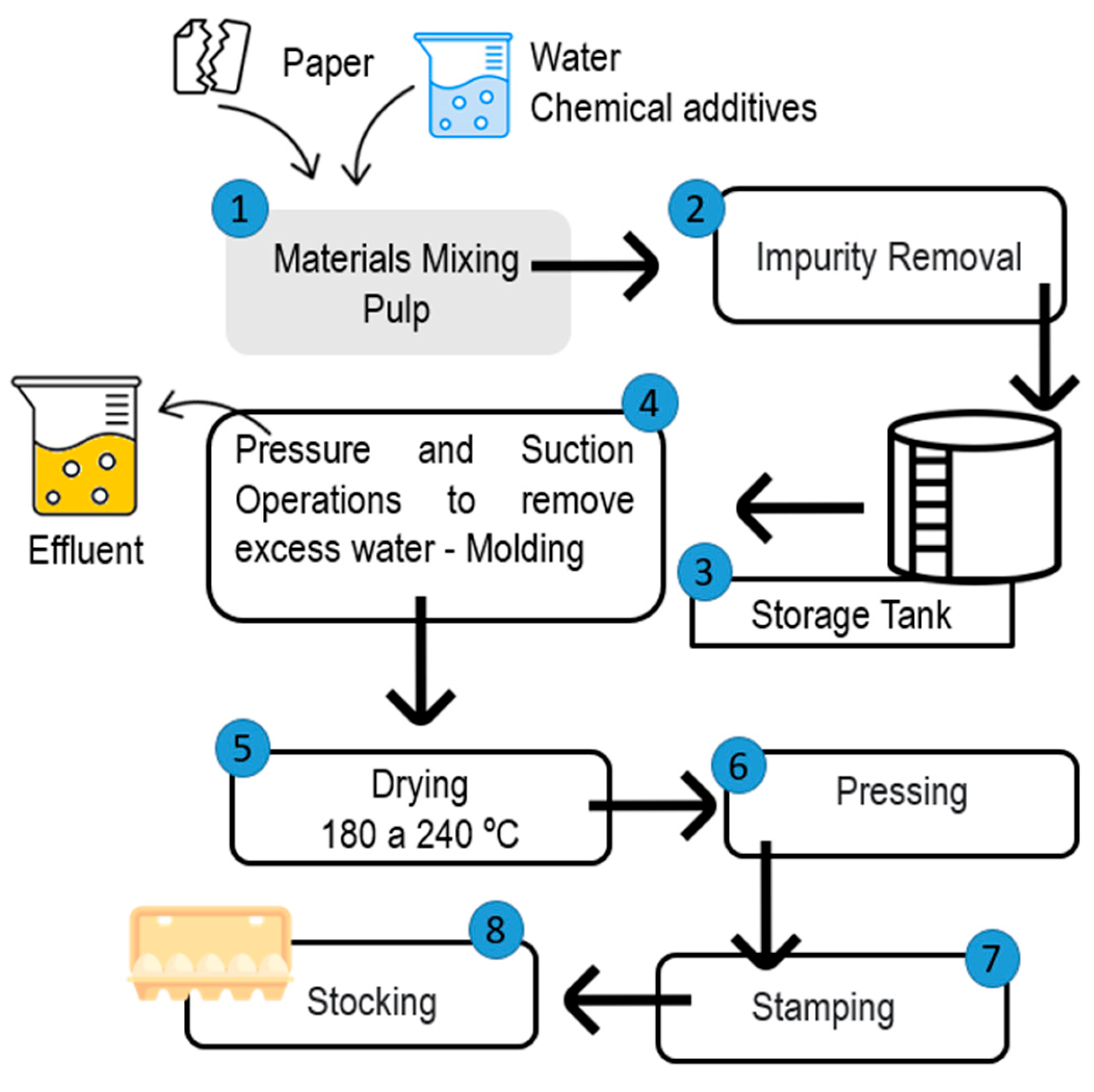
Clarification of Effluents Industry Using Nb2O5
Abstract
:1. Introduction
2. Materials and Methods
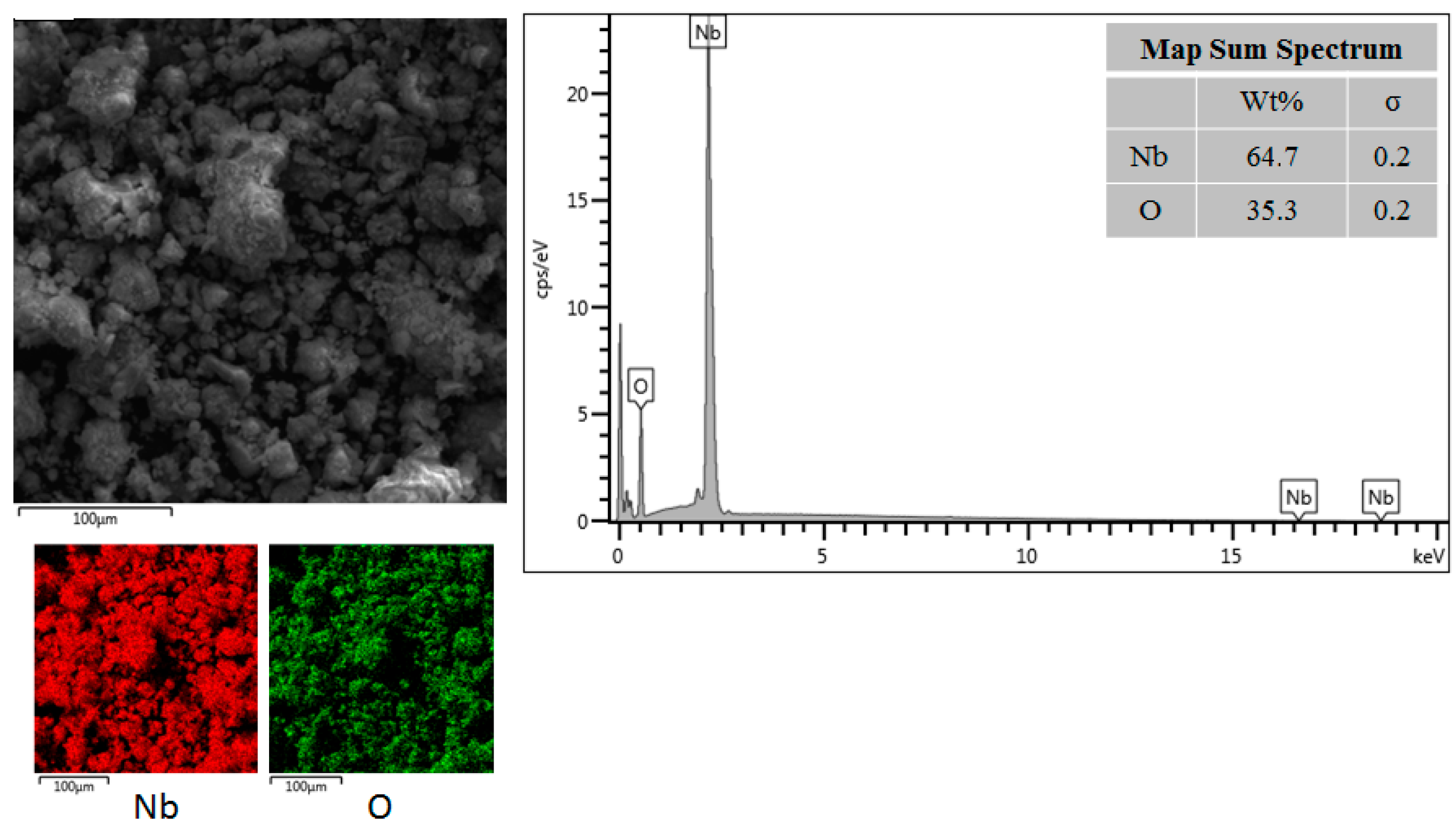
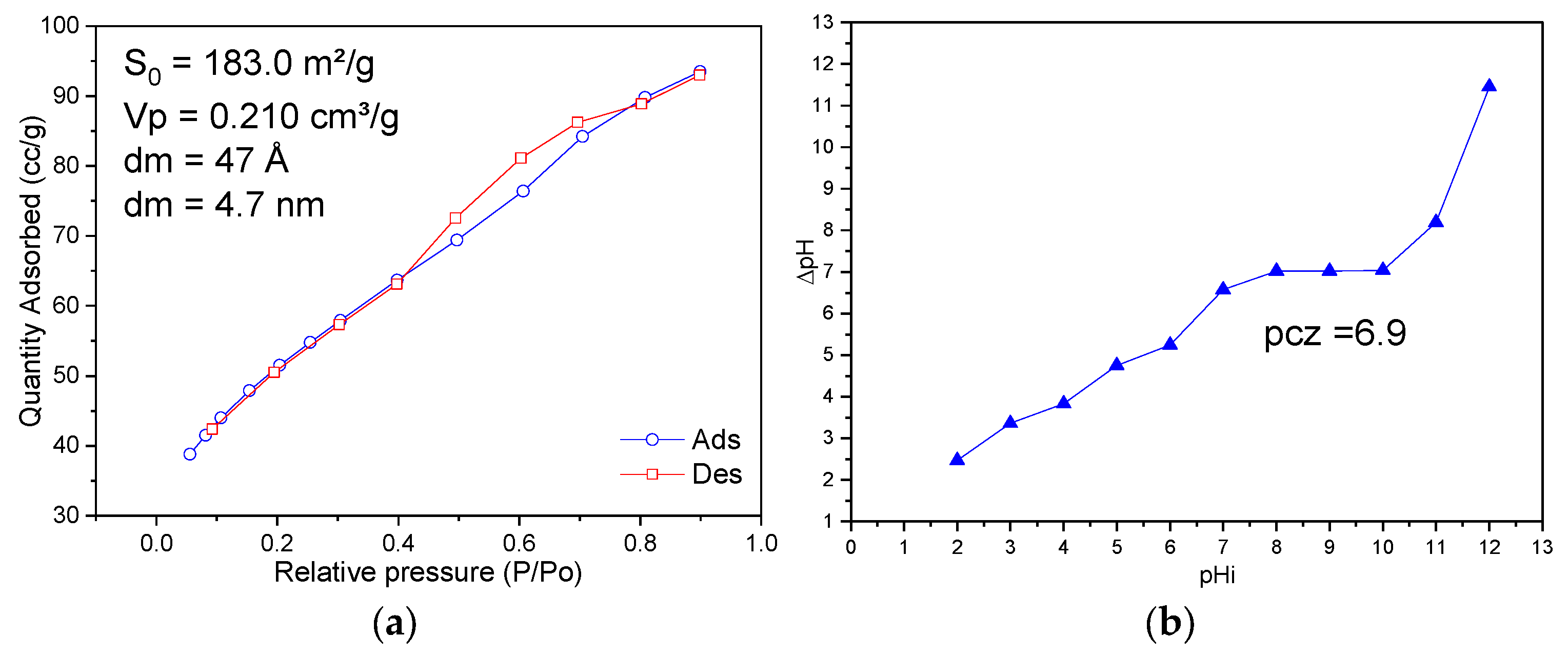
2.1. Catalyst and Characterization
2.2. Photocatalytic Tests
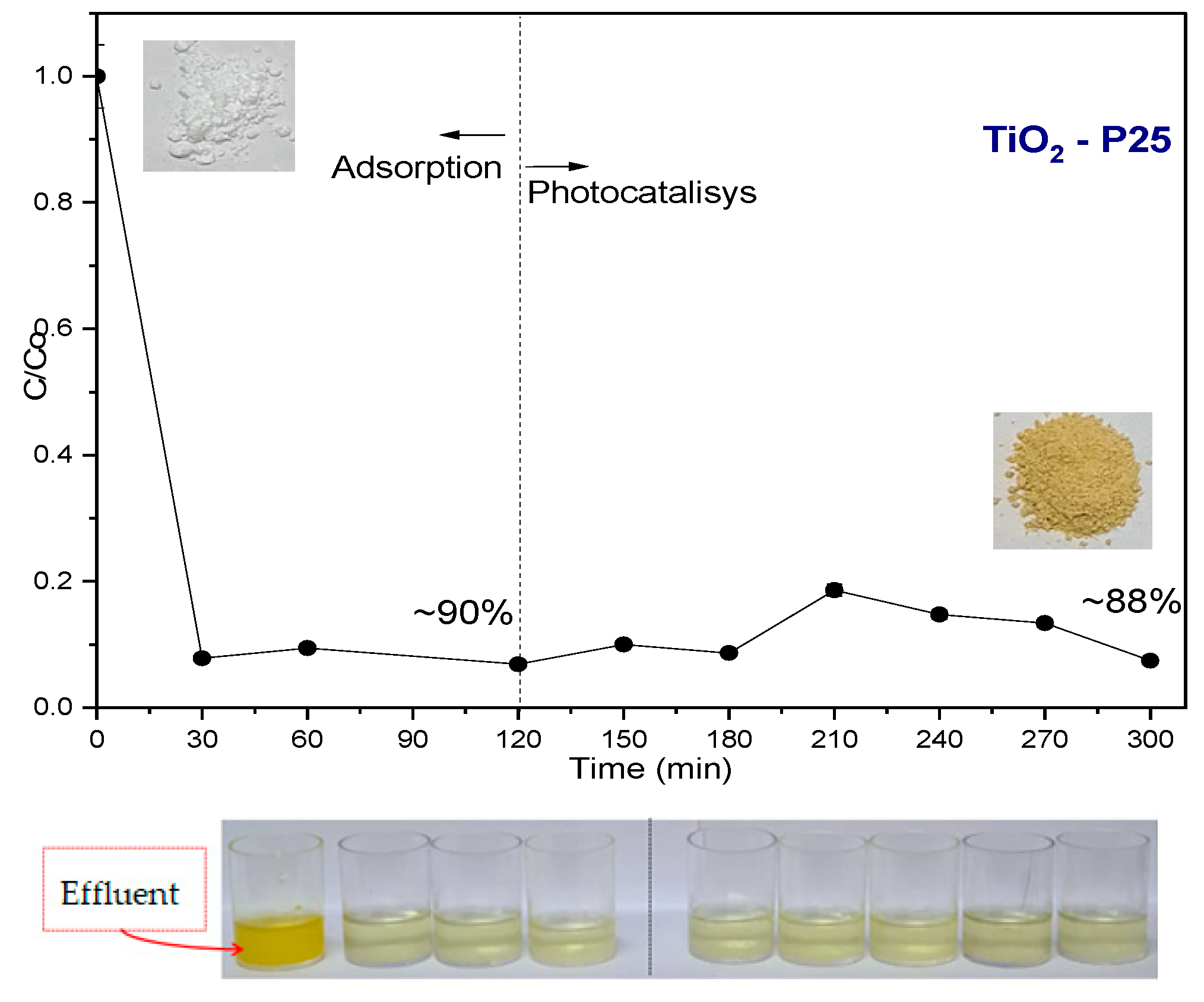
Effluent
2.3. Mathematical Modeling
