Microbial Culture Condition Optimization and Fiber Reinforcement on Microbial-Induced Carbonate Precipitation for Soil Stabilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Sandy Soil and Fiber
2.1.2. Bacteria and Cementation Medium
2.2. Experimental Design
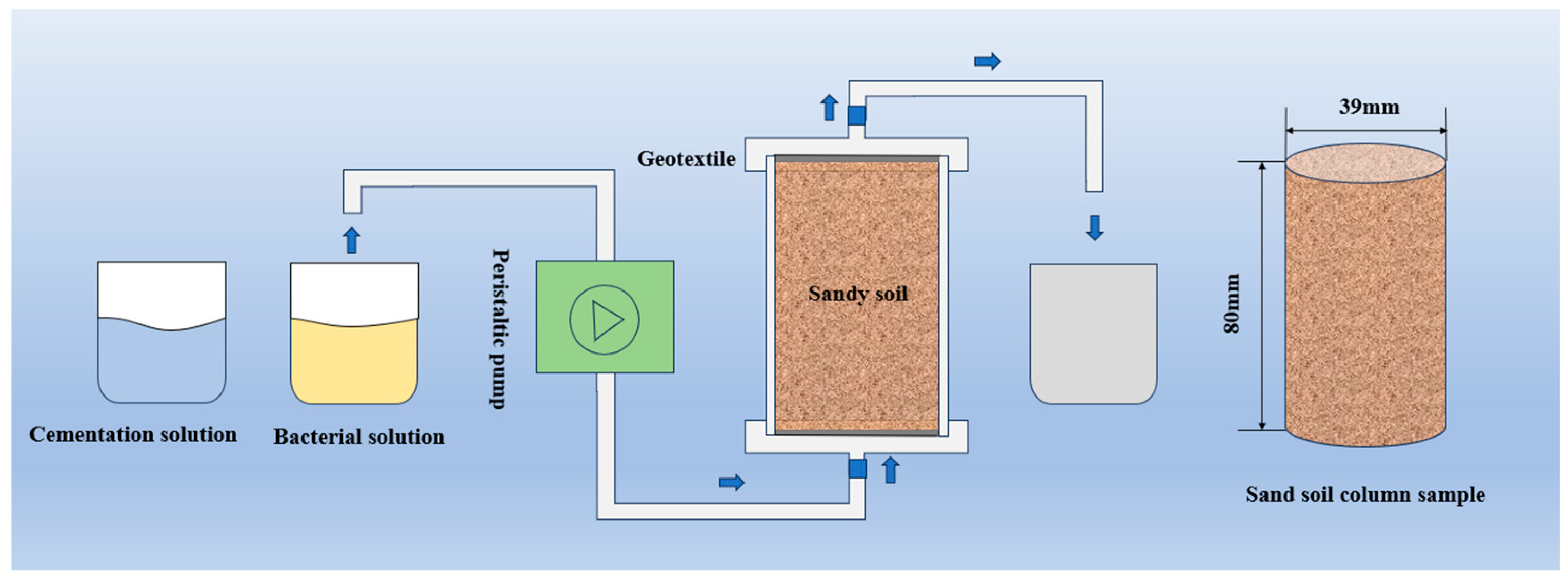
2.3. Sample Preparation
2.4. Test Methods
2.4.1. Microbial Activity Test
2.4.2. Calcium Carbonate Production Rate
2.4.3. UCS
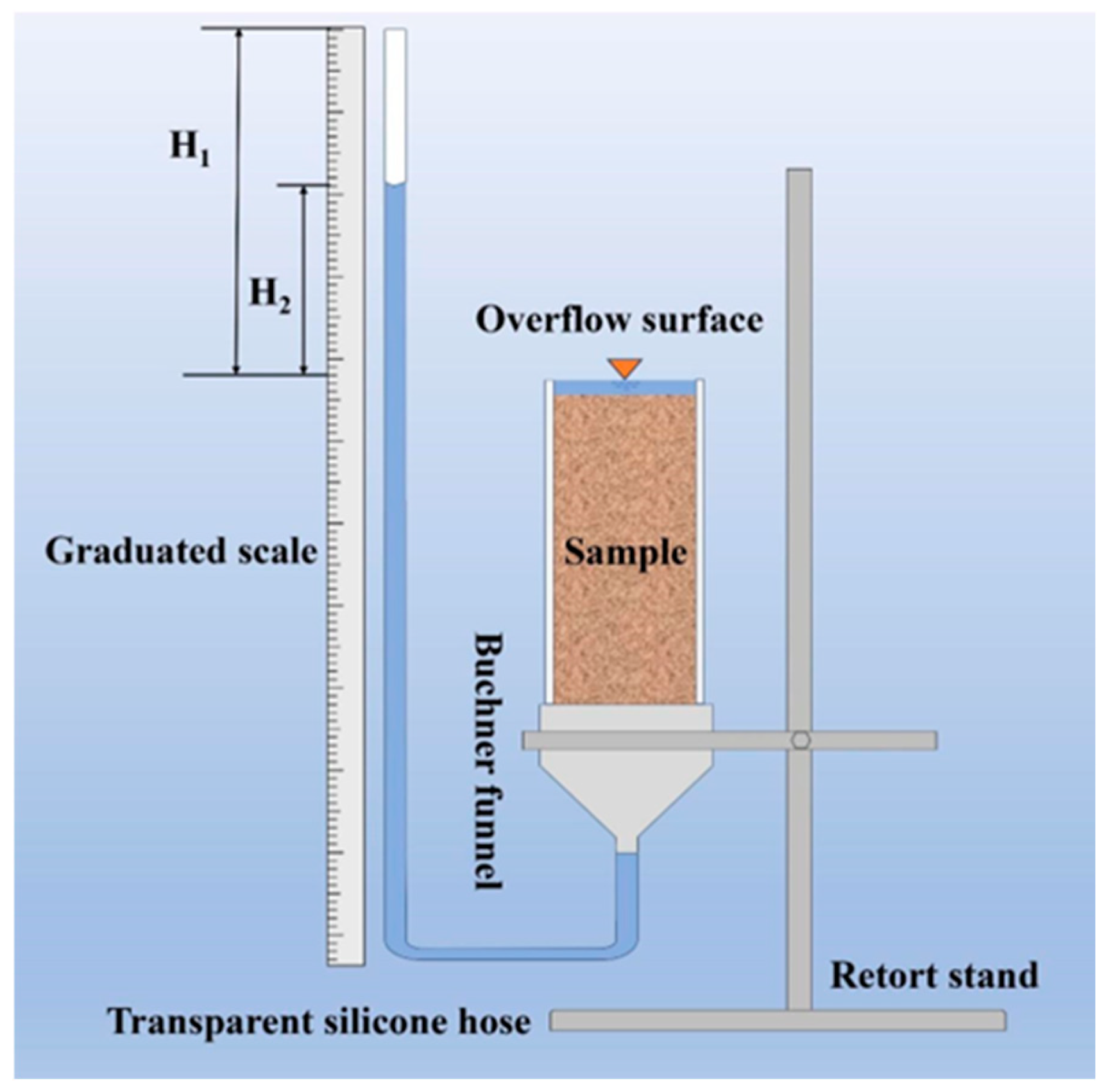
2.4.4. Permeability
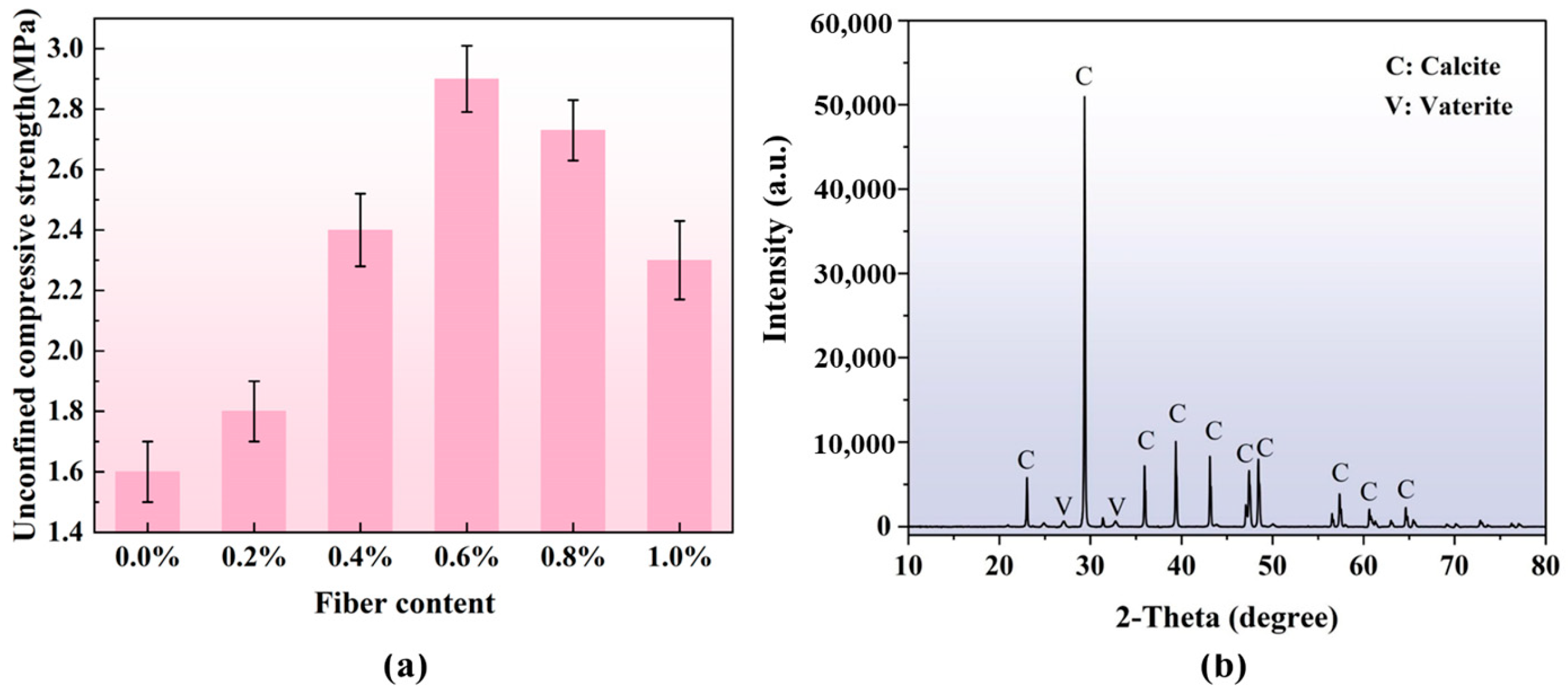
2.4.5. XRD
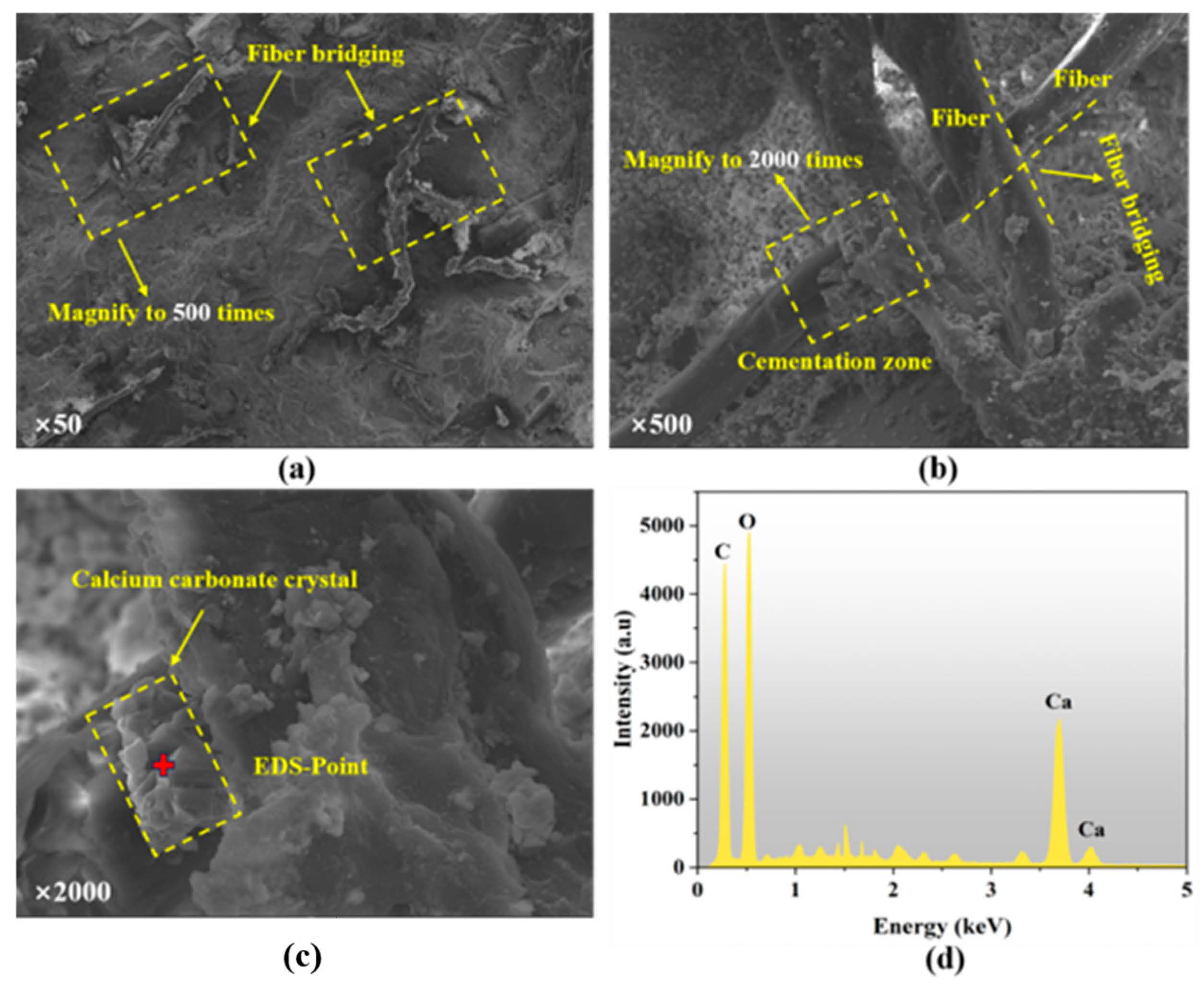
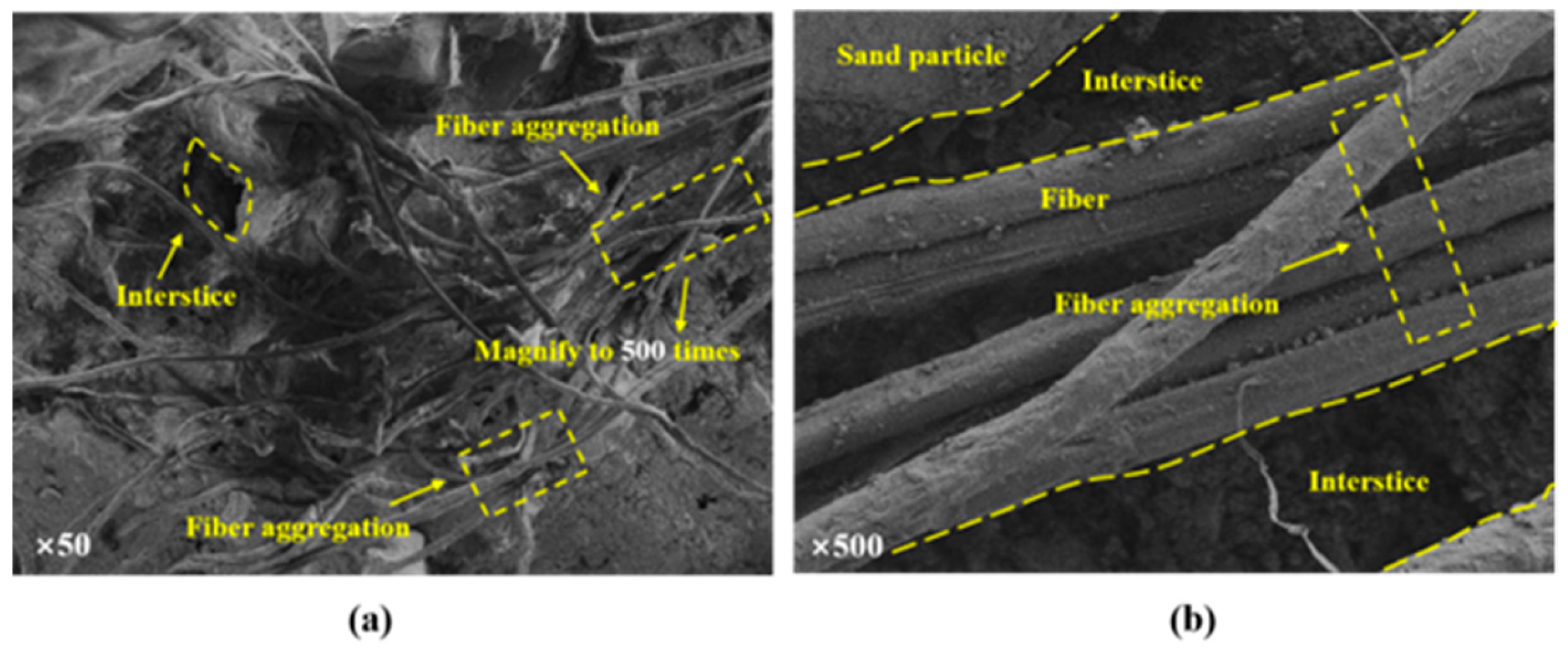
2.4.6. SEM–EDS
3. Results
3.1. Optimization of Microbial Culture Conditions
3.1.1. Influence of Urea Concentration
3.1.2. Influence of pH
3.1.3. Influence of Inoculum Size
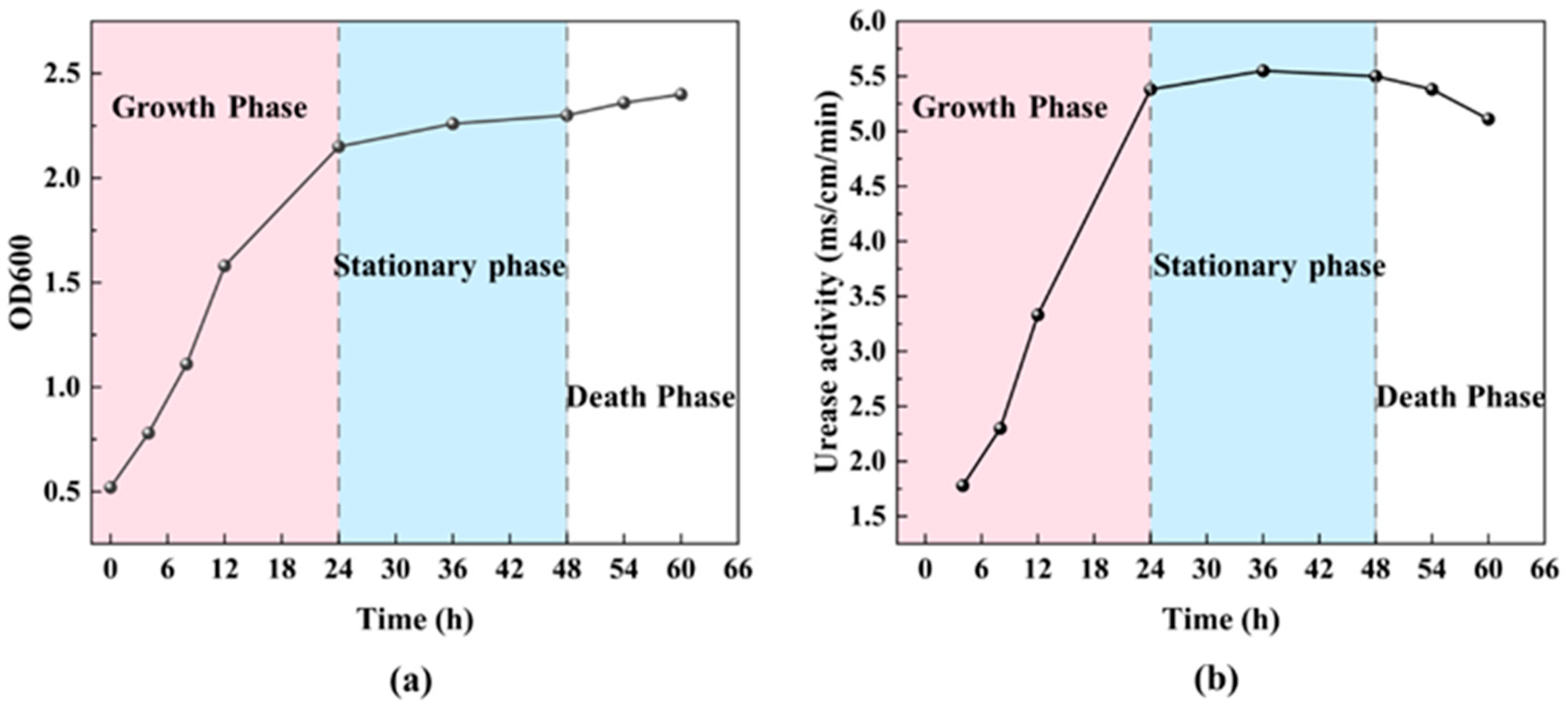
3.1.4. Growth Curve Under Optimal Conditions
3.2. Optimization of the Cementing Medium in the Mineralization Reaction
3.3. UCS Results
3.4. Permeability Results
4. Discussion
4.1. Optimization of Microbial Culture Conditions
4.1.1. Influence of Urea Concentration
4.1.2. Influence of pH
4.1.3. Influence of Inoculum Size
4.2. Optimization of the Cementing Medium in the Mineralization Reaction
4.3. UCS Results
4.4. Permeability Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, M.; Zheng, J.; Dong, J.; Miao, C. Foundation improvement with lattice-shaped diaphragm wall treatment for high embankment culverts on soft foundation. Tunn. Undergr. Space Technol. 2020, 104, 103535. [Google Scholar]
- Long, G.; Li, L.; Li, W.; Ma, K.; Dong, W.; Bai, C.; Zhou, J.L. Enhanced mechanical properties and durability of coal gangue reinforced cement-soil mixture for foundation treatments. J. Clean. Prod. 2019, 231, 468–482. [Google Scholar]
