Non-Celiac Gluten Sensitivity in the Context of Functional Gastrointestinal Disorders
Abstract
:1. Introduction
2. The Boom of Gluten-Free Diets
3. Ancient and Modern Wheats
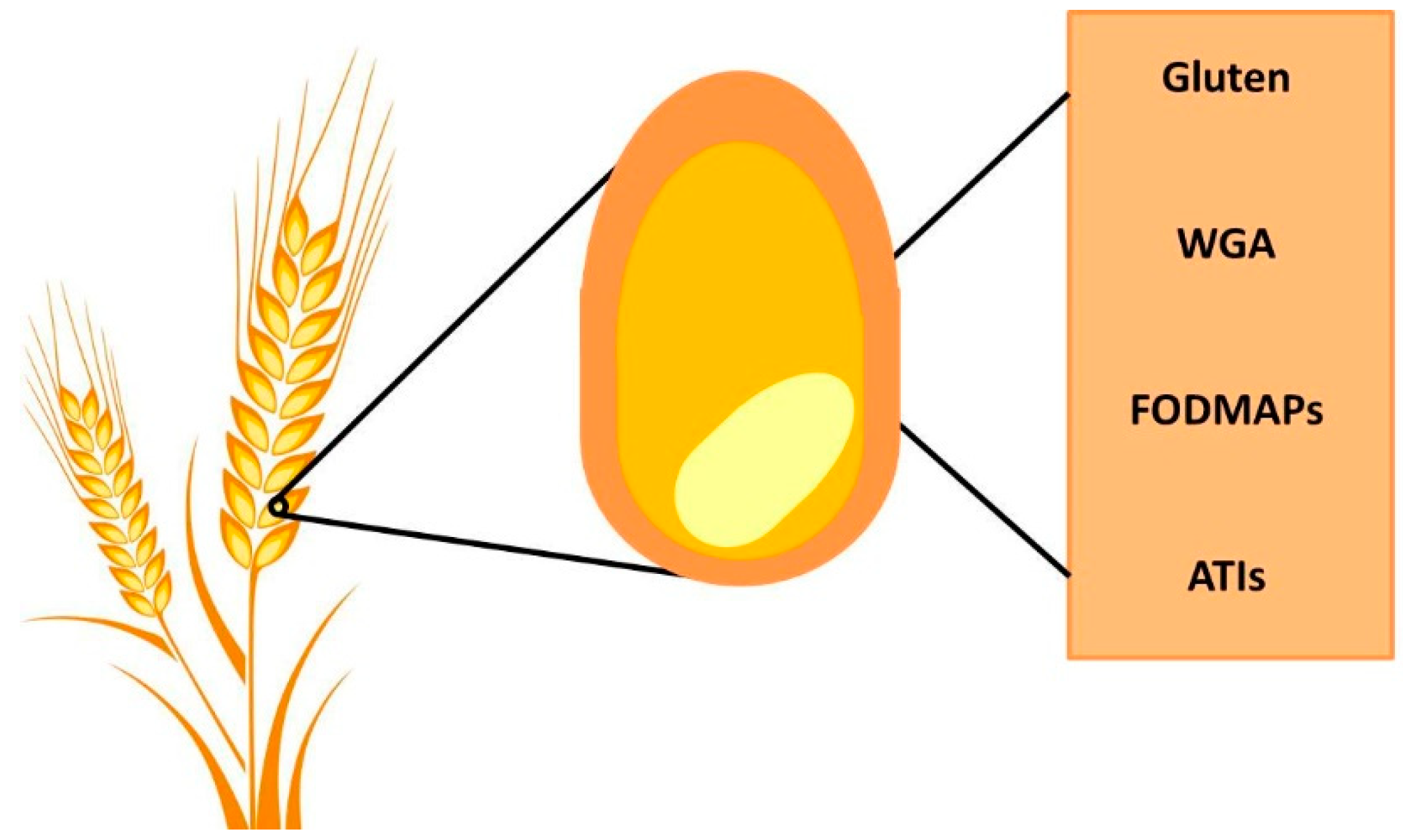
4. Wheat Components and their Impact on Gut Physiology
4.1. Gluten
4.2. α-Amylase/Trypsin Inhibitors (ATIs)
4.3. FODMAPs
4.4. Wheat Germ Agglutinin (WGA)
5. Non-Celiac Gluten Sensitivity
5.1. Epidemiology
5.2. Clinical Characteristics
5.3. Diagnosis
5.4. Pathophysiology
6. Overlap between Gluten Sensitivity and Disorders of Gut–Brain Interaction
6.1. Symptoms of DGBI in Patients with NCGS
6.2. NCGS among Patients Diagnosed as IBS
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bohn, L.; Storsrud, S.; Tornblom, H.; Bengtsson, U.; Simren, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera-Chavez, F.; Dezar, G.V.; Islas-Zamorano, A.P.; Espinoza-Alderete, J.G.; Vergara-Jimenez, M.J.; Magana-Ordorica, D.; Ontiveros, N. Prevalence of Self-Reported Gluten Sensitivity and Adherence to a Gluten-Free Diet in Argentinian Adult Population. Nutrients 2017, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Chavez, F.; Granda-Restrepo, D.M.; Aramburo-Galvez, J.G.; Franco-Aguilar, A.; Magana-Ordorica, D.; Vergara-Jimenez Mde, J.; Ontiveros, N. Self-Reported Prevalence of Gluten-Related Disorders and Adherence to Gluten-Free Diet in Colombian Adult Population. Gastroenterol. Res. Pract. 2016, 2016, 4704309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Gils, T.; Nijeboer, P.; CE, I.J.; Sanders, D.S.; Mulder, C.J.; Bouma, G. Prevalence and Characterization of Self-Reported Gluten Sensitivity in The Netherlands. Nutrients 2016, 8, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golley, S.; Corsini, N.; Topping, D.; Morell, M.; Mohr, P. Motivations for avoiding wheat consumption in Australia: Results from a population survey. Public Health Nutr. 2015, 18, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Ontiveros, N.; Lopez-Gallardo, J.A.; Vergara-Jimenez, M.J.; Cabrera-Chavez, F. Self-Reported Prevalence of Symptomatic Adverse Reactions to Gluten and Adherence to Gluten-Free Diet in an Adult Mexican Population. Nutrients 2015, 7, 6000–6015. [Google Scholar] [CrossRef] [Green Version]
