Gaseous Mercury Limit Values: Definitions, Derivation, and the Issues Related to Their Application
Abstract
:1. Introduction
2. Mercury Forms, Sources and Health Effects
3. Limit Values: Definition, Classification and Derivation Methods
| TLV Acronym/Name (Extended Name) | Definition | TLV Type/Note | Bibliography |
|---|---|---|---|
| Action level | “Indoor air concentration of mercury vapor that should prompt public health and environmental officials to consider implementing response actions” | No TWA. Action level could refer to residential or workplace settings. | [42] |
| AEGLs (Acute Exposure Guideline Levels) | “Threshold exposure limits for the general public, applicable to emergency exposure periods ranging from 10 min to 8 h.” | Three levels (AEGL-1, AEGL-2, AEGL-3) are developed for each of five exposure periods (10 and 30 min, 1 h, 4 h, and 8 h) and are distinguished by varying degrees of severity of toxic effects. | [40] |
| Ceiling limit | “Ceiling concentrations that must not be exceeded during any part of the workday” | If instantaneous monitoring is not feasible, the ceiling must be assessed as a 15-min TWA exposure. Regulatory limit. | [44] |
| IDLH (Immediately dangerous to life or health) | “A condition that pose an immediate threat to life or health, or conditions that pose an immediate threat of severe exposure to contaminants (…) which are likely to have adverse cumulative or delayed effects on health” | Based on the effects that might occur as a consequence of a 30-min exposure | [45] |
| IOELV (Indicative occupational exposure limits) | “Health-based, non-binding values, derived from the most recent scientific data available and taking into account the availability of measurement techniques” | IOELVs are established by the European Commission, assisted by the Scientific Committee for Occupational Exposure Limits to Chemical Agents (SCOEL). | [46] |
| LOAEL (Lowest Observable Effect Levels) | “The lowest exposure (or dose) level of a chemical at which there are statistically or biologically significant increases in frequency or severity of adverse effects between the exposed population and its appropriate control group” | [41] | |
| MRL (Minimal Risk Level) | “An estimate of daily human exposure to a substance that is likely to be without an appreciable risk of adverse effects (noncarcinogenic) over a specified duration of exposure” | [30] | |
| NOAEL (No Observed Adverse Effect Level) | “The highest exposure (or dose) level of a chemical at which there are no statistically or biologically significant increases in frequency or severity of adverse effects seen between the exposed population and its appropriate control” | [41] | |
| PEL (Permissible Exposure Limit) | “The maximum permitted 8-h time-weighted average concentration of an airborne contaminant” | Regulatory limit. | [39] |
| REL (Reference Exposure Level) | “A concentration at or below which adverse health effects are not likely to occur in the general human population” | REL could refer to chronic expositions or TWA. | [43] |
| REL (Recommended Exposure Limit) | “A time-weighted average concentration for up to a 10-h workday during a 40-h workweek to protect workers from hazardous substances and conditions in the workplace” | Refer to a TWA exposition. | [47] |
| RfC (Reference Concentration) | “An estimate (with uncertainty spanning perhaps an order of magnitude) of a daily inhalation exposure of the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime” | [41] | |
| TCL (Lowest Toxic Concentration) | “The lowest concentrations known to cause any level of harm to humans” | [42] |
4. Threshold Limit Values for Hg
4.1. Chronic Hg TLVs
4.2. TWA Hg TLVs
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Takeuchi, T. A pathological study on the fetal Minamata disease diagnosed clinically so-called infantile cerebral palsy. Adv. Neurol. Sci. 1964, 8, 145–161. [Google Scholar]
- Matsumoto, H.; Koya, G.; Takeuchi, T. Fetal Minamata disease: A neuropathological study of two cases of intrauterine intoxication by a methyl mercury compound. J. Neuropat. Exp. Neurol. 1965, 24, 563–574. [Google Scholar] [CrossRef]
- UNEP. Report of the Global Mercury Assessment Working Group on the Work of Its First Meeting; UNEP: Geneva, Switzerland, 2002. [Google Scholar]
- UNEP. Global Mercury Assessment 2018. UN-Environment Programme; Chemicals and Health Branch: Geneva, Switzerland, 2019; 59p. [Google Scholar]
- Jitaru, P.; Adams, F. Toxicity, sources and biogeochemical cycle of mercury. In Journal de Physique IV (Proceedings); EDP Sciences: Les Ulis, France, 2004; Volume 121, pp. 185–193. [Google Scholar]
- Garetano, G.; Stern, A.H.; Robson, M.; Gochfeld, M. Mercury vapor in residential building common areas in communities where mercury is used for cultural purposes versus a reference community. Sci. Total Environ. 2009, 397, 131–139. [Google Scholar] [CrossRef]
- EPA. Inventory of Mercury Supply, Use, and Trade in the United States—2023 Report; Office of Chemical Safety and Pollution Prevention: Washington, DC, USA, 2023.