- Keybondori, S.; Abdi, E.; Deljouei, A.; Cislaghi, A.; Shakeri, Z.; Etemad, V. Soil-bioengineering to stabilize gravel roadside slopes in the steep Hyrcanian Forests of Northern Iran. Ecol. Eng. 2025, 214, 107569. [Google Scholar]
- Löbmann, M.T.; Geitner, C.; Wellstein, C.; Zerbe, S. The influence of herbaceous vegetation on slope stability—A review. Earth-Sci. Rev. 2020, 209, 103328. [Google Scholar]
- Naveed, M.; Duan, J.; Uddin, S.; Suleman, M.; Hui, Y.; Li, H. Application of microbially induced calcium carbonate precipitation with urea hydrolysis to improve the mechanical properties of soil. Ecol. Eng. 2020, 153, 105885. [Google Scholar]
- Al-Sanad, H.; Ismael, N.; Nayfeh, A. Geotechnical properties of dune sands in Kuwait. Eng. Geol. 1993, 34, 45–52. [Google Scholar]
- Fabozzi, S.; Porchia, A.; Fierro, T.; Peronace, E.; Pagliaroli, A.; Moscatelli, M. Seismic compression susceptibility in dry loose sandy and silty soil in a seismic microzonation perspective. Eng. Geol. 2020, 264, 105324. [Google Scholar]
- Elipe, M.G.; López-Querol, S. Aeolian sands: Characterization, options of improvement and possible employment in construction—The State-of-the-art. Constr. Build. Mater. 2014, 73, 728–739. [Google Scholar]
- Sánchez-Garrido, A.J.; Navarro, I.J.; Yepes, V. Evaluating the sustainability of soil improvement techniques in foundation substructures. J. Clean. Prod. 2022, 351, 131463. [Google Scholar] [CrossRef]
- Bagriacik, B. Utilization of alkali-activated construction demolition waste for sandy soil improvement with large-scale laboratory experiments. Constr. Build. Mater. 2021, 302, 124173. [Google Scholar]
- Jia, Z.; Yan, C.; Li, B.; Bao, H.; Lan, H.; Liang, Z.; Shi, Y.; Ren, J. Performance test and effect evaluation of guar gum-stabilized loess as a sustainable slope protection material. J. Clean. Prod. 2023, 408, 137085. [Google Scholar] [CrossRef]