3. Results and Discussion
3.1. Characterization
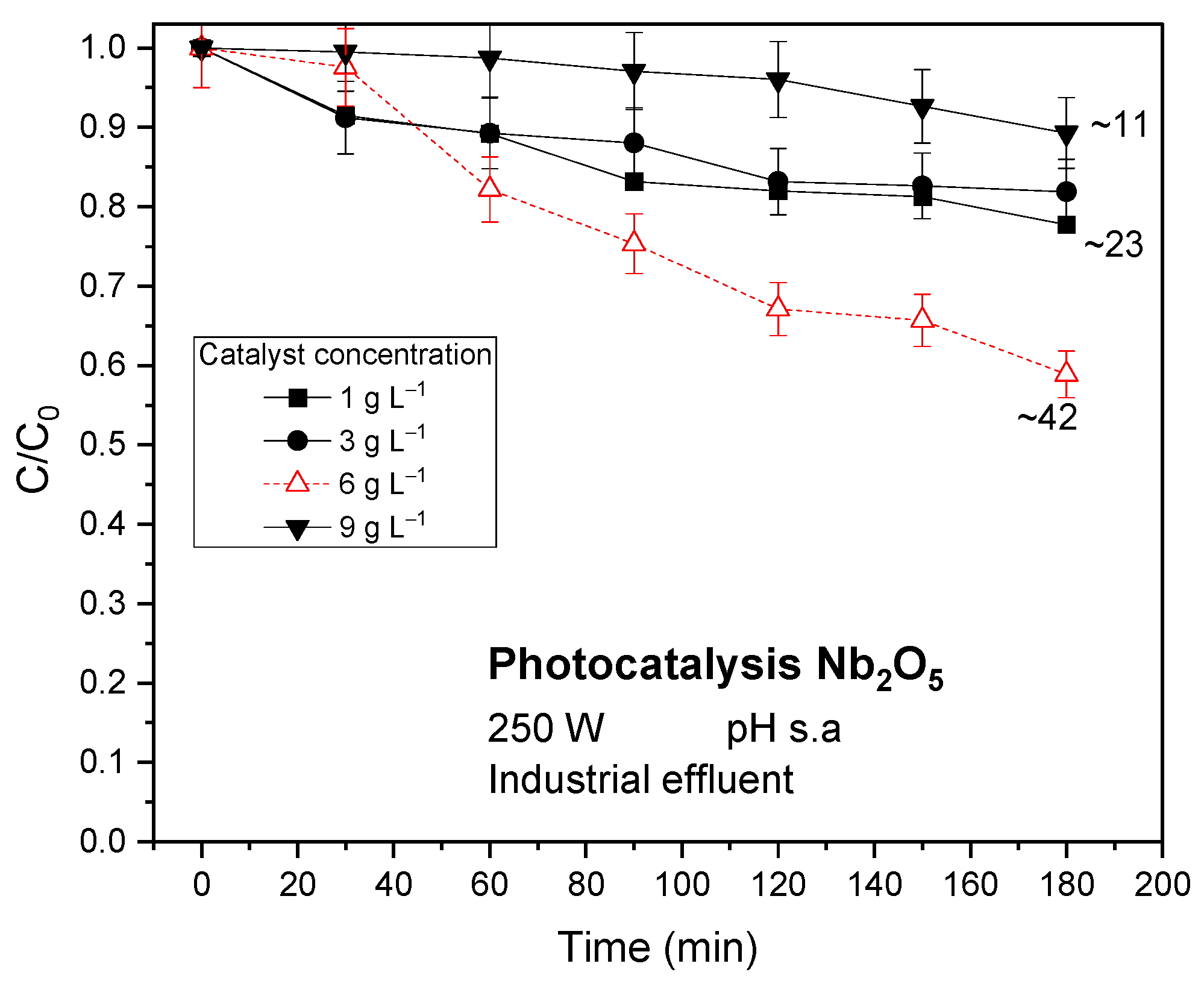
3.1.1. Photocatalytic Tests Results
Synthetic Solution
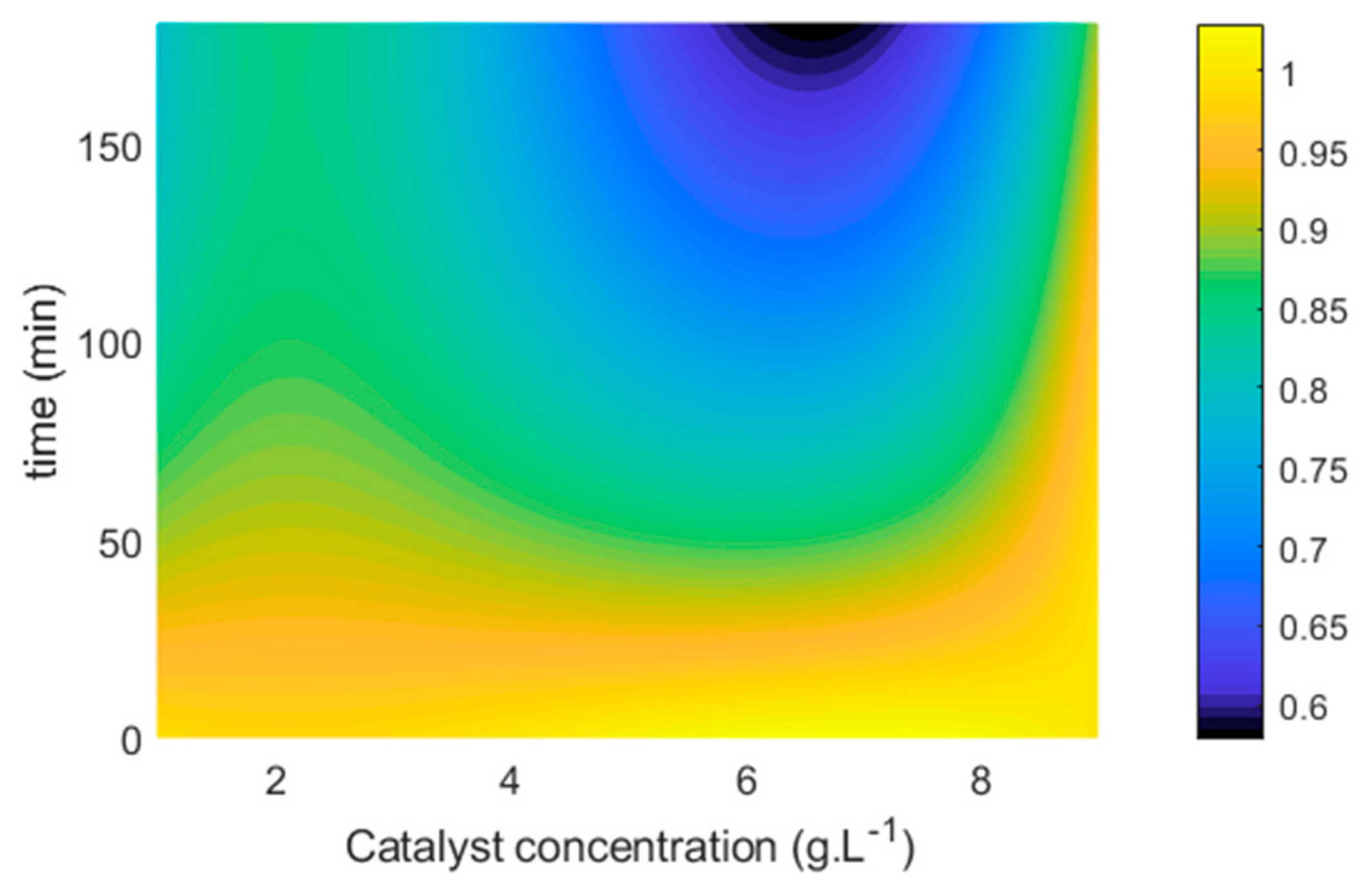
- Figure 5 indicates the results obtained for the photocatalytic reactions to evaluate the catalyst concentration.
- 2.
- It is possible to observe that, with the lowest catalyst concentration (0.5 g L−1), a slower kinetic curve was obtained in relation to the other concentrations evaluated for all catalysts studied. As the catalyst concentration increased from 0.5 to 1.0 g L−1, a notable increase in the reaction rate occurred, and this may be related to the greater availability of active sites and, therefore, greater formation of the electron-hole pair and greater generation of hydroxyl radical, a compound that participates in the degradation of organic pollutants such as the dye under study [31].
- 3.
- The concentration of 1.0 g L−1 was defined as optimal for all catalysts, and, when this condition is reached, if an excess of photocatalyst is added, the efficiency of the reaction may be impaired. This most likely occurs due to a decrease in suspension homogeneity and an increase in turbidity, which hinders the passage of radiation and reduces the availability of active sites for photocatalysis [20,32,33]. For concentrations above 1 g L−1 of Nb2O5, it was clearly found that the reaction was impaired in terms of speed.
- 4.
