Coconut Waste: Discovering Sustainable Approaches to Advance a Circular Economy
Abstract
1. Introduction
2. Research Method and Approach
3. Coconut History, Cultivation, and Value Chain
3.1. Historical Records of Coconut
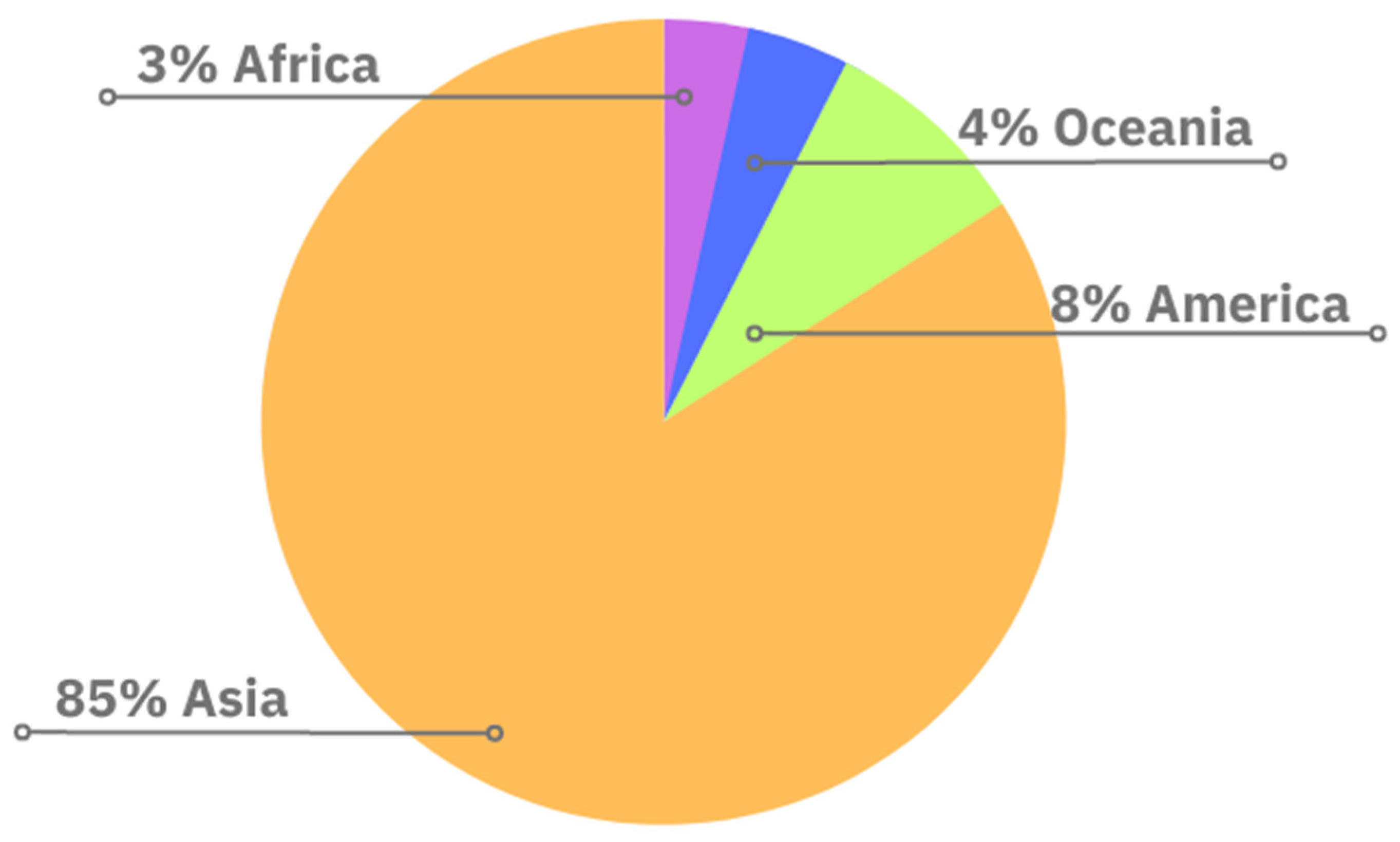
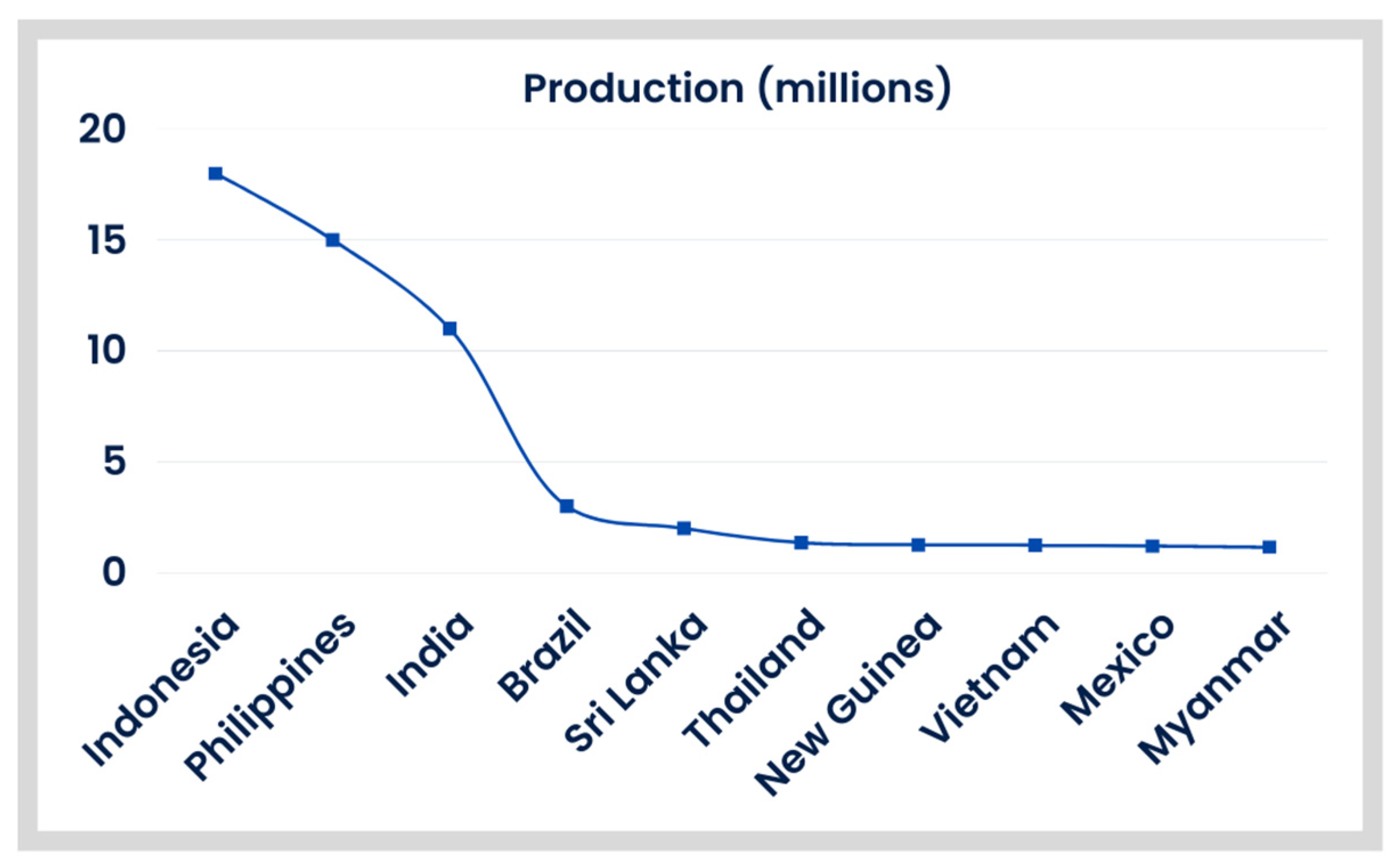
3.2. The Main Aspects of Coconut Cultivation
3.3. Coconut’s Main Products
4. Coconut Residues
4.1. Lignocellulosic Composition
4.2. Coconut Residues Characterization
4.3. From Waste to Value: Byproducts from Coconut Lignocellulosic Residues
4.3.1. Civil Constructions
4.3.2. Filtering Material and Adsorbent
4.3.3. Ethanol Production
4.3.4. Pyrolysis Products
4.3.5. Other Applications
5. Discussion
5.1. Circular Economy
5.2. Insights from Coconut Residue Value Chain
- (i)
- Value addition through product diversification and improvement of lignocellulosic chemical and functional properties in coconut residues;
- (ii)
- Integration of the whole coconut value chain, from main coconut products to byproduct generation;
- (iii)
- Strategic partnerships with other coconut industries or sectors;
- (iv)
- Boosting investments in the research and development of innovative technologies for processing coconut residues;
- (v)
- Identification and exploration of market opportunities for products derived from coconut waste at the national and international levels.
