Implementing Digital Multispectral 3D Scanning Technology for Rapid Assessment of Hemp (Cannabis sativa L.) Weed Competitive Traits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
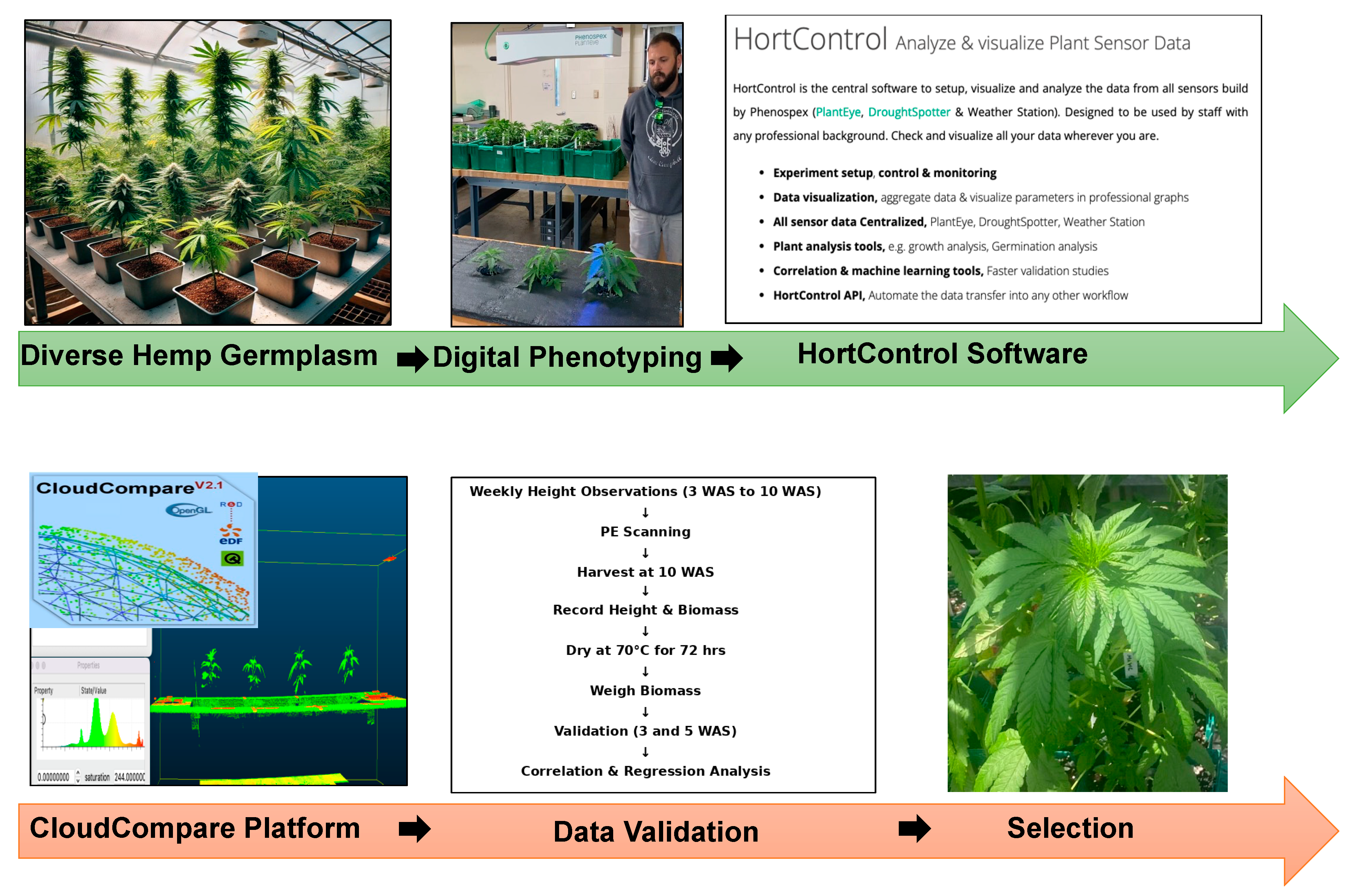
2.2. Experimental Setup and Hemp Plants Scanning
2.3. Description of Remote Sensing Approach Using PlantEye
2.4. PE Data Collection, HortControl, and CloudCompare Software
2.5. Manual Observations, Destructive Harvesting, and Data Validation
2.6. Statistical Analysis
3. Results
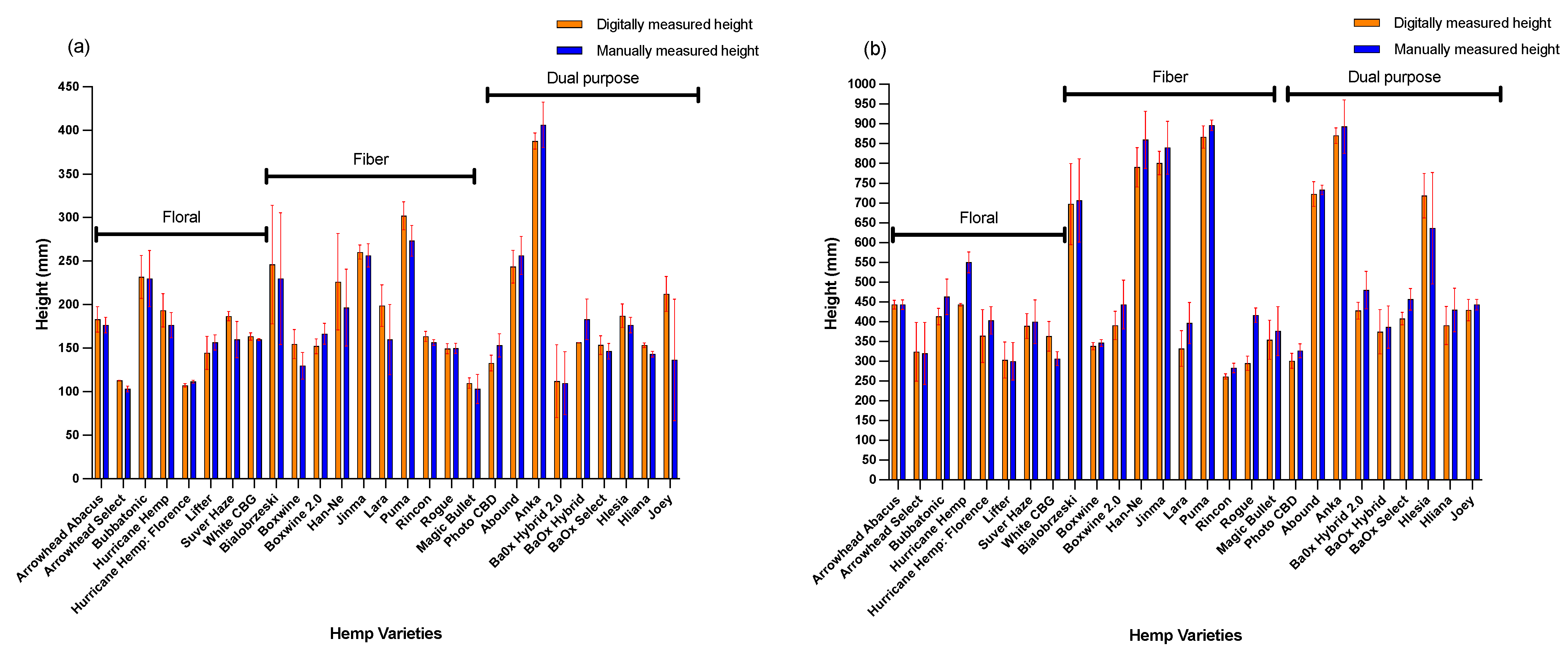
3.1. Digital Height Estimation
