Abstract
This paper provides a review of toxic algal blooms in the Philippine and Malaysian coastal and marine systems, considering relevant available knowledge, including climate change dimension/s in the assessment of their recorded recent expansion. The first record of human toxicity in the Philippines associated with HABs/toxic algal blooms specifically was during the bloom of Pyrodinium bahamense in the Sorsogon, Samar, and Leyte waters in 1983. Since then, the species has been identified to occur and cause blooms in about 44 sites/areas in the country. Recent government reports, i.e., 2021, 2022, and 2023, have also identified other paralytic shellfish poisoning (PSP) causative organisms (Gymnodinium catenatum, Alexandrium spp.) in the country. New records indicate that the presence of PSP causative species has been reported almost year-round in the Philippines. In Malaysia, PSP caused by P. bahamense was initially confined in 1981 to the state of Sabah, Malaysia Borneo, but since then, blooms of this species have been reported almost annually at different scales across the coastal waters of Sabah. P. bahamense and other cyst-forming dinoflagellates could be transported naturally or through human activities. Other eco-physiological and environment factors from the field and the laboratory have been used to study the bloom dynamics and transport of PSP causative species in several areas in the Philippines and Malaysia. More recently, plastics and other marine litter have been considered potential vectors of invasion/transport or expansion of dinoflagellates with other microorganisms. ENSO events have been observed to be stronger since 1950 compared with those recorded from 1850 to 1950. The extreme phases of the ENSO phenomenon have a strong modulating effect based on seasonal rainfall in the Philippines, with extreme ENSO warm events (El Niño) often associated with drought and stresses on water resources and agriculture/aquaculture. In contrast, cold events (La Niña) often result in excessive rainfall. The La Nina Advisories from 2021 to 2023 (18 advisories) showed the persistence of this part of ENSO, particularly in regions with recurrent and new records of HABs/toxic algal blooms. More studies and monitoring of another type of toxic algal bloom, Ciguatera Fish Poisoning (CFP), are recommended in tropical countries such as the Philippines and Malaysia, which have extensive reef areas that harvest and culture marine fish for local and export purposes, as accelerating reports of this type of poisoning have apparently increased and causative organisms have been identified in several areas. There is an urgent need to enhance HAB/toxic algal bloom research and monitoring, particularly those related to climate change, which has apparently impacted these blooms/occurrences directly or indirectly. Local researchers and managers should be made aware of the knowledge and tools already available for their utilization and enhancement to meet local conditions and challenges for potential recurrence and expansion of HABs/toxic algal blooms. Regional and international HAB research and collaboration should be further advanced for the protection of public health and marine resources.
1. Introduction
Harmful algal blooms (HABs), i.e., referring collectively to toxic algal blooms and fish-killing blooms/red tides, are not new to the Philippine Archipelago and Malaysia, similar to many other countries in the world. A “Harmful Algae Event Database (HAEDAT) is now being implemented through the UNESCO-Intergovernmental Oceanographic Commission (IOC) International Oceanographic Data and Information Exchange (IODE), which provides a global map of the different HABs. The availability of HAB monitoring and research data and information depends on national priorities, funding, and human resource support.
Globally, the 2021 Global HAB status report [1,2] illustrated varying patterns for trends in HABs. After correcting for levels of monitoring effort, areas such as Central America/Caribbean, South America, the Mediterranean, and North Asia are experiencing increasing HABs, while the incidence of HABs has decreased on the West Coast of America and Australia/New Zealand. No significant change was observed on the East Coast of America, Europe, and Southeast Asia. Despite these varying trends, HABs have caused significant negative impacts on humans and were correlated with increased aquaculture production across regions. This latter relationship could be due to increased HAB monitoring and/or nutrient pollution. It was difficult to ascribe changes to climate change in particular.
The Mediterranean, which has a long history of HAB studies and monitoring, is experiencing increased HABs. Zingone et al. [3], based on Ocean Biodiversity Information System (OBIS) data, observed that in the Mediterranean, reports of toxic species and HABs apparently increased in the region over the last half-century and this is likely related to the “increased awareness and monitoring operation, without an actual increase in toxic or noxious events so far emerging in intensively studied areas, such as the French and Spanish coasts or the Adriatic Sea”. They also reported a decrease in the occurrence of some HAB species, e.g., Alexandrium minutum blooms disappearing from the Harbor of Alexandria. Generally, Zingone et al. [3] state that the main HAB risks derive from cases of massive microalgal biomass production and consequent impacts on coastal water quality and tourism, which represents the largest part of the marine economy in the region. Using the SeaWIFs (1998–2003) dataset to look into algal bloom patterns in the Mediterranean Sea, Barale et al. [4] interpreted the patterns of high chl a/positive trend of chl anomalies at near-coastal hotspots to be linked to continental runoff and a growing biological dynamism at these sites, referring to the “intensification of noxious or harmful algal blooms, in the north-west, and of coastal fisheries, in the south “of the Mediterranean Sea”.
Moreira [5], in a recent review and editorial, concludes that in the current global climate changes, characterized by heavy rainfall or heat waves that have been attributed as probable causes of HABs with impacts on species diversity and consequently toxin profiles, “discussions on HABs should be considered a primary issue by national governments, international agencies, and scientists. Its understanding and risk evaluation can permit the achievement of safer ecosystems, minimize economic losses, and improve water management strategies without overlooking water quality”.
In the Philippines, the earliest report of an HAB was in 1908 on the coast of Bataan, when a fish kill event was attributed to Peridinium blooms [6]. It was in 1983 that the first outbreak of HABs was recorded in Samar, Philippines, and was caused by the toxic dinoflagellate Pyrodinium bahamense [7]. Since then, the occurrence of HABs has been more frequent and has expanded to several coastal waters in the Philippines [8,9,10]. Filipinos are familiar with the term “red tide,” a misnomer that the populace uses to refer only to toxic algal bloom/HAB events (i.e., those resulting in paralytic shellfish poisoning or PSP). The term “red tide” technically refers to the discoloration of the water due to dense plankton/phytoplankton, which does not necessarily cause human toxicity. So far, the most common reported toxic HAB in the Philippines is paralytic shellfish poisoning (PSP) caused by consuming shellfish that accumulated Pyrodinium bahamense, Gymnodinium catenatum, or toxic Alexandrium spp. from affected waters [11,12,13]. A review of HABs in the Philippines and Malaysia has been published recently [14].
This paper provides an update on this review with a focus on toxic algal blooms in Philippine and Malaysian coastal and marine food systems, considering climate change dimension(s) in the assessment of their sustainability as sources of safe seafood supply in the future. This is significant since these two countries have coastal communities that are dependent on marine resources for their survival and livelihood.
A special issue on Climate Change and Harmful Algal Blooms was recently published [15] and mentioned that “climate change through ocean warming has received the greatest attention in HAB research in mid- and higher latitudes” [16,17,18]. In general, there is a growing belief in a future scenario that increasing sea temperature could lead to possible events wherein HABs migrate toward the poles with progressive warming, a hypothesis put forward and affirmed by some earlier studies [15,19]. HABs that could migrate to new ecosystems may create significant risks to these ecosystems and human communities. How certain HAB species could respond to climate change interests HAB scientists, while HAB managers and the public are concerned with the harm they may cause or manifest within the new coastal zones/habitat.
2. Toxic Harmful Algal Bloom Events in the Philippines and Malaysia
Numerous toxic, harmful microalgal species that can cause human poisonings, such as paralytic shellfish poisoning (PSP), amnesic shellfish poisoning (ASP), diarrhetic shellfish poisoning (DSP), and Ciguatera fish poisoning (CFP), have been documented so far and identified from monitoring and research collections [8,14]. However, only PSP and CFP are the human poisonings that have been well documented. Fish kill events, which are technically referred to as red tides because of the massive discoloration of the water due to the algal bloom, have also been noted in many areas, including aquaculture sites. This review will focus on the two more significant human poisonings (i.e., PSP and CFP) in the two countries.
2.1. Paralytic Shellfish Poisoning
2.1.1. Blooms of Paralytic Shellfish Poisoning (PSP) Causative Organisms in the Philippines
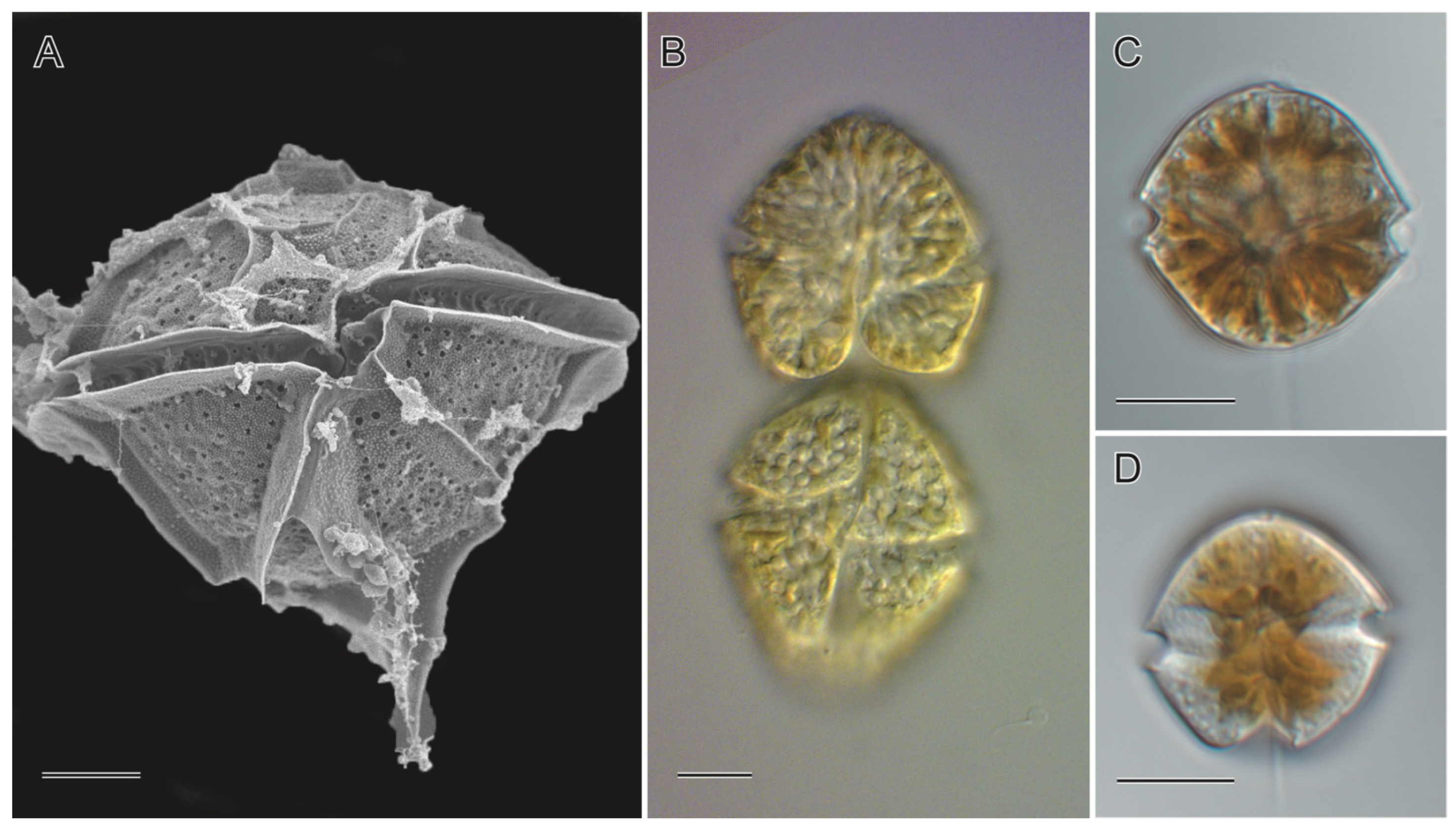
The first record of human toxicity associated with HABs in the Philippines was during the bloom of Pyrodinium bahamense in the Sorsogon, Samar, and Leyte waters in 1983. Since then, the coastal waters of these provinces have been affected by the blooms of this tropical organism, which was first described in the Bahamas and has also been recorded in other waters in Southeast Asia and Central and South America [20,21,22,23,24]. The species and some other PSP causative organisms (Figure 1) have been identified as occurring and causing blooms in about 44 sites/areas in the country since 1983 [8,11]. The Philippine embayment and coastal areas, with records of the presence of PSP causative organisms, are shown in Table 1.