5.3. Coconut Industry: A Sustainable Pathway towards the 2030 Agenda Goals
- a.
- Goal 2: Zero Hunger
- b.
- Goal 3: Good Health and Well-being
- c.
- Goal 7: Affordable and Clean Energy
- d.
- Goal 9: Industry, Innovation, and Infrastructure
- e.
- Goal 12: Responsible Consumption and Production
- f.
- Goal 13: Climate Action
- g.
- Goal 15: Life on Land
- h.
- Goal 17: Partnerships for the Goals
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nampoothiri, K.U.K.; Krishnakumar, V.; Thampan, P.K.; Nair, M.A. World Coconut Economy: Sectoral Issues, Markets and Trade; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9789811327544. [Google Scholar]
- Subramanian, P.; Thamban, C.; Josephrajkumar, A.; Vinayaka, H.; Hebbar, K.B.; Ravi, B.; Niral, V. Coconut Development Board; Ministry of Agriculture & Farmers Welfare: Kerala, India, 2020. [Google Scholar]
- Chan, E.; Elevitch, C.R. Species Profiles for Pacific Island Agroforestry: Cocos nucifera (Coconut). 2006. Available online: https://raskisimani.files.wordpress.com/2013/01/cocos-nucifera-coconut.pdf/ (accessed on 4 December 2023).
- Borel, L.D.M.S.; de Lira, T.S.; Ataíde, C.H.; de Souza Barrozo, M.A. Thermochemical Conversion of Coconut Waste: Material Characterization and Identification of Pyrolysis Products. J. Therm. Anal. Calorim. 2021, 143, 637–646. [Google Scholar] [CrossRef]
- Kalina, S.; Navaratne, S.B. Developing an Edible Food Product from Tender Coconut Mesocarp and Analyzing Its Sensory Parameters. Pharma Innov. J. 2018, 7, 62–66. [Google Scholar]
- Siriphanich, J.; Saradhuldhat, P.; Romphophak, T.; Krisanapook, K.; Pathaveerat, S.; Tongchitpakdee, S. Coconut (Cocos nucifera L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Woodhead Publishing: Sawston, UK, 2011; pp. 8–33. [Google Scholar]
- Adeloye, J.B.; Osho, H.; Idris, L.O. Defatted Coconut Flour Improved the Bioactive Components, Dietary Fibre, Antioxidant and Sensory Properties of Nixtamalized Maize Flour. J. Agric. Food Res. 2020, 2, 100042. [Google Scholar] [CrossRef]
- Beegum, P.P.S.; Nair, J.P.; Manikantan, M.R.; Pandiselvam, R.; Shill, S.; Neenu, S.; Hebbar, K.B. Effect of Coconut Milk, Tender Coconut and Coconut Sugar on the Physico-Chemical and Sensory Attributes in Ice Cream. J. Food Sci. Technol. 2022, 59, 2605–2616. [Google Scholar] [CrossRef] [PubMed]
- Brainer, M.S.C.P. Coco: Produção e Mercado; Caderno Setorial ETENE; Banco do Nordeste do Brasil: Fortaleza, Brazil, 2021; pp. 1–13. [Google Scholar]
- ABIR—Associação Brasileira das Indústrias de Refrigerantes e de Bebidas Não Alcoólicas. Água de Coco. 2022. Available online: https://abir.org.br/o-setor/bebidas/agua-de-coco/ (accessed on 15 November 2023).
- Xu, S.; Ma, Z.; Chen, Y.; Li, J.; Jiang, H.; Qu, T.; Zhang, W.; Li, C.; Liu, S. Characterization of the Flavor and Nutritional Value of Coconut Water Vinegar Based on Metabolomics. Food Chem. 2022, 369, 130872. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, H.; Raji, M.; Abdellaoui, H.; Essabir, H.; Bouhfid, R.; Qaiss, A.e.K. Thermo-Mechanical Properties of Low-Cost “Green” Phenolic Resin Composites Reinforced with Surface Modified Coir Fiber. Int. J. Adv. Manuf. Technol. 2021, 112, 1917–1930. [Google Scholar] [CrossRef]
- Li, N.; Jiang, H.; Yang, J.; Wang, C.; Wu, L.; Hao, Y.; Liu, Y. Characterization of Phenolic Compounds and Anti-Acetylcholinase Activity of Coconut Shells. Food Biosci. 2021, 42, 101204. [Google Scholar] [CrossRef]
- Nunes, M.U.C. Coprodutos—Ccoco; EMBRAPA: Aracaju, Brazil, 2021; Available online: https://www.embrapa.br/en/agencia-de-informacao-tecnologica/cultivos/coco/pos-producao/coprodutos (accessed on 15 November 2023).