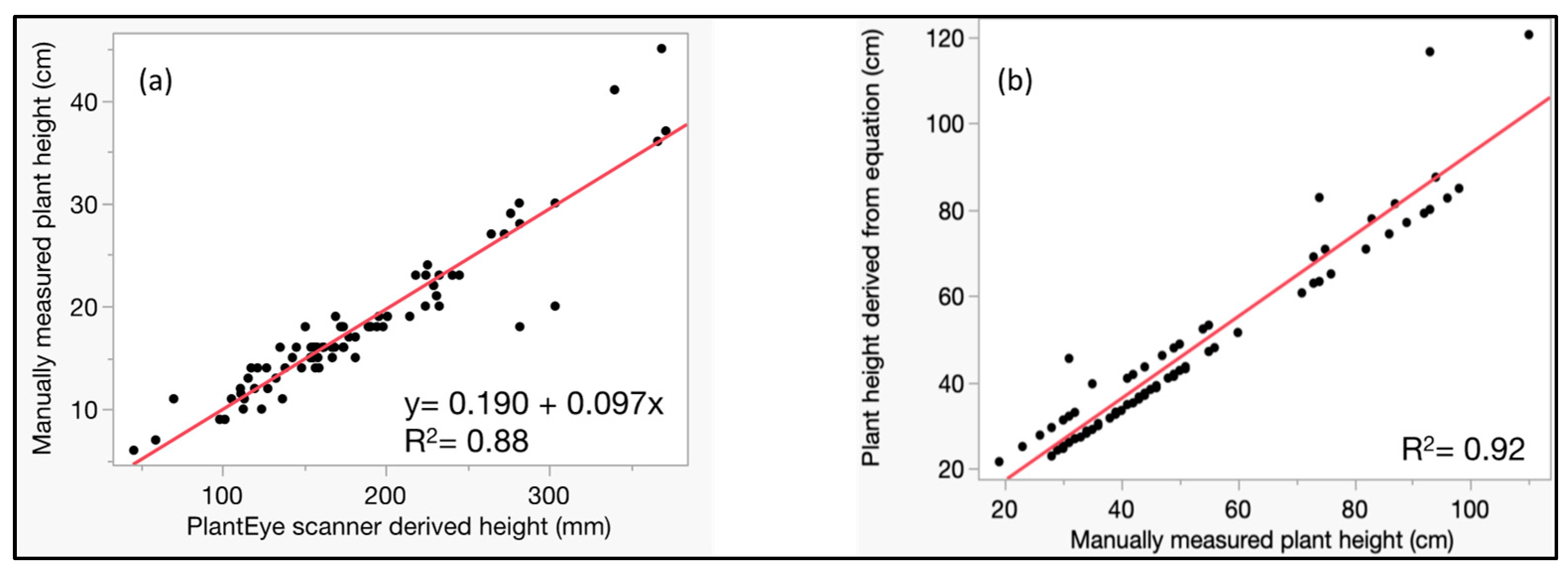
3.2. Method Validation of Digital Plant Height Estimation Techniques
3.3. Leaf Area Index (LAI), Leaf Angle (LA), and Light Penetration Depth (LPD)
3.3.1. Leaf Area Index (LAI)
3.3.2. Leaf Angle (LA)
3.3.3. Light Penetration Depth (LPD)
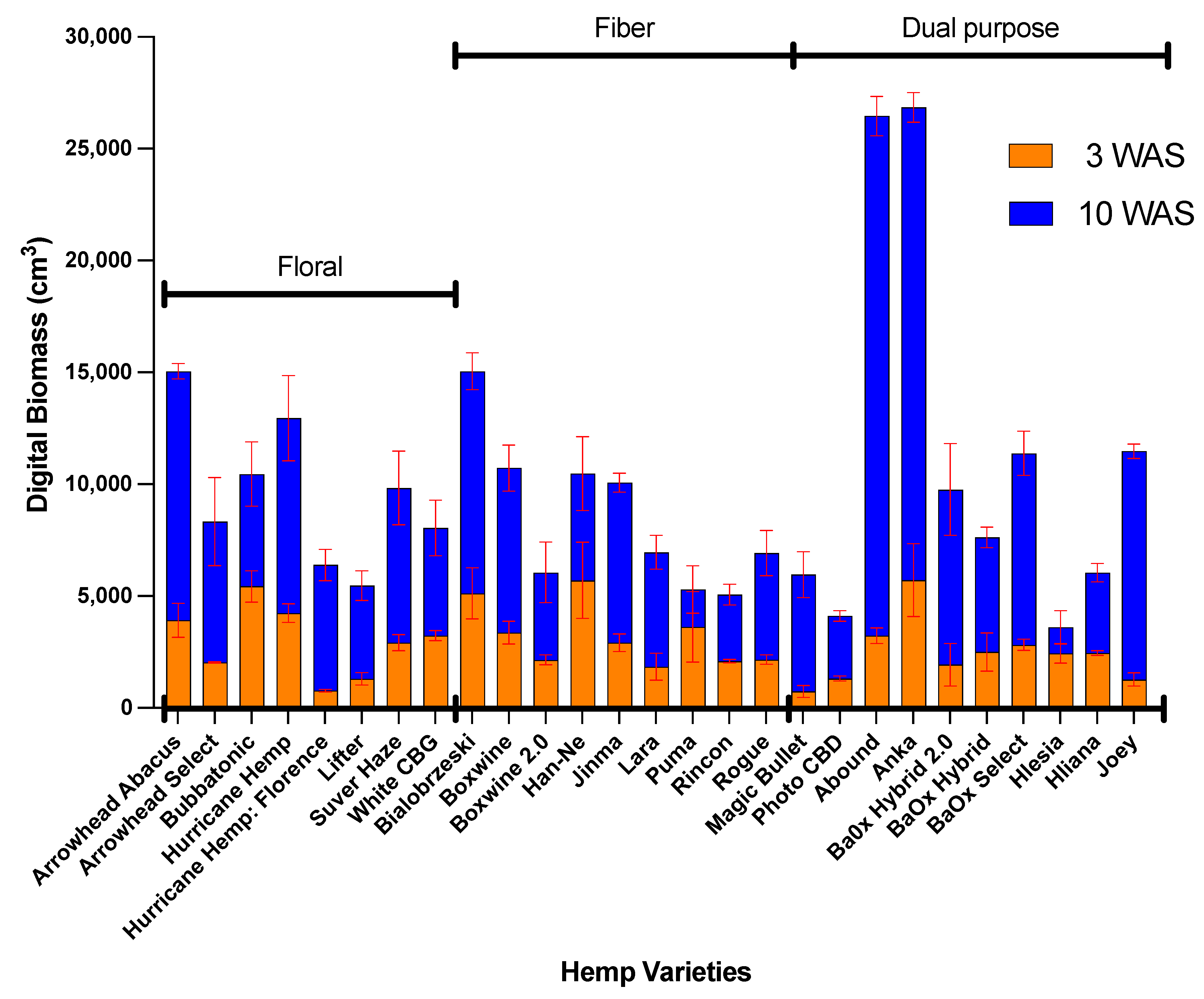
3.4. Digital Biomass
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montgomery, B. The bast fibers. In Matthew’s Textile Fibers, 6th ed.; Mausberger, H.R., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 1954; pp. 257–359. [Google Scholar]