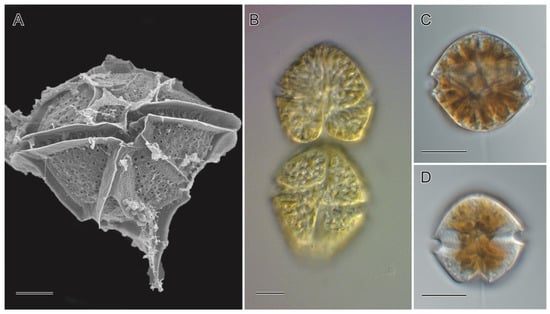
Figure 1.
Cyst-forming PSP causative organisms in the Philippines: (A) Pyrodinium bahamense L. Plate, (B) Gymnodinium catenatum Graham, (C,D) Alexandrium spp. Halim. (A). SEM micrograph captured using S-4800 SEM (JEOL, Tokyo, Japan) (B–D). Light micrographs captured using a BX60 (Olympus, Tokyo, Japan). Scale bar = 10 µm.

Table 1.
Coastal and marine areas in the Philippines reported paralytic shellfish poisoning (PSP) causative organisms (updated from Azanza [8]. Shellfish Bulletins, in red, are recent/new records [25,26]).
2.1.2. Paralytic Shellfish Poisoning Events and Causative Organisms in Malaysia
In Malaysia, paralytic shellfish poisoning caused by P. bahamense was confined to the state of Sabah, Malaysia Borneo. Blooms of P. bahamense have been reported almost annually at different scales across the coastal waters of Sabah. With numerous sentinel sites identified and monitoring programs established, incidents of PSP have been reduced significantly [27]. PSP in Peninsular Malaysia was primarily caused by two species of the genus Alexandrium, A. tamiyavanichii and A. minutum. Alexandrium tamiyavanichii is a euryhaline species that is widely distributed in the Asia Pacific [28]. The first PSP event was recorded in Sebatu, Malacca in 1991 [29,30,31,32], and later in 2013 and 2014 in Kuantan Port [33,34]. The cellular toxin content of this species is considerably high, reaching up to 180 fmol cell−1 in Malaysian strains [35], which is sufficient to cause poisoning at a low cell density. As such, it is important to monitor the species, and molecular techniques such as real-time PCR [36,37] have provided a reliable tool for early detection.
Alexandrium minutum is an euryhaline species whose distribution is known to be confined to estuarine, with salinity fluctuating diurnally, depending on tidal cycles and freshwater plumes. The occurrences of A. minutum reported in the Asia Pacific region were recorded from brackish environments or areas with strong influences of freshwater. The species found in this region are also genetically different from those reported from the temperate region [38]. The Malaysian strains of A. minutum are a toxic species that form high biomass blooms, with cell abundances reaching up to 4 × 107 cells/L. This species was known to cause shellfish contamination and is not safe for consumption. The species produces a suite of PSTs, with GTX4 and GTX1 as the dominant toxin congeners [35]. On rare occasions, the species was reported to cause mass mortality of lobster (South Africa), but the actual cause of mortality due to the toxicity or induced hypoxia conditions during the blooms was not determined.
Lim et al. [35] reported that A. minutum exhibited a shade-adapted strategy that allowed the species to proliferate in turbid estuarine waters. Furthermore, the species can tolerate high ambient ammonia nitrogen concentrations, and uptake of this form of nitrogen [39,40] provides an edge compared with other bloom-forming dinoflagellates. With increasing coastal eutrophication due to mariculture, chemical fertilizer from agricultural activities, and discharge derived from land or sea-based activities, the increase in nutrients in coastal environments and nutrient imbalance will be driving factors for the increased occurrence of algal blooms, in particular species that have an ecological advantage and cellular adaptation toward this type of environmental changes. The life cycle and bloom dynamics of A. minutum were fully clarified during a 2015 prolonged bloom event [41]. The species tends to induce gamete formation to form planozygotes once the populations are subjected to unfavorable conditions [41]. Laboratory studies also confirm the heterothallism of A. minutum, of which compatible gametes are required to have successful fusion and formation of zygotes [33].
With the rapid expansion of mariculture, especially in coastal embayment, these species should be assessed and monitored not only with the conventional microscopy method but also with advanced molecular detection methods (e.g., [36,37]) and in the event of the presence of these species, it should be included in the shellfish monitoring program in the area.
2.2. Ciguatera Fish Poisoning (CFP)
The causative species of Ciguatera fish poisoning (CFP) or Ciguatera poisoning (CP) in the genus Gambierdiscus have been well-studied, with representatives widely distributed in the Pacific, western Indian Ocean, Caribbean waters, Mediterranean Sea, Western Atlantic waters [42,43]. The causative organisms grow on seaweeds and other benthic substrates, where marine fish feed on them and accumulate the toxins in their tissues. Human poisoning, namely CFP, occurs upon consumption of affected fish.
The incidence of CFP is estimated at approximately 10,000 to 50,000 cases annually worldwide [44], and many are believed to go unreported or misdiagnosed. The increase in demand for live reef fish trades from Southeast Asian countries and other regions in the Indo–Pacific to the Global hub of live reef fishes in Hong Kong has contributed to an increase in the occurrence of ciguatera poisoning cases outside the region of origin or the hotspot of CFP [45].
Yogi et al. [46] documented two reports of ciguatera caused by barracuda in 2001 (50 subjects affected) and 2006 (33 subjects affected) and four reports of unknown types of fish poisoning. Further, Yogi et al. [46] observed that based on an extensive search of journal databases, the Internet, and government websites, no reports of ciguatera in Brunei, Cambodia, Indonesia, Myanmar, and North Korea, were encountered and hence, included in their publication are the following countries with at least one report of ciguatera: China including Hong Kong and Macau, East Timor, Japan, Malaysia, Philippines, Singapore, South Korea, Taiwan, Thailand, and Vietnam. Based on their extensive literature research, Yogi et al. [46] found that the earliest Philippines CFP ciguatera incidence could have been in Basilan Province in August 1988, involving 19 subjects from four families: further, the Philippines dataset reviewed by the Department of Health website showed that of the 60 foodborne disease outbreaks in 1995–2004, 38 ciguatera cases were identified. The authors concluded that “In the coastal countries of East Asia and Southeast Asia, ciguatera should be common as well for several reasons. There are extensive tropical and subtropical coral reefs along the coasts and in the neighboring seas”.
Transboundary CFP occurred for various reasons, including the absence of an effective ciguatoxin detection method and the unavailability of ciguatoxin reference materials. Furthermore, high toxicity variation in individual fishes makes toxicity screening a challenging task. Only about 5% of reef fishes from 600 individuals tested positive and no clear relationship was observed among the species or size of fishes [47]. In comparison to the monitoring of planktonic harmful algae species, where a good correlation in plankton cells and toxicity in vector shellfish was observed, monitoring of benthic Gambierdiscus cell numbers and the toxicity of vector reef fishes using conventional approaches seem unreachable.
2.2.1. CFP Events and Causative Organisms in the Philippines
In the Philippines, the presence of the two toxic benthic dinoflagellates, i.e., Gambierdiscus carpenteri and G. balechii, was definitively established for the first time through the examination of samples collected in Bolinao, Pangasinan, and Guian Eastern Samar, employing morphological and phylogenetic analyses [48,49]. It is noteworthy that these two coastal areas area had no historical records of Ciguatera fish poisoning (CFP) incidents. In contrast, four verified CFP cases were documented across the country from 1989 to 2017, involving barracuda-related poisonings in Metro Manila in 2001, Iloilo City in 2006 (central Philippines), and Zamboanga in 2014 (southern Philippines) [50]. Additionally, CFP linked to snapper consumption was identified in Iloilo, with confirmation by the Bureau of Fisheries and Aquatic Resources (BFAR) using the Cigua-Check Kit (Oceanit Test System, Inc, Honolulu, HI, USA). The 2014 Zamboanga CFP case was confirmed using mass spectrometry methods [50].
