The Green Indium Patented Technology SCRIPT, for Indium Recovery from Liquid Crystal Displays: Bench Scale Validation Driven by Sustainability Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
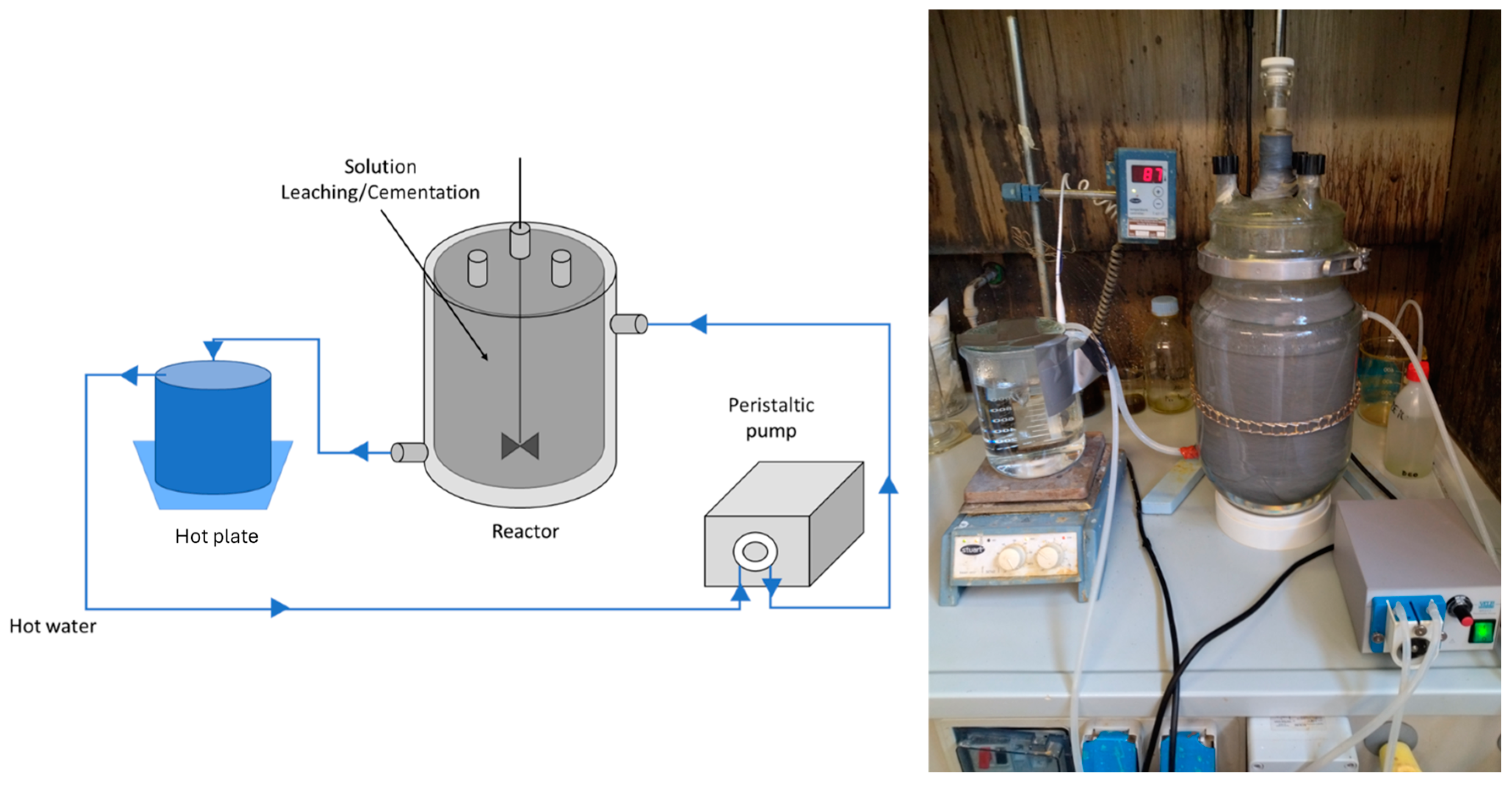
2.2. The Bench Scale Experimental System
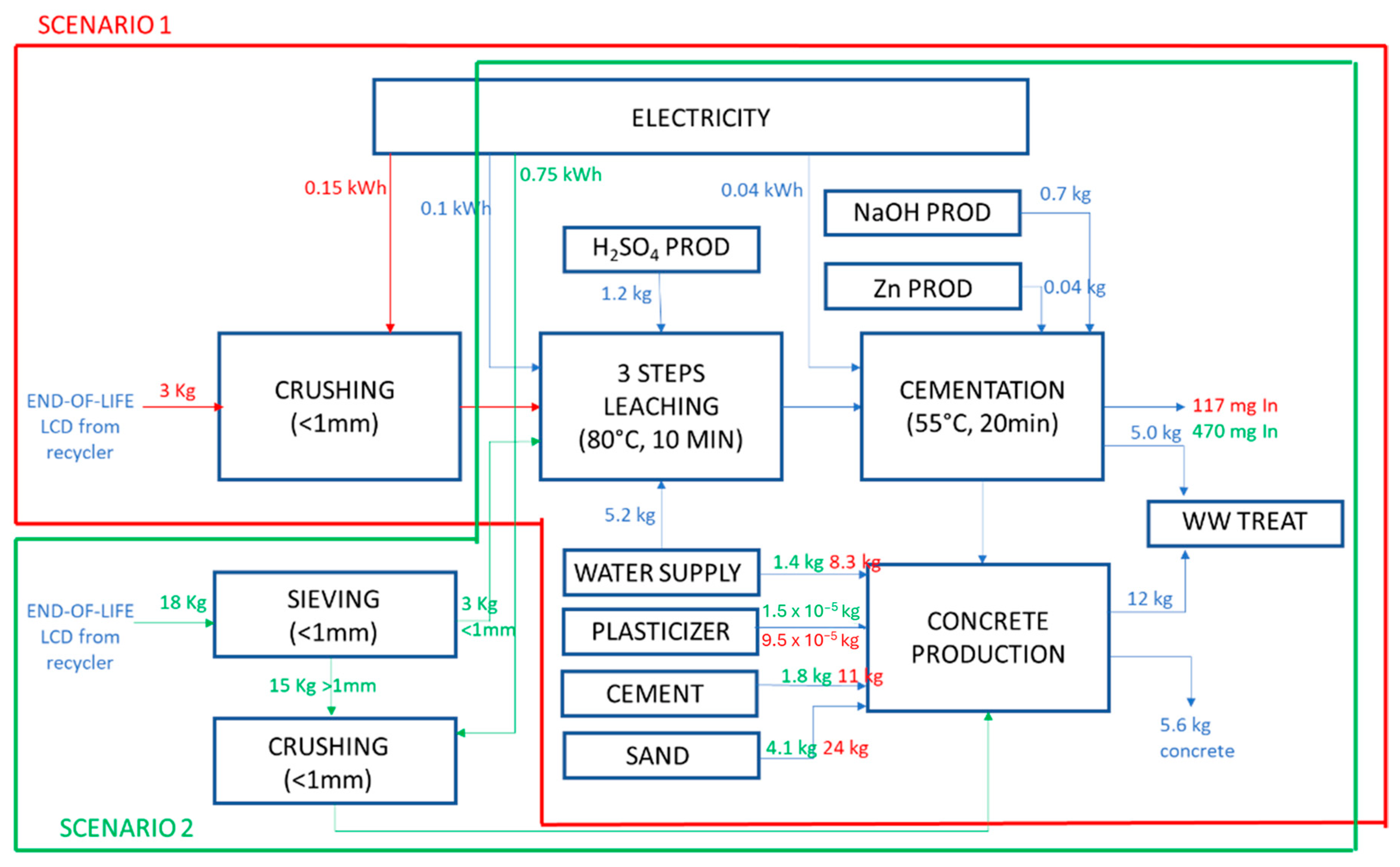
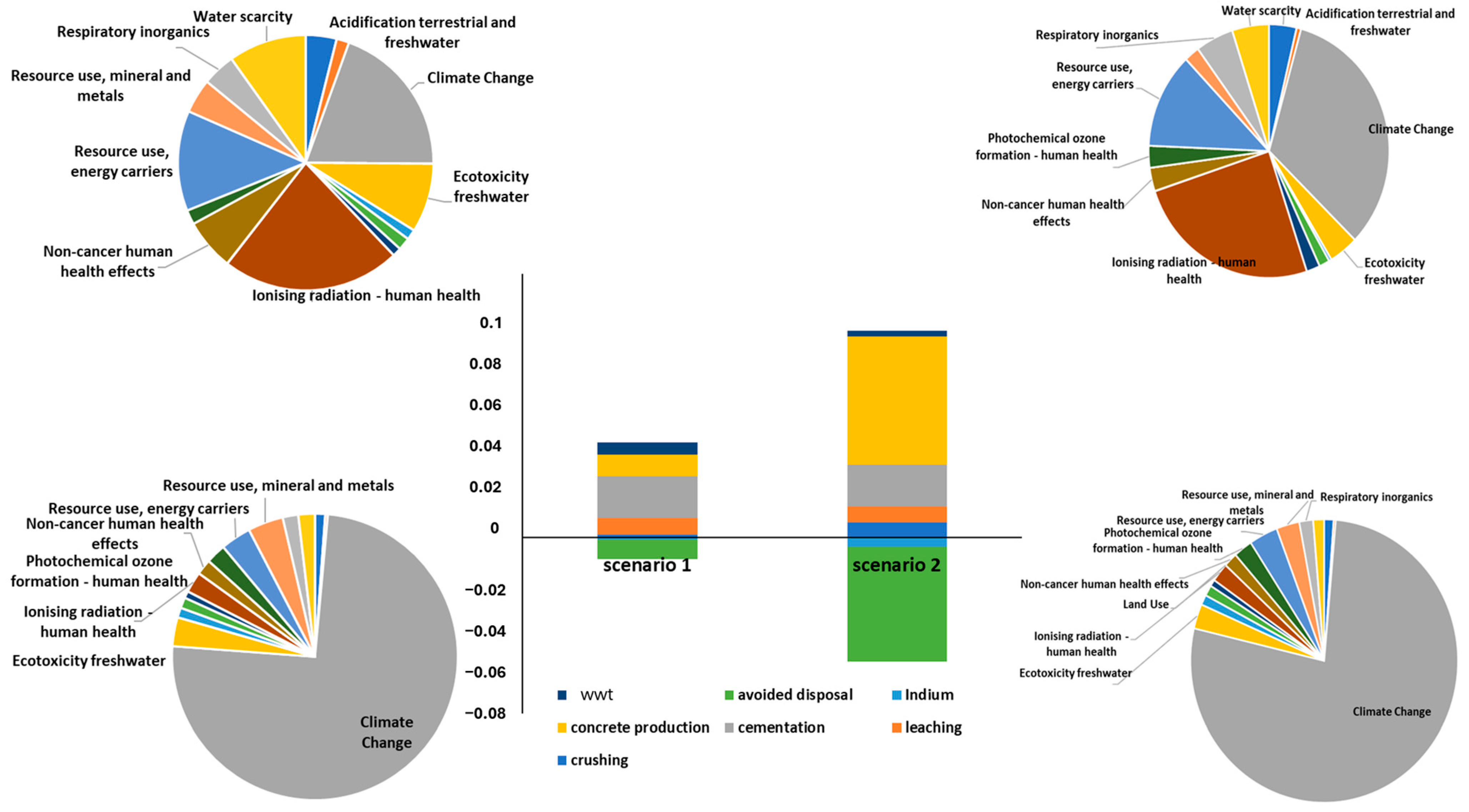
2.3. Assessment of the Advantageous Process Configuration through the Life Cycle Methodology
- Scenario 1: waste from recycler, with an average In concentration around 40 ppm, is crushed and sent to the patented technology SCRIPT.
- Scenario 2: waste from recycler facility is sieved and only the finest fraction (<1 mm) with the highest In concentration (157 ppm) is sent to the patented technology SCRIPT. The <1 mm fraction represents about 17% of the waste; therefore, 18 kg of LCDs are sieved to separate 3 kg, necessary for the 3-step process. The remaining 83% can be crushed and mixed with the scraps for building material production. This option allows an In pre-concentration in the waste flow but also metal loss in the >1 mm fraction (with an In content around 15 ppm).
- An environmental credit due to the avoided disposal in landfilling site has been included considering the current classification of end-of-life LCD panels as urban waste.
- The environmental credit related to the recovered In has been estimated by the allocation of Zn metal value on economic basis (240 USD/kg for In vs. 4 USD/kg for Zn). This assumption was considered acceptable since the two elements are extracted from the same ore, sphalerite [37].