- Almajed, A.; Lateef, M.A.; Moghal, A.A.B.; Lemboye, K. State-of-the-art review of the applicability and challenges of microbial-induced calcite precipitation (MICP) and enzyme-induced calcite precipitation (EICP) techniques for geotechnical and geoenvironmental applications. Crystals 2021, 11, 370. [Google Scholar] [CrossRef]
- Niu, C.; Lin, Z.; Fu, Q.; Xu, Y.; Chen, Y.; Lu, L. An eco-friendly versatile superabsorbent hydrogel based on sodium alginate and urea for soil improvement with a synchronous chemical loading strategy. Carbohydr. Polym. 2024, 327, 121676. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Wang, C.J.; Yue, W.V.; Zhang, Z.J.; Yue, Z.Q. In situ digital testing method for quality assessment of soft soil improvement with polyurethane. J. Rock Mech. Geotech. Eng. 2023, 16, 1732–1748. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, M. Review of ground improvement technical and its application in China. China Civ. Eng. J. 2016, 49, 96–115. [Google Scholar]
- Zhang, Z.; Shi, Z.; Yang, J.; Hao, B.; Hao, L.; Diao, F.; Wang, L.; Bao, Z.; Guo, W. A new strategy for evaluating the improvement effectiveness of degraded soil based on the synergy and diversity of microbial ecological function. Ecol. Indic. 2021, 120, 106917. [Google Scholar] [CrossRef]
- Tan, C.; Yu, X.; Guan, Y. A technology-driven pathway to net-zero carbon emissions for China’s cement industry. Appl. Energy 2022, 325, 119804. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Effect of curing condition on the mechanical properties of fly ash-based geopolymer concrete. Appl. Sci. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Busch, P.; Kendall, A.; Murphy, C.W.; Miller, S.A. Literature review on policies to mitigate GHG emissions for cement and concrete. Resour. Conserv. Recycl. 2022, 182, 106278. [Google Scholar] [CrossRef]
