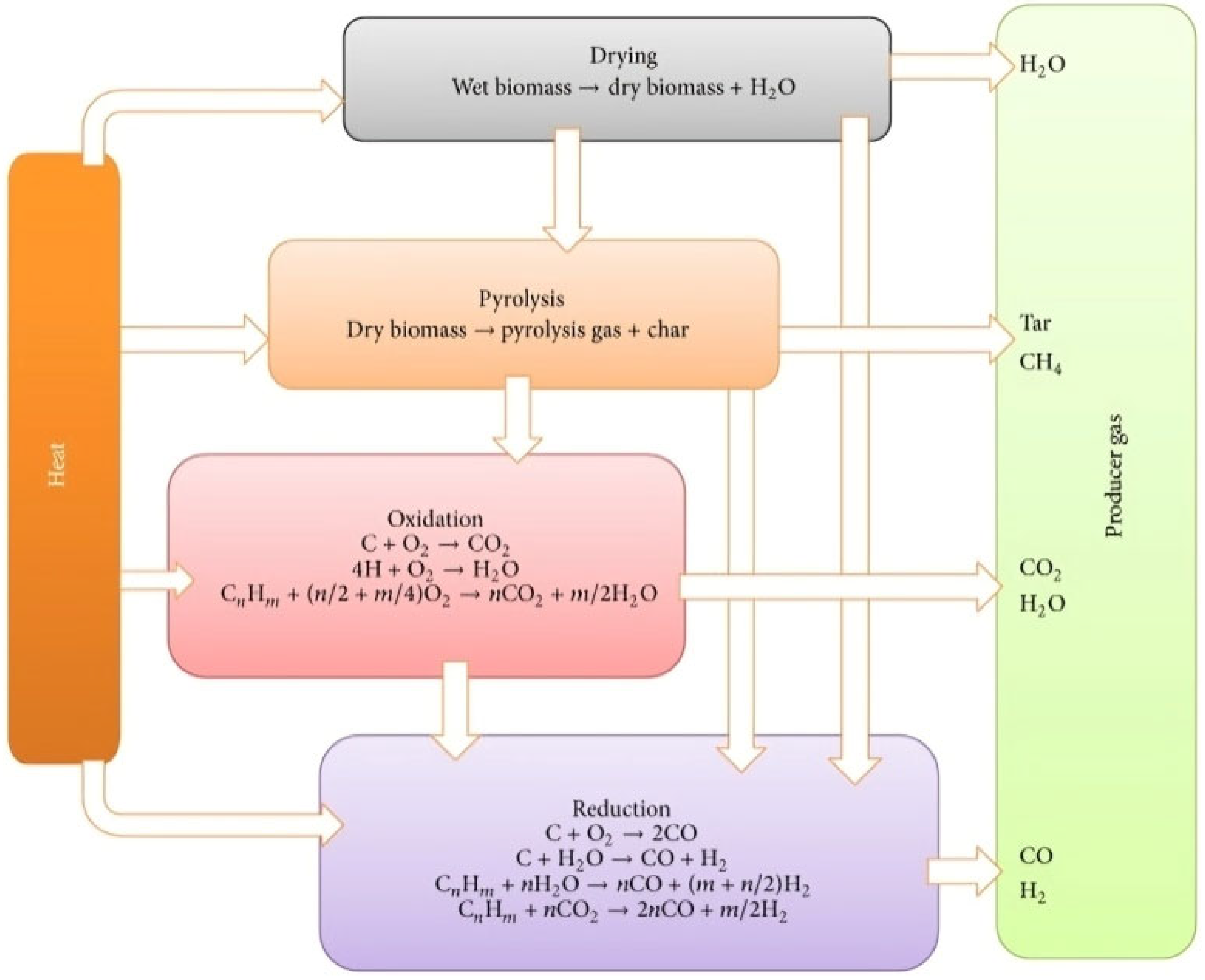
3.2.2. Gasification
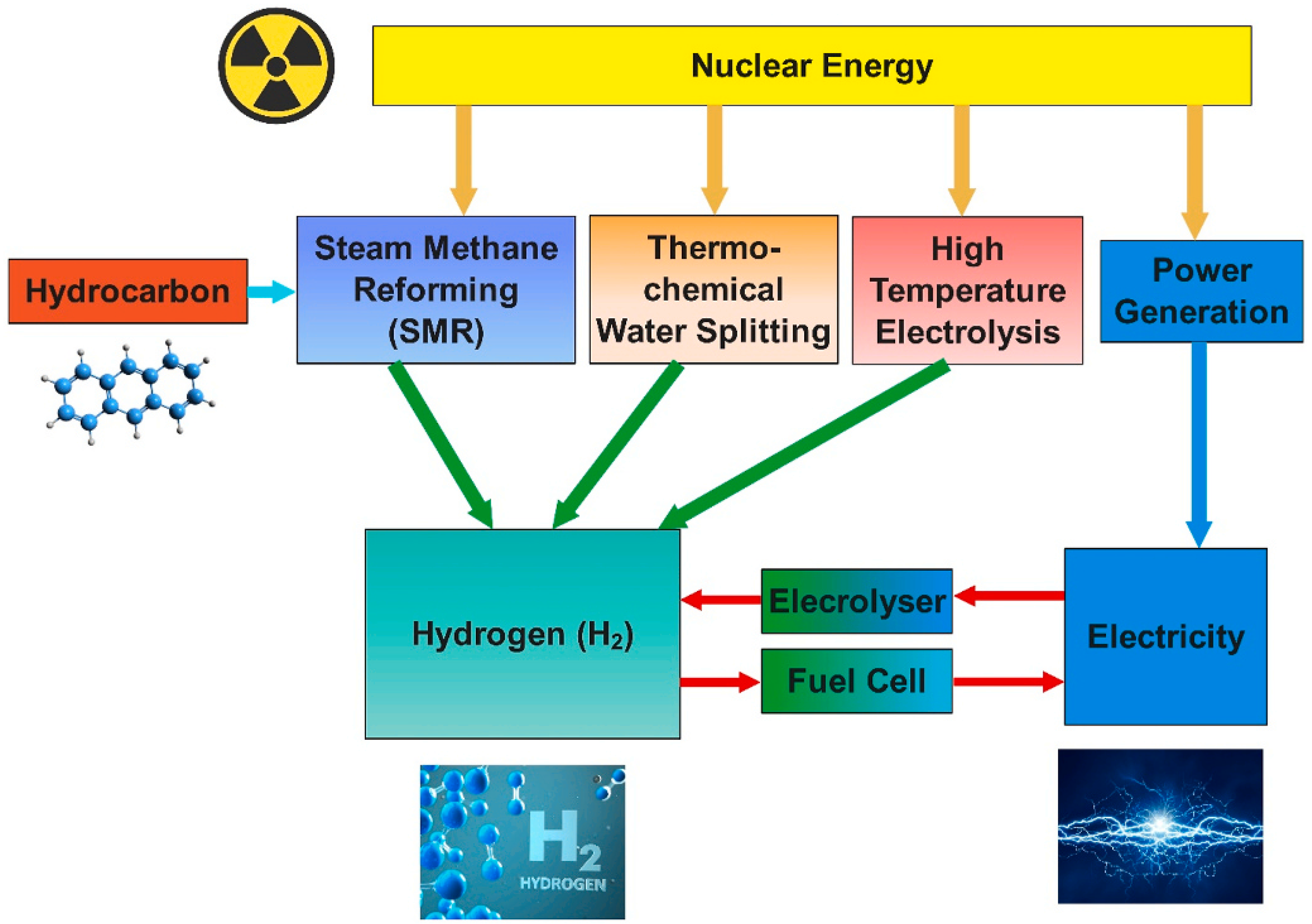
3.2.3. Nuclear Energy
4. Future Prospects/Challenges
5. Conclusions
- While considerable investigations have been conducted on H2 generation from wastewater, numerous works have taken place in laboratory settings.
- Although there are commercially established techniques (electrodialysis and electrolysis), research and development are still ongoing about their potential applications in treating wastewater to produce hydrogen.
- The working circumstances of supercritical water gasification result in a significant energy need. It can produce much more hydrogen, though, if it is backed by waste heat from other businesses. The purity of the generated gas is also crucial as it may need further separation procedures because it contains CO2 and CH4.
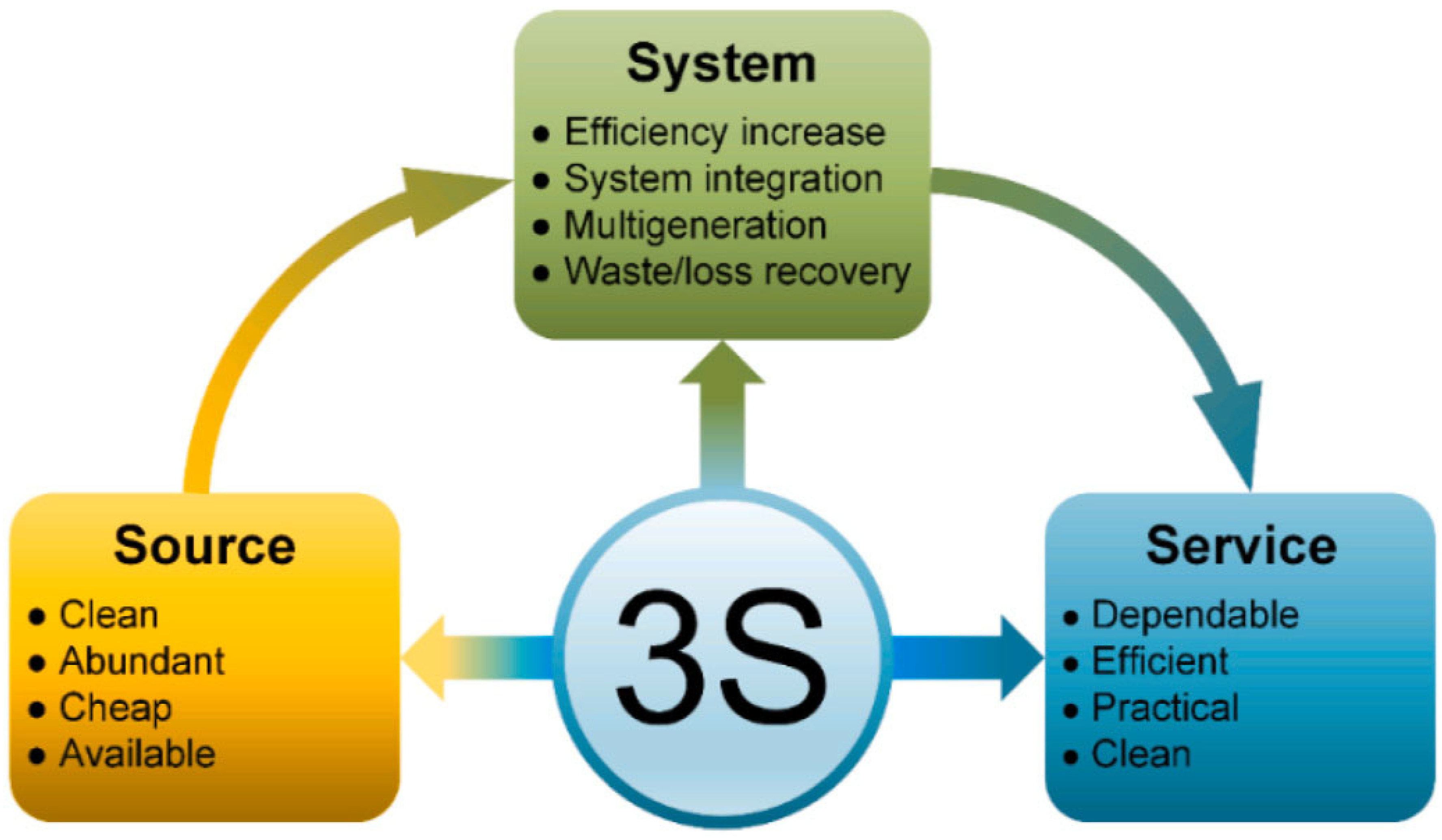
- The price of lower-carbon hydrogen per kilogram should be about $1–1.5 for hydrogen generation to be profitable and comparable with other sources of hydrogen production. Considering the ways that were previously evaluated in the study, further technical optimization and system integrations need to be taken into consideration.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waqas Alam, M.; Khatoon, U.; Qurashi, A. Synthesis and Characterization of Cu-SnO2 Nanoparticles Deposited on Glass Using Ultrasonic Spray Pyrolysis and Their H2S Sensing Properties. Curr. Nanosci. 2012, 8, 919–924. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Elsaid, K.; Wilberforce, T.; Kamil, M.; Sayed, E.T.; Olabi, A. Environmental Aspects of Fuel Cells: A Review. Sci. Total Environ. 2021, 752, 141803. [Google Scholar] [CrossRef] [PubMed]
- Woods, P.; Bustamante, H.; Aguey-Zinsou, K.F. The Hydrogen Economy—Where Is the Water? Energy Nexus 2022, 7, 100123. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Özcan, H. Comprehensive Review on the Techno-Economics of Sustainable Large-Scale Clean Hydrogen Production. J. Clean. Prod. 2019, 220, 593–609. [Google Scholar] [CrossRef]
- Souayeh, B.; Bhattacharyya, S.; Hdhiri, N.; Hammami, F.; Yasin, E.; Raju, S.S.K.; Alam, M.W.; Alsheddi, T.; Al Nuwairan, M. Effect of Magnetic Baffles and Magnetic Nanofluid on Thermo-Hydraulic Characteristics of Dimple Mini Channel for Thermal Energy Applications. Sustainability 2022, 14, 10419. [Google Scholar] [CrossRef]
- Messai, R.; Ferhat, M.F.; Belmekki, B.; Alam, M.W.; Al-Othoum, M.A.S.; Sadaf, S. GAD Plasma-Assisted Synthesis of ZnO Nanoparticles and Their Photocatalytic Activity. Mater. Res. Express 2024, 11, 015006. [Google Scholar] [CrossRef]
- Song, H.; Luo, S.; Huang, H.; Deng, B.; Ye, J. Solar-Driven Hydrogen Production: Recent advances, Challenges, and Future Perspectives. ACS Energy Lett. 2022, 7, 1043–1065. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, G.; Chen, J. Evaluation and Calculation on the Efficiency of a Water Electrolysis System for Hydrogen Production. Int. J. Hydrogen Energy 2010, 35, 10851–10858. [Google Scholar] [CrossRef]