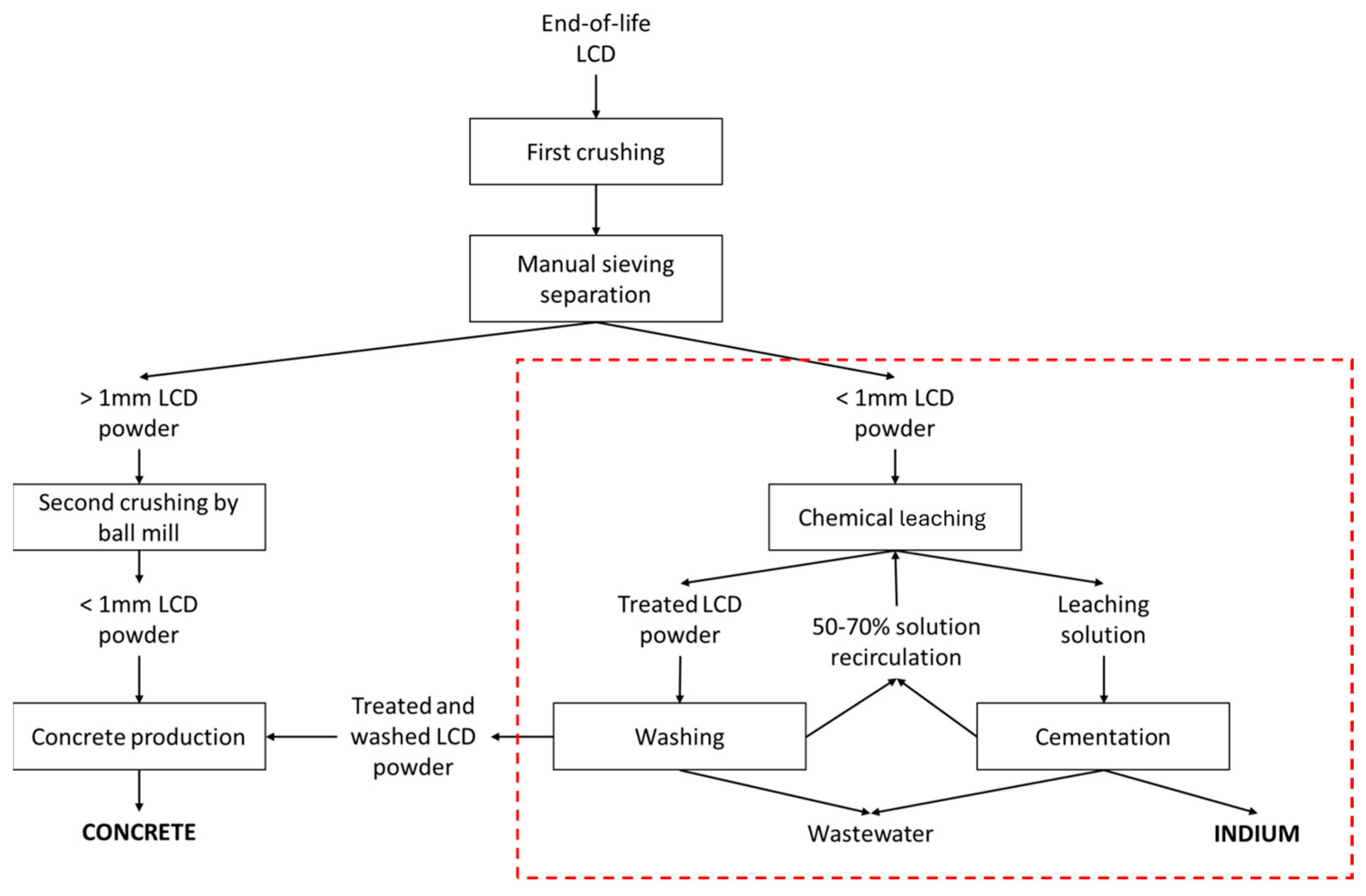
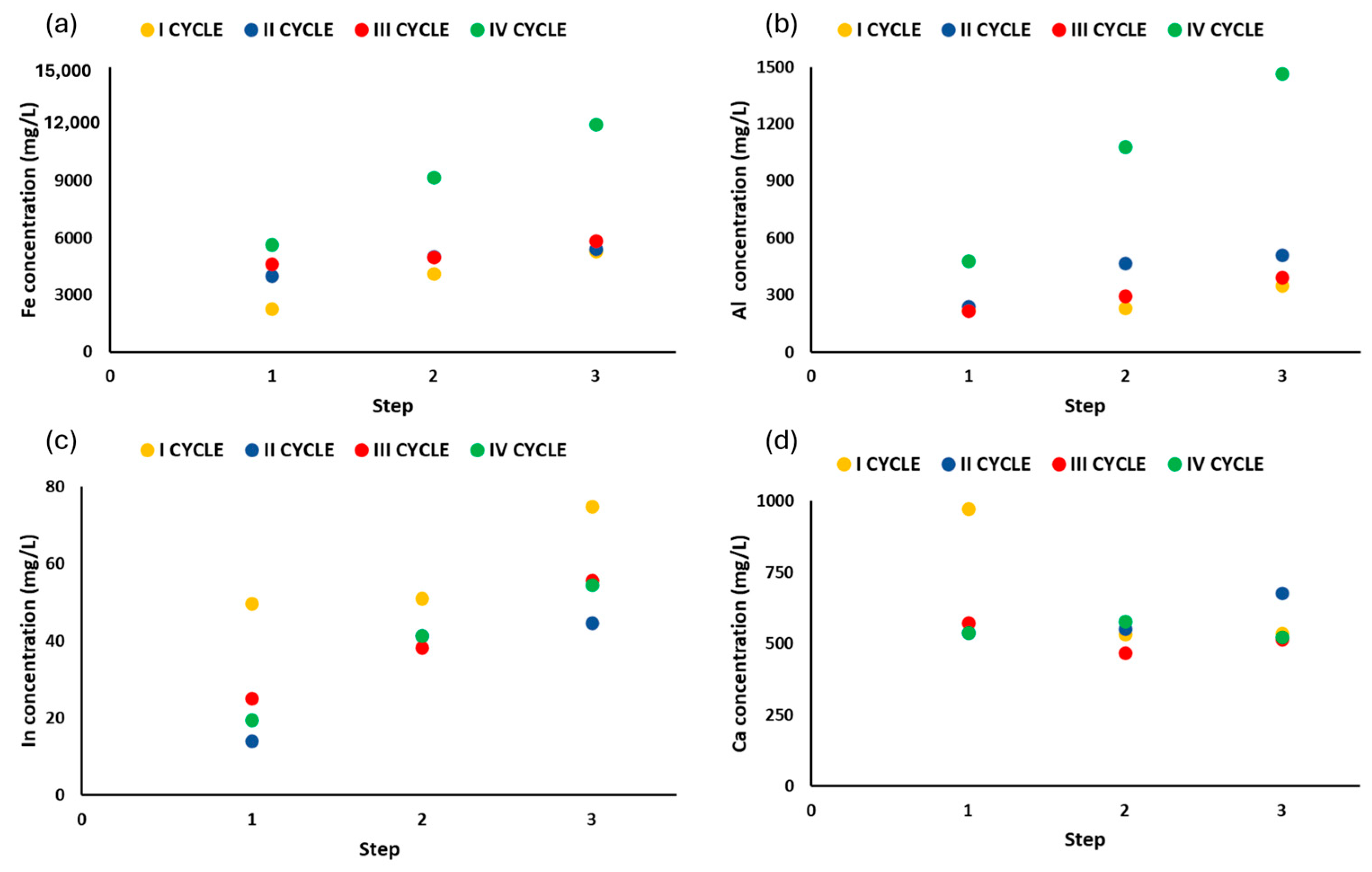
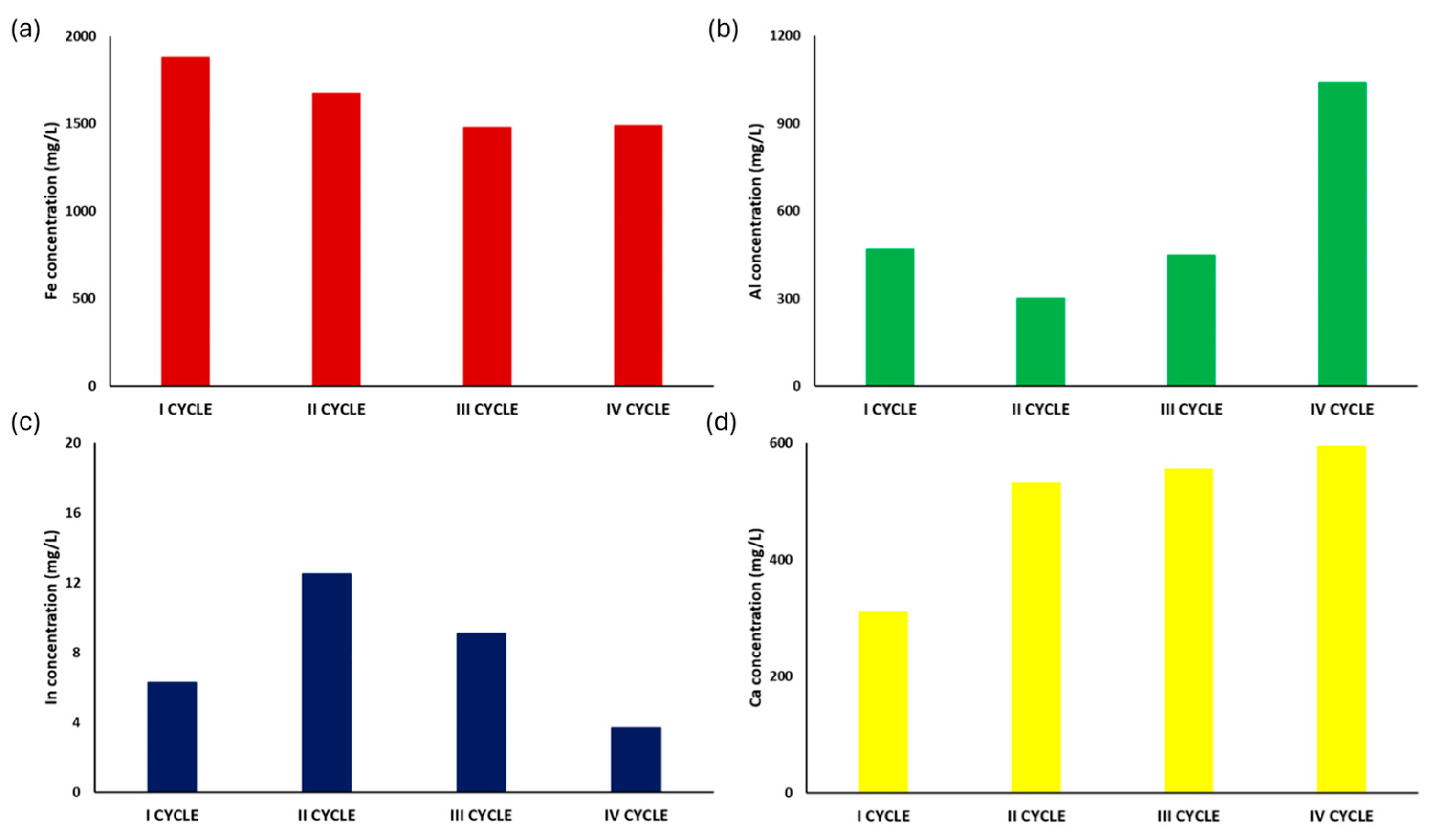
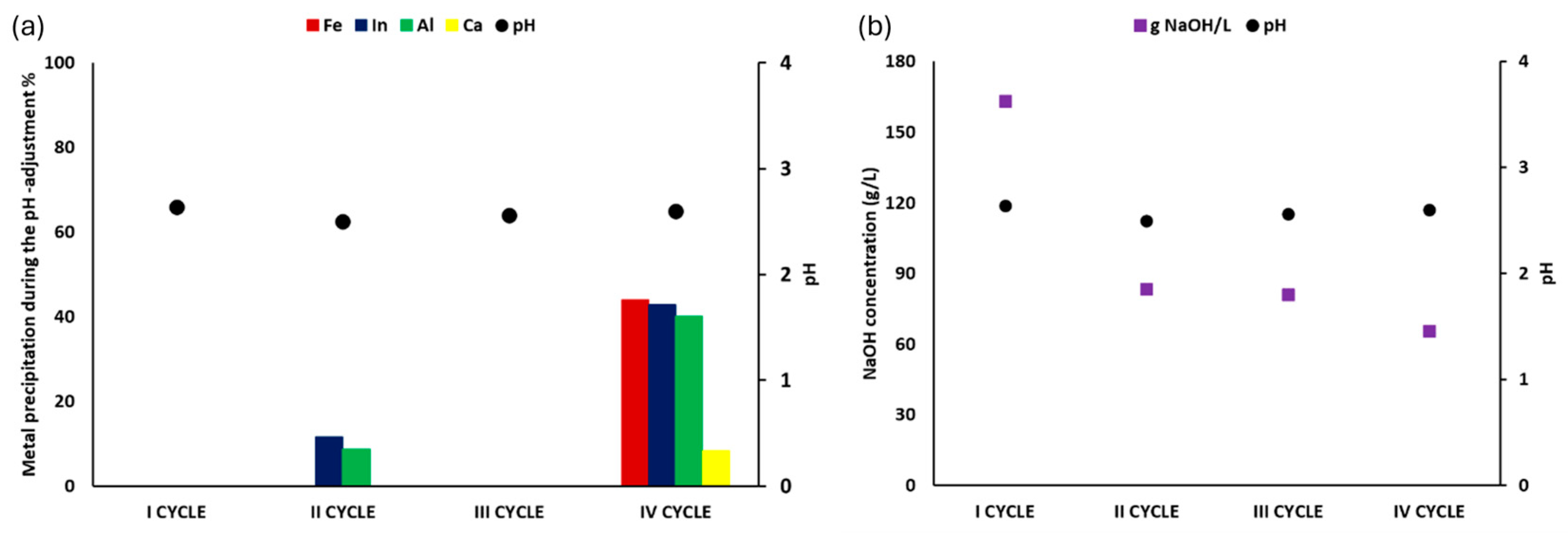
2.4. Optimization of the Patented SCRIPT Technology at Bench Scale
2.5. The Economic Assessment
3. Results and Discussion
3.1. Sample Characterizations
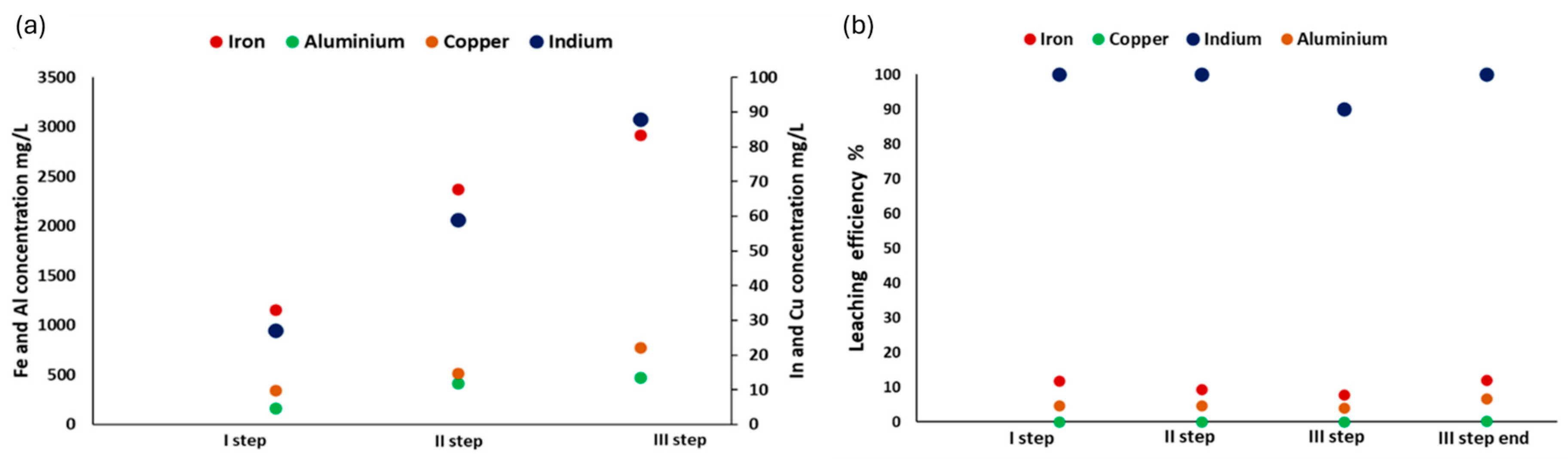