Difficulty in preventing CFP comes from the lack of reliable methods for analysis of Ciguatoxins (CTXs) in contaminated fish, aggravated by the normal appearance, taste, and smell of CTX-contaminated fish. Tsumuraya et al. [51] successfully developed protocols using Monoclonal antibodies (mAbs) specific against major CTX congeners (CTX1B, 54-deoxyCTX1B, CTX3C, and 51-hydroxyCTX3C) using rationally designed synthetic haptens-KLH conjugates instead of the natural CTXs. Haptenic groups with a surface area greater than 400 Å2 are required to produce mAbs that can strongly bind to CTXs. Furthermore, a highly sensitive fluorescence-based sandwich enzyme-linked immunosorbent assay (ELISA) was developed. The protocol can detect and quantify four major CTX congeners (CTX1B, 54-deoxyCTX1B, CTX3C, and 51-hydroxyCTX3C) with a limit of detection (LOD) of less than 1 pg/mL. No cross-reactivity was observed against other marine toxins tested (Brevetoxinx A and B, Okadaic Acid, and Maitotoxin). In countries such as the Philippines, where fish are a staple food, particularly in coastal communities, CFP detection and management using reliable methods such as the protocol developed by Tsumuraya et al. [51] should be given more attention.
In our recent publication authored by Yñiguez et al. [14], multiple instances of CFP were detailed, resulting in 123 confirmed cases and 274 suspected cases or affected individuals. It is important to highlight that a significant portion of the information concerning these cases is sourced from newspaper articles or referenced within previously published works, particularly the study conducted by Mendoza et al. [50], where two entire families became ill in 2010. The limited availability of data regarding the presence of causative organisms and authenticated medical reports associated with CFP poses a challenge in identifying discernible patterns or trends in the associated hazards or risks.
2.2.2. CFP Events and Causative Organisms in Malaysia
In Malaysia, the first suspected CFP case was recorded in 2010, with 22 victims from five families feeling sick after consuming red snapper on 8 September 2010; 11 were admitted with symptoms that resembled those of CP poisoning [52]. The batch of contaminated red snapper was imported, frozen, and sold at a local market. Unfortunately, no fish samples from this poisoning event were available for chemical confirmation.
CFP cases reported in the state of Sabah before 2000 were not investigated or never confirmed, partly because of no clinical information on the victim and a lack of fish samples for chemical confirmation. The first CFP incident with chemical confirmation was documented by Lee et al. [53], which occurred in 2017 in Sabah and involved two victims from a family admitted after consuming two species of red snapper, Lutjanus sebae, and Lutjanus bohar. One of the victims showed severe symptoms, including cardiovascular and muscle weakness and itchiness that lasted nearly two months, whereas other family members showed mild poisoning [53]. The fish samples collected from the victim’s leftovers were confirmed later to contain Pacific-Ciguatoxins (P-CTXs) [54]. Since then, new CP incidents have been reported from the state, involving local folks and tourists visiting Sabah (Department of Fisheries Malaysia). Other reef fishes, including coral groupers, were confirmed to be positive and responsible for one of the CP cases in the state (unpublished data).
The state of Sabah is a popular tourist destination because of its rich marine biodiversity and seafood delicacy. Sabah is also popular for enthusiasts of deep-sea sport fishing. The rich marine resources also contributed to the export of live reef fish valued at USD50 million (estimated about 1700 MT in 2010). Live reef fish from Sabah have been previously reported to cause CFP cases in Hong Kong [55,56]. Delays in the diagnosis of CFP will lead to unnecessary investigations and management, including delayed public health intervention. As a public health measure, people are advised to avoid eating large coral reef fish that were previously known to be associated with CFP incidents in the Pacific region. However, this advisory might not help to resolve the risk posed by CFP in seafood products in Sabah since some small reef fishes can be highly contaminated with ciguatoxins. Therefore, identifying the CP hotspots in the state has been an urgent issue.
In Malaysia, three species of Gambierdiscus, G. balechii, G. caribaeus, G. pacificus, and a new ribotype Gambierdiscus sp. type 7 have been documented [57,58,59]. The strains of G. balechii, G. caribaeus, and Gambierdiscus sp. type 7 analyzed by cytotoxicity assay (neuro-2a and hemolytic assay of fish erythrocytes) and high-resolution mass spectrometry showed detectable ciguatoxicity [60].
The distribution and abundance of benthic harmful dinoflagellate species, including Gambierdiscus species, were known to be influenced by the microhabitats in coral reef ecosystems [29,58,59,61]. The relationships between benthic harmful algal blooms (BHABs) and microhabitat preferences, based on non-quantitative anecdotal observations, are inconsistent or contradictory [62], owing to the differences in the selection of macroalgal substrates and inconsistency in the quantification of cell abundance that was normalized to either surface areas or biomass (dry or wet weight) of macroalgal substrates. A non-destructive sampling method using artificial substrates has been introduced to overcome the limitations [63,64]. Although the sampling method is laborious and requires SCUBA diving to deploy the artificial substrate (fiberglass), it has been demonstrated to be a promising tool in routine monitoring programs. Gambierdiscus exhibited the most restricted range of microhabitats, primarily inhabiting warm-water reefs dominated by turf algae, hard coral, and fleshy macroalgae [58]. It is apparent that bottom substrate perturbations, such as the degradation of coral reefs and climate change, will have a significant impact on the abundance of Gambierdiscus.
3. Early Explanations on the Expansion and Origin of Philippine Toxic Blooms
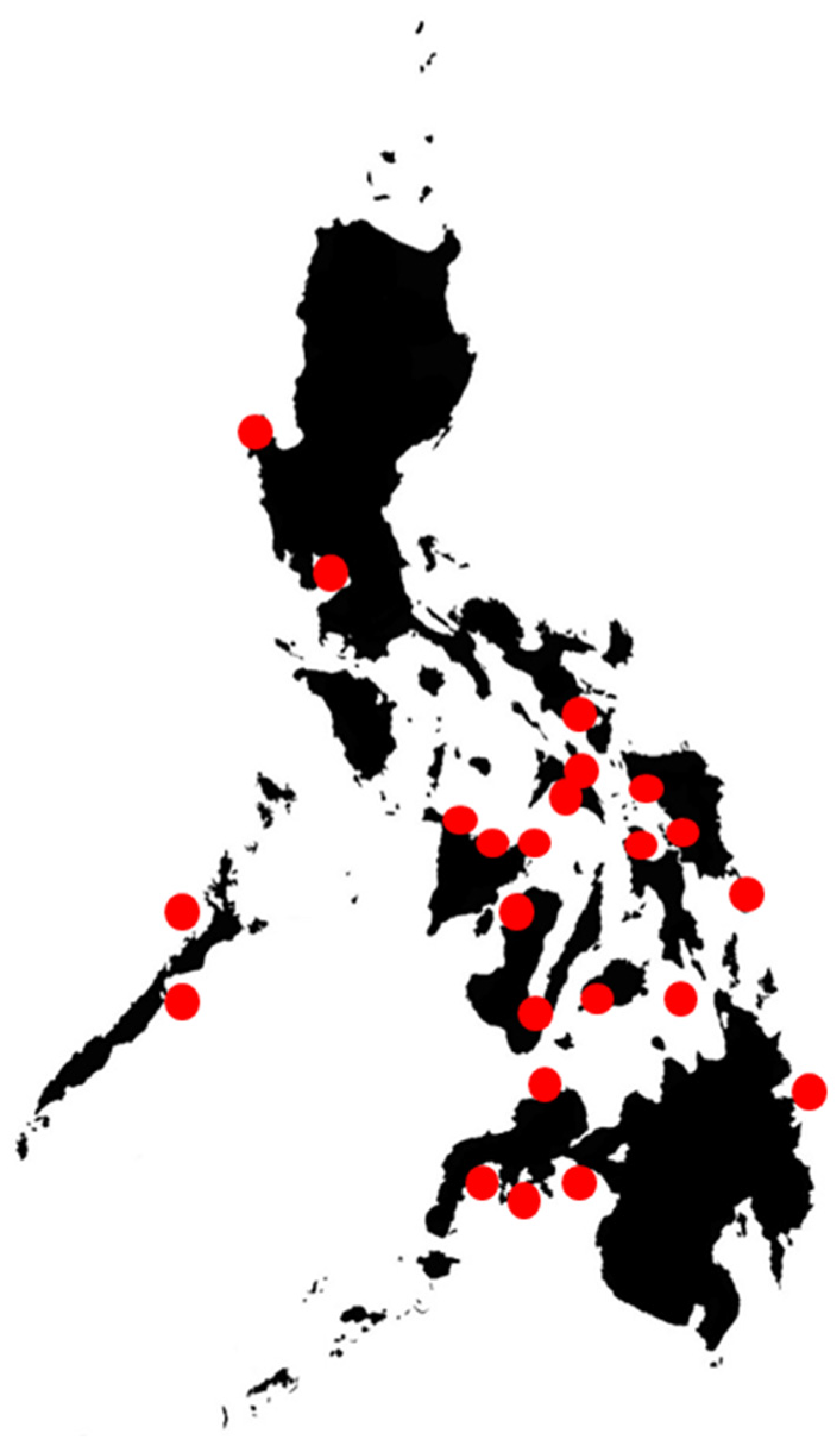
The expansion of the mussel industry in the Philippines beginning in the 1970s [65] could have likely transported shellfish with HAB causative species from affected sites to new mariculture sites, thus serving as vectors for the cysts of P. bahamense [66]. However, many of the HABs in these new areas have not been studied to verify this hypothesis since relatively few sites have been sampled and studied (Figure 2). The presence of the species’ cysts in the sediments in some of these areas [67] could mean that the potential for their bloom could have been there long before the introduction of the mussel industry. The viability of dinoflagellate cysts and the role they play in the life cycle of P. bahamense, the main PSP culprit in the country and many tropical areas, have been elucidated both in the laboratory and the field [68,69]. The role, behavior, or physiological–ecology and potential for transport of dinoflagellate cysts have also been well studied in other areas in North America [68,70,71]. These seeds can be carried from their “seed banks” through natural and artificial means and initiate new blooms in other areas. Marine litter and boats can also serve as HAB transport vectors [72].