- Dai, Z.; Heidrich, E.S.; Dol, J.; Jarvis, A.P. Determination of the Relationship between the Energy Content of Municipal Wastewater and Its Chemical Oxygen Demand. Environ. Sci. Technol. Lett. 2019, 6, 396–400. [Google Scholar] [CrossRef]
- Meg, P.J.; Vizca, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Islam, A.K.M.K.; Dunlop, P.S.M.; Hewitt, N.J.; Lenihan, R.; Brandoni, C. Bio-Hydrogen Production from Wastewater: A Comparative Study of Low Energy Intensive Production Processes. Clean Technol. 2021, 3, 156–182. [Google Scholar] [CrossRef]
- Usman, T.M.M.; Banu, J.R.; Gunasekaran, M.; Kumar, G. Bioresource Technology Reports Biohydrogen Production from Industrial Wastewater: An Overview. Bioresour. Technol. Rep. 2019, 7, 100287. [Google Scholar]
- Banu, J.R.; Kavitha, S.; Kannah, R.Y.; Bhosale, R.R.; Kumar, G. Bioresource Technology Industrial Wastewater to Biohydrogen: Possibilities towards Successful Biorefinery Route. Bioresour. Technol. 2020, 298, 122378. [Google Scholar]
- Islam, A.K. ScienceDirect Hydropower Coupled with Hydrogen Production from Wastewater: Integration of Micro-Hydropower Plant (MHP) and Microbial Electrolysis Cell (MEC). Int. J. Hydrogen Energy 2023, 49, 1–14. [Google Scholar] [CrossRef]
- Kadier, A.; Singh, R.; Song, D.; Ghanbari, F.; Syamimi, N.; Teta, P.; Aryanti, P.; Jadhav, D.A.; Islam, M.A.; Sahaid, M.; et al. ScienceDirect A Novel Pico-Hydro Power (PHP)-Microbial Electrolysis Cell (MEC) Coupled System for Sustainable Hydrogen Production during Palm Oil Mill Effluent (POME) Wastewater Treatment. Int. J. Hydrogen Energy 2022, 8, 21066–21087. [Google Scholar]
- Wang, B.; Jiang, H.; Gu, D.; Li, C.; Nie, C.; Yan, C.; Yuan, D.; Wang, X. An Insight into Solar Thermo-Assisted and Organic-Molecule Alternated Water Splitting Chemistry for Hydrogen Production and Wastewater Treatment by Elucidating Redox Model and Thermodynamics. Energy Convers. Manag. 2020, 226, 113551. [Google Scholar] [CrossRef]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and Regional Potential of Wastewater as a Water, Nutrient and Energy Source. Nat. Resour. Forum 2020, 44, 40–51. [Google Scholar] [CrossRef]
- Brack, W.; Dulio, V.; Ågerstrand, M.; Allan, I.; Altenburger, R.; Brinkmann, M.; Bunke, D.; Burgess, R.M.; Cousins, I.; Escher, B.I.; et al. Towards the Review of the European Union Water Framework Management of Chemical Contamination in European Surface Water Resources. Sci. Total Environ. 2017, 576, 720–737. [Google Scholar] [CrossRef]
- Lavrnić, S.; Zapater-Pereyra, M.; Mancini, M.L. Water Scarcity and Wastewater Reuse Standards in Southern Europe: Focus on Agriculture. Water. Air. Soil Pollut. 2017, 228, 251. [Google Scholar] [CrossRef]
- Sarkar, J.; Bhattacharyya, S. Application of Graphene and Graphene-Based Materials in Clean Energy-Related Devices Minghui. Arch. Thermodyn. 2012, 33, 23–40. [Google Scholar] [CrossRef]
- Aydin, M.I.; Karaca, A.E.; Qureshy, A.M.M.I.; Dincer, I. A Comparative Review on Clean Hydrogen Production from Wastewaters. J. Environ. Manage. 2021, 279, 111793. [Google Scholar] [CrossRef] [PubMed]