- Aziz, I.; Lewis, N.R.; Hadjivassiliou, M.; Winfield, S.N.; Rugg, N.; Kelsall, A.; Newrick, L.; Sanders, D.S. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur. J. Gastroenterol. Hepatol. 2014, 26, 33–39. [Google Scholar] [CrossRef]
- Tanpowpong, P.; Ingham, T.R.; Lampshire, P.K.; Kirchberg, F.F.; Epton, M.J.; Crane, J.; Camargo, C.A., Jr. Coeliac disease and gluten avoidance in New Zealand children. Arch. Dis. Child. 2012, 97, 12–16. [Google Scholar] [CrossRef]
- Carroccio, A.; Giambalvo, O.; Blasca, F.; Iacobucci, R.; D’Alcamo, A.; Mansueto, P. Self-Reported Non-Celiac Wheat Sensitivity in High School Students: Demographic and Clinical Characteristics. Nutrients 2017, 9, 771. [Google Scholar] [CrossRef] [Green Version]
- Potter, M.D.E.; Walker, M.M.; Jones, M.P.; Koloski, N.A.; Keely, S.; Talley, N.J. Wheat Intolerance and Chronic Gastrointestinal Symptoms in an Australian Population-based Study: Association Between Wheat Sensitivity, Celiac Disease and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2018, 113, 1036–1044. [Google Scholar] [CrossRef]
- Potter, M.; Jones, M.P.; Walker, M.M.; Koloski, N.A.; Keely, S.; Holtmann, G.; Talley Ac, N.J. Incidence and prevalence of self-reported non-coeliac wheat sensitivity and gluten avoidance in Australia. Med. J. Aust. 2020, 212, 126–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, I. The Global Phenomenon of Self-Reported Wheat Sensitivity. Am. J. Gastroenterol. 2018, 113, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Foschia, M.; Horstmann, S.; Arendt, E.K.; Zannini, E. Nutritional therapy—Facing the gap between coeliac disease and gluten-free food. Int. J. Food Microbiol. 2016, 239, 113–124. [Google Scholar] [CrossRef]
- Research, G.V. Gluten-Free Products Market Size, Share & Trends Analysis Report by Product (Bakery Products, Dairy/Dairy Alternatives), by Distribution Channel (Grocery Stores, Mass Merchandiser), by Region, and Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/gluten-free-products-market (accessed on 1 July 2020).