- Lyman, S.N.; Cheng, I.; Gratz, L.E.; Weiss-Penzias, P.; Zhang, L. An updated review of atmospheric mercury. Sci. Total Environ. 2020, 707, 135575. [Google Scholar] [CrossRef] [PubMed]
- Selin, N.E. Global biogeochemical cycling of mercury: A review. Annu. Rev. Environ. Resour. 2009, 34, 43–63. [Google Scholar] [CrossRef]
- Weiss-Penzias, P.; Amos, H.M.; Selin, N.E.; Gustin, M.S.; Jaffe, D.A.; Obrist, D.; Sheu, G.R.; Giang, A. Use of a global model to understand speciated atmospheric mercury observations at five high-elevation sites. Atmos. Chem. Phys. 2015, 15, 1161–1173. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Ghoshdastidar, A.J.; Ariya, P.A. The existence of airborne mercury nanoparticles. Sci. Rep. 2019, 9, 10733. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.F.; Engstrom, D.R.; Lamborg, C.H.; Tseng, C.; Balcom, P.H.; Hammerschmidt, C.R. Modern and historic atmospheric mercury fluxes in northern Alaska: Global sources and arctic depletion. Environ. Sci. Technol. 2005, 2, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, O.; Rodhe, H. Atmospheric mercury—A review. Tellus B 1985, 37, 136–159. [Google Scholar] [CrossRef]
- Schroeder, W.H.; Munthe, J. Atmospheric mercury—An overview. Atmos. Environ. 1998, 29, 809–822. [Google Scholar] [CrossRef]
- Xiu, G.L.; Jin, Q.; Zhang, D.; Shi, S.; Huang, X.; Zhang, W.; Bao, L.; Gao, P.; Chen, B. Characterization of size-fractionated particulate mercury in Shanghai ambient air. Atmos. Environ. 2005, 39, 419–427. [Google Scholar] [CrossRef]
- Munthe, J.; Wängberg, I.; Pirrone, N.; Iverfeldt, Å.; Ferrara, R.; Ebinghaus, R.; Feng, X.; Gardfeldt, K.; Keeler, G.; Lanzillotta, E.; et al. Intercomparison of methods for sampling and analysis of atmospheric mercury species. Atmos. Environ. 2001, 35, 3007–3017. [Google Scholar] [CrossRef]
- Lindberg, S.A.; Stratton, W.J. Atmospheric mercury speciation: Concentrations and behavior of reactive gaseous mercury in ambient air. Environ. Sci. Technol. 1998, 32, 49–57. [Google Scholar] [CrossRef]
- Bank, M.S. Mercury in the Environment: Pattern and Process; University of California Press: Berkeley, CA, USA, 2012. [Google Scholar]
- Lynam, M.M.; Keeler, G.J. Comparison of methods for particulate phase mercury analysis: Sampling and analysis. Anal. Bioanal. Chem. 2002, 374, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. A review on the distribution of Hg in the environment and its human health impacts. J. Hazard. Mat. 2016, 306, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Selin, N.E.; Selin, H. Global politics of mercury pollution: The need for multi-scale governance. Rev. Eur. Community Int. Environ. Law 2006, 15, 258–269. [Google Scholar] [CrossRef]
- Esdaile, L.J.; Chalker, J.M. The mercury problem in artisanal and small-scale gold mining. Chem. Eur. J. 2018, 24, 6905–6916. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.S.; Osman, A.I.; Hosny, M.; Elgarahy, A.M.; Eltaweil, A.S.; Rooney, D.W.; Chen, Z.; Rahim, N.S.; Sekar, M.; Gopinat, S.C.B.; et al. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review. ACS Omega 2024, 9, 5100–5126. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344. [Google Scholar] [CrossRef]
- Graeme, K.A.; Pollack, C.V., Jr. Heavy metal toxicity, part I: Arsenic and mercury. J. Emerg. Med. 1998, 16, 45–56. [Google Scholar] [CrossRef]