- Acar, C.; Dincer, I. Review and Evaluation of Hydrogen Production Options for Better Environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Sciences, L.; States, U.; Force, A.; States, U.; Lazaro, C.Z. 3.10 Biohydrogen. Life Sci. 2019, 3, 128–139. [Google Scholar] [CrossRef]
- Program, E.E.; Haven, N. Challenges in Developing Biohydrogen as a Sustainable Energy Source: Implications for a Research Agenda. Environ. Sci. Technol. 2023, 44, 2243–2254. [Google Scholar]
- Antal, T.K.; Lindblad, P. Production of H2 by Sulphur-Deprived Cells of the Unicellular Cyanobacteria Gloeocapsa Alpicola and Synechocystis Sp. PCC 6803 during Dark Incubation with Methane or at Various Extracellular PH. J. Appl. Microbiol. 2005, 98, 114–120. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A Comprehensive Review on Biological Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Tao, Y.; He, Y.; Wu, Y.; Liu, F.; Li, X.; Zong, W.; Zhou, Z. Characteristics of a New Photosynthetic Bacterial Strain for Hydrogen Production and Its Application in Wastewater Treatment. Int. J. Hydrogen Energy 2008, 33, 963–973. [Google Scholar] [CrossRef]
- Chemistry, O. ACS Paragon Plus Environment. Interface 2010, 5, 1–23. [Google Scholar]
- Ghosh, S.; Dairkee, U.K.; Chowdhury, R.; Bhattacharya, P. Hydrogen from Food Processing Wastes via Photofermentation Using Purple Non-Sulfur Bacteria (PNSB)—A Review. Energy Convers. Manag. 2017, 141, 299–314. [Google Scholar] [CrossRef]
- Liu, B.F.; Jin, Y.R.; Cui, Q.F.; Xie, G.J.; Wu, Y.N.; Ren, N.Q. Photo-Fermentation Hydrogen Production by Rhodopseudomonas Sp. Nov. Strain A7 Isolated from the Sludge in a Bioreactor. Int. J. Hydrogen Energy 2015, 40, 8661–8668. [Google Scholar] [CrossRef]
- Biohydrogen and Poly-β-Hydroxybutyrate Production by Winery Wastewater. Available online: https://www.researchgate.net/publication/342569370_Biohydrogen_and_poly-b-hydroxybutyrate_production_by_winery_wastewater_photofermentation_Effect_of_substrate_concentration_and_nitrogen_source (accessed on 10 August 2024).
- Hitam, C.N.C.; Jalil, A.A. A Review on Biohydrogen Production through Photo-Fermentation of Lignocellulosic Biomass. Biomass Convers. Biorefinery 2023, 13, 8465–8483. [Google Scholar] [CrossRef]
- Balat, M. Production of Hydrogen via Biological Processes. Energy Sources Part A Recover. Util. Environ. Eff. 2009, 31, 1802–1812. [Google Scholar] [CrossRef]
- Laurinavichene, T.; Tekucheva, D.; Laurinavichius, K.; Tsygankov, A. Utilization of Distillery Wastewater for Hydrogen Production in One-Stage and Two-Stage Processes Involving Photofermentation. Enzyme Microb. Technol. 2018, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chookaew, T.; O-Thong, S.; Prasertsan, P. Biohydrogen Production from Crude Glycerol by Two Stage of Dark and Photo Fermentation. Int. J. Hydrogen Energy 2015, 40, 7433–7438. [Google Scholar] [CrossRef]
- Hitit, Z.Y.; Zampol Lazaro, C.; Hallenbeck, P.C. Increased Hydrogen Yield and COD Removal from Starch/Glucose Based Medium by Sequential Dark and Photo-Fermentation Using Clostridium Butyricum and Rhodopseudomonas Palustris. Int. J. Hydrogen Energy 2017, 42, 18832–18843. [Google Scholar] [CrossRef]