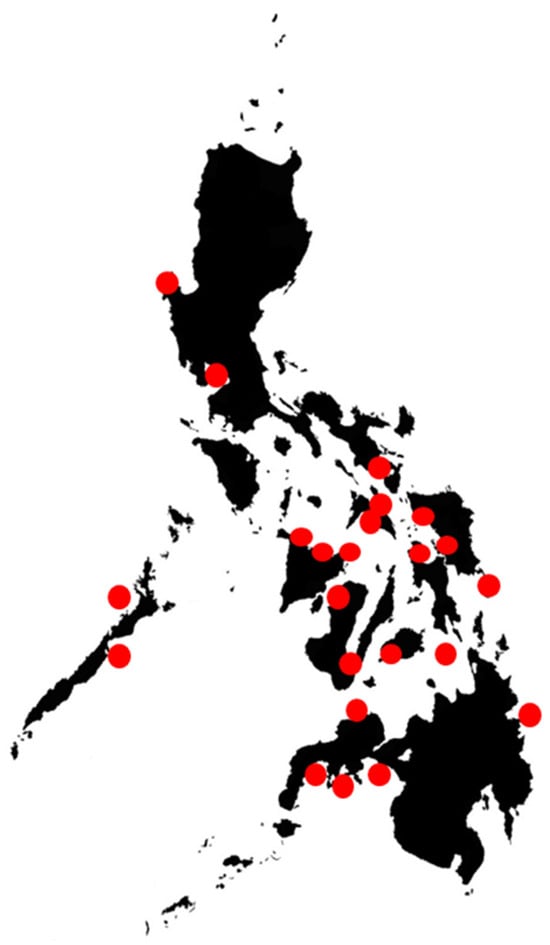
Figure 2.
Areas in the Philippines where HAB monitoring and research have been performed or initiated, from [8].
Advection plays an important role in the development and expansion of HABs across sites in many different parts of the world [73,74]. Previous studies have shown the role of advection in the blooms of cyst-based dinoflagellates such as Pyrodinium bahamense and Alexandrium [75,76]. Cysts such as those of Pyrodinium (Figure 1) could withstand long-distance transport [68] and act as progenitors of bloom in new areas. In previously affected areas, the presence of seed beds has been well documented for P. bahamense, Alexandrium spp., and other species here and in other countries [67,68,77,78,79]. In Manila Bay, the advection of cells and/or cysts from cyst beds could spread the bloom of P. bahamense around the bay following oceanographic and/or environmental conditions [80]. A similar dynamic has also been shown in other embayments in the Philippines, such as Sorsogon Bay [81] and Murcielagos Bay [82]. A recent study illustrated for the first time the important role of advection across a larger seascape in the Philippines [83]. They showed that blooms across adjacent embayments in the Samar and Leyte areas are likely due to the transport of cells across this area, and certain sites could be where cysts are situated and develop local blooms, which then serve as the source of blooms for other bays.
The linkage between the eutrophication in Philippine coastal waters and the occurrences and apparent expansion of harmful algal blooms (HABs) remains insufficiently substantiated or not thoroughly grasped. As a rapidly developing nation in the region with over half of its population residing in close proximity to the coastline, the pace of coastal development in the Philippines has been rapid. Notably, the management of wastewater, especially its discharge into the ocean, lacks comprehensive strategies, and routine surveillance of water quality is notably absent, as seen in instances such as Manila Bay and the renowned tourist destination Boracay [84].
The impact of eutrophication on HAB occurrences has been more robustly established in Bolinao–Anda, an area with a prolonged history of challenges related to the proliferation of fish pens/cages, stocking densities, and the quantity and quality of feed [85,86,87,88]. Unregulated practices in mariculture and the expansion of culture sites contribute to the accumulation of unconsumed feeds and waste rich in phosphorus, rendering the culture systems/sites vulnerable to blooms when nitrogen is introduced through river run-off, particularly during the rainy season [87]. In some areas in the Philippines, decreased DO is usually observed when fish-killing blooms/red tides are common, such as in Bolinao, Pangasinan fish pens, and fish cages [89]. Recent use of more sensitive and culture-independent high throughput methods (i.e., sequencing) has also revealed a previously unappreciated diversity of dinoflagellates and diatoms occurring in many areas in western Luzon [90,91]. Within this group, many previously unreported taxa or those that had never been reported to bloom before had been detected. Their detection posits potential threats, with several studies suggesting that they may be favored under climate change scenarios [5].
Plastic is now considered a major pollutant in marine ecosystems, which mainly enter via major riverine bodies [92]. These new floating substrates can be colonized and host-microbial biofilms that sustain the growth of different attaching organisms [93]. Their attachment will allow them to disperse and be transported to areas far from where they originated, thus increasing the dispersal of harmful species. For example, Masó et al. [72] showed the presence of viable vegetative cells and cysts of harmful algal species, such as Ostreopsis sp., Coolia sp., and Alexandrium sp. in the Mediterranean Sea, while Zettler et al. [93] reported Alexandrium sp. in the Atlantic, and Reisser et al. [94] with Ceratium sp. in Australian waters in the Pacific. Pasqualini et al. [95] further that such attaching communities are also influenced by season, habitat, and polymer types. In the Philippines, the HAB-causing species Gymnodinium sp. and Protoperidinium sp. cells were found on floating plastics collected from the coasts of Manila Bay [96]. This makes the plastics and other marine litter capable of serving as vectors for the dispersal of many microorganisms, including HAB-causing species [72,96], and posits a new and emerging threat to different marine environments.
4. Climate Change Hypothesis/Predictions on Toxic Algal Blooms
In 2021, the IPCC stated that ENSO events have been observed as stronger since 1950 compared with those recorded from 1850 to 1950. El Niño and La Niña episodes typically occur every two to seven years and usually last nine to 12 months, not necessarily alternating with one another with La Niña events are less common than El Niño episodes. The IPCC concluded that there is no clear evidence that climate change has affected these events [97]. The extreme phases of the ENSO phenomenon have a strong modulating effect on seasonal rainfall in the Philippines, with mature ENSO warm events (El Niño) often associated with drought and stresses on water resources and agriculture, while cold events (La Niña) often result in excessive rainfall. There are also impacts on the onset and length of the rainy season and, importantly, the number of tropical cyclones. Since 1949, there have been 17 El Niño events based on the National Oceanic and Atmospheric Administration’s classification [97,98].
The Philippine Atmospheric, Geophysical, and Atmospheric Services and Administration (PAGASA) issues Advisories about ENSO considering both the El Niño and La Nina parts. The La Nina Advisories from 2021 to 2023 (18 advisories) showed persistence of this part of ENSO, particularly in regions 6 (Western Visayas), 7 (Central Visayas), 8 (Eastern Visayas), 9 (Zamboanga Peninsula), 10 (Northern Mindanao), 11 (Davao Region), and the CARAGA region and MIMAROPA. La Niña Advisory No. 8 mentioned that in April 2022, La Niña strengthened, and three rainfall stations in the Visayas, namely Baybay Leyte; Mambual, Capiz, and Guiuan, Samar, recorded 536.0 mm, 338.0 mm, and 207.8 mm of rain, respectively. Advisory No. 14 again advised that the La Niña strengthened and will continue up to December 2022 or even up to January 2023, affecting most parts of the country, particularly Western Visayas and the Bicol Region, which both face the Pacific Ocean. El Niño Advisories (for January 2021 to December 2023) reports in Advisory No. 4 that the tropical Pacific still shows warmer-than-normal sea surface temperatures, signifying a moderate El Niño; however, a strong El Niño was predicted for late 2023, an event likely to continue until the second quarter of 2024. El Niño Advisory No. 5 A moderate-to-strong El Niño is present in the tropical Pacific, showing signs of further intensification in the coming months as sea surface temperature anomalies (SSTAs) reach more than 1.5 °C. Recent analyses from global climate models suggest that El Niño will likely continue until the second quarter of 2024 (PAGASA).
The influence of the El Niño Southern Oscillation (ENSO) suggests that conditions during prolonged and stronger El Niño (e.g., warmer sea surface temperatures) seem to be correlated with HAB occurrences in Southeast Asia [14,20]. However, this needs to be further substantiated with more studies in the region. Climate, which is a significant contributor to periodic shifts in temperature and rainfall in the region [99,100], could influence phytoplankton/plankton ecology/eco-physiology. For example, considering the mechanisms promoting Pyrodinium blooms, it has been shown that transitions from dry to rainy conditions, which promote increased nutrient run-off and a more stable water column, can be the triggering factors that initiate and promote Pyrodinium blooms [8,14,80,101].
The climate of the Philippines is highly influenced by the El Niño Southern Oscillation (ENSO). El Niño is associated with an increased chance of drier conditions, and La Niña is associated with an increased chance of wetter conditions. Changes in rainfall are associated with changes to tropical cyclone activity in the western equatorial Pacific, the strength of the monsoon, and changes in the onset and/or termination of monsoon rains.
The tropical monsoonal system is strongly influenced by the El Niño and La Niña events [102]. In the Southeast Asian regions, coastal blooms are often regulated by tropical monsoonal shifts, with distinctive precipitation patterns and wind-driven coastal upwelling events affected by the wet northeast and dry southwest monsoons. During strong El Niño episodes, maximum air temperature increased, and rainfall decreased significantly. In Malaysia, phytoplankton blooms have been consistently observed during the dry season [103,104,105]; during this period, the water was strongly associated with elevated water temperatures. In addition, more bloom episodes with high biomass were observed in the El Niño period of 2015/2016 concurrently with the positive Indian Ocean Dipole phenomenon [106].
Hallegraeff [19] has made predictions for other HAB species in connection with climate change. He stated that we can expect (1) range expansion of warm-water species at the expense of cold-water species, which are driven poleward; (2) species-specific changes in the abundance and seasonal window of growth of HAB taxa; (3) earlier timing of peak production of some phytoplankton; and (4) secondary effects for marine food webs, notably when individual zooplankton and fish grazers are differentially impacted (“match-mismatch”) by climate change.
HABs are site-specific and species-specific, which generally means that HABs occur only in areas where the causative organism could adjust to the environmental conditions based on their inherent or genetic capacity. Whether an organism, Pyrodinium bahamense, for example, would be able to bloom in a bay or coastal area depends on how it can successfully adjust to the conditions of the bay [68]. Understanding the bloom dynamics (what, when, and how specific HABs occur or recur) is based on scientific understanding/knowledge about the organism’s pre-bloom, bloom, and post-bloom behavior in a specific area [68,70,71].