- UN Environment; Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Xu, D.; Cui, Y.; Li, H.; Yang, K.; Xu, W.; Chen, Y. On the future of Chinese cement industry. Cem. Concr. Res. 2015, 78, 2–13. [Google Scholar] [CrossRef]
- Achal, V.; Kawasaki, S.J. Biogrout: A Novel Binding Material for Soil Improvement and Concrete Repair. In Frontiers in Microbiology; Frontiers Media SA: Lausanne, Switzerland, 2016; p. 314. [Google Scholar]
- Ramos, O.; Kwon, T.-H. Development of bio-grout injection strategy and design guide using reactive transport model for field-scale soil improvement based on microbially induced calcium carbonate precipitation (MICP). Geomech. Energy Environ. 2023, 36, 100509. [Google Scholar]
- Zheng, R.; Feng, X.; Zou, W.; Wang, R.; Yang, D.; Wei, W.; Li, S.; Chen, H. Converting loess into zeolite for heavy metal polluted soil remediation based on “soil for soil-remediation” strategy. J. Hazard. Mater. 2021, 412, 125199. [Google Scholar] [PubMed]
- Payan, M.; Sangdeh, M.K.; Salimi, M.; Ranjbar, P.Z.; Arabani, M.; Hosseinpour, I. A comprehensive review on the application of microbially induced calcite precipitation (MICP) technique in soil erosion mitigation as a sustainable and environmentally friendly approach. Results Eng. 2024, 24, 103235. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Miao, L.; Wang, H.; Wu, L.; Shi, W.; Kawasaki, S. State-of-the-art review of soil erosion control by MICP and EICP techniques: Problems, applications, and prospects. Sci. Total Environ. 2024, 912, 169016. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Liu, Y.; Sun, X.D.; Zeng, W.; Xing, H.P.; Lin, J.Z.; Kang, S.B.; Yu, L. Application of microbially induced calcium carbonate precipitation (MICP) technique in concrete crack repair: A review. Constr. Build. Mater. 2024, 411, 134313. [Google Scholar]