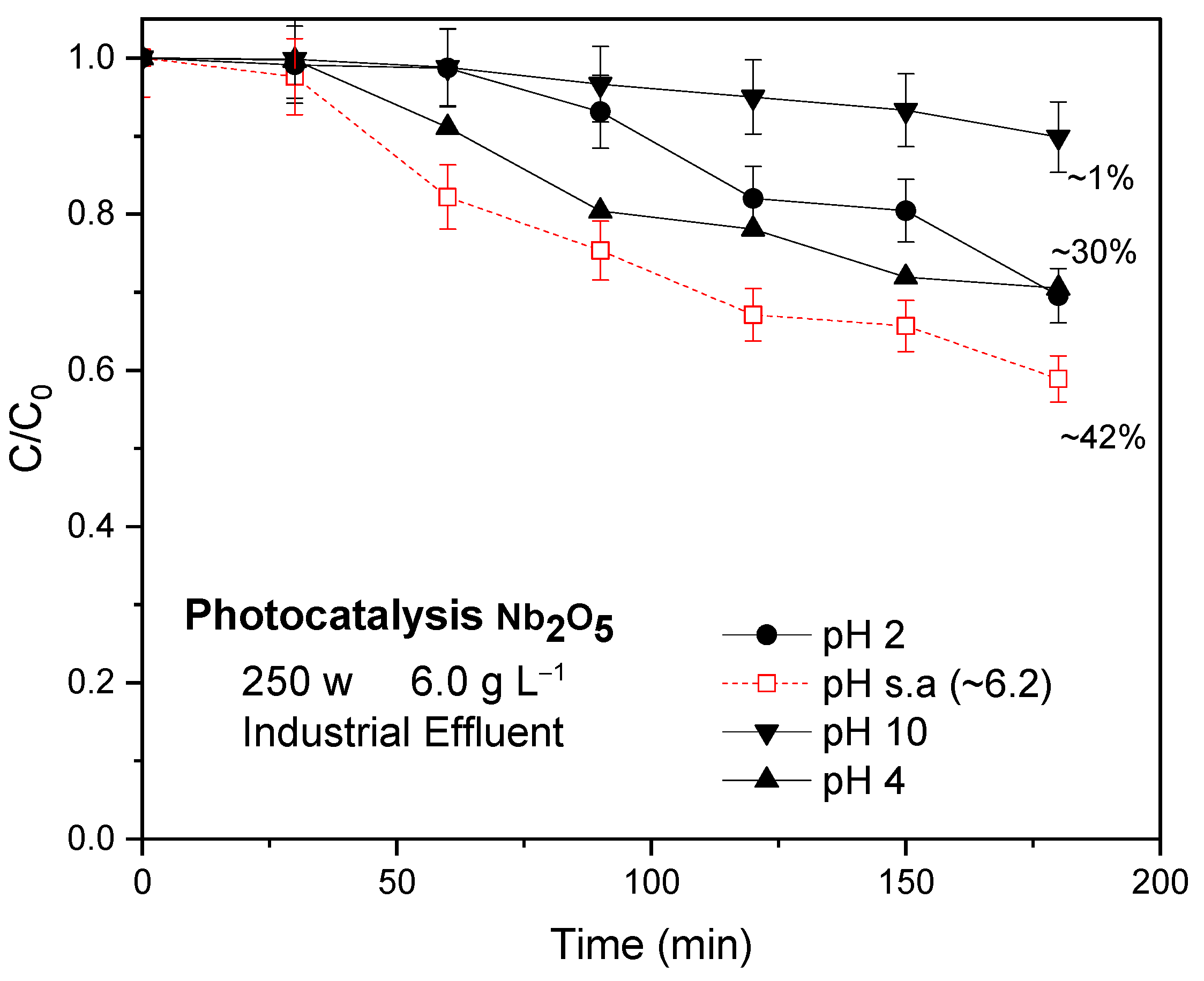
- The tests to verify the influence of pH are shown in Figure 6. It can be seen that the unadjusted pH (natural pH of the synthetic solution itself, 4.2) was determined as the most suitable regardless of which catalyst was used. It can be seen that any variation in pH, whether to a more acidic or basic medium, affected the reaction rate, which also leads to considering the unadjusted pH as the most advantageous for the development of subsequent reactions. A change in the pH value can cause changes in the surface properties of the catalytic materials as well as in the surface properties of the organic pollutant, thus modifying the behavior of the photocatalytic reactions [14]. The dye studied here as a pollutant is, as previously mentioned, classified as a basic or cationic dye [34]. Cationic dyes are easily adsorbed in solutions whose reaction medium pH is basic because the surface of the adsorbent/catalyst will be negatively charged and consequently will attract the cationic dye, which has a positive charge [35]. However, if only this phenomenon were involved during the reactions, regardless of the catalyst, the behavior would be the same, because it is also worth remembering the materials characterizations, in particular, the analysis of the point of zero charge in which a value of 6.9 was obtained.