- Robinson, B.B. Abaca, a cordage fiber. USDA Agricultural Monograph No. 21. 1953. Available online: https://www.nal.usda.gov/collections/special-collections/usda-crop-fiber-research-collection (accessed on 2 February 2024).
- Clarke, R.C.; Merlin, M.D. Cannabis domestication, breeding history, present-day genetic diversity, and future prospects. Crit. Rev. Plant Sci. 2016, 35, 293–327. [Google Scholar] [CrossRef]
- Johnson, R. Hemp as an Agricultural Commodity; Congressional Research Service Report 7-57000 RL32725; Congressional Research Service: Washington, DC, USA, 2017.
- Fike, J. Industrial hemp: Renewed opportunities for an ancient crop. Crit. Rev. Plant Sci. 2016, 35, 406–424. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Yual, L. Challenges towards revitalizing hemp: A multifaceted crop. Trends Plant Sci. 2017, 22, 917–929. [Google Scholar] [CrossRef] [PubMed]
- H.R.2—An act to Provide for the Reform and Continuation of Agricultural and Other Programs of the Department of Agriculture through Fiscal Year 2023, and for Other Purposes. Agriculture Improvement Act of 2018. Available online: https://www.congress.gov/bill/115th-congress/house-bill/2/text?format=txt%26overview=closed (accessed on 20 February 2024).