- Food and Agriculture Organization of the United Nations. FAOSTAT—Food and Agriculture Data Database 2021; FAO: Rome, Italy, 2021. [Google Scholar]
- Schena, T.; Lazzari, E.; Primaz, C.; Canielas Krause, L.; Machado, M.E.; Bastos Caramão, E. Upgrading of Coconut Fibers Bio-Oil: An Investigation By Gc×Gc/Tofms. J. Environ. Chem. Eng. 2020, 8, 103662. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Conradie, J.; Ohoro, C.R.; Amaku, J.F.; Oyedotun, K.O.; Maxakato, N.W.; Akpomie, K.G.; Okeke, E.S.; Olisah, C.; Malloum, A.; et al. Biochar from Coconut Residues: An Overview of Production, Properties, and Applications. Ind. Crops Prod. 2023, 204, 117300. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Tan, L.; Li, Q.; Zhang, C.; Wei, X.; Wang, Q.; Zheng, X.; Xu, Y. Coconut Shell and Its Biochar as Fertilizer Amendment Applied with Organic Fertilizer: Efficacy and Course of Actions on Eliminating Antibiotic Resistance Genes in Agricultural Soil. J. Hazard. Mater. 2022, 437, 129322. [Google Scholar] [CrossRef]
- Cabral, M.M.S.; Abud, A.K.d.S.; Silva, C.E.d.F.; Almeida, R.M.R.G. Bioethanol Production from Coconut Husk Fiber. Cienc. Rural 2016, 46, 1872–1877. [Google Scholar] [CrossRef]
- Bui, H.; Boutouil, M.; Levacher, D.; Sebaibi, N. Evaluation of the Influence of Accelerated Carbonation on the Microstructure and Mechanical Characteristics of Coconut Fibre-Reinforced Cementitious Matrix. J. Build. Eng. 2021, 39, 102269. [Google Scholar] [CrossRef]
- James, A.; Yadav, D. Valorization of Coconut Waste for Facile Treatment of Contaminated Water: A Comprehensive Review (2010–2021). Environ. Technol. Innov. 2021, 24, 102075. [Google Scholar] [CrossRef]
- Babatabar, M.A.; Yousefian, F.; Mousavi, V.M.; Hosseini, M.; Tavasoli, A. Pyrolysis of Lignocellulosic and Algal Biomasses in a Fixed-Bed Reactor: A Comparative Study on the Composition and Application Potential of Bioproducts. Int. J. Energy Res. 2022, 46, 9836–9850. [Google Scholar] [CrossRef]
- Doe, B.; Aboagye, P.D.; Osei-Owusu, P.K.; Amoah, T.; Aidoo, A.; Amponsah, N.Y. Towards Circular Economy and Local Economic Development in Ghana: Insights from the Coconut Waste Value Chain. Circ. Econ. Sustain. 2023, 3, 347–372. [Google Scholar] [CrossRef]
- Ricciardi, P.; Cillari, G.; Carnevale Miino, M.; Collivignarelli, M.C. Valorization of Agro-Industry Residues in the Building and Environmental Sector: A Review. Waste Manag. Res. 2020, 38, 487–513. [Google Scholar] [CrossRef]
- Nascimento, R.J.M.; Pereira, K.R.A.; Avelino, F. Parametric and Modelling Studies of Rhodamine-B Adsorption Using Coconut Coir-Based Materials as Eco-Friendly Adsorbents. J. Environ. Chem. Eng. 2021, 9, 105943. [Google Scholar] [CrossRef]
- Padilha, C.E.A.; da Costa Nogueira, C.; de Santana Souza, D.F.; de Oliveira, J.A.; dos Santos, E.S. Valorization of Green Coconut Fibre: Use of the Black Liquor of Organolsolv Pretreatment for Ethanol Production and the Washing Water for Production of Rhamnolipids by Pseudomonas aeruginosa ATCC 27583. Ind. Crops Prod. 2019, 140, 111604. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Horváth, P.G.; Bak, M.; Alpár, T. A State-of-the-Art Review on Coir Fiber-Reinforced Biocomposites. RSC Adv. 2021, 11, 10548–10571. [Google Scholar] [CrossRef]
- Prasetyo, I.; Permatasari, P.R.; Laksmana, W.T.; Rochmadi, R.; Oh, W.-C.; Ariyanto, T. Lignin Refinery Using Organosolv Process for Nanoporous Carbon Synthesis. Molecules 2020, 25, 3428. [Google Scholar] [CrossRef]
- Marciano, S.J.; Avelino, F.; da Silva, L.R.R.; Mazzetto, S.E.; Lomonaco, D. Microwave-Assisted Phosphorylation of Organosolv Lignin: New Bio-Additives for Improvement of Epoxy Resins Performance. Biomass Convers. Biorefin. 2022, 12, 619–631. [Google Scholar] [CrossRef]
- Marafon, A.C.; Nunes, M.U.C.; Amaral, A.F.C.; dos Santos, J.P. Aproveitamento de Cascas de Coco Para Geração de Energia Térmica: Potencialidades e Desafios; EMBRAPA: Brasília, Brazil, 2019. [Google Scholar]
- Elroi, H.; Zbigniew, G.; Agnieszka, W.-C.; Piotr, S. Enhancing Waste Resource Efficiency: Circular Economy for Sustainability and Energy Conversion. Front. Environ. Sci. 2023, 11, 1303792. [Google Scholar] [CrossRef]
- Maloney, B.K. Palaeoecology and the Origin of the Coconut. GeoJournal 1993, 31, 355–362. [Google Scholar] [CrossRef]
- Gunn, B.F.; Baudouin, L.; Olsen, K.M. Independent Origins of Cultivated Coconut (Cocos nucifera L.) in the Old World Tropics. PLoS ONE 2011, 6, e21143. [Google Scholar] [CrossRef] [PubMed]
- Nayar, N.M. The Coconut Phylogeny, Origins, and Spread, 4th ed.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Batugal, P.; Rao, V.R.; Oliver, J. Coconut Genetic Resources Network Its History and Achievements. CORD 2005, 21, 34. [Google Scholar] [CrossRef]
- FAO. FAOSTAT—Crops and Livestock Products 2021; FAO: Rome, Italy, 2021. [Google Scholar]
- IBGE. Levantamento Sistemático da Produção Agrícola; IBGE: Rio de Janeiro, Brazil, 2022. [Google Scholar]
- Liyanage, V.D. Varieties and Forms of the Coconut Palm Grown in Ceylon. Ceylon Coconut Q. 1958, 9, 1–10. [Google Scholar]
- Niral, V.; Jerard, B.A. Botany, Origin and Genetic Resources of Coconut. Coconut Palm (Cocos nucifera L.)—Research and Development Perspectives; Springer: Singapore, 2019; pp. 57–111. [Google Scholar] [CrossRef]
- Donadio, L.C.; Lederman, I.E.; Roberto, S.R.; Stucchi, E.S. Dwarfing-Canopy and Rootstock Cultivars for Fruit Trees. Rev. Bras. Frutic. 2019, 41, e997. [Google Scholar] [CrossRef]
- Fontes, H.R.; Ferreira, J.M.S. A Cultura do Coco; Área de Informação da Sede-Col Criar Plantar ABC 500p/500r Saber (INFOTECA-E); Embrapa Tabuleiros Costeiros: Aracaju, Brazil, 2006; 500p. [Google Scholar]