5. National and International Networking on HAB Research and Monitoring
The Scientific Committee on Oceanic Research (SCOR) and the Intergovernmental Oceanographic Commission (IOC) of UNESCO developed the program Global Ecology and Oceanography of Harmful Algal Blooms (GEOHAB), the first international research program on harmful algal blooms. A country-level HAB research program was part of GEOHAB and was implemented in the Philippines in 2009–2014. The research results of the Philippine program called Philippine Harmful Algal Blooms (PhilHABs) were useful inputs into the Asian component of the GEOHAB Program. Global Harmful Algal Blooms (GlobalHAB) is the current program being implemented by the UNESCO IOC and SCOR. Whereas GEOHAB aimed to understand the biology and chemistry of causative organisms and the environmental conditions before, during, and after the blooms and use this information to manage these blooms, the GlobalHAB adopts the partially accomplished GEOHAB objectives and extends them into brackish and freshwater systems and to a variety of harmful groups, including benthic microalgae, cyanobacteria, and macroalgae [107]. GlobalHAB also addresses several issues related to the effects of HABs on human societies, such as health, socio-cultural aspects, and economic impacts, under collaboration with other international programs (e.g., [108]).
A number of areas (Figure 2) in the Philippines have been studied through the PhilHABs project using several tools, and some applications have been initiated. In order to obtain more in-depth results, the PhilHABs concentrated in areas where HAB incidences were highest, such as Anda and Bolinao, Pangasinan, Manila Bay, Juag Lagoon, and Sorsogon Bay, Sorsogon. The recently concluded Hazard Detection and Mitigation Tools for Algal Blooms in a Changing Environment (HABHazard) program built on the PhilHABs program and previous efforts. HABHazard enabled the initial development of a HAB early-warning system through enhanced HAB detection methods, a better understanding of dynamics, operational HAB models, and stakeholder engagement [109]. HAB detection methods involved the development of cost-effective sensors for real-time ocean condition monitoring, the use of biotoxin adsorption toxin tracking techniques (BATTs) for cheaper and more rapid toxin measurements, and the development of local toxin standards. Machine learning models for fish kills and toxic blooms were also developed for forecasting. This information and these components were integrated into the HABHub informatics system (https://habhub.philhabs.net/#/) (accessed on 27 November 2023), which helped disseminate the information from real-time sensors, standardized databases, and models. These, together with the BFAR HAB monitoring and management program, are the seeds for a more comprehensive and operational HAB early-warning system in the country [109].
Past and current efforts in HAB research and monitoring in the Philippines focus on the long-affected sites and some newly affected ones. Research collaboration and networking to correlate HABs with the rampant coastal and marine pollution have started, for example, in the ongoing work on plastic litter that serves as vectors for the transfer of harmful algae and other organisms [96,110].
Synthesis and Recommendations
HABs, which have a long recorded history, could persist or would always be part of coastal communities’/humanity’s existence. Their recurrence is complicated by heightened anthropogenic activities in the coastal areas, and climate change will only exacerbate their impacts. Some tropical dinoflagellate species benefitting from anthropogenic inputs (i.e., nutrient run-off from land and rain, increased seawater temperatures, coral reef destruction) are expected to become more successful and can continue to recur and expand even in colder areas [19,42,43]. Cold water-loving species may be pushed or driven pole-ward (i.e., they would become more limited by temperature rise brought about by climate change).
Blooms of the tropical species Pyrodinium bahamense have been recurring in several areas. The species’ cysts have been found almost globally; hence, it could be the organism that would most likely have expanded blooms in time and space with the predicted climate change-induced increase in temperature. The presence of cysts in the geologically dated sediment is evidence indicating that they existed or could have bloomed in these environments in the past and that they may once again bloom and cause problems. This PSP causative organism also dominated the phytoplankton in areas of Southeast Asia, South America, and the South Pacific [111], and there is evidence that the organism exhibited expansion during prolonged/strong El Niño/ENSO [20,101]. This species has been reported in Malaysia and Indonesia together with other PSP-causative organisms in the Southeast Asian region, including Alexandrium spp. and Gymnodinium catenatum.
Another public health hazard caused by HABs, i.e., ciguatera fish poisoning (CFP), has been sparsely studied in the Philippines and Malaysia. The causative organisms for ciguatera fish poisoning have been reported in both countries, especially in mariculture areas such as Sorsogon and Bolinao, Pangasinan. However, CFP detection and management have to be seriously considered for implementation by the national and local governments with the support of relevant international bodies. As rightfully stated by Tsumuraya et al. [51], where fish are a staple diet in local populations or commercially sold to other communities, CFP could pose a great health problem.
There is an increasing need to define the niche (specific role in its living space) of HAB species, remembering the fact that HABs are species-specific and area-specific, which are important in the prediction of “winners” and “losers” in a changing environment. Long-range data sets about the organisms and their bloom/growth preference should be gathered and analyzed/modeled for forecasting and hind casting.
Aligazaki [112] discussed “microalgal invasions” and “tropicalization“ of environments favoring CFP-causing organisms in the warmer southern areas (e.g., Egypt, Tunis, and Lebanon, as shown in the increased records in recent years, in number of species, abundance, and geographical distribution. The author believes that the situation is most probably related to climate change, but many issues have to be examined to come to accurate conclusions and cooperation among Mediterranean countries to understand the environmental changes occurring to protect human health and other human activities.
Since many of the toxins produced by HAB organisms are new or not yet identified, they must be characterized to make them more available as standards for testing of samples, monitoring, and research. Efforts for innovation can also be accelerated with the isolation and culture of causative organisms, where culturing in large amounts can result in the application of knowledge and development of technologies.
The multi-media identification of HAB species through electronic information systems can provide guided identification of causative organisms. For example, Linnaeus II, a software created by the Expert Center for Taxonomic Identification (ECTI) of UNESCO, is available on CD and online (HAEDAT).
Local researchers and managers should be made aware of the knowledge and tools already available for their utilization and enhancement to meet local conditions and challenges for potential recurrence and expansion of HABs/toxic algal blooms vital to the life of coastal communities and over-all economies of countries such as the Philippines and Malaysia.
Funding
HAB research in the Philippines was funded by the Department of Science and Technology-Philippine Council for Agriculture and Aquatic Resources Research and Development (DOST-PCAARRD) under Project “Philippine Harmful Algal Blooms” (PhilHABs) 2012–2017, and Project “Hazard Detection and Mitigation Tools for Algal Blooms in Changing Environment” 2018–2022. The National Academy of Science and Technology (NAST) provided a fellowship grant (NAST RF 2021–2022) to the senior author. HAB Research in Malaysia was funded by the Malaysian Government, Ministry of Higher Education HI CoE grant (HI CoES-2023B) to Po Teen Lim.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We gratefully acknowledge the varied kinds of assistance provided by the Marine Science Institute, University of the Philippines, Diliman. We also wish to acknowledge our Philippine, Malaysian, ASEAN, and other international collaborators and co-workers in the laboratory and the field who have helped or influenced our research. Positive recommendations from the editor/s and reviewers enhanced this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.Y.D.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Hallegraeff, G.; Enevoldsen, H.; Zingone, A. Global Harmful Algal Bloom Status Reporting. Harmful Algae 2021, 102, 101992. [Google Scholar] [CrossRef]
- Zingone, A.; Escalera, L.; Aligizaki, K.; Fernández-Tejedor, M.; Ismael, A.; Montresor, M.; Mozetič, P.; Taş, S.; Totti, C. Toxic Marine Microalgae and Noxious Blooms in the Mediterranean Sea: A Contribution to the Global HAB Status Report. Harmful Algae 2021, 102, 101843. [Google Scholar] [CrossRef] [PubMed]
- Barale, V.; Jaquet, J.M.; Ndiaye, M. Algal Blooming Patterns and Anomalies in the Mediterranean Sea as Derived from the SeaWiFS Data Set (1998–2003). Remote Sens. Environ. 2008, 112, 3300–3313. [Google Scholar] [CrossRef]
- Moreira, C. Editorial: Harmful Algal Blooms in Marine Environments: From Biologically Active Compounds to Species Diversity. Front. Mar. Sci. 2022, 9, 1–3. [Google Scholar] [CrossRef]
- Smith, A.M. Peridinium (Editorial). Philipp. J. Sci. Ser. 1908, A3, 187–188. [Google Scholar]
- Estudillo, R.A.; Gonzales, C.L. Red Tides and Paralytic Shellfish Poisoning in the Philippines. In Toxic Red Tides and Shellfish Toxicity in Southeast Asia; White, A.W., Anraku, M., Hooi, K.K., Eds.; Asian Fisheries Development Research Center: Singapore, 1984; pp. 52–79. [Google Scholar]
- Azanza, R.V. When the Blue Sea Turns Red; The Marine Science Institute, University of the Philippines Diliman: Quezon City, Philippines, 2017; 64p. [Google Scholar]
- Bajarias, F.F.A.; Relox, J.R., Jr.; Fukuyo, Y. PSP in the Philippines: Three decades of monitoring disaster. Coast. Mar. Sci. 2006, 30, 104–106. [Google Scholar]
- Ravelo, S.F.; Yap-Dejeto, L.G.; Silaras, M.L.S.; Amparado, M.L.L.; Ocampo, J.A.; Abria, E.G.; Albina, M.B. A Snapshot on the Distribution of Coastal Phytoplankton Communities in Five HAB-Affected Bays in Eastern Visayas, Philippines. Front. Mar. Sci. 2022, 9, 1–14. [Google Scholar] [CrossRef]
- Azanza, R.V.; Benico, G.A.; Iwataki, M.; Fukuyo, Y. Harmful Marine Dinoflagellates in the Philippines; The Marine Science Institute, University of the Philippines Diliman: Quezon City, Philippines, 2017; 96p. [Google Scholar]
- Azanza, R.V.; Benico, G.A. Toxic Alexandrium blooms in fish farming sites in Bolinao, Pangasinan. J. Environ. Sci. Manag. 2013, 1, 44–49. [Google Scholar]
- Subong, B.; Lluisma, A.; Azanza, R.; Miranda, L. Differentiating two closely related Alexandrium species using comparative quantitative proteomics. Toxin 2020, 13, 7. [Google Scholar] [CrossRef]
- Yñiguez, Y.; Lim, P.; Leaw, C.; Jipanin, S.; Iwataki, M.; Benico, G.; Azanza, R. Over 30 years of HABs in the Philippines and Malaysia: What have we learned? Harmful Algae 2021, 102, 101776. [Google Scholar] [CrossRef]
- Gobler, J. Climate Change and Harmful Algal Blooms: Insights and Perspectives. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding Harmful Algal Blooms: Paradigm Shifts and New Techniques for Research, Monitoring and Management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Anderson, D.M.; Gentien, P.; Granéli, E.; Sellner, K.G. The Global, Complex Phenomena of Harmful Algal Blooms. Oceanography 2005, 18, 131–141. [Google Scholar] [CrossRef]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of Climate Variability and Future Climate Change on Harmful Algal Blooms and Human Health. In Environmental Health; BioMed Central: London, UK, 2008; Volume 7. [Google Scholar]
- Hallegraeff, G.M. Ocean Climate Change, Phytoplankton Community Responses, and Harmful Algal Blooms: A Formidable Predictive Challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Azanza, R.; Taylor, J. Are Pyrodinium Blooms in the Southeast East Asian Region Recurring and Spreading? A View at the End of the Millennium. AMBIO A J. Hum. Environ. 2001, 30, 356–364. [Google Scholar] [CrossRef]
- IOC/WESTPAC-HAB Project. IOC/WESTPAC-HAB PROJECT Brochure: Harmful Algal Blooms in the Western Pacific; IOC Sub-Commission for the Western Pacific-Harmful Algal Bloom Programme: Bangkok, Thailand, 2017. [Google Scholar]
- IOC/WESTPAC-HAB Project. Current Status and Future Perspectives of HAB Science. 2017. Available online: http://iocwestpac.org/harmful-algal-bloom/67.html (accessed on 5 April 2017).