- Vaskevicius, L.; Malunavicius, V.; Jankunec, M.; Lastauskiene, E.; Talaikis, M.; Mikoliunaite, L.; Maneikis, A.; Gudiukaite, R. Insights in MICP dynamics in urease-positive Staphylococcus sp. H6 and Sporosarcina pasteurii bacterium. Environ. Res. 2023, 234, 116588. [Google Scholar]
- Prajapati, N.K.; Agnihotri, A.K.; Basak, N. Microbial induced calcite precipitation (MICP) a sustainable technique for stabilization of soil: A review. Mater. Today Proc. 2023, 93, 357–361. [Google Scholar] [CrossRef]
- Chu, J.; Ivanov, V.; Naeimi, M.; Stabnikov, V.; Liu, H.-L. Optimization of calcium-based bioclogging and biocementation of sand. Acta Geotech. 2014, 9, 277–285. [Google Scholar]
- Jiang, N.-J.; Soga, K.; Kuo, M. Microbially induced carbonate precipitation for seepage-induced internal erosion control in sand–clay mixtures. J. Geotech. Geoenviron. Eng. 2017, 143, 04016100. [Google Scholar] [CrossRef]
- Chen, Y.; Han, Y.; Zhang, X.; Sarajpoor, S.; Zhang, S.; Yao, X. Experimental study on permeability and strength characteristics of MICP-treated calcareous sand. Biogeotechnics 2023, 1, 100034. [Google Scholar] [CrossRef]
- Shan, Y.; Zhao, J.; Tong, H.; Yuan, J.; Lei, D.; Li, Y. Effects of activated carbon on liquefaction resistance of calcareous sand treated with microbially induced calcium carbonate precipitation. Soil Dyn. Earthq. Eng. 2022, 161, 107419. [Google Scholar] [CrossRef]
- Ivanov, V.; Stabnikov, V. Construction Biotechnology: Biogeochemistry, Microbiology and Biotechnology of Construction Materials and Processes; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Dejong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; Van Paassen, L.; Al Qabany, A.; Aydilek, A.; Bang, S.; Burbank, M.; Caslake, L.F. Biogeochemical Processes and Geotechnical Applications: Progress, Opportunities and Challenges. In Proceedings of the Bio- and Chemo-Mechanical Processes in Geotechnical Engineering: Géotechnique Symposium in Print 2013, London, UK, 3 June 2013. [Google Scholar]