- Hursh, J.B.; Clarkson, T.W.; Miles, E.F.; Goldsmith, L.A. Percutaneous absorption of mercury vapor by man. Arch. Environ. Health Int. J. 1989, 44, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Risher, J.F.; Nickle, R.A.; Amler, S.N. Elemental mercury poisoning in occupational and residential settings. Int. J. Hyg. Environ. Health 2003, 206, 371–379. [Google Scholar] [CrossRef]
- Rowland, A.S.; Baird, D.D.; Weinberg, C.R.; Shore, D.L.; Shy, C.M.; Wilcox, A.J. The effect of occupational exposure to mercury vapour on the fertility of female dental assistants. Occup. Environ. Med. 1994, 51, 28–34. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Mercury (Draft for Public Comment); U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2022.
- Broussard, L.A.; Hammett-Stabler, C.A.; Winecker, R.E.; Ropero-Miller, J.D. The toxicology of mercury. Lab. Med. 2002, 33, 614–625. [Google Scholar] [CrossRef]
- SCOEL. Recommendation from the SCOEL/SUM/84; Scientific Committee on Occupational Exposure Limits for Elemental Mercury and Inorganic Divalent Mercury Compounds: 2007. Available online: https://circabc.europa.eu (accessed on 23 September 2022).
- Ziem, G.E.; Castleman, B.I. Threshold limit values: Historical perspectives and current practice. J. Occup. Med. 1989, 31, 910–918. [Google Scholar] [CrossRef] [PubMed]
- ACGIH Website. 2022. Available online: https://www.acgih.org/science/tlv-bei-guidelines/tlv-chemical-substances-introduction (accessed on 4 October 2022).
- Caira, M.; Fargione, P.; La Pegna, P. L’esposizione professionale ad agenti chimici pericolosi: Lacune, carenze e problematiche nell’uso dei valori limite. In Proceedings of the Conference on Risk Assessment and Management (VGR 2006), Pisa, Italy, 19 October 2006. [Google Scholar]
- EPA. Health Effects Notebook Glossary. 2021. Available online: https://www.epa.gov/haps/health-effects-notebook-glossary (accessed on 4 October 2022).
- Code of Federal Regulations, Title 29, Subtitle B, Chapter Xvii, Part 1910, Subpart Z—Toxic and Hazardous Substances. Available online: https://www.ecfr.gov/current/title-29/subtitle-B/chapter-XVII/part-1910/subpart-Z (accessed on 26 October 2022).
- NIOSH. NIOSH Pocket Guide to Chemical Hazards. 2019. Department of Health and Human Services. Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health (NIOSH). Available online: https://www.cdc.gov/niosh/npg (accessed on 18 October 2019).
- OSHA. OSHA Annotated PELs. 2015. Available online: www.osha.gov (accessed on 4 October 2023).
- US EPA. Acute Exposure Guideline Levels (AEGLs) for Mercury Vapor (Hg0) (CAS Reg. No. 7439-97-6); NAC/Interim: 09/2010; US EPA: Washington, DC, USA, 2010.
- US EPA. A Review of the Reference Dose and Reference Concentration Processes; US EPA/630/P-02/002F, December 1, 2002; Risk Assessment Forum: Washington, DC, USA, 2003; 192p. [Google Scholar]
- ATSDR Action Levels for Elemental Mercury Spills: Chemical-Specific Health Consultation for Joint EPA/ATSDR National Mercury Cleanup Policy Workgroup. United States Agency for Toxic Substances and Disease Registry. Division of Toxicology and Environmental Medicine. 22 March 2012. Available online: https://stacks.cdc.gov/view/cdc/37505 (accessed on 23 September 2022).