- Maclean, J.L. Indo-Pacific red tide occurrences, 1972–1984. In Toxic Red Tides and Shellfish Toxicity in Southeast Asia; White, A.W., Anraku, M., Hooi, K.K., Eds.; Southeast Asian Fisheries Development Center: Bangkok, Thailand; Singapore and International Development Research Centre: Ottawa, ON, USA, 1984; pp. 92–98. [Google Scholar]
- Usup, G.; Ahmad, A.; Matsuoka, K.; Lim, P.T.; Leaw, C.P. Biology, Ecology and Bloom Dynamics of the Toxic Marine Dinoflagellate Pyrodinium bahamense. Harmful Algae 2012, 14, 301–312. [Google Scholar] [CrossRef]
- BFAR (Bureau of Fisheries and Aquatic Resources). Philippine Fisheries Profile; BFAR: Quezon City, Philippines, 2015. [Google Scholar]
- BFAR (Bureau of Fisheries and Aquatic Resources). “Shellfish Bulletin”. 2016. Available online: https://www.bfar.da.gov.ph/red-tide-archives/ (accessed on 27 November 2023).
- Jipanin, S.J.; Muhamad Shaleh, S.R.; Lim, P.T.; Leaw, C.P.; Mustapha, S. The monitoring of harmful algae blooms in Sabah, Malaysia. J. Phys. Conf. Ser. 2019, 1358, 012114. [Google Scholar] [CrossRef]
- Lim, P.T.; Leaw, C.P.; Ogata, T. Morphological variation of two Alexandrium species responsible for paralytic shellfish poisoning in Southeast Asia. Bot. Mar. 2007, 50, 14–21. [Google Scholar] [CrossRef]
- Leaw, C.P.; Lim, P.T.; Ng, B.K.; Cheah, M.Y.; Ahmad, A.; Usup, G. Phylogenetic analysis of Alexandrium species and Pyrodinium bahamense (Dinophyceae) based on theca morphology and nuclear ribosomal gene sequence. Phycologia 2005, 44, 550–565. [Google Scholar] [CrossRef]
- Lim, H.C.; Lim, P.T.; Su, S.N.P.; Kotaki, Y.; Leaw, C.P. Morphological Observation of Two Species of Pseudo-Nitzschia (Bacillariophyceae). Coast. Mar. Sci. 2012, 35, 52–57. [Google Scholar]
- Usup, G.; Leaw, C.P.; Ahmad, A.; Lim, P.T. Alexandrium (Dinophyceae) species in Malaysian waters. Harmful Algae 2002, 1, 265–275. [Google Scholar] [CrossRef]
- Usup, G.; Leaw, C.P.; Lim, P.T.; Ahmad, A. Probable toxin producer responsible for the first occurrence of paralytic shellfish poisoning on the east coast of Peninsula Malaysia. Malays. Appl. Biol. 2002, 31, 29–35. [Google Scholar]
- Liow, G.R.; Lau, W.L.S.; Law, I.K.; Mohammad Noor, N.; Leaw, C.P.; Lim, P.T. Phytoplankton community changes in Kuantan Port (Malaysia), with emphasis on the paralytic-shellfish toxin-producing dinoflagellate Alexandrium tamiyavanichii. Reg. Stud. Mar. Sci. 2019, 26, 100504. [Google Scholar] [CrossRef]
- Mohammad-Noor, N.; Adam, A.; Lim, P.T.; Leaw, C.P.; Lau, W.L.S.; Liow, G.R.; Mohammad-Bunnori, N.; Hamdan, N.-A.; Md-Noor, A.; Kemat, N.; et al. First report of paralytic shellfish poisoning (PSP) caused by Alexandrium tamiyavanichii in Kuantan port, Pahang, east coast of Malaysia. Phycol. Res. 2018, 66, 37–44. [Google Scholar] [CrossRef]
- Lim, P.T.; Leaw, C.P.; Usup, G.; Kobiyama, A.; Koike, K.; Ogata, T. Effects of light and temperature on growth, nitrate uptake, and toxin production of two tropical dinoflagellates: Alexandrium tamiyavanichii and Alexandrium minutum (Dinophyceae). J. Phycol. 2006, 42, 786–799. [Google Scholar] [CrossRef]
- Kon, N.F.; Lau, W.L.S.; Law, I.K.; Lim, P.T.; Leaw, C.P. On-site rapid detection of toxic Alexandrium tamiyavanichii: Integrating the species-specific hydrolysis probe in insulated isothermal polymerase chain reaction (iiPCR). J. Appl. Phycol. 2016, 28, 2815–2820. [Google Scholar] [CrossRef]
- Kon, N.F.; Teng, S.T.; Hii, K.S.; Yek, L.H.; Aazani, M.; Lim, H.C.; Lim, P.T.; Leaw, C.P. Spatial distribution of toxic Alexandrium tamiyavanichii (Dinophyceae) in the southeastern South China Sea-Sulu Sea: A molecular-based assessment using real-time quantitative PCR (qPCR) assay. Harmful Algae 2015, 50, 8–20. [Google Scholar] [CrossRef]
- Liu, M.; Krock, B.; Yu, R.; Leaw, C.P.; Lim, P.T.; Ding, G.; Wang, N.; Zheng, J.; Gu, H. Co-occurrence of Alexandrium minutum (Dinophyceae) ribotypes from the Chinese and Malaysian coastal waters and their toxin production. Harmful Algae 2022, 115, 102238. [Google Scholar] [CrossRef]
- Hii, K.S.; Lim, P.T.; Kon, N.F.; Takata, Y.; Usup, G.; Leaw, C.P. Physiological and transcriptional responses to inorganic nutrition in a tropical Pacific strain of Alexandrium minutum: Implications for the saxitoxin genes and toxin production. Harmful Algae 2016, 56, 921. [Google Scholar] [CrossRef] [PubMed]
- Hii, K.S.; Lim, P.T.; Kon, N.F.; Usup, G.; Gu, H.; Leaw, C.P. Transcriptional and physiological responses to inorganic nutrition in a tropical Pacific strain of Alexandrium minutum: Implications for nutrient uptakes and assimilation. Gene 2019, 711, 143950. [Google Scholar] [CrossRef]
- Lau, W.L.S.; Law, I.K.; Liow, G.R.; Hii, K.S.; Usup, G.; Lim, P.T.; Leaw, C.P. Life-history stages of natural bloom populations and the bloom dynamics of a tropical Asian ribotype of Alexandrium minutum. Harmful Algae 2017, 70, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J. The changing face of ciguatera. Toxicon 2001, 39, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Tester, P.A.; Litaker, R.W.; Berdalet, E. Climate change and harmful benthic microalgae. Harmful Algae 2020, 91, 101655. [Google Scholar] [CrossRef] [PubMed]
- Chinain, M.; Gatti, C.M.I.; Darius, H.T.; Quod, J.P.; Tester, P.A. Ciguatera poisonings: A global review of occurrences and trends. Harmful Algae 2021, 102, 101873. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y.K. Ciguatera Fish Poisoning in East Asia and Southeast Asia. Mar. Drugs 2015, 13, 3466–3478. [Google Scholar] [CrossRef] [PubMed]
- Yogi, K.; Oshiro, N.; Inafuku, Y.; Hirama, M.; Yasumoto, T. Detailed LC-MS/MS Analysis of Ciguatoxins Revealing Distinct Regional and Species Characteristics in Fish and Causative Alga from the Pacific. Anal. Chem. 2011, 83, 8886–8891. [Google Scholar] [CrossRef] [PubMed]
- Montojo, U.; Tanyag, B.E.; Rerelonia, K.B.S.; Cambia, F.D.; Oshiro, N. Ciguatera in the Philippines: Examining reef fish vectors and its causative benthic dinoflagellates in Visayan and Sibuyan Seas. Philipp. J. Fish. 2020, 27, 19–29. [Google Scholar] [CrossRef]
- Malto, Z.B.L.; Benico, G.A.; Batucan, J.D.; Dela Cruz, J.; Romero, M.L.J.; Azanza, R.V.; Salvador-Reyes, L.A. Global Mass Spectrometric Analysis Reveals Chemical Diversity of Secondary Metabolites and 44-Methylgambierone Production in Philippine Gambierdiscus Strains. Front. Mar. Sci. 2022, 8, 1–17. [Google Scholar] [CrossRef]
- Vacarizas, J.; Benico, G.; Austero, N.; Azanza, R. Taxonomy and toxin production of Gambierdiscus carpenteri (Dinophyceae) in a tropical marine ecosystem: The first record from the Philippines. Mar. Pollut. Bull. 2018, 137, 430–443. [Google Scholar] [CrossRef]
- Mendoza, C.O.; Rabanes, A.C.; Jimenez, E.C.; Azanza, R.V.; Cortez-Akhunzadah, J.; Cruz, L.J. Detection of ciguatera fish poisoning in the Philippines. J. Environ. Sci. Manag. 2013, 16, 50–55. [Google Scholar]
- Tsumuraya, T.; Hirama, M. Rationally Designed Synthetic Haptens to Generate Anti-Ciguatoxin Monoclonal Antibodies, and Development of a Practical Sandwich ELISA to Detect Ciguatoxins. Toxins 2019, 11, 533. [Google Scholar] [CrossRef] [PubMed]
- Nik Khairol Reza, B.M.; Wan Mansor, B.H.; Anita, B.S.; Fauziah, B.M.; Mat Ghani, B.M.; Sahari, B.C. Ciguatera poisoning after imported red snapper fish ingestion in Jeli, Kelantan, Malaysia, 8–10 September 2010. In Proceedings of the 7th Kelantan Health Conference, Kota Bharu, Kelantan, Malaysia, 15–16 June 2011. [Google Scholar]
- Lee, H.; Leaw, C.P.; Lim, P.T.; Jipanin, S. Ciguatera fish poisoning: First reported case in Sabah, Malaysia. Med. J. Malaysia 2019, 74, 545–546. [Google Scholar] [PubMed]