- Liufu, Z.; Yuan, J.; Shan, Y.; Cui, J.; Tong, H.; Zhao, J. Effect of particle size and gradation on compressive strength of MICP-treated calcareous sand. Appl. Ocean Res. 2023, 140, 103723. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Guo, Z.; Xu, Q. Durability of MICP-reinforced calcareous sand in marine environments: Laboratory and field experimental study. Biogeotechnics 2023, 1, 100018. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; He, J. A highly effective strain screened from soil and applied in cementing fine sand based on MICP-bonding technology. J. Biotechnol. 2022, 350, 55–66. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Wang, Y.; Wang, J.; Cao, J.; Zhang, G. Improved methods, properties, applications and prospects of microbial induced carbonate precipitation (MICP) treated soil: A review. Biogeotechnics 2024, 100123. [Google Scholar] [CrossRef]
- Shan, Y.; Liang, J.; Tong, H.; Yuan, J.; Zhao, J. Effect of different fibers on small-strain dynamic properties of microbially induced calcite precipitation–fiber combined reinforced calcareous sand. Constr. Build. Mater. 2022, 322, 126343. [Google Scholar] [CrossRef]
- GB/T 50145-2007; Standard for Engineering Classification of Soil. China Architecture & Building Press (CABP): Beijing, China, 2007.
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University Perth, Perth, Australia, 2004. [Google Scholar]
- Chen, L.; Song, Y.; Fang, H.; Feng, Q.; Lai, C.; Song, X. Systematic optimization of a novel, cost-effective fermentation medium of Sporosarcina pasteurii for microbially induced calcite precipitation (MICP). Constr. Build. Mater. 2022, 348, 128632. [Google Scholar]
- GB/T 50123-2019; Standard for Geotechnical Testing Method. China Planning Publishing House: Beijing, China, 2019.