Industrial Effluent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez, K.Y.P.; Toso, E.A.V.; Morabito, R. Production Planning in the Molded Pulp Packaging Industry. Comput. Ind. Eng. 2016, 98, 554–566. [Google Scholar] [CrossRef]
- Jamil, A.; Bokhari, T.H.; Javed, T.; Mustafa, R.; Sajid, M.; Noreen, S.; Zuber, M.; Nazir, A.; Iqbal, M.; Jilani, M.I. Photocatalytic Degradation of Disperse Dye Violet-26 Using TiO2 and ZnO Nanomaterials and Process Variable Optimization. J. Mater. Res. Technol. 2020, 9, 1119–1128. [Google Scholar] [CrossRef]
- Periyasamy, A.P. Recent Advances in the Remediation of Textile-Dye-Containing Wastewater: Prioritizing Human Health and Sustainable Wastewater Treatment. Sustainability 2024, 16, 495. [Google Scholar] [CrossRef]
- Mcyotto, F.; Wei, Q.; Macharia, D.K.; Huang, M.; Shen, C.; Chow, C.W.K. Effect of Dye Structure on Color Removal Efficiency by Coagulation. Chem. Eng. J. 2021, 405, 126674. [Google Scholar] [CrossRef]
- Shabil Sha, M.; Anwar, H.; Musthafa, F.N.; Al-Lohedan, H.; Alfarwati, S.; Rajabathar, J.R.; Khalid Alahmad, J.; Cabibihan, J.-J.; Karnan, M.; Kumar Sadasivuni, K. Photocatalytic Degradation of Organic Dyes Using Reduced Graphene Oxide (rGO). Sci. Rep. 2024, 14, 3608. [Google Scholar] [CrossRef]
- Braslavsky, S.E.; Braun, A.M.; Cassano, A.E.; Emeline, A.V.; Litter, M.I.; Palmisano, L.; Parmon, V.N.; Serpone, N. Glossary of Terms Used in Photocatalysis and Radiation Catalysis (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 931–1014. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Photocatalysis Fundamentals Revisited to Avoid Several Misconceptions. Appl. Catal. B Environ. 2010, 99, 461–468. [Google Scholar] [CrossRef]
- Oliveira, I.E.; Silva, R.M.; Girão, A.V.; Faria, J.L.; Silva, C.G.; Silva, R.F. Facile Preparation of ZnO/CNTs Nanocomposites via ALD for Photocatalysis Applications. Eur. J. Inorg. Chem. 2020, 2020, 1743–1750. [Google Scholar] [CrossRef]
- Ziolli, R.L.; Jardim, W.F. Mecanismo de fotodegradação de compostos orgânicos catalisada por TiO2. Quím. Nova 1998, 21, 319–325. [Google Scholar] [CrossRef]
- Silva, M.K.; Marques, R.G.; Machado, N.R.C.F.; Santos, O.A.A. Evaluation of Nb2O5 and Ag/Nb2O5 in the Photocatalytic Degradation of Dyes from Textile Industries. Braz. J. Chem. Eng. 2002, 19, 359–363. [Google Scholar] [CrossRef]
- Zarrin, S.; Heshmatpour, F. Photocatalytic Activity of TiO2/Nb2O5/PANI and TiO2/Nb2O5/RGO as New Nanocomposites for Degradation of Organic Pollutants. J. Hazard. Mater. 2018, 351, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Abreu, E.; Fidelis, M.Z.; Fuziki, M.E.; Malikoski, R.M.; Mastsubara, M.C.; Imada, R.E.; Diaz De Tuesta, J.L.; Gomes, H.T.; Anziliero, M.D.; Baldykowski, B.; et al. Degradation of Emerging Contaminants: Effect of Thermal Treatment on Nb2O5 as Photocatalyst. J. Photochem. Photobiol. Chem. 2021, 419, 113484. [Google Scholar] [CrossRef]
- Almeida, L.N.; Lenzi, G.G.; Pietrobelli, J.M.T.A.; Santos, O.A. Performance Evaluation of Catalysts of ZnO in Photocatalytic Degradation of Caffeine Solution. Chem. Eng. Trans. 2017, 57, 667–672. [Google Scholar] [CrossRef]
- Josué, T.G.; Almeida, L.N.B.; Lopes, M.F.; Santos, O.A.A.; Lenzi, G.G. Cr (VI) Reduction by Photocatalyic Process: Nb2O5 an Alternative Catalyst. J. Environ. Manag. 2020, 268, 110711. [Google Scholar] [CrossRef]
- Rani, S.; Sanghi, S.; Agarwal, A.; Khasa, S. Influence of Nb2O5 on the optical band gap and electrical conductivity of Nb2O5·BaO·B2O3. IOP Conf. Ser. Mater. Sci. Eng. 2009, 2, 2–6. [Google Scholar]
- Boruah, B.D. Zinc Oxide Ultraviolet Photodetectors: Rapid Progress from Conventional to Self-Powered Photodetectors. Nanoscale Adv. 2019, 1, 2059–2085. [Google Scholar] [CrossRef]
- Santos, A.J.; Batista, L.M.B.; Martínez-Huitle, C.A.; Alves, A.P.D.M.; Garcia-Segura, S. Niobium Oxide Catalysts as Emerging Material for Textile Wastewater Reuse: Photocatalytic Decolorization of Azo Dyes. Catalysts 2019, 9, 1070. [Google Scholar] [CrossRef]
- Teixeira, C.P.A.B.; Jardim, W.F. Influence of Several Factors on the Heterogeneous Photocatalytic Degradation of Phenol. Rev. Quím. 2004, 18, 3–10. [Google Scholar]