- CalEPA. Technical Support Document for the Derivation of Noncancer Reference Exposure Levels; Office of Environmental Health Hazard Assessmen, California Environmental Protection Agency: San Francisco, CA, USA, 2014.
- OSHA. Occupational Safety and Health Standards, Toxic and Hazardous Substances. All Contaminants, Final Rule. Code of Federal Regulations; 29 CFR 1910.1000; OSHA: Washington, DC, USA, 1989.
- NIOSH. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHS); Publication Number PB-94-195047; US Department of Health and Human Service: Washington, DC, USA, 1994.
- EUROPEA Commissione. Commission Directive 2009/161/EU of 17 December 2009 Establishing a Third List of Indicative Occupational Exposure Limit Values in Implementation of Council Directive 98/24/EC and Amending Commission Directive 2000/39/EC (Text with EEA Relevance); EUROPEA Commissione: Brussels, Belgium, 2010. [Google Scholar]
- NIOSH. Criteria for a Recommended Standard—Occupational Exposure to Inorganic Mercury; NIOSH: Washington, DC, USA, 1973. [Google Scholar]
- Hogan, T.J.; Nalbone, J.T. TLV Development: Threshold Limit Values Are Not Just Numbers. Prof. Safe. 2016, 61, 52–56. [Google Scholar]
- Luttrell, W.E.; Jederberg, W.W.; Still, K.R. (Eds.) Toxicology Principles for the Industrial Hygienist; Aiha: Falls Church, VA, USA, 2008. [Google Scholar]
- WHO, World Health Organization, Regional Office for Europe. Air Quality Guidelines for Europe, 2nd ed.; World Health Organization, Regional Office for Europe: Geneva, Switzerland, 2000; Available online: https://apps.who.int/iris/handle/10665/107335 (accessed on 23 September 2021).
- ACGIH. ACGIH: Documentation of the Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs)—Mercury [7439-97-6], All Forms Except Alkyl, as Hg, Elemental and Inorganic Forms; ACGIH: Sharonville, OH, USA, 2009. [Google Scholar]
- WHO, World Health Organization. Recommended Health-Based Limits in Occupational Exposure to Heavy Metals; Report of a WHO Study Group; World Health Organization Technical Report Series 647; World Health Organization: Geneva, Switzerland, 1980; ISBN 9241206470. [Google Scholar]
- California Code of Regulations. Title 8: Division 1, Chapter 4, Subchapter 7, Article 107, Section 5155, Table AC-1 of the General Industry Safety Orders. Airborne Contaminants. 2019. Available online: https://www.dir.ca.gov/title8/5155.html (accessed on 21 March 2023).
- Fawer, R.F.; DeRibaupierre, Y.; Guillemin, M.; Berode, M.; Lob, M. Measurement of hand tremor induced by industrial exposure to metallic mercury. Br. J. Ind. Med. 1983, 40, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Piikivi, L.; Hanninen, H.; Martelin, T.; Mantere, P. Psychological performance and long-term exposure to mercury vapours. Scand. J. Work. Environ. Health 1984, 10, 35–41. [Google Scholar] [CrossRef]
- Piikivi, L.; Tolonen, U. Eeg findings in chlor-alkali workers subjected to low long Term exposure to mercury vapor. Br. J. Ind. Med. 1989, 46, 370–375. [Google Scholar]
- Piikivi, L. Cardiovascular reflexes and low long-term exposure to mercury vapor. Int. Arch. Occup. Environ. Health 1989, 61, 391–395. [Google Scholar] [CrossRef]
- Ngim, C.H.; Foo, S.C.; Boey, K.W.; Jeyaratnam, J. Chronic neurobehavioural effects of elemental mercury in dentists. Occup. Environ. Med. 1992, 49, 782–790. [Google Scholar] [CrossRef] [PubMed]
- US EPA IRIS (Integrated Risk Information System). Mercury, Elemental. CASRN 7439-97-6. 1995. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr=370 (accessed on 11 October 2022).