- Vishweshwar, S.; Meti, S.; Champa, B.V.; Nagaraja, M.S. Climate Based Coconut Yield Model for Arsikere Taluk of Hassan District in Karnataka. J. Plant. Crops 2020, 48, 27–35. [Google Scholar]
- Hebbar, K.B.; Neethu, P.; Sukumar, P.A.; Sujithra, M.; Santhosh, A.; Ramesh, V.S.; Niral, V.; Hareesh, G.S.; Nameer, P.O.; Prasad, V.P.V. Understanding Physiology and Impacts of High Temperature Stress on the Progamic Phase of Coconut (Cocos nucifera L.). Plants 2020, 9, 1651. [Google Scholar] [CrossRef]
- Foale, M. The Coconut Odyssey: The Bounteous Possibilities of the Tree of Life; Australian Centre for International Agricultural Research Canberra: Canberra, Australia, 2003. [Google Scholar]
- Das, B.; Nair, B.; Arunachalam, V.; Reddy, K.V.; Venkatesh, P.; Chakraborty, D.; Desai, S. Comparative Evaluation of Linear and Nonlinear Weather-Based Models for Coconut Yield Prediction in the West Coast of India. Int. J. Biometeorol. 2020, 64, 1111–1123. [Google Scholar] [CrossRef]
- Naresh Kumar, S.; Aggarwal, P.K. Climate Change and Coconut Plantations in India: Impacts and Potential Adaptation Gains. Agric. Syst. 2013, 117, 45–54. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Puchades, R.; Maquieira, A.; Noguera, V. Physico-chemical and chemical properties of some coconut coir dusts for use as a peat substitute for containerised ornamental plants. Bioresour. Technol. 2002, 82, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Worku, L.A.; Bachheti, R.K.; Tadesse, M.G. Isolation and Characterization of Natural Cellulose from Oxytenanthera abyssinica (Lowland Ethiopian Bamboo) Using Alkali Peroxide Bleaching Stages Followed by Aqueous Chlorite in Buffer Solution. Int. J. Polym. Sci. 2022, 2022, 5155552. [Google Scholar] [CrossRef]
- van Dam, J.E.; Oever, M.J.v.D.; Keijsers, E.R.; van der Putten, J.C.; Anayron, C.; Josol, F.; Peralta, A. Process for production of high density/high performance binderless boards from whole coconut husk: Part 2: Coconut husk morphology, composition and properties. Ind. Crop. Prod. 2006, 24, 96–104. [Google Scholar] [CrossRef]
- Kuram, E. Degradation Effects of Completely Biodegradable Composites to Moisture Absorption and Water Aging. In Aging Effects on Natural Fiber-Reinforced Polymer Composites: Durability and Life Prediction; Springer Nature: Singapore, 2022; pp. 85–113. [Google Scholar] [CrossRef]
- Rethinam, P.; Krishnakumar, V. Coconut Water: A Promising Natural Health Drink-Distribution, Processing and Nutritional Benefits; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–507. [Google Scholar] [CrossRef]
- Corradini, E.; Rosa, M.d.F.; de Macedo, B.P.; Paladin, P.D.; Mattoso, L.H.C. Composição Química, Propriedades Mecânicas e Térmicas da Fibra de Frutos de Cultivares de Coco Verde. Rev. Bras. Frutic. 2009, 31, 837–846. [Google Scholar] [CrossRef]
- EMBRAPA. Recomendações Técnicas Para o Cultivo do Coqueiro; EMBRAPA: Aracaju, Brazil, 1993. [Google Scholar]
- Galvão, E.U.P. Coqueiro: Recomendações de Cultivo. Available online: http://www.infoteca.cnptia.embrapa.br/handle/doc/697390 (accessed on 19 November 2023).
- Santos, J.L.A.; Bispo, V.S.; Filho, A.B.C.; Pinto, I.F.D.; Dantas, L.S.; Vasconcelos, D.F.; Abreu, F.F.; Melo, D.A.; Matos, I.A.; Freitas, F.P.; et al. Evaluation of Chemical Constituents and Antioxidant Activity of Coconut Water (Cocus nucifera L.) and Caffeic Acid in Cell Culture. Acad. Bras. Cienc. 2013, 85, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.; Sunil, C.K.; Rawson, A.; Venkatachalapathy, N. Tender Coconut Water: A Review on Recent Advances in Processing and Preservation. Food Rev. Int. 2020, 38, 1215–1236. [Google Scholar] [CrossRef]
- Kim, T.K.; Lee, M.H.; Kim, S.M.; Kim, M.J.; Jung, S.; Yong, H.I.; Choi, Y.S. Physiochemical Properties of Reduced-Fat Duck Meat Emulsion Systems: Effects of Preemulsification with Vegetable Oils and Duck Skin. Poult. Sci. 2021, 100, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.R.; Sivaprakasam, T.O.; Arumugam, I.; Dilip, N.; Raghuraman, M.; Pavan, K.B.; Rafiq, M.; Paramesh, R.; Varma, S.R.; Sivaprakasam, T.O.; et al. In Vitro Anti-Inflammatory and Skin Protective Properties of Virgin Coconut Oil Author Links Open Overlay Panel. J. Tradit. Complement. Med. 2019, 9, 5–14. [Google Scholar] [CrossRef]
- Ghani, N.A.A.; Channip, A.A.; Chok Hwee Hwa, P.; Ja’afar, F.; Yasin, H.M.; Usman, A. Physicochemical Properties, Antioxidant Capacities, and Metal Contents of Virgin Coconut Oil Produced by Wet and Dry Processes. Food Sci. Nutr. 2018, 6, 1298–1306. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Illam, S.P.; Raghavamenon, A.C. Health Impacts of Different Edible Oils Prepared from Coconut (Cocos nucifera): A Comprehensive Review. Trends Food Sci. Technol. 2018, 80, 1–7. [Google Scholar] [CrossRef]
- Neto, A.S.d.S.; Silva, L.M.S.; Neto, B.M. Use of Coconut Oil in the Production of Cosmetics: A Bibliographic Review. Res. Soc. Dev. 2020, 9, e75491110397. [Google Scholar] [CrossRef]
- Manikantan, M.R.; Pandiselvam, R.; Beegum, S.; Mathew, A.C. Harvest and Postharvest Technology. In Coconut Palm (Cocos nucifera L.)—Research and Development Perspectives; Springer: Singapore, 2019; pp. 635–722. [Google Scholar] [CrossRef]
- Lekshmi Sheela, D.; Nazeem, P.A.; Narayanankutty, A.; Manalil, J.J.; Raghavamenon, A.C. In Silico and Wet Lab Studies Reveal the Cholesterol Lowering Efficacy of Lauric Acid, a Medium Chain Fat of Coconut Oil. Plant Foods Hum. Nutr. 2016, 71, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Naresh Kumar, S.; Balakrishna, A. Seasonal Variations in Fatty Acid Composition of Oil in Developing Coconut. J. Food Qual. 2009, 32, 410. [Google Scholar] [CrossRef]