- Loddo, V.; Bellardita, M.; Camera-Roda, G.; Parrino, F.; Palmisano, L. Heterogeneous Photocatalysis. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–43. ISBN 978-0-12-813549-5. [Google Scholar]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An Overview of Solar/Visible Light-Driven Heterogeneous Photocatalysis for Water Purification: TiO2- and ZnO-Based Photocatalysts Used in Suspension Photoreactors. J. Ind. Eng. Chem. 2019, 71, 19–49. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters Affecting the Photocatalytic Degradation of Dyes Using TiO2: A Review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar]
- Fidelis, M.Z. Degradação de Triclosan e 2,8-Diclorodibenzeno-p-Dioxina via Sistema Fe/Nb2O5/UV. Master’s Thesis, Universidade Tecnológica Federal do Paraná, Ponta Grossa, Brazil, 2019. [Google Scholar]
- Burden, R.L.; Faires, J.D. Numerical Analysis; Cengage Learning: Stamford, CT, USA, 2007. [Google Scholar]
- Zhang, P.; Wang, M.; Wang, J.; Teng, X.; Zhang, S.; Xie, H.; Ding, S. Facile Synthesis and Characterization of Low Crystalline Nb2O5 Ultrafine Nanoparticles as a New Efficient Photocatalyst. J. Non-Cryst. Solids 2018, 500, 371–376. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, T.; Mai, Y.; Wang, X.; Chen, J.; Liao, B. Selective Catalytic Oxidation of Sulfides to Sulfoxides or Sulfones over Amorphous Nb2O5/AC Catalysts in Aqueous Phase at Room Temperature. Catal. Commun. 2019, 127, 10–14. [Google Scholar] [CrossRef]
- Nunes, S.C.; Brackmann, R. Catalisadores Cu-Fe/Nb2O5 Para Aplicação Na Redução Catalítica Seletiva de NO Por CO. In Proceedings of the “20º CBCat: Expandindo as Fronteiras da Catálise”, o 20º Congresso Brasileiro de Catálise, São Paulo, Brazil, 1–7 September 2019. [Google Scholar]
- Costa, G.P.; Rafael, R.A.; Soares, J.C.S.; Gaspar, A.B. Synthesis and Characterization of ZnO-Nb2O5 Catalysts for Photodegradation of Bromophenol Blue. Catal. Today 2020, 344, 240–246. [Google Scholar] [CrossRef]
- Bolzon, L.B.; Prado, A.G.S. Effect of Protonation and Deprotonation on Surface Charge Density of Nb2O5: A Thermodynamic Approach. J. Therm. Anal. Calorim. 2011, 106, 427–430. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Bui, H.M.; Nguyen, H.Q. Heterogeneous Photocatalysis for the Removal of Pharmaceutical Compounds. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–183. ISBN 978-0-12-819594-9. [Google Scholar]
- Mangrulkar, P.A.; Joshi, M.M.; Tijare, S.N.; Polshettiwar, V.; Labhsetwar, N.K.; Rayalu, S.S. Nano Cobalt Oxides for Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2012, 37, 10462–10466. [Google Scholar] [CrossRef]
- Taffarel, S.R.; Lansarin, M.A.; Moro, C.C. Styrene Photocatalytic Degradation Reaction Kinetics. J. Braz. Chem. Soc. 2011, 22, 1872–1879. [Google Scholar] [CrossRef]
- Knutson, K.P. Enzymatic Biobleaching of Recalcitrant Paper Dyes; Georgia Institute of Technology: Atlanta, GA, USA, 2004. [Google Scholar]
- Zanoni, M.V.B.; Yamanaka, H. Corantes: Caracterização Química, Toxicológica, Métodos de Detecção e Tratamento, 1st ed.; Cultura Acadêmica Editora: São Paulo, Brazil, 2016; ISBN 978-85-7983-780-7. [Google Scholar]
- Mills, A.; Davies, R.H.; Worsley, D. Water Purification by Semiconductor Photocatalysis. Chem. Soc. Rev. 1993, 22, 417. [Google Scholar] [CrossRef]
- Jada, A.; Akbour, R.A. Adsorption and Removal of Organic Dye at Quartz Sand-Water Interface. Oil Gas Sci. Technol. 2014, 69, 405–413. [Google Scholar] [CrossRef]
- Ceretta, M.B.; Vieira, Y.; Wolski, E.A.; Foletto, E.L.; Silvestri, S. Biological degradation coupled to photocatalysis by ZnO/polypyrrole composite for the treatment of real textile wastewater. J. Water Process. Eng. 2020, 35, 101230. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endo, G.Y.; Tusset, A.M.; Almeida, L.N.B.d.; Santos, O.A.A.d.; Lenzi, G.G. Clarification of Effluents Industry Using Nb2O5. Sustainability 2025, 17, 3204. https://doi.org/10.3390/su17073204
Endo GY, Tusset AM, Almeida LNBd, Santos OAAd, Lenzi GG. Clarification of Effluents Industry Using Nb2O5. Sustainability. 2025; 17(7):3204. https://doi.org/10.3390/su17073204
Chicago/Turabian StyleEndo, Gustavo Yuho, Angelo M. Tusset, Lariana Negrão Beraldo de Almeida, Onélia A. A. dos Santos, and Giane G. Lenzi. 2025. "Clarification of Effluents Industry Using Nb2O5" Sustainability 17, no. 7: 3204. https://doi.org/10.3390/su17073204
APA StyleEndo, G. Y., Tusset, A. M., Almeida, L. N. B. d., Santos, O. A. A. d., & Lenzi, G. G. (2025). Clarification of Effluents Industry Using Nb2O5. Sustainability, 17(7), 3204. https://doi.org/10.3390/su17073204