3.2. Validation of the Patented SCRIPT Technology at Bench Scale
3.3. Assessment of Advantageous Process Configuration through the Life Cycle Methodology—Life Cycle Impact Assessment (LCIA)
3.3.1. Classification and Characterization
3.3.2. Normalization and Weighing
3.4. Results of Optimization of the Patented Technology SCRIPT at Bench Scale
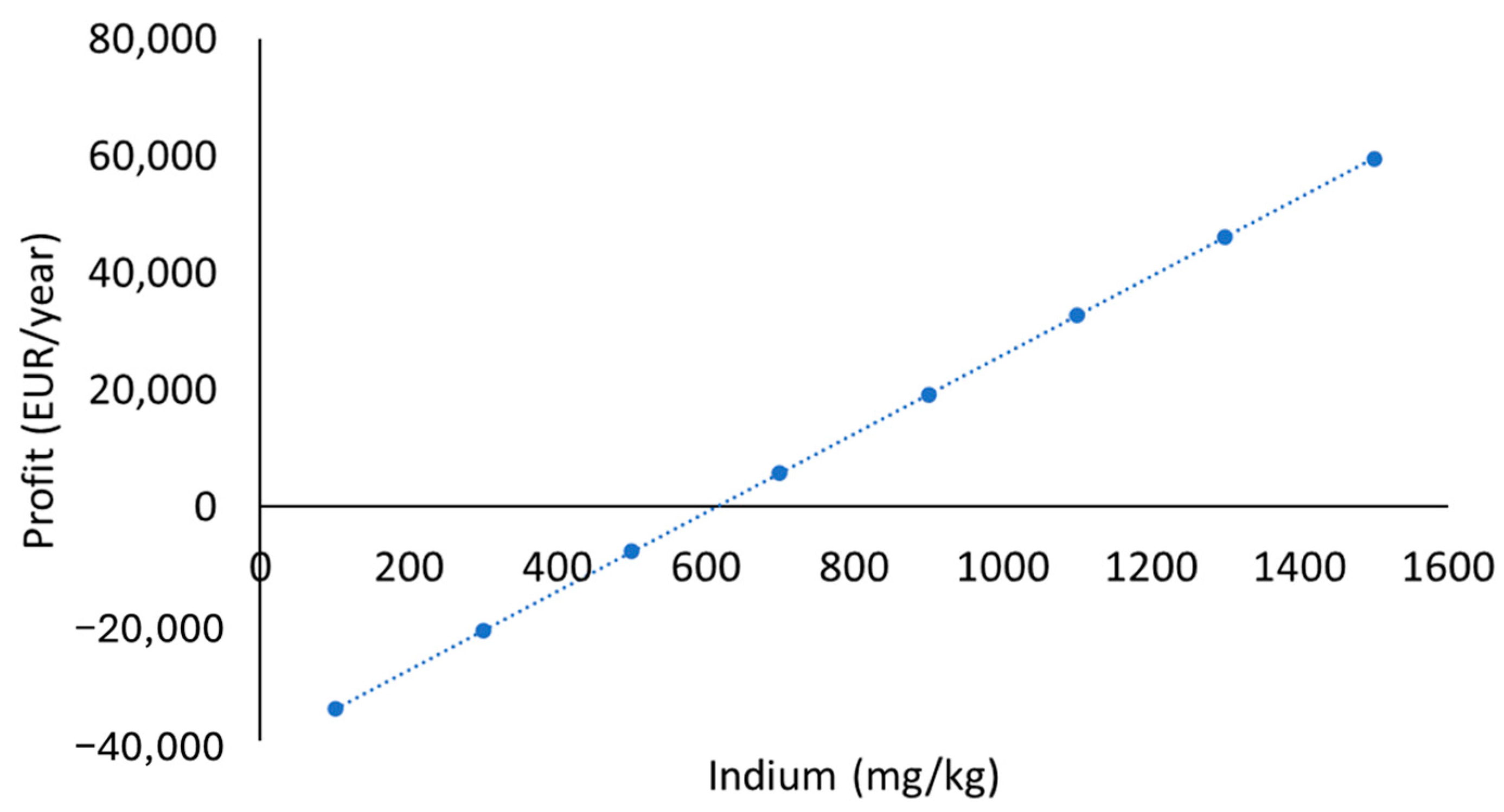
3.5. Economic Sustainability
3.6. Obstacles to Scaling Up
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lokanc, M.; Eggert, R.; Redlinger, M. The Availability of Indium: The Present, Medium Term, and Long Term; National Renewable Energy Laboratory: Golden, CO, USA, 2015; pp. 1–90. Available online: https://www.nrel.gov/docs/fy16osti/62409.pdf (accessed on 30 July 2024).