- Bast-Pettersen, R.; Ellingsen, D.G.; Efskind, J.; Jordskogen, R.; Thomassen, Y. A neurobehavioral study of chloralkali workers after the cessation of exposure to mercury vapor. Neurotoxicology 2005, 26, 427–437. [Google Scholar] [CrossRef]
- Boogaard, P.J.; Houtsma, A.-T.A.J.; Journée, H.L.; Van Sittert, N.J. Effects of exposure to elemental mercury on the nervous system and the kidneys of workers producing natural gas. Arch. Environ. Health 1996, 51, 108–115. [Google Scholar] [CrossRef]
- Chapman, L.J.; Sauter, S.L.; Henning, R.A.; Dodson, V.N.; Reddan, W.G.; Matthews, C.G. Differences in frequency of finger tremor in otherwise asymptomatic mercury workers. Br. J. Ind. Med. 1990, 47, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, D.G.; Bast-Pettersen, R.; Efskind, J.; Thomassen, Y. Neuropsychological effects of low mercury vapor exposure in chloralkali workers. Neurotoxicology 2001, 22, 249–258. [Google Scholar] [CrossRef]
- Langworth, S.; Almkvist, O.; Soderman, E.; Wikstrom, B.O. Effects of occupational exposure to mercury vapour on the central nervous system. Br. J. Ind. Med. 1992, 49, 545–555. [Google Scholar] [CrossRef]
- Wastensson, G.; Lamoureux, D.; Sällsten, G.; Beuter, A.; Barregard, L. Quantitative tremor assessment in workers with current low exposure to mercury vapor. Neurotoxicol. Teratol. 2006, 28, 681–693. [Google Scholar] [CrossRef]
- Wastensson, G.; Lamoureux, D.; Sallsten, G.; Beuter, A.; Barregard, L. Quantitative assessment of neuromotor function in workers with current low exposure to mercury vapor. Neurotoxicology 2008, 29, 596–604. [Google Scholar] [CrossRef]
- Cárdenas, A.; Roels, H.; Bernard, A.M.; Barbon, R.; Buchet, J.P.; Lauwerys, R.R.; Rosello, J.; Hotter, G.; Mutti, A.; Franchini, I. Markers of early renal changes induced by industrial pollutants. I. Application to workers exposed to mercury vapour. Br. J. Ind. Med. 1993, 50, 17–27. [Google Scholar] [CrossRef] [PubMed]
- WHO, World Health Organization. Inorganic Mercury; Environmental Health Criteria, No. 118; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Danielsson, B.R.; Fredriksson, A.; Dahlgren, L.; Gardlund, A.T.; Olsson, L.; Dencker, L.; Archer, T. Behavioural effects of prenatal metallic mercury inhalation exposure in rats. Neurotoxicol. Teratol. 1993, 15, 391–396. [Google Scholar] [CrossRef]
- Williamson, A.M.; Teo, R.K.C.; Sanderson, J. Occupational mercury exposure and its consequences for behavior. Int. Arch. Occup. Environ. Health 1982, 50, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Roels, H.; Gennart, J.P.; Lauwerys, R.; Buchet, J.P.; Malchaire, J.; Bernard, A. Surveillance of workers exposed to mercury vapour: Validation of a previously proposed biological threshold limit value for mercury concentration in urine. Am. J. Ind. Med. 1985, 7, 45–71. [Google Scholar] [CrossRef] [PubMed]
- Rentos, P.G.; Seligman, E.J. Relationship between environmental exposure to mercury and clinical observation. Arch. Environ. Health Int. J. 1968, 16, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.G.; Vorwald, A.J.; Patil, L.S.; Mooney, T.F. Effects of exposure to mercury in the manufacture of chlorine. Am. Ind. Hyg. Assoc. J. 1970, 31, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Danziger, S.J.; Possick, P.A. Metallic mercury exposure in scientific glassware manufacturing plants. J. Occup. Med. Off. Publ. Ind. Med. Assoc. 1973, 15, 15–20. [Google Scholar]
- IRIS Glossary. Available online: https://www.epa.gov/iris/iris-glossary#c (accessed on 4 October 2023).