- Wu, S.C.; Liou, S.Z.; Lee, C.M. Correlation between Bio-Hydrogen Production and Polyhydroxybutyrate (PHB) Synthesis by Rhodopseudomonas Palustris WP3-5. Bioresour. Technol. 2012, 113, 44–50. [Google Scholar] [CrossRef]
- Nortez, K.B.; Movillon, J.L.; Alfafara, C.G.; Sevilla-Nastor, J.B.; Ventura, R.L.G.; Ventura, J.R.S. Optimization of Photofermentative Biohydrogen Production in a Mixed Volatile Fatty Acid Medium by Rhodobacter Sp. MAY2: A Response Surface Methodology (RSM) Approach. Int. J. Hydrogen Energy 2024, 56, 844–852. [Google Scholar] [CrossRef]
- Al-Mohammedawi, H.H.; Znad, H.; Eroglu, E. Synergistic Effects and Optimization of Photo-Fermentative Hydrogen Production of Rhodobacter Sphaeroides DSM 158. Int. J. Hydrogen Energy 2018, 43, 15823–15834. [Google Scholar] [CrossRef]
- Policastro, G.; Carraturo, F.; Compagnone, M.; Guida, M.; Fabbricino, M. Enhancing Hydrogen Production from Winery Wastewater through Fermentative Microbial Culture Selection. Bioresour. Technol. Reports 2022, 19, 101196. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Thomas, I. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef]
- Alam, M.W.; Wang, Z.; Naka, S.; Okada, H. Top Contact Pentacene Based Organic Thin Film Transistor with Bi-Layer TiO 2/Au Electrodes. J. Photopolym. Sci. Technol. 2012, 25, 659–664. [Google Scholar] [CrossRef]
- Waqas Alam, M.; Wang, Z.; Naka, S.; Okada, H. Mobility Enhancement of Top Contact Pentacene Based Organic Thin Film Transistor with Bi-Layer GeO/Au Electrodes. Appl. Phys. Lett. 2013, 102, 2–5. [Google Scholar] [CrossRef]
- Nozik, A.J. Photoelectrochemical Cells. Int. Telemetering Conf. 1978, 414, 6–12. [Google Scholar]
- Manuscript, A. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Takanabe, K. Photocatalytic Water Splitting: Quantitative Approaches toward Photocatalyst by Design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- Joseph, M.; Kumar, M.; Haridas, S.; Subrahmanyam, C.; Remello, S.N. A Review on the Advancements of Graphitic Carbon Nitride-Based Photoelectrodes for Photoelectrochemical Water Splitting. Energy Adv. 2023, 3, 30–59. [Google Scholar] [CrossRef]
- Liu, J.; Lu, L.; Wood, D.; Lin, S. New Redox Strategies in Organic Synthesis by Means of Electrochemistry and Photochemistry. ACS Cent. Sci. 2020, 6, 1317–1340. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; Raja, K.S.; Mahajan, V.K.; Misra, M. Efficient Photoelectrolysis of Water Using TiO2 Nanotube Arrays by Minimizing Recombination Losses with Organic Additives. J. Phys. Chem. C 2008, 112, 11007–11012. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I.; Naterer, G.F. Analysis of a Photochemical Water Splitting Reactor with Supramolecular Catalysts and a Proton Exchange Membrane. Int. J. Hydrogen Energy 2011, 36, 11273–11281. [Google Scholar] [CrossRef]
- Grimm, A.; de Jong, W.A.; Kramer, G.J. Renewable Hydrogen Production: A Techno-Economic Comparison of Photoelectrochemical Cells and Photovoltaic-Electrolysis. Int. J. Hydrogen Energy 2020, 45, 22545–22555. [Google Scholar] [CrossRef]