- Boateng, L.; Ansong, R.; Owusu, W.B.; Steiner-Asiedu, M. Coconut Oil and Palm Oil’s Role in Nutrition, Health and National Development: A Review. Ghana Med. J. 2016, 50, 189. [Google Scholar] [CrossRef] [PubMed]
- Pinho, A.P.S.; Souza, A.F. Extração e Caracterização do Óleo de Coco (Cocos nucifera L.). Biol. Saúde 2018, 8, 9–18. [Google Scholar] [CrossRef][Green Version]
- Sundrasegaran, S.; Hui Mah, S.; Taylor, J.; Jaya, S. Extraction Methods of Virgin Coconut Oil and Palm-Pressed Mesocarp Oil and Their Phytonutrients. eFood 2020, 1, 381–391. [Google Scholar] [CrossRef]
- Patil, U.; Benjakul, S. Coconut Milk and Coconut Oil: Their Manufacture Associated with Protein Functionality. J. Food Sci. 2018, 83, 2019–2027. [Google Scholar] [CrossRef]
- Trinidad, T.P.; Mallillin, A.C.; Valdez, D.H.; Loyola, A.S.; Askali-Mercado, F.C.; Castillo, J.C.; Encabo, R.R.; Masa, D.B.; Maglaya, A.S.; Chua, M.T. Dietary Fiber from Coconut Flour: A Functional Food. Innov. Food Sci. Emerg. Technol. 2006, 7, 309–317. [Google Scholar] [CrossRef]
- Kumar, S.; Senanayake, G.; Visvanathan, C.; Basu, B. Desiccated Coconut Industry of Sri Lanka: Opportunities for Energy Efficiency and Environmental Protection. Energy Convers. Manag. 2003, 44, 2205–2215. [Google Scholar] [CrossRef]
- Hebbar, K.B.; Ramesh, S.V.; Ghosh, D.K.; Beegum, P.P.S.; Pandiselvam, R.; Manikantan, M.R.; Mathew, A.C. Coconut Sugar—A Potential Storehouse of Nutritive Metabolites, Novel Bio-Products and Prospects. Sugar Tech. 2022, 24, 841–856. [Google Scholar] [CrossRef]
- Saraiva, A.; Carrascosa, C.; Ramos, F.; Raheem, D.; Lopes, M.; Raposo, A. Coconut Sugar: Chemical Analysis and Nutritional Profile; Health Impacts; Safety and Quality Control; Food Industry Applications. Int. J. Environ. Res. Public Health 2023, 20, 3671. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Ulven, C.A.; Johnson, M.A.; Durant, C.; Hossain, K.G. Pretreatment of Wheat Bran for Suitable Reinforcement in Biocomposites. J. Renew. Mater. 2017, 5, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.G.; Franklin, J.N. The Pulp of Wood (Pulp and Paper Manufacture); MacGraw-Hill: New York, NY, USA, 1969. [Google Scholar]
- Magagula, L.P.; Masemola, C.M.; Ballim, M.A.; Tetana, Z.N.; Moloto, N.; Linganiso, E.C. Lignocellulosic Biomass Waste-Derived Cellulose Nanocrystals and Carbon Nanomaterials: A Review. Int. J. Mol. Sci. 2022, 23, 4310. [Google Scholar] [CrossRef] [PubMed]
- Barros, D.; Fernandes, É.; Jesus, M.; Barros, L.; Alonso-Esteban, J.I.; Pires, P.; Vaz Velho, M. The Chemical Characterisation of the Maritime Pine Bark Cultivated in Northern Portugal. Plants 2023, 12, 3940. [Google Scholar] [CrossRef]
- Souza, A.G.; Junqueira, M.T.; de Lima, G.F.; Rangari, V.K.; Rosa, D.S. A New Proposal of Preparation of Different Polymorphs of Nanocellulose from Eucalyptus Citriodora. J. Polym. Environ. 2020, 28, 1150–1159. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Louis, H.; Akakuru, O.U.; Joseph, I.; Enudi, O.C.; Michael, D.P. A Review on the Conversion of Levulinic Acid and Its Esters to Various Useful Chemicals. AIMS Energy 2019, 7, 165–185. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood—Chemistry, Ultrastructure, Reactions, 2nd ed.; De Gruyter: Berlin, Germany, 1989; Volume 1. [Google Scholar]
- Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Zimonin, D.V.; Skripnikov, A.M.; Miroshnikova, A.V.; Ionin, V.A.; Kazachenko, A.S.; Sychev, V.V.; et al. Composition and Structure of Aspen (Pópulus trémula) Hemicelluloses Obtained by Oxidative Delignification. Polymer 2022, 14, 4521. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.L.P.; Jesus, M.S.; Mata, F.; Prado, A.A.O.S.; Vieira, I.M.M.; Ramos, L.C.; López, J.A.; Vaz-Velho, M.; Ruzene, D.S.; Silva, D.P. Use of Agro-Industrial Waste for Biosurfactant Production: A Comparative Study of Hemicellulosic Liquors from Corncobs and Sunflower Stalks. Sustainability 2023, 15, 6341. [Google Scholar] [CrossRef]
- Wolf, M.; Berger, F.; Hanstein, S.; Weidenkaff, A.; Endreß, H.U.; Oestreich, A.M.; Ebrahimi, M.; Czermak, P. Hot-Water Hemicellulose Extraction from Fruit Processing Residues. ACS Omega 2022, 7, 13436–13447. [Google Scholar] [CrossRef]
- Baucher, M.; Halpin, C.; Petit-Conil, M.; Boerjan, W. Critical Reviews in Biochemistry and Molecular Biology Lignin: Genetic Engineering and Impact on Pulping Lignin: Genetic Engineering and Impact on Pulping. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 305–350. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose Biomass Pyrolysis for Bio-Oil Production: A Review of Biomass Pre-Treatment Methods for Production of Drop-in Fuels. Renew. Sustain. Energy Rev. 2020, 123, 106763. [Google Scholar] [CrossRef]
- Jesus, M.; Romaní, A.; Mata, F.; Domingues, L. Current Options in the Valorisation of Vine Pruning Residue for the Production of Biofuels, Biopolymers, Antioxidants, and Bio-Composites following the Concept of Biorefinery: A Review. Polymers 2022, 14, 1640. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.A.H.; Hirai, S.; Tuan, H.A.; Akioka, S.; Shoji, W. Effects of Chemical Composition, Mild Alkaline Pretreatment and Particle Size on Mechanical, Thermal, and Structural Properties of Binderless Lignocellulosic Biopolymers Prepared by Hot-Pressing Raw Microfibrillated Phoenix Dactylifera and Cocos nucifera. Polym. Test. 2020, 84, 106384. [Google Scholar] [CrossRef]
- Guleria, A.; Kumari, G.; Lima, E.C.; Ashish, D.K.; Thakur, V.; Singh, K. Removal of Inorganic Toxic Contaminants from Wastewater Using Sustainable Biomass: A Review. Sci. Total Environ. 2022, 823, 153689. [Google Scholar] [CrossRef]
- Marafon, A.C.; Amaral, A.F.C.; De Lemos, E.E.P. Characterization of Bamboo Species and Other Biomasses with Potential for Thermal Energy Generation. Pesqui. Agropecu. Trop. 2019, 49, e55282. [Google Scholar] [CrossRef]