- Dieterich, W.; Zopf, Y. Gluten and FODMAPS-Sense of a Restriction/When Is Restriction Necessary? Nutrients 2019, 11, 1957. [Google Scholar] [CrossRef] [Green Version]
- Lerner, B.A.; Green, P.H.R.; Lebwohl, B. Going against the Grains: Gluten-Free Diets in Patients without Celiac Disease-Worthwhile or Not? Dig. Dis. Sci. 2019, 64, 1740–1747. [Google Scholar] [CrossRef]
- The Hartman Group, Inc. Eating Gluten-Free. Available online: https://www.hartman-group.com/acumenPdfs/gluten-free-9_13_18.pdf (accessed on 1 July 2020).
- Lis, D.M. Exit Gluten-Free and Enter Low FODMAPs: A Novel Dietary Strategy to Reduce Gastrointestinal Symptoms in Athletes. Sports Med. 2019, 49, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Croall, I.D.; Trott, N.; Rej, A.; Aziz, I.; O’Brien, D.J.; George, H.A.; Hossain, M.Y.; Marks, L.J.S.; Richardson, J.I.; Rigby, R.; et al. A Population Survey of Dietary Attitudes towards Gluten. Nutrients 2019, 11, 1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, M.D.E.; Brienesse, S.C.; Walker, M.M.; Boyle, A.; Talley, N.J. Effect of the gluten-free diet on cardiovascular risk factors in patients with coeliac disease: A systematic review. J. Gastroenterol. Hepatol. 2018, 33, 781–791. [Google Scholar] [CrossRef]
- Hallert, C.; Grant, C.; Grehn, S.; Granno, C.; Hulten, S.; Midhagen, G.; Strom, M.; Svensson, H.; Valdimarsson, T. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment. Pharmacol. Ther. 2002, 16, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Makovicky, P.; Makovicky, P.; Caja, F.; Rimarova, K.; Samasca, G.; Vannucci, L. Celiac disease and gluten-free diet: Past, present, and future. Gastroenterol. Hepatol. Bed Bench 2020, 13, 1–7. [Google Scholar]
- Bulka, C.M.; Davis, M.A.; Karagas, M.R.; Ahsan, H.; Argos, M. The Unintended Consequences of a Gluten-free Diet. Epidemiology 2017, 28, e24–e25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludvigsson, J.F.; Lebwohl, B.; Chen, Q.; Broms, G.; Wolf, R.L.; Green, P.H.R.; Emilsson, L. Anxiety after coeliac disease diagnosis predicts mucosal healing: A population-based study. Aliment. Pharmacol. Ther. 2018, 48, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Croall, I.D.; Aziz, I.; Trott, N.; Tosi, P.; Hoggard, N.; Sanders, D.S. Gluten Does Not Induce Gastrointestinal Symptoms in Healthy Volunteers: A Double-Blind Randomized Placebo Trial. Gastroenterology 2019, 157, 881–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spisni, E.; Imbesi, V.; Giovanardi, E.; Petrocelli, G.; Alvisi, P.; Valerii, M.C. Differential Physiological Responses Elicited by Ancient and Heritage Wheat Cultivars Compared to Modern Ones. Nutrients 2019, 11, 2879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prandi, B.; Tedeschi, T.; Folloni, S.; Galaverna, G.; Sforza, S. Peptides from gluten digestion: A comparison between old and modern wheat varieties. Food Res. Int. 2017, 91, 92–102. [Google Scholar] [CrossRef]