- Alfano, A.; Mezzetti, A.; Fumagalli, F.; Tao, C.; Rovera, E.; Petrozza, A.; Di Fonzo, F. Photoelectrochemical Water Splitting by Hybrid Organic-Inorganic Systems: Setting the Path from 2% to 20% Solar-to-Hydrogen Conversion Efficiency. iScience 2021, 24, 102463. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhao, B.; Chen, M.; Wang, L.; Fu, X.Z.; Luo, J.L. Electrolysis of Waste Water Containing Aniline to Produce Polyaniline and Hydrogen with Low Energy Consumption. Int. J. Hydrogen Energy 2020, 45, 22419–22426. [Google Scholar] [CrossRef]
- Yao, J.; Pan, B.; Shen, R.; Yuan, T.; Wang, J. Differential Control of Anode/Cathode Potentials of Paired Electrolysis for Simultaneous Removal of Chemical Oxygen Demand and Total Nitrogen. Sci. Total Environ. 2019, 687, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Vijapur, S.H.; Wang, Y.; Botte, G.G. NiCo2O4 Nanosheets Grown on Current Collectors as Binder-Free Electrodes for Hydrogen Production via Urea Electrolysis. Int. J. Hydrogen Energy 2017, 42, 3987–3993. [Google Scholar] [CrossRef]
- Hu, S.; Tan, Y.; Feng, C.; Wu, H.; Zhang, J.; Mei, H. Synthesis of N Doped NiZnCu-Layered Double Hydroxides with Reduced Graphene Oxide on Nickel Foam as Versatile Electrocatalysts for Hydrogen Production in Hybrid-Water Electrolysis. J. Power Sources 2020, 453, 227872. [Google Scholar] [CrossRef]
- Chen, C.; Bai, Q.; Liu, J.; Wang, Z.; Cen, K. Characteristics and Anode Reaction of Organic Wastewater-Assisted Coal Electrolysis for Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 20894–20903. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nahrin, M.; Chowdhury, S.N.; Nuzhat, S.; Alherek, M.; Rafa, N.; Ong, H.C.; Nghiem, L.D.; Mahlia, T.M.I. Biohydrogen Production from Wastewater-Based Microalgae: Progresses and Challenges. Int. J. Hydrogen Energy 2022, 47, 37321–37342. [Google Scholar] [CrossRef]
- Zakaria, B.S.; Lin, L.; Dhar, B.R. Shift of Biofilm and Suspended Bacterial Communities with Changes in Anode Potential in a Microbial Electrolysis Cell Treating Primary Sludge. Sci. Total Environ. 2019, 689, 691–699. [Google Scholar] [CrossRef]
- Khan, M.Z.; Nizami, A.S.; Rehan, M.; Ouda, O.K.M.; Sultana, S.; Ismail, I.M.; Shahzad, K. Microbial Electrolysis Cells for Hydrogen Production and Urban Wastewater Treatment: A Case Study of Saudi Arabia. Appl. Energy 2017, 185, 410–420. [Google Scholar] [CrossRef]
- Jayabalan, T.; Matheswaran, M.; Preethi, V.; Naina Mohamed, S. Enhancing Biohydrogen Production from Sugar Industry Wastewater Using Metal Oxide/Graphene Nanocomposite Catalysts in Microbial Electrolysis Cell. Int. J. Hydrogen Energy 2020, 45, 7647–7655. [Google Scholar] [CrossRef]
- Shen, R.; Jiang, Y.; Ge, Z.; Lu, J.; Zhang, Y.; Liu, Z.; Ren, Z.J. Microbial Electrolysis Treatment of Post-Hydrothermal Liquefaction Wastewater with Hydrogen Generation. Appl. Energy 2018, 212, 509–515. [Google Scholar] [CrossRef]
- Int, S.G.; Biotech, J.B. Biohydrogen Production—Sources and Methods: A Review. Int. J. Bioprocess Biotech. 2018, 2018. Available online: https://www.gavinpublishers.com/article/view/biohydrogen-production-sources-and-methods-a-review (accessed on 10 August 2024).