- Droepenu, E.K.; Asare, E.A.; Dampare, S.B.; Adotey, D.K.; Gyampoh, A.O.; Kumi-Arhin, E. Laboratory and Commercial Synthesized Zinc Oxide Nanoparticles Adsorption onto Coconut Husk: Characterization, Isotherm, Kinetic, and Thermodynamic Studies. Biointerface Res. Appl. Chem. 2021, 11, 7871–7889. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of Nanocrystalline Cellulose from Lignocellulosic Biomass: Technology and Applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Brown, M.E.; Chang, M.C.Y. Exploring Bacterial Lignin Degradation. Curr. Opin. Chem. Biol. 2014, 19, 1–7. [Google Scholar] [CrossRef]
- Nascimento, D.M.; Dias, A.F.; de Araújo Junior, C.P.; de Freitas Rosa, M.; Morais, J.P.S.; de Figueirêdo, M.C.B. A Comprehensive Approach for Obtaining Cellulose Nanocrystal from Coconut Fiber. Part II: Environmental Assessment of Technological Pathways. Ind. Crops Prod. 2016, 93, 58–65. [Google Scholar] [CrossRef]
- Anuchi, S.O.; Campbell, K.L.S.; Hallett, J.P. Effective Pretreatment of Lignin-Rich Coconut Wastes Using a Low-Cost Ionic Liquid. Sci. Rep. 2022, 12, 6108. [Google Scholar] [CrossRef]
- Andrade, S.N.; Veloso, C.M.; Fontan, R.C.I.; Bonomo, R.C.F.; Santos, L.S.; Brito, M.J.P.; Diniz, G.A. Chemical-Activated Carbon from Coconut (Cocos nucifera) Endocarp Waste and Its Application in the Adsorption of β-Lactoglobulin Protein. Rev. Mex. De. Ing. Quim. 2018, 17, 463–475. [Google Scholar] [CrossRef]
- Jose, S.; Sajeena Beevi, B. Optimization of Ultrasonication Assisted Alkaline Delignification of Coir Pith Using Response Surface Methodology. Bioresour. Technol. Rep. 2023, 21, 101330. [Google Scholar] [CrossRef]
- TAPPI, Acid-Insoluble Lignin in Wood and Pulp, Test Method T 222 Om-21. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T222.aspx (accessed on 3 April 2024).
- TAPPI, Alpha-, Beta- and Gamma-Cellulose in Pulp, Test Method T 203 Cm-22. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T203.aspx (accessed on 3 April 2024).
- NREL; Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Analytical Procedure—Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; NREL: Golden, CO, USA, 2012. [Google Scholar]
- Kochova, K.; Schollbach, K.; Gauvin, F.; Brouwers, H.J.H. Effect of Saccharides on the Hydration of Ordinary Portland Cement. Constr. Build. Mater. 2017, 150, 268–275. [Google Scholar] [CrossRef]
- Mariano, A.P.B.; Unpaprom, Y.; Ramaraj, R. Hydrothermal Pretreatment and Acid Hydrolysis of Coconut Pulp Residue for Fermentable Sugar Production. Food Bioprod. Process. 2020, 122, 31–40. [Google Scholar] [CrossRef]
- Danso, H.; Martinson, D.B.; Ali, M.; Williams, J.B. Physical, Mechanical and Durability Properties of Soil Building Blocks Reinforced with Natural Fibres. Constr. Build. Mater. 2015, 101, 797–809. [Google Scholar] [CrossRef]
- Robert, U.W.; Etuk, S.E.; Umoren, G.P.; Agbasi, O.E. Assessment of Thermal and Mechanical Properties of Composite Board Produced from Coconut (Cocos nucifera) Husks, Waste Newspapers, and Cassava Starch. Int. J. Thermophys. 2019, 40, 83. [Google Scholar] [CrossRef]
- Pereira, T.G.T.; Silva, D.W.; Eugênio, T.M.C.; Scatolino, M.V.; de Carvalho Terra, I.C.; Fonseca, C.S.; Bufalino, L.; Mendes, R.F.; Mendes, L.M. Coconut Fibers and Quartzite Wastes for Fiber-Cement Production by Extrusion. Mater. Today Proc. 2019, 31, S309–S314. [Google Scholar] [CrossRef]
- Fonseca, C.S.; Silva, M.F.; Mendes, R.F.; Hein, P.R.G.; Zangiacomo, A.L.; Savastano, H.; Tonoli, G.H.D. Jute Fibers and Micro/Nanofibrils as Reinforcement in Extruded Fiber-Cement Composites. Constr. Build. Mater. 2019, 211, 517–527. [Google Scholar] [CrossRef]
- Wagle, A.; Angove, M.J.; Mahara, A.; Wagle, A.; Mainali, B.; Martins, M.; Goldbeck, R.; Raj Paudel, S. Multi-Stage Pre-Treatment of Lignocellulosic Biomass for Multi-Product Biorefinery: A Review. Sustain. Energy Technol. Assess. 2022, 49, 101702. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent Updates on Different Methods of Pretreatment of Lignocellulosic Feedstocks: A Review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging Technologies for the Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Umoren, S.A.; Obot, I.B.; Israel, A.U.; Asuquo, P.O.; Solomon, M.M.; Eduok, U.M.; Udoh, A.P. Inhibition of Mild Steel Corrosion in Acidic Medium Using Coconut Coir Dust Extracted from Water and Methanol as Solvents. J. Ind. Eng. Chem. 2014, 20, 3612–3622. [Google Scholar] [CrossRef]
- Ramasubramani, R.; Gunasekaran, K. Sustainable Alternate Materials for Concrete Production from Renewable Source and Waste. Sustainability 2021, 13, 1204. [Google Scholar] [CrossRef]
- Narciso, C.R.P.; Reis, A.H.S.; Mendes, J.F.; Nogueira, N.D.; Mendes, R.F. Potential for the Use of Coconut Husk in the Production of Medium Density Particleboard. Waste Biomass Valoriz. 2021, 12, 1647–1658. [Google Scholar] [CrossRef]
- Souza, M.J.C.; de Melo, R.R.; Guimarães, J.B.; Carnaval, T.K.B.d.A.; Pimenta, A.S.; Mascarenhas, A.R.P. Wood–Cement Boards with Addition of Coconut Husk. Wood Mater. Sci. Eng. 2021, 17, 617–626. [Google Scholar] [CrossRef]
- Hoang, L.P.; Van, H.T.; Hang Nguyen, T.T.; Nguyen, V.Q.; Thang, P.Q. Coconut Shell Activated Carbon/CoFe2O4 Composite for the Removal of Rhodamine B from Aqueous Solution. J. Chem. 2020, 2020, 9187960. [Google Scholar] [CrossRef]
- Padilha, C.E.A.; da Costa Nogueira, C.; de Santana Souza, D.F.; de Oliveira, J.A.; dos Santos, E.S. Organosolv Lignin/Fe3O4 Nanoparticles Applied as a β-Glucosidase Immobilization Support and Adsorbent for Textile Dye Removal. Ind. Crops Prod. 2020, 146, 112167. [Google Scholar] [CrossRef]