- Amaducci, S.; Scordia, D.; Liu, F.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S. Key cultivation techniques for hemp in Europe and China. Ind. Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Hooks, P.J. Production of Industrial Hemp in Kentucky. Master’s Thesis, Murray State University, Murray, KY, USA, 2018. [Google Scholar]
- Tang, K.; Struik, P.; Yin, X.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Amaducci, S. Comparing hemp (Cannabis sativa L.) varieties for dual-purpose production under contrasting environments. Ind. Crops Prod. 2016, 87, 33–44. [Google Scholar] [CrossRef]
- Cutulle, M.; Ward, B.; Campbell, H.T.; and Horry, M. Varieties and Weed-Free Duration is Important for Hemp Bud Yield in Coastal South Carolina. Clemson (SC): Clemson Cooperative Extension, Land-Grant Press by Clemson Extension; 2020 Jun. LGP 1074. Available online: https://lgpress.clemson.edu/publication/varieties-and-weed-free-duration-is-important-for-hemp-bud-yield-in-coastal-south-carolina/ (accessed on 21 February 2024).
- Harrison, H.F., Jr.; Jackson, D.M. Response of two sweet potato varieties to weed interference. Crop Prot. 2011, 20, 1291–1296. [Google Scholar] [CrossRef]
- Seavers, G.P.; Wright, K.J. Crop canopy development and structure influence weed suppression. Weed Res. 1999, 39, 319–328. [Google Scholar] [CrossRef]
- Ramesh, K.; Rao, A.; Chauhan, B.S. Role of crop competition in managing weeds in rice, wheat, and maize in India: A review. Crop Prot. 2017, 95, 14–21. [Google Scholar] [CrossRef]
- Saito, K.; Azoma, K.; Rodenburg, J. Plant characteristics associated with weed competitiveness of rice under upland and lowland conditions in West Africa. Field Crops Res. 2010, 116, 308–317. [Google Scholar] [CrossRef]
- Sardana, V.; Mahajan, G.; Jabran, K.; Chauhan, B.S. Role of competition in managing weeds: An introduction to the special issue. Crop Prot. 2017, 95, 1–7. [Google Scholar] [CrossRef]
- Kjær, K.H.; Ottosen, C.-O. 3D Laser triangulation for plant phenotyping in challenging environments. Sensors 2015, 15, 13533–13547. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V.; Kholová, J.; Hummel, G.; Zhokhavets, U.; Gupta, S.K.; Hash, C.T. LeasyScan: A novel concept combining 3D imaging and lysimetry for high-throughput phenotyping of traits controlling plant water budget. J. Exp. Bot. 2015, 66, 5581–5593. [Google Scholar] [CrossRef] [PubMed]
- Maphosa, L.; Thoday-Kennedy, E.; Vakani, J.; Phelan, A.; Badenhorst, P.; Slater, A.; Spangenberg, G.; Kant, S. Phenotyping wheat under salt stress conditions using a 3D laser scanner. Isr. J. Plant Sci. 2017, 64, 55–62. [Google Scholar] [CrossRef]
- Laxman, R.H.; Hemamalini, P.; Bhatt, R.M.; Sadashiva, A.T. Non-invasive quantification of tomato (Solanum lycopersicum L.) plant biomass through digital imaging using phenomics platform. Indian J. Plant Physiol. 2018, 23, 369–375. [Google Scholar] [CrossRef]
- Manavalan, L.P.; Cui, I.; Ambrose, K.V.; Panjwani, S.; DeLong, S.; Mleczko, M.; Spetsieris, K. Systematic approach to validate and implement digital phenotyping tool for soybean: A case study with PlantEye. Plant Phenome J. 2021, 4, e20025. [Google Scholar] [CrossRef]