- Sällsten, G.; Barregård, L.; Schütz, A. Clearance half-life of mercury in urine after the cessation of long term occupational exposure: Influence of a chelating agent (DMPS) on excretion of mercury in urine. Occup. Environ. Med. 1994, 51, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Hursh, J.B.; Clarkson, T.W.; Cherian, M.G.; Vostal, J.J.; Mallie, R.V. Clearance of mercury (Hg-197, Hg-203) vapor inhaled by human subjects. Arch. Environ. Health Int. J. 1976, 31, 302–309. [Google Scholar] [CrossRef]
- Akerstrom, M.; Barregard, L.; Lundh, T.; Sallsten, G. Relationship between mercury in kidney, blood, and urine in environmentally exposed individuals, and implications for biomonitoring. Toxicol. Appl. Pharmacol. 2017, 320, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Richardson, G.M.; Brecher, R.W.; Scobie, H.; Hamblen, J.; Samuelian, J.; Smith, C. Mercury vapour (Hg0): Continuing toxicological uncertainties, and establishing a Canadian reference exposure level. Regul. Toxicol. Pharmacol. 2009, 53, 32–38. [Google Scholar] [CrossRef]
- Beate, L.; Gustav, D. Proposal for a revised reference concentration (RfC) for mercury vapour in adults. Sci. Total Environ. 2010, 408, 3530–3535. [Google Scholar] [CrossRef]
- Salthammer, T.; Uhde, E.; Omelan, A.; Lüdecke, A.; Moriske, H.J. Estimating human indoor exposure to elemental mercury from broken compact fluorescent lamps (CFLs). Indoor Air 2012, 22, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Carpi, A.; Chen, Y.F. Gaseous elemental mercury as an indoor air pollutant. Environ. Sci. Technol. 2001, 35, 4170–4173. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Y.; Chen, H.; Xiao, G.Q.; Wei, S.Q. Sourcing contributions of gaseous mercury in indoor and outdoor air in China. Environ. Forensics 2010, 11, 154–160. [Google Scholar] [CrossRef]
- Pohl, H.R.; Abadin, H.G. Utilizing uncertainty factors in minimal risk levels derivation. Regul. Toxicol. Pharmacol. 1995, 22, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Alexeeff, G.V.; Broadwin, R.; Liaw, J.; Dawson, S.V. Characterization of the LOAEL-to-NOAEL uncertainty factor for mild adverse effects from acute inhalation exposures. Regul. Toxicol. Pharmacol. 2002, 36, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Hassett-Sipple, B.; Swartout, J.; Schoeny, R. Mercury Study Report to Congress. Health Effects of Mercury and Mercury Compounds (No. PB-98-124779/XAB.; EPA-452/R-97/007); Environmental Protection Agency, Office of Air Quality Planning and Standards: Research Triangle Park, NC, USA, 1997; Volume 5.
- Ratcliffe, H.E.; Swanson, G.M.; Fischer, L.J. Human exposure to mercury: A critical assessment of the evidence of adverse health effects. J. Toxic. Environ. Health 1996, 49, 221–270. [Google Scholar] [CrossRef]
- Dourson, M.L.; Felter, S.P.; Robinson, D. Evolution of science-based uncertainty factors in noncancer risk assessment. Regul. Toxicol. Pharmacol. 1996, 24, 108–120. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directorate-General for Employment, Social Affairs and Inclusion, Methodology for Derivation of Occupational Exposure Limits of Chemical Agents—The General Decision-Making Framework of the Scientific Committee on Occupational Exposure Limits (SCOEL) 2017, Publications Office, 2018. Available online: https://data.europa.eu/doi/10.2767/435199 (accessed on 4 October 2023).
- Dankovic, D.A.; Naumann, B.D.; Maier, A.; Dourson, M.L.; Levy, L.S. The scientific basis of uncertainty factors used in setting occupational exposure limits. J. Occup. Environ. Hyg. 2015, 12 (Suppl. S1), S55–S68. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Wang, X.; Wang, D.; Duan, Y.; Cheng, N.; Xiu, G. Atmospheric mercury speciation in Shanghai, China. Sci. Total Environ. 2017, 578, 460–468. [Google Scholar] [CrossRef]
- Legislative Decree n. 81/2008, Gazzetta Ufficiale n. 218, 2012. Attuazione Dell’articolo 1 Della Legge 3 Agosto 2007, n. 123, in Materia di Tutela Della Salute e Della Sicurezza nei Luoghi di Lavoro. Available online: https://www.gazzettaufficiale.it/eli/id/2008/04/30/008G0104/sg (accessed on 31 July 2022).