- Esfandiar, N.; Suri, R.; McKenzie, E.R. Simultaneous Removal of Multiple Polycyclic Aromatic Hydrocarbons (PAHs) from Urban Stormwater Using Low-Cost Agricultural/Industrial Byproducts as Sorbents. Chemosphere 2021, 274, 129812. [Google Scholar] [CrossRef]
- Marín-Velásquez, T.D.; Cóndor-Salvatierra, E.J. Capacidad de Retención de Hidrocarburos del Endocarpio de Coco en Aguas Aceitosas. Tecnol. Cienc. Agua 2021, 12, 1–36. [Google Scholar] [CrossRef]
- Thongsamer, T.; Vinitnantharat, S.; Pinisakul, A.; Werner, D. Fixed-Bed Biofilter for Polluted Surface Water Treatment Using Chitosan Impregnated-Coconut Husk Biochar. Environ. Pollut. 2023, 334, 122137. [Google Scholar] [CrossRef] [PubMed]
- Pettit, T.; Irga, P.J.; Torpy, F.R. Functional Green Wall Development for Increasing Air Pollutant Phytoremediation: Substrate Development with Coconut Coir and Activated Carbon. J. Hazard. Mater. 2018, 360, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Caparanga, A.R.; Ordono, E.E.; Villaflores, O.B. Evaluation of Organosolv Pretreatment on the Enzymatic Digestibility of Coconut Coir Fibers and Bioethanol Production via Simultaneous Saccharification and Fermentation. Renew. Energy 2017, 109, 41–48. [Google Scholar] [CrossRef]
- Arantes, H.T.L.; Machado, M.A.; Santoro, M.C.; Freitas, J.C.C.; Ronconi, C.M.; Ligiero, C.B.P.; Cassini, S.T.A.; Sampaio, I.C.F.; Luz, P.P. Effect of Activated Biochar as a Low-Cost Catalyst on the Quality of Catalytic Intermediate Co-Pyrolysis Oil from Waste Polystyrene and Green Coconut Pericarp. Fuel Process. Technol. 2023, 240, 107539. [Google Scholar] [CrossRef]
- Sahoo, S.; Basu, D.; Kumar, A.; Nawale, M.; Kadam, S.; Bhujbal, A.; Rajkumar, K.; Bhowmick, A.; Chattopadhyay, S. Bio-Based Oil Derived from Waste Coconut Shell: A Potential Additive for Enhancing Silanization in Silica Filled Styrene Butadiene Copolymer. J. Polym. Res. 2022, 29, 311. [Google Scholar] [CrossRef]
- Chaos-Hernández, D.; Reynel-Avila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Aguayo-Villarreal, I.A. Functionalization and Activation of Carbon-Based Catalysts with KOH and Calcium and Their Application in Transesterification to Produce Biodiesel: Optimization of Catalytic Properties and Kinetic Study. Fuel 2022, 310, 122066. [Google Scholar] [CrossRef]
- Gopal, M.; Gupta, A.; Shahul Hameed, K.; Sathyaseelan, N.; Khadeejath Rajeela, T.H.; Thomas, V.G. Biochars Produced from Coconut Palm Biomass Residues Can Aid Regenerative Agriculture by Improving Soil Properties and Plant Yield in Humid Tropics. Biochar 2020, 2, 211–226. [Google Scholar] [CrossRef]
- Oliveira, M.B.S.; Valentim, I.B.; Santos, T.R.; Xavier, J.A.; Ferro, J.N.S.; Barreto, E.O.; Santana, A.E.G.; Melo, V.L.; Bottoli, C.B.G.; Goulart, M.O.F. Photoprotective and Antiglycation Activities of Non-Toxic Cocos nucifera Linn. (Arecaceae) Husk Fiber Ethanol Extract and Its Phenol Chemical Composition. Ind. Crops Prod. 2021, 162, 113246. [Google Scholar] [CrossRef]
- Tang, P.L.; Hassan, O.; Md-Jahim, J.; Mustapha, W.A.W.; Maskat, M.Y. Fibrous Agricultural Biomass as a Potential Source for Bioconversion to Vanillic Acid. Int. J. Polym. Sci. 2014, 2014, 509035. [Google Scholar] [CrossRef]
- Luis-Zarate, V.H.; Rodriguez-Hernandez, M.C.; Alatriste-Mondragon, F.; Chazaro-Ruiz, L.F.; Rangel-Mendez, J.R. Coconut Endocarp and Mesocarp as Both Biosorbents of Dissolved Hydrocarbons in Fuel Spills and as a Power Source When Exhausted. J. Environ. Manag. 2018, 211, 103–111. [Google Scholar] [CrossRef]
- Lasmini, S.A.; Rosmini, R.; Lakani, I.; Hayati, N.; Nasir, B.H. Increasing Shallot Production in Marginal Land Using Mulches and Coconut Husk Fertilizer. Int. J. Des. Nat. Ecodyn. 2021, 16, 105–110. [Google Scholar] [CrossRef]
- Zubiolo, C.; de Santana, H.E.P.; Pereira, L.L.; Ruzene, D.S.; Silva, D.P.; Freitas, L.S. Bio-Oil Production and Characterization from Corn Cob and Sunflower Stem Pyrolysis. Ind. Eng. Chem. Res. 2024, 63, 65–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Chen, P.; Liu, S.; Zhou, N.; Ding, K.; Fan, L.; Peng, P.; Min, M.; Cheng, Y.; et al. Gasification Technologies and Their Energy Potentials; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444642004. [Google Scholar]
- Gupta, S.; Patel, P.; Mondal, P. Biofuels Production from Pine Needles via Pyrolysis: Process Parameters Modeling and Optimization through Combined RSM and ANN Based Approach. Fuel 2022, 310, 122230. [Google Scholar] [CrossRef]
- Grams, J.; Jankowska, A.; Goscianska, J. Advances in Design of Heterogeneous Catalysts for Pyrolysis of Lignocellulosic Biomass and Bio-Oil Upgrading. Microporous Mesoporous Mater. 2023, 362, 112761. [Google Scholar] [CrossRef]
- Sørmo, E.; Krahn, K.M.; Flatabø, G.Ø.; Hartnik, T.; Arp, H.P.H.; Cornelissen, G. Distribution of PAHs, PCBs, and PCDD/Fs in Products from Full-Scale Relevant Pyrolysis of Diverse Contaminated Organic Waste. J. Hazard. Mater. 2023, 461, 132546. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Muñoz, J.F.; Aznar-Sánchez, J.A.; López-Felices, B.; Román-Sánchez, I.M. Circular Economy in Agriculture. An Analysis of the State of Research Based on the Life Cycle. Sustain. Prod. Consum. 2022, 34, 257–270. [Google Scholar] [CrossRef]
- Scarano, P.; Sciarrillo, R.; Tartaglia, M.; Zuzolo, D.; Guarino, C. Circular Economy and Secondary Raw Materials from Fruits as Sustainable Source for Recovery and Reuse. A Review. Trends Food Sci. Technol. 2022, 122, 157–170. [Google Scholar] [CrossRef]
- United Nations. Sustainable Development Goals 2024. United Nations SDG12: Ensure Sustainable Consumption and Production Patterns; United Nations: New York, NY, USA, 2024; Available online: https://sdgs.un.org/goals/goal12 (accessed on 3 January 2024).