- Brandenburg, R.; Brandenburg, R. Corrigendum: Dielectric Barrier Discharges: Progress on Plasma Sources and on the Understanding of Regimes and Single Fi Laments. Plasma Sources Sci. Technol. 2017, 26, 053001. [Google Scholar] [CrossRef]
- Rehman, F.; Lozano-parada, J.H.; Zimmerman, W.B. A Kinetic Model for H2 Production by Plasmolysis of Water Vapours at Atmospheric Pressure in a Dielectric Barrier Discharge Microchannel Reactor. Int. J. Hydrogen Energy 2012, 37, 17678–17690. [Google Scholar] [CrossRef]
- Rehman, F.; Majeed, W.S.A.; Zimmerman, W.B. Hydrogen Production from Water Vapor Plasmolysis Using DBD- Corona Hybrid Reactor. Energy Fuels 2013, 27, 2748–2761. [Google Scholar] [CrossRef]
- Ramesh, K.; Honorio, H.; Chandra, D.; Lesueur, M. Comprehensive Review of Geomechanics of Underground Hydrogen Storage in Depleted Reservoirs and Salt Caverns. J. Energy Storage 2023, 73, 108912. [Google Scholar] [CrossRef]
- Alam, M.W.; BaQais, A.; Nahvi, I.; Yasin, A.; Mir, T.A.; Shajahan, S. Hydrothermally Synthesized Fluorine Added O3-NaFe1-XMgxO2 Cathodes for Sodium Ion Batteries. Inorganics 2023, 11, 37. [Google Scholar] [CrossRef]
- Jayachitra, J.; Richards Joshua, J.; Balamurugan, A.; Sivakumar, N.; Sharmila, V.; Shanavas, S.; Abu Haija, M.; Waqas Alam, M.; BaQais, A. High Electrode Performance of Hydrothermally Developed Activated C Coated O3–NaFeO2 Electrode for Na-Ion Batteries Applications. Ceram. Int. 2023, 49, 48–56. [Google Scholar] [CrossRef]
- Ross, D.K. Hydrogen Storage: The Major Technological Barrier to the Development of Hydrogen Fuel Cell Cars. Vacuum 2006, 80, 1084–1089. [Google Scholar] [CrossRef]
- Chen, X.; Suib, S.L.; Hayashi, Y.; Matsumoto, H. H2O Splitting in Tubular PACT (Plasma and Catalyst Integrated Technologies) Reactors. J. Catal. 2001, 201, 198–205. [Google Scholar] [CrossRef]
- Conde, J.J.; Maroño, M.; Sánchez-hervás, J.M. Pd-Based Membranes for Hydrogen Separation: Review of Alloying Elements and Their Influence on Membrane Properties; Taylor & Francis: Abingdon, UK, 2016; Volume 46. [Google Scholar] [CrossRef]
- Souayeh, B.; Bhattacharyya, S.; Hdhiri, N.; Alam, M.W. Heat and Fluid Flow Analysis and Ann-Based Prediction of a Novel Spring Corrugated Tape. Sustainability 2021, 13, 3023. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Challenges and Opportunities for Mixed-Matrix Membranes for Gas Separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Rehman, F.; Medley, G.J.D.; Bandulasena, H.; Zimmerman, W.B.J. Fluidic Oscillator-Mediated Microbubble Generation to Provide Cost Effective Mass Transfer and Mixing Efficiency to the Wastewater Treatment Plants. Environ. Res. 2015, 137, 32–39. [Google Scholar] [CrossRef] [PubMed]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y.; Miura, T. Preliminary Results of Hydrogen Production from Water Vapor Decomposition Using DBD Plasma in a PMCR Reactor. Int. J. Hydrogen Energy 2019, 44, 20239–20248. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Becher, M.; Haluska, M.; Hirscher, M.; Quintel, A.; Skakalova, V.; Dettlaff-Weglikovska, U.; Chen, X.; Hulman, M.; Choi, Y.; Roth, S.; et al. Hydrogen Storage in Carbon Nanotubes. Comptes Rendus Phys. 2003, 4, 1055–1062. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Bellosta von Colbe, J.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for Hydrogen-Based Energy Storage—Past, Recent Progress and Future Outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. ScienceDirect Catalytic Conversion of Lignocellulosic Biomass into Chemicals and Fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Furkan, H. Isikgor and C. Remzi Becer Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497. [Google Scholar]
- Lange, J.; Solutions, S.G. Lignocellulose Conversion: An Introduction to Chemistry, Process and Economics. Biofuels Bioprod. Biorefining: Innov. A Sustain. Econ. 2007, 1, 39–48. [Google Scholar] [CrossRef]
- Kasap, H.; Achilleos, D.S.; Huang, A.; Reisner, E. Engineered Carbon Nitride under Benign Conditions Photoreforming of Lignocellulose into H2 Using Nano-Engineered Carbon Nitride under Benign Conditions; American Chemical Society: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Bonabi, S.; Zhong, J.; Fang, P. Applied Catalysis B: Environmental Visible Light Driven Hydrogen Evolution by Photocatalytic Reforming of Lignin and Lactic Acid Using One-Dimensional NiS/CdS Nanostructures. Appl. Catal. B Environ. 2018, 227, 229–239. [Google Scholar] [CrossRef]
- Wakerley, D.W.; Kuehnel, M.F.; Orchard, K.L.; Ly, K.H.; Rosser, T.E.; Reisner, E. Solar-driven reforming of lignocellulose to H2 with a CdS/CdOx Photocatalyst. Nat. Energy 2017, 2, 17021. [Google Scholar] [CrossRef]
- Suleman, F.; Dincer, I.; Agelin-Chaab, M. Environmental Impact Assessment and Comparison of Some Hydrogen Production Options. Int. J. Hydrogen Energy 2015, 40, 6976–6987. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.S.; Park, J.; Kang, B.M.; Cho, C.; Lim, H.; Won, W. Applied Sciences Scenario-Based Techno-Economic Analysis of Steam Methane Reforming Process for Hydrogen Production No Research Hydrogen Emission. Appl. Sci. 2021, 11, 6021. [Google Scholar] [CrossRef]