- Lazarević, B.; Šatović, Z.; Nimac, A.; Vidak, M.; Gunjača, J.; Politeo, O.; Carović-Stanko, K. Application of Phenotyping Methods in Detection of Drought and Salinity Stress in Basil (Ocimum basilicum L.). Front. Plant Sci. 2021, 12, 629441. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Kumar, V.; Godara, R.K.; Chauhan, B.S. Weed management using crop competition in the United States: A review. Crop Prot. 2017, 95, 31–37. [Google Scholar] [CrossRef]
- Holt, J.S. Plant Responses to Light: A Potential Tool for Weed Management. Weed Sci. 1995, 43, 474–482. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Wadl, P.A.; Campbell, H.T.; Rutter, W.B.; Williams, L.H.; Murphey, V.; Culbreath, J.; Cutulle, M. A sustainable approach for weed and insect management in sweetpotato: Breeding for weed and insect tolerant/resistant clones. Weed Technol. 2023, 37, 60–66. [Google Scholar] [CrossRef]

| Varieties | Type (Fiber, Floral or Dual Purpose) |
|---|---|
| Arrowhead Abacus | Floral |
| Arrowhead Select | Floral |
| Bubbatonic | Floral |
| Hurricane Hemp | Floral |
| Hurricane Hemp: Florence | Floral |
| Lifter | Floral |
| Suver Haze | Floral |
| White CBG | Floral |
| Bialobrzeski | Fiber |
| Boxwine | Fiber |
| Boxwine 2.0 | Fiber |
| Han-Ne | Fiber |
| Jinma | Fiber |
| Lara | Fiber |
| Puma | Fiber |
| Rincon | Fiber |
| Rogue | Fiber |
| Magic Bullet | Dual-purpose |
| Photo CBD | Dual-purpose |
| Abound | Dual Purpose |
| Anka | Dual Purpose |
| Ba0x Hybrid 2.0 | Dual purpose |
| BaOx Hybrid | Dual purpose |
| BaOx Select | Dual purpose |
| Hlesia | Dual purpose |
| Hliana | Dual Purpose |
| Joey | Dual Purpose |
| Digital Biomass | Digital Height | Leaf Angle | Leaf Area Index | Light Penetration | Manual Plant Height | |
|---|---|---|---|---|---|---|
| Digital biomass | 1.00 | |||||
| Digital height | 0.72 * | 1.00 | ||||
| Leaf angle | 0.04 | −0.19 | 1.00 | |||
| Leaf area index | 0.77 * | 0.27 | 0.25 | 1.00 | ||
| Light penetration depth | 0.61 * | 0.71 * | 0.01 | 0.44 | 1.00 | |
| Manual plant height | 0.59 * | 0.90 * | −0.05 | 0.19 | 0.68 * | 1.00 |
| Digital Biomass | Digital Height | Leaf Angle | Leaf Area Index | Light Penetration Depth | Manual Plant Height | Fresh Biomass | |
|---|---|---|---|---|---|---|---|
| Digital biomass | 1.00 | ||||||
| Digital height | 0.68 * | 1.00 | |||||
| Leaf angle | 0.39 | −0.06 | 1.00 | ||||
| Leaf area index | 0.74 * | −0.27 | 0.47 | 1.00 | |||
| Light penetration depth | 0.64 * | 0.79 * | −0.13 | −0.11 | 1.00 | ||
| Manual plant height | 0.15 | 0.94 * | −0.18 | 0.31 | 0.52 * | 1.00 | |
| Fresh biomass | 0.89 * | 0.69 * | 0.08 | 0.67 * | 0.62 * | −0.02 | 1.00 |
| Varieties | Type (Fiber, Floral or Dual Purpose) | Digital Biomass (cm3) | Digital Height (mm) | Leaf Angle (°) | Leaf Area Index | Light Penetration Depth (mm) | Manual Plant Height (cm) |
|---|---|---|---|---|---|---|---|
| Arrowhead Abacus | Floral | 3915 abc | 183 bcde | 54 ab | 0.123 abcd | 153.13 bc | 17.67 bcde |
| Arrowhead Select | Floral | 2035 abc | 113 de | 53 ab | 0.1046 abcdef | 104.88 bc | 10.33 f |