- Illés, I.B.; Nagy, S.; Kékesi, T. The recycling of pure metallic indium from waste LCD screens by a combined hydro-electrometallurgical method. Hydrometallurgy 2022, 213, 105945. [Google Scholar] [CrossRef]
- Lin, K.L.; Chang, W.K.; Chang, T.C.; Lee, C.H.; Lin, C.H. Recycling thin film transistor liquid crystal display (TFT-LCD) waste glass produced as glass-ceramics. J. Clean. Prod. 2009, 17, 1499–1503. [Google Scholar] [CrossRef]
- Yuan, Z.; Shi, L. Improving enterprise competitive advantage with industrial symbiosis: Case study of a smeltery in China. J. Clean. Prod. 2009, 17, 1295–1302. [Google Scholar] [CrossRef]
- Rocchetti, L.; Amato, A.; Beolchini, F. Recovery of indium from liquid crystal displays. J. Clean. Prod. 2016, 116, 299–305. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Kucuker, M.A.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes—A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Zheng, K.; Benedetti, M.F.; van Hullebusch, E.D. Recovery technologies for indium, gallium, and germanium from end-of-life products (electronic waste)—A review. J. Environ. Manag. 2023, 347, 119043. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, Y.; Wang, W.; Li, B.; Zhang, Y.; Zuo, T. Recycling indium from waste LCDs: A review. Resour. Conserv. Recycl. 2015, 104, 276–290. [Google Scholar] [CrossRef]
- Rocchetti, L.; Amato, A.; Fonti, V.; Vegliò, F.; Beolchini, F. Innovative method to extract indium from LCD panels. Chem. Eng. Trans. 2015, 43, 1–6. [Google Scholar] [CrossRef]
- Ma, E.; Xu, Z. Technological process and optimum design of organic materials vacuum pyrolysis and indium chlorinated separation from waste liquid crystal display panels. J. Hazard. Mater. 2013, 263, 610–617. [Google Scholar] [CrossRef]
- Amato, A.; Rocchetti, L.; Beolchini, F. Evaluation of different strategies for end-of-life liquid crystal displays (LCD) management. Environ. Eng. Manag. J. 2017, 16, 1651. [Google Scholar] [CrossRef]
- Rocchetti, L.; Amato, A.; Fonti, V.; Ubaldini, S.; De Michelis, I.; Kopacek, B.; Vegliò, F.; Beolchini, F. Cross-current leaching of indium from end-of-life LCD panels. Waste Manag. 2015, 42, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Fontana, D.; Forte, F.; Pietrantonio, M.; Pucciarmati, S. Recent developments on recycling end-of-life flat panel displays: A comprehensive review focused on indium. Crit. Rev. Environ. Sci. Technol. 2021, 51, 429–456. [Google Scholar] [CrossRef]
- Gabriel, A.P.; Kasper, A.C.; Veit, H.M. Acid leaching of indium from the screens of obsolete LCD monitors. J. Environ. Chem. Eng. 2020, 8, 103758. [Google Scholar] [CrossRef]
- Zhang, K.; Li, B.; Wu, Y.; Wang, W.; Li, R.; Zhang, Y.N.; Zuo, T. Recycling of indium from waste LCD: A promising non-crushing leaching with the aid of ultrasonic wave. Waste Manag. 2017, 64, 236–243. [Google Scholar] [CrossRef]
- Pereira, E.B.; Suliman, A.L.; Tanabe, E.H.; Bertuol, D.A. Recovery of indium from liquid crystal displays of discarded mobile phones using solvent extraction. Miner. Eng. 2018, 119, 67–72. [Google Scholar] [CrossRef]
- Qin, J.; Ning, S.; Fujita, T.; Wei, Y.; Zhang, S.; Lu, S. Leaching of indium and tin from waste LCD by a time-efficient method assisted planetary high energy ball milling. Waste Manag. 2021, 120, 193–201. [Google Scholar] [CrossRef]
- Silveira, A.V.M.; Fuchs, M.S.; Pinheiro, D.K.; Tanabe, E.H.; Bertuol, D.A. Recovery of indium from LCD screens of discarded cell phones. Waste Manag. 2015, 45, 334–342. [Google Scholar] [CrossRef]
- Yen, F.C.; Chang, T.C.; Laohaprapanon, S.; Chen, Y.L.; You, S.J. Recovery of indium from LCD waste by solvent extraction and the supported liquid membrane with strip dispersion using D2EHPA as the extractant. Solvent Extr. Res. Dev. Jpn. 2016, 23, 63–73. [Google Scholar] [CrossRef]
- Savvilotidou, V.; Hahladakis, J.N.; Gidarakos, E. Leaching capacity of metals-metalloids and recovery of valuable materials from waste LCDs. Waste Manag. 2015, 45, 314–324. [Google Scholar] [CrossRef]
- Assefi, M.; Maroufi, S.; Nekouei, R.K.; Sahajwalla, V. Selective recovery of indium from scrap LCD panels using macroporous resins. J. Clean. Prod. 2018, 180, 814–822. [Google Scholar] [CrossRef]
- Lee, C.H.; Jeong, M.K.; Kilicaslan, M.F.; Lee, J.H.; Hong, H.S.; Hong, S.J. Recovery of indium from used LCD panel by a time efficient and environmentally sound method assisted HEBM. Waste Manag. 2013, 33, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhu, N.; Luo, D.; Li, Y.; Wu, P.; Dang, Z.; Hu, X. The Role of Oxalic Acid in the Leaching System for Recovering Indium from Waste Liquid Crystal Display Panels. ACS Sustain. Chem. Eng. 2019, 7, 3849–3857. [Google Scholar] [CrossRef]