- Iribarren, D.; Susmozas, A.; Petrakopoulou, F.; Dufour, J. Environmental and Exergetic Evaluation of Hydrogen Production via Lignocellulosic Biomass Gasification. J. Clean. Prod. 2014, 69, 165–175. [Google Scholar] [CrossRef]
- Zhang, C.; Yue, H.; Huang, Z.; Li, S.; Wu, G.; Ma, X.; Gong, J. Hydrogen Production via Steam Reforming of Ethanol on Phyllosilicate- Derived Ni/SiO2: Enhanced Metal-Support Interaction and Catalytic Stability. ACS Sustain. Chem. Eng. 2013, 1, 161–173. [Google Scholar] [CrossRef]
- Compagnoni, M.; Tripodi, A.; Rossetti, I. Parametric Study and Kinetic Testing for Ethanol Steam Reforming. Appl. Catal. B Environ. 2017, 203, 899–909. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The Role of Renewable Energy in the Global Energy Transformation. Energy Strateg. Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Xu, T.; Xiao, B.; Liu, S.; Hu, Z.; Li, J. CO2 Sorption-Enhanced Steam Reforming of Phenol Using Ni–M/CaO–Ca12Al14O33 (M = Cu, Co, and Ce) as Catalytic Sorbents. Chem. Eng. J. 2020, 393, 124769. [Google Scholar] [CrossRef]
- Sang, S.; Zhao, Z.J.; Tian, H.; Sun, Z.; Li, H.; Assabumrungrat, S.; Muhammad, T.; Zeng, L.; Gong, J. Promotional Role of MgO on Sorption-Enhanced Steam Reforming of Ethanol over Ni/CaO Catalysts. AIChE J. 2020, 66, e16877. [Google Scholar] [CrossRef]
- Franchi, G.; Capocelli, M.; De Falco, M.; Piemonte, V. Hydrogen Production via Steam Reforming: A Critical Analysis of MR and RMM Technologies. Membranes 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment Methods to Enhance Anaerobic Digestion of Organic Solid Waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Kothari, R.; Buddhi, D.; Sawhney, R.L. Comparison of Environmental and Economic Aspects of Various Hydrogen Production Methods. Renew. Sustain. Energy Rev. 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Proost, J. Critical Assessment of the Production Scale Required for Fossil Parity of Green Electrolytic Hydrogen. Int. J. Hydrogen Energy 2020, 45, 17067–17075. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Kavitha, S.; Preethi; Parthiba Karthikeyan, O.; Kumar, G.; Dai-Viet, N.V.; Rajesh Banu, J. Techno-Economic Assessment of Various Hydrogen Production Methods—A Review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef]
- Luo, M.; Yi, Y.; Wang, S.; Wang, Z.; Du, M.; Pan, J.; Wang, Q. Review of Hydrogen Production Using Chemical-Looping Technology. Renew. Sustain. Energy Rev. 2018, 81, 3186–3214. [Google Scholar] [CrossRef]
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam Gasification of Biomass with Subsequent Syngas Adjustment Using Shift Reaction for Syngas Production: An Aspen Plus Model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Xu, P.; Liu, B.; Shuai, Y.; Li, B. Hydrogen Production through Biomass Gasification in Supercritical Water: A Review from Exergy Aspect. Int. J. Hydrogen Energy 2019, 44, 15727–15736. [Google Scholar] [CrossRef]
- Yilmaz, F.; Ozturk, M.; Selbas, R. Design and Thermodynamic Analysis of Coal-Gasification Assisted Multigeneration System with Hydrogen Production and Liquefaction. Energy Convers. Manag. 2019, 186, 229–240. [Google Scholar] [CrossRef]
- Hyder, Z.; Ripepi, N.S.; Karmis, M.E. A Life Cycle Comparison of Greenhouse Emissions for Power Generation from Coal Mining and Underground Coal Gasification. Mitig. Adapt. Strateg. Glob. Change 2016, 21, 515–546. [Google Scholar] [CrossRef]
- Maka, A.O.M.; Mubbashar Mehmood, M. Green hydrogen energy production: Current status and potential. Clean Energy 2024, 8, 1–7. [Google Scholar] [CrossRef]
- Wu, T.; Shi, K.; Song, M. Biomass Gasification: An Overview of Technological Technological Barriers Barriers and and Socio-Environmental Socio-Environmental Impact. In Gasification for Low-Grade Feedstock; BoD: Norderstedt, Germany, 2018; pp. 3–18. [Google Scholar] [CrossRef]
- Jin, C.; Wu, Z.; Wang, S.; Cai, Z.; Chen, T. Economic Assessment of Biomass Gasification and Pyrolysis: A Review. Energy Sources Part B Econ. Plan. Policy 2017, 12, 1030–1035. [Google Scholar] [CrossRef]
- Kamble, A.D.; Kumar, V.; Dhondiram, P.; Atmaram, V. International Journal of Mining Science and Technology Co-Gasification of Coal and Biomass an Emerging Clean Energy Technology: Status and Prospects of Development in Indian Context. Int. J. Min. Sci. Technol. 2019, 29, 171–186. [Google Scholar] [CrossRef]
- Das, B.K.; Hoque, S.M.N. Assessment of the Potential of Biomass Gasification for Electricity Generation in Bangladesh. J. Renew. Energy 2014, 2014, 429518. [Google Scholar] [CrossRef]
- Ali, I.; Imanova, G.; Alharbi, O.M.L.; Hameed, A.M.; Nahid, M. Recent Updates in Direct Radiation Water—Splitting Methods of Hydrogen Production. J. Umm Al-Qura Univ. Appl. Sci. 2023, 10, 567–578. [Google Scholar] [CrossRef]
- Mathew, M.D. Progress in Nuclear Energy Nuclear Energy: A Pathway towards Mitigation of Global Warming. Prog. Nucl. Energy 2022, 143, 104080. [Google Scholar] [CrossRef]