| Bubbatonic | Floral | 5436 ab | 232 abcde | 55 a | 0.135 ab | 185.69 abc | 23.00 bc |
| Hurricane Hemp | Floral | 4235 abc | 193 bcde | 51 abc | 0.1283 abc | 185.41 abc | 17.67 bcde |
| Hurricane Hemp: Florence | Floral | 777 c | 107 e | 51 abc | 0.042 ef | 42.27 c | 11.17 ef |
| Lifter | Floral | 1303 bc | 144 cde | 52 abc | 0.051 def | 49.93 c | 15.67 cde |
| Suver Haze | Floral | 2912 abc | 166 bcde | 51 abc | 0.101 abcdef | 160.57 abc | 16.00 cde |
| White CBG | Floral | 3231 abc | 163 cde | 53 ab | 0.114 abcde | 158.17 abc | 16.00 cde |
| Bialobrzeski | Fiber | 5121 ab | 246 abc | 56 a | 0.125 abcd | 232.87 ab | 23.00 bc |
| Boxwine | Fiber | 3372 abc | 154 cde | 55 a | 0.126 abc | 132.45 bc | 13.00 ef |
| Boxwine 2.0 | Fiber | 2143 abc | 152 cde | 51 abc | 0.081 abcdef | 68.72 c | 16.67 cde |
| Han-Ne | Fiber | 5693 a | 226 abcde | 45 bc | 0.140 a | 110.28 bc | 19.67 bcde |
| Jinma | Fiber | 2910 abc | 260 abc | 48 abc | 0.065 bcdef | 128.33 bc | 25.67 b |
| Lara | Fiber | 1840 abc | 165 bcde | 47 abc | 0.070 abcdef | 163.16 abc | 16.00 cde |
| Puma | Fiber | 3625 abc | 225 ab | 51 abc | 0.079 abcdef | 140.44 bc | 23.67 b |
| Rincon | Fiber | 2092 abc | 163 cde | 55 a | 0.074 abcdef | 104.24 bc | 15.67 cde |
| Rogue | Fiber | 2155 abc | 149 cde | 49 abc | 0.084 abcdef | 124.3 bc | 15.00 de |
| Magic Bullet | Dual purpose | 739 c | 96 e | 52 abc | 0.041 ef | 34.74 c | 10.33 f |
| Photo CBD | Dual purpose | 1314 bc | 133 cde | 51 abc | 0.057 cdef | 53.25 c | 15.33 cde |
| Abound | Dual purpose | 3232 abc | 243 abcd | 54 ab | 0.077 abcdef | 120.54 bc | 25.67 b |
| Anka | Dual purpose | 5713 a | 358 a | 51 abc | 0.094 abcdef | 308.98 a | 40.67 a |
| Ba0x Hybrid 2.0 | Dual purpose | 1938 abc | 112 de | 53 ab | 0.0863 abcdef | 74.37 c | 11.00 f |
| BaOx Hybrid | Dual purpose | 2498 abc | 177 cde | 55 a | 0.078 abcdef | 65.25 c | 18.33 bcde |
| BaOx Select | Dual purpose | 2815 abc | 153 cde | 51 abc | 0.106 abcdef | 139.09 bc | 14.67 de |
| Hlesia | Dual purpose | 2443 abc | 187 bcde | 52 abc | 0.075 abcdef | 182.38 abc | 17.67 bcde |
| Hliana | Dual purpose | 2446 abc | 153 cde | 53 ab | 0.0923 abcdef | 130.71 bc | 14.33 ef |
| Joey | Dual purpose | 1273 bc | 212 bcde | 43 c | 0.033 f | 88.17 bc | 13.67 ef |
| Varieties | Type (Fiber, Floral or Dual Purpose) | Digital Biomass (cm3) | Digital Height (mm) | Leaf Angle (°) | Leaf Area Index | Light Penetration Depth (mm) | Manual Plant Height (cm) |
|---|---|---|---|---|---|---|---|
| Arrowhead Abacus | Floral | 11,141 abc | 443 d | 44 ab | 0.112 ab | 419.76 a | 44.33 cdef |
| Arrowhead Select | Floral | 6296 abc | 324 de | 49 a | 0.088 ab | 198.93 bc | 32.00 ef |
| Bubbatonic | Floral | 5017 abc | 413 de | 46 a | 0.055 ab | 318.06 abc | 46.33 cdef |
| Hurricane Hemp | Floral | 8712 abc | 443 d | 48 a | 0.088 ab | 430.99 a | 55.00 bcdef |
| Hurricane Hemp: Florence | Floral | 5618 abc | 364 de | 46 ab | 0.073 ab | 350.00 abc | 40.33 def |
| Lifter | Floral | 4159 abc | 303 de | 47 a | 0.061 ab | 254.06 abc | 30.00 f |
| Suver Haze | Floral | 6916 abc | 389 de | 43 ab | 0.077 ab | 384.76 abc | 40.00 def |