- Sun, Y.; Liu, K.; Sun, D.; Jiang, N.; Xu, W.; Wang, A. Evaluation of urea hydrolysis for MICP technique applied in recycled aggregate: Concentration of urea and bacterial spores. Constr. Build. Mater. 2024, 419, 135366. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Jiang, X.; Zhao, D.; Liu, X.; Zhou, J.; He, Z.; Zheng, C.; Pan, X. Study on soil physical structure after the bioremediation of Pb pollution using microbial-induced carbonate precipitation methodology. J. Hazard. Mater. 2021, 411, 125103. [Google Scholar] [CrossRef]
- Yi, H.; Zheng, T.; Jia, Z.; Su, T.; Wang, C. Study on the influencing factors and mechanism of calcium carbonate precipitation induced by urease bacteria. J. Cryst. Growth 2021, 564, 126113. [Google Scholar] [CrossRef]
- Martinez, B.; DeJong, J.; Ginn, T.; Montoya, B.; Barkouki, T.; Hunt, C.; Tanyu, B.; Major, D. Experimental optimization of microbial-induced carbonate precipitation for soil improvement. J. Geotech. Geoenvironmental Eng. 2013, 139, 587–598. [Google Scholar] [CrossRef]
- Nemati, M.; Greene, E.A.; Voordouw, G. Permeability profile modification using bacterially formed calcium carbonate: Comparison with enzymic option. Process Biochem. 2005, 40, 925–933. [Google Scholar]
- Lauchnor, E.G.; Topp, D.; Parker, A.; Gerlach, R. Whole cell kinetics of ureolysis by Sporosarcina pasteurii. J. Appl. Microbiol. 2015, 118, 1321–1332. [Google Scholar]
- Gat, D.; Ronen, Z.; Tsesarsky, M. Long-term sustainability of microbial-induced CaCO3 precipitation in aqueous media. Chemosphere 2017, 184, 524–531. [Google Scholar]
- Mobley, H.; Island, M.D.; Hausinger, R.P. Molecular biology of microbial ureases. Microbiol. Rev. 1995, 59, 451–480. [Google Scholar]
- Erşan, Y.Ç.; Belie, N.d.; Boon, N. Microbially induced CaCO3 precipitation through denitrification: An optimization study in minimal nutrient environment. Biochem. Eng. J. 2015, 101, 108–118. [Google Scholar]
- Panikov, N.S. Kinetics of Microbial Processes: General Principles. In Encyclopedia of Soils in the Environment, 2nd ed.; Goss, M.J., Oliver, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 168–185. [Google Scholar]
- Fu, T.; Saracho, A.C.; Haigh, S.K. Microbially induced carbonate precipitation (MICP) for soil strengthening: A comprehensive review. Biogeotechnics 2023, 1, 100002. [Google Scholar]
- Seifan, M.; Samani, A.K.; Berenjian, A. Bioconcrete: Next generation of self-healing concrete. Appl. Microbiol. 2016, 100, 2591–2602. [Google Scholar] [CrossRef]
- Choi, S.G.; Chu, J.; Brown, R.C.; Wang, K.; Wen, Z. Sustainable biocement production via microbially induced calcium carbonate precipitation: Use of limestone and acetic acid derived from pyrolysis of lignocellulosic biomass. Sustain. Chem. 2017, 5, 5183–5190. [Google Scholar]
- Li, D.; Tian, K.; Zhang, H.; Wu, Y.; Nie, K.; Zhang, S. Experimental investigation of solidifying desert aeolian sand using microbially induced calcite precipitation. Constr. Build. Mater. 2018, 172, 251–262. [Google Scholar]
- Tang, C.-S.; Li, H.; Pan, X.-H.; Yin, L.-Y.; Cheng, L.; Cheng, Q.; Liu, B.; Shi, B. Coupling effect of biocementation-fiber reinforcement on mechanical behavior of calcareous sand for ocean engineering. Bull. Eng. Geol. Environ. 2022, 81, 163. [Google Scholar]
- Shu, Y.; Song, Y.; Fang, H.; Wang, D.; Lu, W.; Huang, Y.; Zhao, C.; Chen, L.; Song, X. Fiber-reinforced microbially induced carbonate precipitation (MICP) for enhancing soil stability: Mechanisms, effects, and future prospects. J. Build. Eng. 2024, 94, 109955. [Google Scholar]
- Yetimoglu, T.; Salbas, O. A study on shear strength of sands reinforced with randomly distributed discrete fibers. Geotext. Geomembr. 2003, 21, 103–110. [Google Scholar]
- Gong, L.; Liu, L.; Xu, Y.; Zhu, S.; Hao, T. A discrete element simulation considering calcite crystal shape to investigate the mechanical behaviors of bio-cemented sands. Constr. Build. Mater. 2023, 368, 130398. [Google Scholar]
- Wang, Y.; Soga, K.; Dejong, J.T.; Kabla, A.J. A microfluidic chip and its use in characterising the particle-scale behaviour of microbial-induced calcium carbonate precipitation (MICP). Géotechnique 2019, 69, 1086–1094. [Google Scholar]
- Choi, S.-G.; Wang, K.; Chu, J. Properties of biocemented, fiber reinforced sand. Constr. Build. Mater. 2016, 120, 623–629. [Google Scholar]