- MAK. The MAK Collection for Occupational Health and Safety; WILEY-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2016; Volume 1. [Google Scholar]
- ACGIH. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices (1994–1995); American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 1994. [Google Scholar]
- ATSDR. Toxicological Profile for Mercury; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 1999.
- Tsuji, J.; Williams, P.; Edwards, M.; Allamneni, K.; Kelsh, M.; Paustenbach, D.; Sheehan, P. Evaluation of mercury in urine as an indicator of exposure to low levels of mercury vapor. Environ. Health Perspect. 2003, 111, 623–630. [Google Scholar] [CrossRef]
- Hryhorczuk, D.; Persky, V.; Piorkowski, J.; Davis, J.; Moomey, C.; Krantz, A.; Runkle, K.; Saxer, T.; Baughman, T.; McCann, K. Residential mercury spills from gas regulators. Environ. Health Perspect. 2006, 114, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Piikivi, L.; Hanninen, H. Subjective symptoms and psychological performance of Chlorine-alkali workers. Scand. J. Work Environ. Health 1989, 15, 69–74. [Google Scholar] [CrossRef] [PubMed]
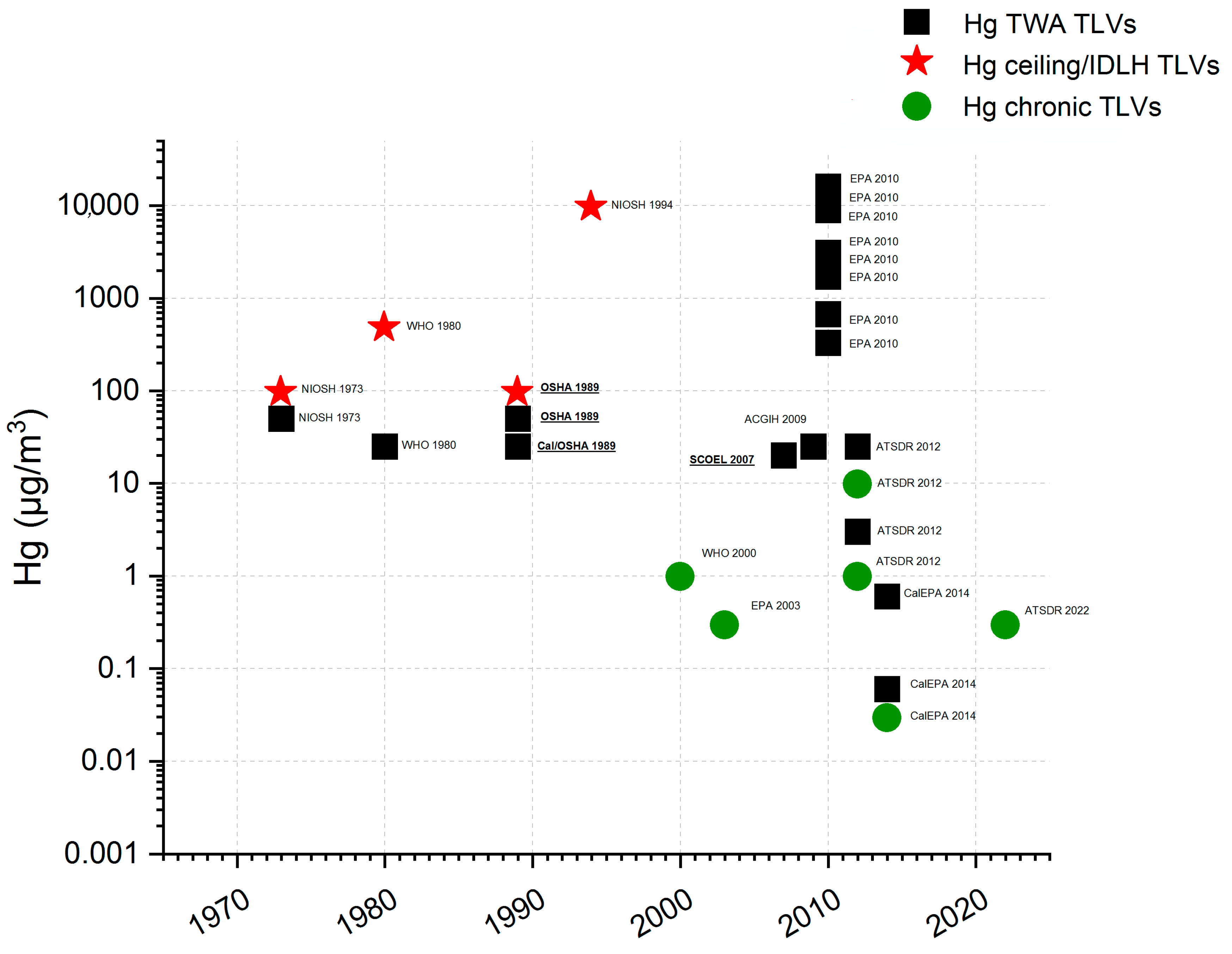
| μg/m3 | Bibliography | |
|---|---|---|
| chronic exposure | ||
| CalEPA—chronic REL for Hg0 | 0.03 | [43] |
| ATSDR—MRL for chronic-duration inhalation | 0.3 | [30] |
| EPA—RfC | 0.3 | [41] |
| WHO—Average annual Hg concentration guideline | 1 | [50] |
| ATSDR—Action level for residential settings (normal occupancy) | 1 | [42] |
| ATSDR—Action level for residential settings (residents’ evacuation/relocation) | 10 | [42] |
| TWA exposure | ||
| CalEPA—8 h REL for Hg0 | 0.06 | [43] |
| CalEPA—acute REL for Hg0 | 0.6 | [43] |