| White CBG | Floral | 4815 abc | 363 de | 47 a | 0.058 ab | 346.30 abc | 30.67 f |
| Bialobrzeski | Fiber | 9932 abc | 698 c | 46 ab | 0.231 a | 259.01 abc | 70.67 abcd |
| Boxwine | Fiber | 7352 abc | 338 de | 51 a | 0.097 ab | 279.93 abc | 34.67 ef |
| Boxwine 2.0 | Fiber | 3912 abc | 391 de | 45 ab | 0.046 ab | 260.03 abc | 44.33 cdef |
| Han-Ne | Fiber | 4788 abc | 701 bc | 45 ab | 0.161 ab | 187.41 c | 96.00 a |
| Jinma | Fiber | 7163 bc | 801 abc | 42 ab | 0.051 ab | 252.02 abc | 84.00 ab |
| Lara | Fiber | 5113 abc | 332 de | 47 a | 0.068 ab | 328.64 abc | 39.67 def |
| Puma | Fiber | 1675 c | 867 ab | 34 b | 0.021 b | 382.37 abc | 91.67 a |
| Rincon | Fiber | 2969 bc | 261 e | 48 a | 0.051 ab | 251.36 abc | 28.33 f |
| Rogue | Fiber | 4767 abc | 295 de | 45 ab | 0.071 ab | 291.88 abc | 41.67 cdef |
| Magic Bullet | Dual purpose | 5220 abc | 354 de | 49 a | 0.065 ab | 322.78 abc | 37.67 ef |
| Photo CBD | Dual purpose | 2800 c | 301 de | 46 ab | 0.042 ab | 201.65 bc | 32.67 ef |
| Abound | Dual purpose | 23,227 a | 723 abc | 45 ab | 0.186 ab | 374.64 abc | 73.33 abc |
| Anka | Dual purpose | 21,139 ab | 871 a | 44 ab | 0.192 ab | 292.15 abc | 89.33 a |
| BaOX Hybrid | Dual purpose | 7820 abc | 428 de | 46 a | 0.081 ab | 410.51 ab | 48.00 cdef |
| BaOX Hybrid 2.0 | Dual purpose | 5122 abc | 375 de | 46 ab | 0.066 ab | 302.25 abc | 38.67 def |
| BaOX Select | Dual purpose | 8566 abc | 408 de | 47 a | 0.093 ab | 401.60 ab | 45.67 cdef |
| Hlesia | Dual purpose | 1158 c | 819 abc | 44 ab | 0.013 b | 241.52 abc | 63.67 abcde |
| Hliana | Dual purpose | 3599 bc | 391 de | 47 a | 0.043 ab | 239.57 abc | 43.00 cdef |
| Joey | Dual purpose | 10,207 abc | 430 d | 49 a | 0.106 ab | 421.87 a | 44.33 cdef |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, G.; Slonecki, T.; Wadl, P.; Flessner, M.; Sosnoskie, L.; Hatterman-Valenti, H.; Gage, K.; Cutulle, M. Implementing Digital Multispectral 3D Scanning Technology for Rapid Assessment of Hemp (Cannabis sativa L.) Weed Competitive Traits. Remote Sens. 2024, 16, 2375. https://doi.org/10.3390/rs16132375
Singh G, Slonecki T, Wadl P, Flessner M, Sosnoskie L, Hatterman-Valenti H, Gage K, Cutulle M. Implementing Digital Multispectral 3D Scanning Technology for Rapid Assessment of Hemp (Cannabis sativa L.) Weed Competitive Traits. Remote Sensing. 2024; 16(13):2375. https://doi.org/10.3390/rs16132375
Chicago/Turabian StyleSingh, Gursewak, Tyler Slonecki, Philip Wadl, Michael Flessner, Lynn Sosnoskie, Harlene Hatterman-Valenti, Karla Gage, and Matthew Cutulle. 2024. "Implementing Digital Multispectral 3D Scanning Technology for Rapid Assessment of Hemp (Cannabis sativa L.) Weed Competitive Traits" Remote Sensing 16, no. 13: 2375. https://doi.org/10.3390/rs16132375
APA StyleSingh, G., Slonecki, T., Wadl, P., Flessner, M., Sosnoskie, L., Hatterman-Valenti, H., Gage, K., & Cutulle, M. (2024). Implementing Digital Multispectral 3D Scanning Technology for Rapid Assessment of Hemp (Cannabis sativa L.) Weed Competitive Traits. Remote Sensing, 16(13), 2375. https://doi.org/10.3390/rs16132375