- Dao, H.V.; Le, H.H.K.; Le, T.T.T.; Pham, K.X.; Bui, M.Q.; Chan, L.L. Ciguatoxin in Moray Eels Raising the Risk for Seafood Safety in Viet Nam. Fish. Sci. 2022, 88, 821–830. [Google Scholar] [CrossRef]
- Sadovy, Y. Ciguatera hits Hong Kong live reef fish trade. SPC Fish. Newslett. 1997, 83, 26–28. [Google Scholar]
- Sadovy, Y. Ciguatera hits Hong Kong live food-fish trade. SPC Live Reef Fish. Inf. Bull. 1998, 4, 51–53. [Google Scholar]
- Dai, X.; Mak, Y.L.; Lu, C.-K.; Mei, H.-H.; Wu, J.J.; Lee, W.H.; Chan, L.L.; Lim, P.T.; Mustapa, N.I.; Lim, H.C.; et al. Taxonomic assignment of the benthic toxigenic dinoflagellate Gambierdiscus sp. type 6 as Gambierdiscus balechii (Dinophyceae), including its distribution and ciguatoxicity. Harmful Algae 2017, 67, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.K.; Lim, Z.F.; Gu, H.; Chan, L.L.; Litaker, R.W.; Tester, P.A.; Leaw, C.P.; Lim, P.T. Effects of substratum and depth on benthic harmful dinoflagellate assemblages. Sci. Rep. 2020, 10, 11251. [Google Scholar] [CrossRef] [PubMed]
- Mustapa, N.I.; Yong, H.L.; Lee, L.K.; Lim, Z.F.; Lim, H.C.; Teng, S.T.; Luo, Z.; Gu, H.; Leaw, C.P.; Lim, P.T. Growth and epiphytic behavior of three Gambierdiscus species (Dinophyceae) associated with various macroalgal substrates. Harmful Algae 2019, 89, 101671. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lee, W.H.; Yip, K.C.; Wu, Z.; Wu, J.J.; Leaw, C.P.; Lim, P.T.; Lu, C.K.; Chan, L.L. Regional comparison on ciguatoxicity, hemolytic activity, and toxin profile of the dinoflagellate Gambierdiscus from Kiribati and Malaysia. Sci. Total Environ. 2023, 873, 162236. [Google Scholar] [CrossRef]
- Yong, H.L.; Mustapa, N.I.; Lee, L.K.; Lim, Z.F.; Tan, T.H.; Usup, G.; Gu, H.; Litaker, R.W.; Tester, P.A.; Lim, P.T.; et al. Habitat complexity affects benthic harmful dinoflagellate assemblages in the fringing reef of Rawa Island, Malaysia. Harmful Algae 2018, 78, 56–68. [Google Scholar] [CrossRef]
- Tester, P.A.; Litaker, R.W.; Soler-Onís, E.; Fernández-Zabala, J.; Berdalet, E. Using Artificial Substrates to Quantify Gambierdiscus and Other Toxic Benthic Dinoflagellates for Monitoring Purposes. Harmful Algae 2022, 120, 102351. [Google Scholar] [CrossRef]
- Tester, P.A.; Wicklaffe, L.; Jossart, J.; Rhodes, L.L.; Enevoldsen, H.; Adachi, M.; Nishimura, T.; Rodriguez, F.; Chinain, M.; Litaker, R.W. Global distribution of the genera Gambierdiscus and Fukuyoa. In Proceedings of the 18th International Conference on Harmful Algae, Nantes, France, 21–26 October 2018; ISSHA: Nantes, France, 2020. Available online: https://ipt.iobis.org/hab/resource?r=hab-gambierdiscus-fukuyoa (accessed on 27 November 2023).
- Tester, P.A.; Kibler, S.R.; Holland, W.C.; Usup, G.; Vandersea, M.W.; Leaw, C.P.; Lim, P.T.; Larsen, J.; Mohammad-Noor, N.; Faust, M.A.; et al. Sampling harmful benthic dinoflagellates: Comparison of artificial and natural substrate methods. Harmful Algae 2014, 39, 8–25. [Google Scholar] [CrossRef]
- Samonte, G.P.B.; Siar, S.; Ortega, R.; Espada, L. Socio-economics study of oyster (Crassostrea iredalei) and mussel (Perna viridis) farming in Western Visayas, Philippines; AFSSRN-SEAFDEC-AQD Team, Southeast Asian Fisheries Department Center, Aquaculture Department: Iloilo, Philippines, 1992. [Google Scholar]
- PhilHABs. Philippine Harmful Algal Blooms Program: Ecology and Oceanography of Harmful Algal Blooms; PhilHAB Terminal Report; The Marine Science Institute, University of the Philippines: Quezon City, Philippines, 2013. [Google Scholar]
- Furio, E.F.; Azanza, R.V.; Fukuyo, Y.; Matsuoka, K. Review of Geographical distribution of dinoflagellate cysts in Southeast Asian coasts. Coast. Mar. Sci. 2012, 35, 20–33. [Google Scholar]
- Azanza, R.V.; Brosnahan, M.L.; Anderson, D.M.; Hense, I.; Montresor, M. The Role of Life Cycle Characteristics in Harmful Algal Bloom Dynamics. In Global Ecology and Oceanography of Harmful Algal Blooms; Glibert, P., Berdale, E., Burford, M., Pitcher, G., Zhou, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 133–161. [Google Scholar]
- Onda, D.F.; Lluisma, A.; Azanza, R. Development, Morphological Characteristics and Viability of Temporary Cysts of Pyrodinium bahamense var. compressum (Dinophyceae) in Vitro. Eur. J. Phycol. 2014, 49, 265–275. [Google Scholar] [CrossRef]
- Cuellar-Martinez, T.; Morquecho, L.; Alonso-Rodríguez, R.; Ruiz-Fernández, A.C.; Sanchez-Cabeza, J.A. Germination of Pyrodinium bahamense Cysts from a Pristine Lagoon in San José Island, Gulf of California: Implications of Long-Term Survival. Phycology 2023, 3, 65–78. [Google Scholar] [CrossRef]
- Zonneveld, K.; Grotheer, H.; Versteegh, G. Dinoflagellate Cyst Production, Excystment and Transport in Upwelling off Cape Blanc (NW Africa). Front. Mar. Sci. 2022, 9, 915755. [Google Scholar] [CrossRef]
- Masó, M.; Garcés, E.; Pagès, F.; Camp, J. Drifting plastic debris as a potential vector for dispersing Harmful Algal Bloom (HAB) species. Sci. Mar. 2003, 67, 107–111. [Google Scholar] [CrossRef]
- Aleynik, D.; Dale, A.C.; Porter, M.; Davidson, K. A High Resolution Hydrodynamic Model System Suitable for Novel Harmful Algal Bloom Modelling in Areas of Complex Coastline and Topography. Harmful Algae 2016, 53, 102–117. [Google Scholar] [CrossRef]
- Brosnahan, M.L.; Fischer, A.D.; Lopez, C.B.; Moore, S.K.; Anderson, D.M. Cyst-Forming Dinoflagellates in a Warming Climate. Harmful Algae 2020, 91, 101728. [Google Scholar] [CrossRef]
- Anderson, D.M.; Keafer, B.A.; Kleindinst, J.L.; McGillicuddy, D.J.; Martin, J.L.; Norton, K.; Pilskaln, C.H.; Smith, J.L.; Sherwood, C.R.; Butman, B. Alexandrium fundyense Cysts in the Gulf of Maine: Long-Term Time Series of Abundance and Distribution, and Linkages to Past and Future Blooms. Deep. Res. Part II Top. Stud. Oceanogr. 2014, 103, 6–26. [Google Scholar] [CrossRef]
- Stock, C.A.; McGillicuddy, D.J.; Solow, A.R.; Anderson, D.M. Evaluating Hypotheses for the Initiation and Development of Alexandrium Fundyense Blooms in the Western Gulf of Maine Using a Coupled Physical-Biological Model. Deep Res. Part II Top. Stud. Oceanogr. 2005, 52, 2715–2744. [Google Scholar] [CrossRef]
- Anderson, D.M. Toxic algal blooms and red tides: A global perspective. In Red Tides: Biology, Environmental Science and Toxicology; Okaichi, T., Anderson, D.M., Nemoto, T., Eds.; Elsevier: New York, NY, USA, 1989; pp. 11–16. [Google Scholar]
- Borja, V.M.; Furio, E.F.; Gatdula, N.C.; Iwataki, M. Occurrence of harmful algal blooms caused by various phytoplankton species for the last three decades in Manila Bay, Philippines. Philipp. J. Nat. Sci. 2019, 24, 80–90. [Google Scholar]
- Rachman, A.; Thoha, H.; Sianturi, O.R.; Bayu, M.D.; Fitriya, N.; Sidabutar, T.; Witasari, Y.; Adi Wibowo, S.P.; Iwataki, M. Distribution of Pyrodinium bahamense cysts in modern sediments of Sukalila water, Cirebon, Indonesia. Philipp. J. Sci. 2019, 24, 104–115. [Google Scholar]
- Villanoy, C.L.; Azanza, R.V.; Altemerano, A.; Casil, A.L. Attempts to model the bloom dynamics of Pyrodinium, a tropical toxic dinoflagellate. Harmful Algae 2006, 5, 156–183. [Google Scholar] [CrossRef]
- Yñiguez, A.T.; Maister, J.; Villanoy, C.L.; Deauna, J.D.; Peñaflor, E.; Almo, A.; David, L.T.; Benico, G.A.; Hibay, E.; Mora, I.; et al. Insights into the Dynamics of Harmful Algal Blooms in a Tropical Estuary through an Integrated Hydrodynamic-Pyrodinium-Shellfish Model. Harmful Algae 2018, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lumayno, S.D.P.; Benico, G.A.; Yñiguez, A.T.; Alabia, I.D.; Fernandez, I.Q.D.G.; Dianala, R.D.B.; Azanza, R.V.; Villanoy, C.L. Residence Time Models and Pyrodinium Blooms in Matarinao and Murcielagos Bays, Philippines. Philipp. J. Sci. 2022, 151, 79–90. [Google Scholar] [CrossRef]