- Argenta, A.B.; Reis, C.M.; Mello, G.P.; Dotto, G.L.; Tanabe, E.H.; Bertuol, D.A. Supercritical CO2 extraction of indium present in liquid crystal displays from discarded cell phones using organic acids. J. Supercrit. Fluids. 2017, 120, 95–101. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, N.; Wei, X.; Cui, J.; Wu, P.; Li, P.; Wu, J.; Lin, Y. Leaching of indium from waste LCD screens by oxalic acid in temperature-controlled aciduric stirred reactor. Process Saf. Environ. Prot. 2020, 133, 137–148. [Google Scholar] [CrossRef]
- López-Yáñez, A.; Alonso, A.; Vengoechea-Pimienta, A.; Ramírez-Muñoz, J. Indium and tin recovery from waste LCD panels using citrate as a complexing agent. Waste Manag. 2019, 96, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Gupta, B. Cyphos IL 104 assisted extraction of indium and recycling of indium, tin and zinc from discarded LCD screen. Sep. Purif. Technol. 2020, 237, 116407. [Google Scholar] [CrossRef]
- Swain, B.; Mishra, C.; Hong, H.S.; Cho, S.S. Beneficiation and recovery of indium from liquid-crystal-display glass by hydrometallurgy. Waste Manag. 2016, 57, 207–214. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, F.; Sun, X.; Li, J. Recycling Indium from Scraped Glass of Liquid Crystal Display: Process Optimizing and Mechanism Exploring. ACS Sustain. Chem. Eng. 2015, 3, 1306–1312. [Google Scholar] [CrossRef]
- Jowkar, M.J.; Bahaloo-Horeh, N.; Mousavi, S.M.; Pourhossein, F. Bioleaching of indium from discarded liquid crystal displays. J. Clean. Prod. 2018, 180, 417–429. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Nancharaiah, Y.V. Biotechnology in the management and resource recovery from metal bearing solid wastes: Recent advances. J. Environ. Manag. 2018, 211, 138–153. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, S.; Tian, X.; Che, L.; Wu, X.; Zhao, F. Leaching of indium from end-of-life LCD panels via catalysis by synergistic microbial communities. Sci. Total Environ. 2019, 655, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Willner, J.; Fornalczyk, A.; Saternus, M.; Sedlakova-Kadukova, J.; Gajda, B. LCD panels bioleaching with pure and mixed culture of Acidithiobacillus. Physicochem. Probl. Miner. Process. 2022, 58, 15–23. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, N.; Mao, F.; Wu, P.; Dang, Z. Bioleaching of indium from waste LCD panels by Aspergillus niger: Method optimization and mechanism analysis. Sci. Total Environ. 2021, 790, 148151. [Google Scholar] [CrossRef] [PubMed]
- Ruello, M.L.; Amato, A.; Beolchini, F.; Monosi, S. Valorizing end-of-life LCD scraps after indium recovery. Phys. Status Solidi 2016, 13, 1011–1016. [Google Scholar] [CrossRef]
- European Commission. European Platform on Life Cycle Assessment. Available online: https://eplca.jrc.ec.europa.eu/ (accessed on 24 September 2024).
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Ghisellini, P.; Passaro, R.; Ulgiati, S. Environmental and Social Life Cycle Assessment of Waste Electrical and Electronic Equipment Management in Italy According to EU Directives. Environment 2023, 10, 106. [Google Scholar] [CrossRef]
- Eynard, U.; Mancini, L.; Eisfeldt, F.; Ciroth, A.; Pennington, D. Social risk in raw materials extraction: A macro-scale assessment. In Proceedings of the 6th Social LCA Conference, People and Places for Partnership, Pescara, Itlaly, 10–12 September 2018. [Google Scholar]
- Amato, A.; Rocchetti, L.; Beolchini, F. Environmental impact assessment of different end-of-life LCD management strategies. Waste Manag. 2017, 59, 432–441. [Google Scholar] [CrossRef]
- Umair, S.; Björklund, A.; Petersen, E.E. Social impact assessment of informal recycling of electronic ICT waste in Pakistan using UNEP SETAC guidelines. Resour. Conserv. Recycl. 2015, 95, 46–57. [Google Scholar] [CrossRef]
- Abeliotis, K.; Chroni, C.; Lasaridi, K. Social Life Cycle Assessment for WEEE Reuse. In Proceedings of the XI Convegno della Rete Italiana LCA Resource Efficiency e Sustainable Development Goals: Il Ruolo del Life Cycle Thinking, Siena, Italy, 22–23 June 2017; Volume 2017. Available online: http://uest.ntua.gr/athens2017/proceedings/pdfs/Athens2017_Abeliotis_Chroni_Lasaridi.pdf (accessed on 24 September 2024).
- The London Metals Exchange, an HKEX Company. Available online: https://www.lme.com/en/ (accessed on 25 September 2024).