| ATSDR—Action level for workplaces not covered by 29 CFR 1910 Subpart Z | 3-4 | [42] |
| European Union 8-h TWA IOELV (Directive 2009/161/EU) | 20 | [32] |
| ATSDR—Action level for workplaces covered by 29 CFR 1910 Subpart Z | 25 | [42] |
| ACGIH—TLV (8-h TWA for inorganic Hg forms including metallic Hg) | 25 | [51] |
| Cal/OSHA—PEL (8-h TWA for mercury, metallic and inorganic compounds as Hg) | 25 | [44] |
| WHO—Recommended occupational exposure (TWA) | 25 | [52] |
| OSHA PEL (8-h TWA) and NIOSH—REL (10-h TWA) | 50 | [53]; [47] |
| NIOSH and OSHA Ceiling limit for metallic and inorganic Hg | 100 | [44]; [47] |
| EPA AEGL-2—AEGL-3 for 8 h exposure | 330–2200 | [40] |
| WHO—Recommended occupational short-term exposure | 500 | [52] |
| EPA AEGL-2—AEGL-3 for 4 h exposure | 670–2200 | [40] |
| EPA AEGL-2—AEGL-3 for 60 min exposure | 1700–8900 | [40] |
| NIOSH IDLH | 10,000 | [45] |
| EPA AEGL-2—AEGL-3 for 30 min exposure | 2100–11,000 | [40] |
| EPA AEGL-2—AEGL-3 for 10 min exposure | 3100–16,000 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciani, F.; Costagliola, P.; Lattanzi, P.; Rimondi, V. Gaseous Mercury Limit Values: Definitions, Derivation, and the Issues Related to Their Application. Sustainability 2024, 16, 3142. https://doi.org/10.3390/su16083142
Ciani F, Costagliola P, Lattanzi P, Rimondi V. Gaseous Mercury Limit Values: Definitions, Derivation, and the Issues Related to Their Application. Sustainability. 2024; 16(8):3142. https://doi.org/10.3390/su16083142
Chicago/Turabian StyleCiani, Francesco, Pilario Costagliola, Pierfranco Lattanzi, and Valentina Rimondi. 2024. "Gaseous Mercury Limit Values: Definitions, Derivation, and the Issues Related to Their Application" Sustainability 16, no. 8: 3142. https://doi.org/10.3390/su16083142