- Zeng, H.; Yin, L.-Y.; Tang, C.-S.; Zhu, C.; Cheng, Q.; Li, H.; Lv, C.; Shi, B. Tensile behavior of bio-cemented, fiber-reinforced calcareous sand from coastal zone. Eng. Geol. 2021, 294, 106390. [Google Scholar]
- Li, M.; Li, L.; Ogbonnaya, U.; Wen, K.; Tian, A.; Amini, F. Influence of fiber addition on mechanical properties of MICP-treated sand. J. Mater. Civ. Eng. 2016, 28, 04015166. [Google Scholar]
- Wang, D.-L.; Tang, C.-S.; Pan, X.-H.; Liu, B.; Shi, B. Coupling effect of fiber reinforcement and MICP stabilization on the tensile behavior of calcareous sand. Eng. Geol. 2023, 317, 107090. [Google Scholar]
- Feng, K.; Montoya, B. Influence of confinement and cementation level on the behavior of microbial-induced calcite precipitated sands under monotonic drained loading. J. Geotech. Geoenviron. Eng. 2016, 142, 04015057. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.a.; Li, G.; Zhang, J. Experimental study on the mechanical behaviors of aeolian sand treated by microbially induced calcite precipitation (MICP) and basalt fiber reinforcement (BFR). Materials 2023, 16, 1949. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiao, Z.; Fan, C.; Shen, W.; Wang, Q.; Liu, P. Comparative mechanical behaviors of four fiber-reinforced sand cemented by microbially induced carbonate precipitation. Bull. Eng. Geol. Environ. 2020, 79, 3075–3086. [Google Scholar]
- Imran, M.A.; Gowthaman, S.; Nakashima, K.; Kawasaki, S. The influence of the addition of plant-based natural fibers (Jute) on biocemented sand using MICP method. Materials 2020, 13, 4198. [Google Scholar] [CrossRef]
- Yao, D.; Wu, J.; Wang, G.; Wang, P.; Zheng, J.-J.; Yan, J.; Xu, L.; Yan, Y. Effect of wool fiber addition on the reinforcement of loose sands by microbially induced carbonate precipitation (MICP): Mechanical property and underlying mechanism. Acta Geotech. 2021, 16, 1401–1416. [Google Scholar]
- Lv, C.; Zhu, C.; Tang, C.-S.; Cheng, Q.; Yin, L.-Y.; Shi, B. Effect of fiber reinforcement on the mechanical behavior of bio-cemented sand. Geosynth. Int. 2021, 28, 195–205. [Google Scholar]
- Chaduvula, U.; Viswanadham, B.; Kodikara, J. A study on desiccation cracking behavior of polyester fiber-reinforced expansive clay. Appl. Clay Sci. 2017, 142, 163–172. [Google Scholar]
- Li, G.; Liu, J.; Zhang, J.; Yang, Y.; Chen, S. Shear Strength Behaviors of Aeolian Sand Solidified by Microbially Induced Calcite Precipitation and Basalt Fiber Reinforcement. Materials 2023, 16, 5857. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, X.; Wang, J.; Wang, Y.; Feng, D.; Zhu, C. Influence of fiber type and length on mechanical properties of MICP-treated sand. Materials 2022, 15, 4017. [Google Scholar] [CrossRef]











| D10 (µm) | D50 (µm) | D90 (µm) | D [3,2] (µm) | D [4,3] (µm) | Cu | Cc |
|---|---|---|---|---|---|---|
| 254.560 | 395.127 | 601.782 | 372.982 | 413.576 | 1.72 | 0.95 |
| Mean Diameter | Length | Modulus of Elasticity | Tensile Strength | Elongation at Break | Density | ||
|---|---|---|---|---|---|---|---|
| MPa | cN/dtex | MPa | cN/dtex | ||||
| 25 µm | 12 mm | 122,000 | 1230 | 3100 | 32.6 | 3.5% | 0.97 g/cm3 |
| Group | Sandy Soil (g) | Fiber (%) |
|---|---|---|
| 1 | 145 | 0 |
| 2 | 145 | 0.2 |
| 3 | 145 | 0.4 |
| 4 | 145 | 0.6 |
| 5 | 145 | 0.8 |
| 6 | 145 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Li, X.; Zhu, J.; Wei, W.; Qu, X.; Wang, L.; Sun, N.; Zhang, L. Microbial Culture Condition Optimization and Fiber Reinforcement on Microbial-Induced Carbonate Precipitation for Soil Stabilization. Sustainability 2025, 17, 3101. https://doi.org/10.3390/su17073101
Wang C, Li X, Zhu J, Wei W, Qu X, Wang L, Sun N, Zhang L. Microbial Culture Condition Optimization and Fiber Reinforcement on Microbial-Induced Carbonate Precipitation for Soil Stabilization. Sustainability. 2025; 17(7):3101. https://doi.org/10.3390/su17073101
Chicago/Turabian StyleWang, Changjun, Xiaoxiao Li, Jianjun Zhu, Wenzhu Wei, Xinran Qu, Ling Wang, Ninghui Sun, and Lei Zhang. 2025. "Microbial Culture Condition Optimization and Fiber Reinforcement on Microbial-Induced Carbonate Precipitation for Soil Stabilization" Sustainability 17, no. 7: 3101. https://doi.org/10.3390/su17073101
APA StyleWang, C., Li, X., Zhu, J., Wei, W., Qu, X., Wang, L., Sun, N., & Zhang, L. (2025). Microbial Culture Condition Optimization and Fiber Reinforcement on Microbial-Induced Carbonate Precipitation for Soil Stabilization. Sustainability, 17(7), 3101. https://doi.org/10.3390/su17073101