- Hayes, D.J.M. Biomass Composition and Its Relevance to Biorefining. In The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 27–65. [Google Scholar]
- Wagh, M.S.; Chandra Nath, P.; Chakraborty, A.; Amrit, R.; Mishra, B.; Kumar Mishra, A.; Kishore Mohanta, Y. Valorisation of Agro-Industrial Wastes: Circular Bioeconomy and Biorefinery Process—A Sustainable Symphony. Process Saf. Environ. Prot. 2024, 183, 708–725. [Google Scholar] [CrossRef]
- Haque, F.; Fan, C.; Lee, Y.Y. From Waste to Value: Addressing the Relevance of Waste Recovery to Agricultural Sector in Line with Circular Economy. J. Clean. Prod. 2023, 415, 137873. [Google Scholar] [CrossRef]
- Ramírez, J.F.G.; Muñoz, R.C.; Sossa, J.W.Z. Innovations and Trends in the coconut Agroindustry Supply: A Technological and Foresight Analysis. Front. Sustain. Food Syst. 2023, 7, 1048450. [Google Scholar]
- Alan, H.; Köker, A.R. Analyzing and Mapping Agricultural Waste Recycling Research: An Integrative Review for Conceptual Framework and Future Directions. Resour. Policy 2023, 85, 103987. [Google Scholar] [CrossRef]
- United Nations. United Nations Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations Sustainable Knowledge Platform; Division for Sustainable Development Goals: New York, NY, USA, 2015. [Google Scholar]
- Ignacio, I.F.; Miguel, T.S. Research Opportunities on the Coconut (Cocos nucifera L.) Using New Technologies. S. Afr. J. Bot. 2021, 141, 414–420. [Google Scholar] [CrossRef]
- Krishnan, S.; Zulkapli, N.S.; Kamyab, H.; Taib, S.M.; Din, M.F.B.M.; Majid, Z.A.; Chaiprapat, S.; Kenzo, I.; Ichikawa, Y.; Nasrullah, M.; et al. Current Technologies for Recovery of Metals from Industrial Wastes: An Overview. Environ. Technol. Innov. 2021, 22, 101525. [Google Scholar] [CrossRef]
- Azevedo, A.R.G.; Amin, M.; Hadzima-Nyarko, M.; Agwa, I.S.; Zeyad, A.M.; Tayeh, B.A.; Adesina, A. Possibilities for the Application of Agro-Industrial Wastes in Cementitious Materials: A Brief Review of the Brazilian Perspective. Clean. Mater. 2022, 3, 100040. [Google Scholar] [CrossRef]
- Diwan, B.; Mukhopadhyay, D.; Gupta, P. Recent Trends in Biorefinery-Based Valorisation of Lignocellulosic Biomass; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128179512. [Google Scholar]




| Characteristic | Typica | Nana | Hybrid |
|---|---|---|---|
| Height | 30 m | 10–12 m | 20 m |
| Life cycle | 60–80 years | 30 years | 50 years |
| Growth | Fast | Slow | Intermediate |
| Fluorescence time | 5–7 years | 2–3 years | 3–4 years |
| Applications | Agro-industry/food preparation | Water consumption | Water consumption/agro-industry/food preparation |
| Authors | Residue | Hemicellulose (%) | Lignin (%) | Cellulose (%) |
|---|---|---|---|---|
| Nascimento et al. [92] | Mesocarp | 25.5 | 35.1 | 31.6 |
| Borel et al. [4] | Mesocarp | 30 | 32 | 31 |
| Anuchi et al. [93] | Mesocarp | 15 | 41 | 38 |
| Andrade et al. [94] | Endocarp | 15.2 | 33.7 | 10.4 |
| Alharbi et al. [86] | Leaves | 19 | 21 | 33 |
| Jose and Beevi [95] | Coir pith * | 14.2 | 41.3 | 34 |
| Application | Highlights | Challenges |
|---|---|---|
| Civil construction |
|
|
| Adsorbent material |
|
|
| Production of organic solvents |
|
|
| Feedstock for pyrolysis process |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, F.; Santana, H.E.P.; Jesus, M.; Santos, J.; Pires, P.; Vaz-Velho, M.; Silva, D.P.; Ruzene, D.S. Coconut Waste: Discovering Sustainable Approaches to Advance a Circular Economy. Sustainability 2024, 16, 3066. https://doi.org/10.3390/su16073066
Vieira F, Santana HEP, Jesus M, Santos J, Pires P, Vaz-Velho M, Silva DP, Ruzene DS. Coconut Waste: Discovering Sustainable Approaches to Advance a Circular Economy. Sustainability. 2024; 16(7):3066. https://doi.org/10.3390/su16073066
Chicago/Turabian StyleVieira, Fabrícia, Hortência E. P. Santana, Meirielly Jesus, Joana Santos, Preciosa Pires, Manuela Vaz-Velho, Daniel Pereira Silva, and Denise Santos Ruzene. 2024. "Coconut Waste: Discovering Sustainable Approaches to Advance a Circular Economy" Sustainability 16, no. 7: 3066. https://doi.org/10.3390/su16073066
APA StyleVieira, F., Santana, H. E. P., Jesus, M., Santos, J., Pires, P., Vaz-Velho, M., Silva, D. P., & Ruzene, D. S. (2024). Coconut Waste: Discovering Sustainable Approaches to Advance a Circular Economy. Sustainability, 16(7), 3066. https://doi.org/10.3390/su16073066