- Punongbayan, A.T.; Wang, Y.D.; Villanoy, C.L.; Yñiguez, A.T. Connections and Clustering of Paralytic Shellfish Toxin Events among Coastal Embayments in an Archipelago Partly Mediated by Advection. Harmful Algae 2022, 111, 102147. [Google Scholar] [CrossRef]
- Jacinto, G.S.; Velasquez, I.B.; San Diego-McGlone, M.L.; Villanoy, C.L.; Siringan, F.B. Biophysical environment of Manila Bay—Then and now. In The Environment in Asia Pacific Harbours; Wolanski, E., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 293–307. [Google Scholar]
- David, L.T.; Pastor-Rengel, D.; Talaue-McManus, L.; Magdaong, E.; Salalila-Aruelo, R.; Bangi, H.G.; San Diego-McGlone, M.L.; Villanoy, C.; Cordero-Bailey, K. The saga of community learning: Mariculture and the Bolinao experience. Aquat. Ecosyst. Health Manag. 2014, 17, 196–204. [Google Scholar] [CrossRef]
- Escobar, M.T.L.; Sotto, L.P.A.; Jacinto, G.S.; Benico, G.A.; Azanza, R.V. Eutrophic conditions during the 2010 fish kill in Bolinao and Anda, Pangasinan, Philippines. J. Environ. Sci. Manag. 2013, 1, 29–35. [Google Scholar]
- Ferrera, C.M.; Watanabe, A.; Miyajima, T.; San Diego-McGlone, M.L.; Morimoto, N.; Umezawa, Y.; Herrera, E.; Tsuchiya, T.; Yoshikai, M.; Nadaoka, K. Phosphorus as a driver of nitrogen limitation and sustained eutrophic conditions in Bolinao and Anda, Philippines, a mariculture-impacted tropical coastal area. Mar. Pollut. Bull. 2016, 105, 237–248. [Google Scholar] [CrossRef]
- San Diego-McGlone, M.L.; Azanza, R.V.; Villanoy, C.L.; Jacinto, G.S. Eutrophic waters, algal bloom and fish kill in fish farming areas in Bolinao, Pangasinan, Philippines. Mar. Pollut. Bull. 2008, 57, 295–301. [Google Scholar] [CrossRef]
- Albelda, R.; Purganan, D.J.; Gomez, N.C.; Narvarte, B.C.; Calalang, P.C.; Genovia, T.G.; Gernato, E.G.; Bondoc, K.G.; San Diego-McGlone, M.L.; Onda, D.F. Summer phytoplankton community structure and distribution in a mariculture-affected coastal environment. Phil. Sci. Lett. 2019, 2, 157–166. [Google Scholar]
- Dela Cruz, M.A.M.; Hingpit, B.W.; Guillot, L.; Onda, D.F.L. Effects of monsoon and disturbances on the temporal variability microbial picoeukaryotes in a tropical coastal ecosystem. Deep Sea Res. Part II 2023, 209, 105294. [Google Scholar] [CrossRef]
- Dela Peña, L.B.R.O.; Tejada, A.J.P.; Quijano, J.B.; Alonzo, K.H.; Gernato, E.G.; Caril, A.; dela Cruz, M.A.M.; Onda, D.F.L. Diversity of marine eukaryotic picophytoplankton communities with emphasis on mamiellophyceae in northwestern Philippines. Philipp. J. Sci. 2021, 150, 27–42. [Google Scholar] [CrossRef]
- Meijer, L.J.; Van Emmerik, T.; Van Der Ent, R.; Schmidt, C.; Lebreton, L. More than 1000 rivers account for 80% of global riverine plastic emissions into the ocean. Sci. Adv. 2021, 7, eaaz5803. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Reisser, J.; Shaw, J.; Hallegraeff, G.; Proietti, M.; Barnes, D.K.; Thums, M.; Wilcox, C.; Hardesty, B.D.; Pattiaratchi, C. Millimeter-sized marine plastics: A new pelagic habitat for microorganisms and invertebrates. PLoS ONE 2014, 9, e100289. [Google Scholar] [CrossRef]
- Pasqualini, V.; Garrido, M.; Cecchi, P.; Connès, C.; Couté, A.; El Rakwe, M.; Henry, M.; Hervio-Heath, D.; Quilichini, Y.; Simonnet, J.; et al. Harmful Algae and Pathogens on Plastics in Three Mediterranean Coastal Lagoons. Heliyon 2023, 9, e13654. [Google Scholar] [CrossRef]
- Onda, D.F.L.; Gomez, N.C.F.; Purganan, D.J.E.; Tolentino, M.P.S.; Bitalac, J.M.S.; Calpito, J.V.M.; Perez, J.N.O.; Viernes, A.C.A. Marine Microbes and Plastic Debris: Research Status and Opportunities in the Philippines. Philipp. J. Sci. 2020, 149, 71–82. [Google Scholar] [CrossRef]
- Liberto, T. ENSO and Climate Change: What Does the New IPCC Report Say? Available online: https://www.climate.gov/news-features/blogs/enso/enso-and-climate-change-what-does-new-ipcc-report-say (accessed on 7 November 2023).
- Hilario, F.D.; de Guzman, R.; Ortega, D.; Haymena, P.; Alexander, D. El Niño Southern Oscillation in the Philippines: Impacts, forecasts and risk management. Phil. J. Dev. 2009, 36, 9–31. [Google Scholar]
- Juneng, L.; Tangang, F.T. Evolution of ENSO-related rainfall anomalies in Southeast Asia region and its relationship with atmosphere–ocean variations in Indo-Pacific sector. Clim. Dynam. 2005, 25, 337–350. [Google Scholar] [CrossRef]
- Lyon, B.; Camargo, S.J. The seasonally-varying influence of ENSO on rainfall and tropical cyclone activity in the Philippines. Clim. Dyn. 2009, 32, 125–141. [Google Scholar] [CrossRef]
- Azanza, R.V. Harmful Algal Blooms in Tropical Embayments Affected by Monsoons. In Global Ecology and Oceanography of Harmful Algal Blooms, GEOHAB Core Research Project: HABs in Fjords and Coastal Embayments; Cembella, A., Guzmán, L., Roy, S., Diogène, J., Eds.; IOC and SCOR: Paris, France; Newark, DE, USA, 2013; pp. 20–23. [Google Scholar]
- Tangang, F.; Farzanmanesh, M.; Ali, S.; Salimun, E.; Jamaluddin, A.F.; Liew, J. Characteristics of precipitation extremes in Malaysia associated with El Niño and La Niña events. Int. J. Climatol. 2017, 37, 696–716. [Google Scholar] [CrossRef]
- Hii, K.S.; Mohd-Din, M.; Luo, Z.; Tan, S.N.; Lim, Z.F.; Lee, L.K.; Leong, S.C.Y.; Teng, S.T.; Gu, H.; Cao, X.; et al. Diverse harmful microalgal community assemblages in the Johor Strait and the environmental effects on its community dynamics. Harmful Algae 2021, 107, 102077. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.C.; Leaw, C.P.; Tan, T.H.; Kon, N.F.; Yek, L.H.; Hii, K.S.; Teng, S.T.; Razali, R.M.; Usup, G.; Iwataki, M.; et al. A Bloom of Karlodinium australe (Gymnodiniales, Dinophyceae) Associated with Mass Mortality of Cage-Cultured Fishes in West Johor Strait, Malaysia. Harmful Algae 2014, 40, 51–62. [Google Scholar] [CrossRef]
- Mohd-Din, M.; Hii, K.S.; Abdul-Wahab, M.F.; Mohamad, S.E.; Gu, H.; Leaw, C.P.; Lim, P.T. Spatial–temporal variability of microphytoplankton assemblages including harmful microalgae in a tropical semi-enclosed strait (Johor Strait, Malaysia). Mar. Environ. Res. 2022, 175, 105589. [Google Scholar] [CrossRef]
- Law, I.K.; Hii, K.S.; Lau, W.L.S.; Leaw, C.P.; Lim, P.T. Coastal micro-phytoplankton community changes during the toxigenic Alexandrium minutum blooms in a semi-enclosed tropical coastal lagoon (Malaysia, South China Sea). Harmful Algae 2023, 57, 102733. [Google Scholar] [CrossRef]
- GlobalHAB. Global Harmful Algal Blooms, Science and Implementation Plan; Berdalet, E., Banas, N., Bresnan, E., Burford, M., Davidson, K., Gobler, C., Karlson, B., Kudela, R., Lim, P.T., Montresor, M., et al., Eds.; SCOR and IOC: Newark, DE, USA; Paris, France, 2017; 64p. [Google Scholar]
- GlobalHAB. Fish-Killing Marine Algal Blooms: Causative Organisms, Ichthyotoxic Mechanisms, Impacts and Mitigation; Hallegraeff, G.M., Anderson, D.M., Davidson, K., Gianella, F., Hansen, P.J., Hegaret, H., Iwataki, M., Larsen, T.O., Mardones, J., MacKenzie, L., et al., Eds.; UNESCO-IOC/SCOR: Paris, France, 2023; 96p. [Google Scholar]
- FAO; IOC; IAEA. Joint FAO-IOC-IAEA Technical Guidance for the Implementation of Early Warning Systems for Harmful Algal Blooms; Fisheries and Aquaculture Technical Paper, No. 690; FAO: Rome, Italy, 2023. [Google Scholar]
- Omeyer, L.; Duncan, E.; Aisembom, K. Priorities to inform research on marine plastic pollution in Southeast Asia. Sci. Total Environ. 2022, 841, A1656704. [Google Scholar] [CrossRef]
- Maclean, J.A. Indo-Pacific red tides, 1985–1988. Mar. Pollut. Bull. 1989, 20, 304–310. [Google Scholar] [CrossRef]
- Aligasaki, K. Phytoplankton responses to Mediterranean Environmental Changes. In Proceedings of the CIESM Workshop Monographs, Tunis, Tunisia, 7–10 October 2009. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).