- Wang, F.; Li, Y.; Liu, M.; Pang, D.; Du, W.; Zhang, Y.; Cheng, X.; Gu, T.; Guo, W. Comprehensive Evaluation of the Performances of Heat Exchangers with Aluminum and Copper Finned Tubes. Int. J. Chem. Eng. 2023, 2023, 6666947. [Google Scholar] [CrossRef]
- Savvilotidou, V.; Kousaiti, A.; Batinic, B.; Vaccari, M.; Kastanaki, E.; Karagianni, K.; Gidarakos, E. Evaluation and comparison of pre-treatment techniques for recovering indium from discarded liquid crystal displays. Waste Manag. 2019, 87, 51–61. [Google Scholar] [CrossRef]
- Marra, A.; Cesaro, A.; Belgiorno, V. Hydrometallurgical recovery of critical metals from WEEE shredding dust. In Proceedings of the 15th International Conference on Environmental Science and Technology, Rhodes, Greece, 21–23 November 2017. [Google Scholar]
- Chancerel, P.; Meskers, C.E.M.; Hagelüken, C.; Rotter, V.S. Assessment of precious metal flows during preprocessing of waste electrical and electronic equipment. J. Ind. Ecol. 2009, 13, 791–810. [Google Scholar] [CrossRef]
- Yang, J.; Retegan, T.; Ekberg, C. Indium recovery from discarded LCD panel glass by solvent extraction. Hydrometallurgy 2013, 137, 68–77. [Google Scholar] [CrossRef]
- Ardente, F.; Mathieux, F.; Recchioni, M. Recycling of electronic displays: Analysis of pre-processing and potential ecodesign improvements. Resour. Conserv. Recycl. 2014, 92, 158–171. [Google Scholar] [CrossRef]
- Forti, V.; Baldè, C.P.; Kuehr, R.; Bel, G.; Monitor, T.G.E.-W. 2020: Quantities, Flows and the Circular Economy Potential; United Nations University (UNU): Tokyo, Japan; United Nations Institute for Training and Research: Geneva, Switzerland, 2020. [Google Scholar]
- Vučinić, A.A.; Šimunić, S.; Radetić, L.; Presečki, I. Indium Recycling from Waste Liquid Crystal Displays: Is It Possible? Processes 2023, 11, 1662. [Google Scholar] [CrossRef]
- Ferella, F.; Belardi, G.; Marsilii, A.; De Michelis, I.; Vegliò, F. Separation and recovery of glass, plastic and indium from spent LCD panels. Waste Manag. 2017, 60, 569–581. [Google Scholar] [CrossRef]
- Song, Q.; Liu, Y.; Zhang, L.; Xu, Z. Facile indium recovery from waste liquid crystal displays: Chloride-facilitated indium electroreduction and stepwise Cu/MoO2 and indium electrodeposition. J. Hazard. Mater. 2021, 415, 125599. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, L.; Xu, Z. Indium recovery from In-Sn-Cu-Al mixed system of waste liquid crystal display panels via acid leaching and two-step electrodeposition. J. Hazard. Mater. 2020, 381, 120973. [Google Scholar] [CrossRef]







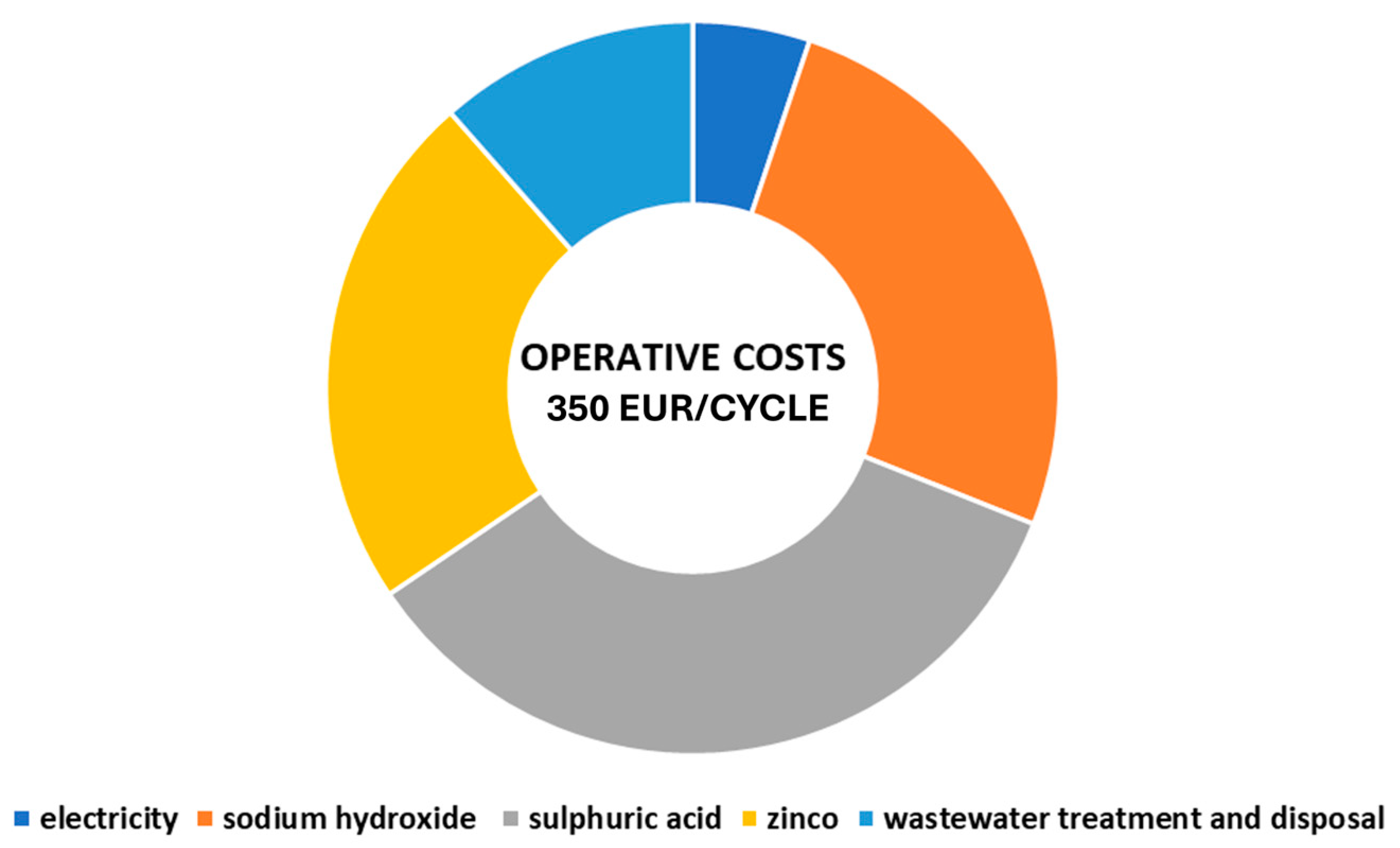
| Flow | Unitary Cost (EUR/kg) |
|---|---|
| Electricity (EUR/kWh) | 0.1 |
| NaOH | 1 |
| H2SO4 | 0.5 |
| Zn | 10 |
| Wastewater treatment and disposal | 0.1 |
| Solid waste disposal | 0.25 |
| In | 400 |
| <1 | 1 < Ø < 5 | >5 | |
| S1 | |||
 |  |  | |
| Waste | Dimension (mm) | Metal Conc. (mg/kg) | |
| In | Ga | ||
| S1 | <1 | 160 ± 30 | 6 ± 1 |
| 1 < Ø < 5 | 17 ± 5 | 6 ± 3 | |
| >5 | 2.5 ± 0.5 | 14 ± 2 | |
| Indium Content | Other Metals | Reference |
|---|---|---|
| 100 | Al, Ca, Fe, Mn, Mo, and Sn | [12] |
| 261 | Sn | [22] |
| 576 | Si, Al, B, Ca, Sr, Fe, Mg, Ba, Sn, Cr, Na, K, and Cu | [23] |
| 120 | Al, Fe, In, Ca, Mg, Sr, and Mo | [15] |
| 30 | n.a. a | [21] |
| n.a. a | Sn, Cu, Pb, and Al | [33] |
| 219 | Sn | [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becci, A.; Amato, A.; Merli, G.; Beolchini, F. The Green Indium Patented Technology SCRIPT, for Indium Recovery from Liquid Crystal Displays: Bench Scale Validation Driven by Sustainability Assessment. Sustainability 2024, 16, 8917. https://doi.org/10.3390/su16208917
Becci A, Amato A, Merli G, Beolchini F. The Green Indium Patented Technology SCRIPT, for Indium Recovery from Liquid Crystal Displays: Bench Scale Validation Driven by Sustainability Assessment. Sustainability. 2024; 16(20):8917. https://doi.org/10.3390/su16208917
Chicago/Turabian StyleBecci, Alessandro, Alessia Amato, Giulia Merli, and Francesca Beolchini. 2024. "The Green Indium Patented Technology SCRIPT, for Indium Recovery from Liquid Crystal Displays: Bench Scale Validation Driven by Sustainability Assessment" Sustainability 16, no. 20: 8917. https://doi.org/10.3390/su16208917
APA StyleBecci, A., Amato, A., Merli, G., & Beolchini, F. (2024). The Green Indium Patented Technology SCRIPT, for Indium Recovery from Liquid Crystal Displays: Bench Scale Validation Driven by Sustainability Assessment. Sustainability, 16(20), 8917. https://doi.org/10.3390/su16208917