- Orhan, M.F.; Dincer, I.; Rosen, M.A.; Kanoglu, M. Integrated Hydrogen Production Options Based on Renewable and Nuclear Energy Sources. Renew. Sustain. Energy Rev. 2012, 16, 6059–6082. [Google Scholar] [CrossRef]
- Aravindan, M.; Hariharan, V.S.; Narahari, T.; Kumar, A.; Madhesh, K.; Kumar, P.; Prabakaran, R. Fuelling the Future: A Review of Non-Renewable Hydrogen Production and Storage Techniques. Renew. Sustain. Energy Rev. 2023, 188, 113791. [Google Scholar] [CrossRef]
- Brook, B.W.; Alonso, A.; Meneley, D.A.; Misak, J.; Blees, T.; Erp, J.B. Van Sustainable Materials and Technologies Why Nuclear Energy Is Sustainable and Has to Be Part of the Energy Mix. Sustain. Mater. Technol. 2014, 2, 8–16. [Google Scholar]
- Navarro-Solís, I.; Villalba-Almendra, L.; Alvarez-Gallegos, A. H2 Production by PEM Electrolysis, Assisted by Textile Effluent Treatment and a Solar Photovoltaic Cell. Int. J. Hydrogen Energy 2010, 35, 10833–10841. [Google Scholar] [CrossRef]
- Du, L.; Xiong, H.; Lu, H.; Yang, L.M.; Liao, R.Z.; Xia, B.Y.; You, B. Electroshock Synthesis of a Bifunctional Nonprecious Multi-Element Alloy for Alkaline Hydrogen Oxidation and Evolution. Exploration 2022, 2, 20220024. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.K.; Kothari, R.; Tyagi, V.V.; Anand, S. Integrated Approach for Textile Industry Wastewater for Efficient Hydrogen Production and Treatment through Solar PV Electrolysis. Int. J. Hydrogen Energy 2020, 45, 25768–25782. [Google Scholar] [CrossRef]
- Arimi, M.M.M. Progress in Applications of Advanced Oxidation Processes for Promotion of Biohydrogen Production by Fermentation Processes. Biomass Convers. Biorefinery 2022, 12, 6033–6057. [Google Scholar]
- Heidrich, E.S.; Edwards, S.R.; Dolfing, J.; Cotterill, S.E.; Curtis, T.P. Performance of a Pilot Scale Microbial Electrolysis Cell Fed on Domestic Wastewater at Ambient Temperatures for a 12month Period. Bioresour. Technol. 2014, 173, 87–95. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Wu, X.; Jiaqiang, E.; Lu, N.; Wang, T.; Zuo, H. System Development and Environmental Performance Analysis of a Pilot Scale Microbial Electrolysis Cell for Hydrogen Production Using Urban Wastewater. Energy Convers. Manag. 2019, 193, 52–63. [Google Scholar] [CrossRef]
- Le, P.A.; Trung, V.D.; Nguyen, P.L.; Bac Phung, T.V.; Natsuki, J.; Natsuki, T. The Current Status of Hydrogen Energy: An Overview. RSC Adv. 2023, 13, 28262–28287. [Google Scholar] [CrossRef]
- Alam, M.W.; Kumar, V.G.D.; Ravikumar, C.R.; Prashantha, S.C.; Murthy, H.C.A.; Kumar, M.R.A. Chromium (III) Doped Polycrystalline MgAl2O4 Nanoparticles for Photocatalytic and Supercapacitor Applications. J. Phys. Chem. Solids 2022, 161, 110491. [Google Scholar] [CrossRef]
- Pratapkumar, C.; Prashantha, S.C.; Dileep Kumar, V.G.; Santosh, M.S.; Ravikumar, C.R.; Anilkumar, M.R.; Shashidhara, T.S.; Nanjunda Swamy, C.; Jahagirdar, A.A.; Alam, M.W.; et al. Structural, Photocatalytic and Electrochemical Studies on Facile Combustion Synthesized Low-Cost Nano Chromium (III) Doped Polycrystalline Magnesium Aluminate Spinels. J. Sci. Adv. Mater. Devices 2021, 6, 462–471. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Liu, S.; Fang, B. Z-Scheme Systems: From Fundamental Principles to Characterization, Synthesis, and Photocatalytic Fuel-Conversion Applications. Phys. Rep. 2022, 983, 1–41. [Google Scholar] [CrossRef]
- Pérez Orosa, L.; Chinarro, E.; Guinea, D.; García-Alegre, M.C. Hydrogen Production by Wastewater Alkaline Electro-Oxidation. Energies 2022, 15, 5888. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Chen, C.; Guo, J.; Bi, R.; Chen, S.; Zhang, L.; Zhu, M. Pt Nanoclusters Anchored on Ordered Macroporous Nitrogen-Doped Carbon for Accelerated Water Dissociation toward Superior Alkaline Hydrogen Production. Chem. Eng. J. 2022, 436, 135186. [Google Scholar] [CrossRef]

| S.No. | Production Technique | Cost | Environmental Impact | Efficiency |
|---|---|---|---|---|
| 1 | Biomass Gasification | Average | Low | 65–69% |
| 2 | Gasification | Moderate | Optimum | 60–80% |
| 3 | Electrolysis | Depends on energy source used | Optimum to high | 56–80% |
| 4 | Photoelectrochemical Water Splitting | High | Low | Less than 25% |
| 5 | Thermochemical Water Splitting | High | Low | 40–50% |
| 6 | Steam Methane Reforming | Low-cost | Higher emissions of CO2 | 65–75% |
| 7 | Coal Gasification | Low | - | 60–70% |
| 8 | Hydrogen From Wind | Optimum | Less | 5–70% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, H.S. Lower-Carbon Hydrogen Production from Wastewater: A Comprehensive Review. Sustainability 2024, 16, 8659. https://doi.org/10.3390/su16198659
Alqahtani HS. Lower-Carbon Hydrogen Production from Wastewater: A Comprehensive Review. Sustainability. 2024; 16(19):8659. https://doi.org/10.3390/su16198659
Chicago/Turabian StyleAlqahtani, Hassan S. 2024. "Lower-Carbon Hydrogen Production from Wastewater: A Comprehensive Review" Sustainability 16, no. 19: 8659. https://doi.org/10.3390/su16198659