- Gianfrani, C.; Camarca, A.; Mazzarella, G.; Di Stasio, L.; Giardullo, N.; Ferranti, P.; Picariello, G.; Rotondi Aufiero, V.; Picascia, S.; Troncone, R.; et al. Extensive in vitro gastrointestinal digestion markedly reduces the immune-toxicity of Triticum monococcum wheat: Implication for celiac disease. Mol. Nutr. Food Res. 2015, 59, 1844–1854. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Prandi, B.; Amaretti, A.; Anfelli, I.; Leonardi, A.; Raimondi, S.; Pecchioni, N.; De Vita, P.; Faccini, A.; Sforza, S.; et al. Comparison of gluten peptides and potential prebiotic carbohydrates in old and modern Triticum turgidum ssp. genotypes. Food Res. Int. 2019, 120, 568–576. [Google Scholar] [CrossRef]
- Shewry, P. What Is Gluten-Why Is It Special? Front. Nutr. 2019, 6, 101. [Google Scholar] [CrossRef]
- Balakireva, A.V.; Zamyatnin, A.A. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxification Capabilities. Nutrients 2016, 8, 644. [Google Scholar] [CrossRef] [Green Version]
- Silano, M.; Vincentini, O.; De Vincenzi, M. Toxic, immunostimulatory and antagonist gluten peptides in celiac disease. Curr. Med. Chem. 2009, 16, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Picarelli, A.; Di Tola, M.; Sabbatella, L.; Anania, M.C.; Di Cello, T.; Greco, R.; Silano, M.; De Vincenzi, M. 31-43 amino acid sequence of the alpha-gliadin induces anti-endomysial antibody production during in vitro challenge. Scand. J. Gastroenterol. 1999, 34, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Lammers, K.M.; Lu, R.; Brownley, J.; Lu, B.; Gerard, C.; Thomas, K.; Rallabhandi, P.; Shea-Donohue, T.; Tamiz, A.; Alkan, S.; et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 2008, 135, 194–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuppan, D. Current concepts of celiac disease pathogenesis. Gastroenterology 2000, 119, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, L.; Ciacci, C.; Ricciardelli, I.; Vacca, L.; Raia, V.; Auricchio, S.; Picard, J.; Osman, M.; Quaratino, S.; Londei, M. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet 2003, 362, 30–37. [Google Scholar] [CrossRef]
- Frossi, B.; Tripodo, C.; Guarnotta, C.; Carroccio, A.; De Carli, M.; De Carli, S.; Marino, M.; Calabro, A.; Pucillo, C.E. Mast cells are associated with the onset and progression of celiac disease. J. Allergy Clin. Immunol. 2017, 139, 1266–1274. [Google Scholar] [CrossRef] [Green Version]
- Andren Aronsson, C.; Lee, H.S.; Hard Af Segerstad, E.M.; Uusitalo, U.; Yang, J.; Koletzko, S.; Liu, E.; Kurppa, K.; Bingley, P.J.; Toppari, J.; et al. Association of Gluten Intake During the First 5 Years of Life With Incidence of Celiac Disease Autoimmunity and Celiac Disease Among Children at Increased Risk. JAMA 2019, 322, 514–523. [Google Scholar] [CrossRef]
- Olivares, M.; Neef, A.; Castillejo, G.; Palma, G.D.; Varea, V.; Capilla, A.; Palau, F.; Nova, E.; Marcos, A.; Polanco, I.; et al. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut 2015, 64, 406–417. [Google Scholar] [CrossRef]
- Dieterich, W.; Schuppan, D.; Schink, M.; Schwappacher, R.; Wirtz, S.; Agaimy, A.; Neurath, M.F.; Zopf, Y. Influence of low FODMAP and gluten-free diets on disease activity and intestinal microbiota in patients with non-celiac gluten sensitivity. Clin. Nutr. 2019, 38, 697–707. [Google Scholar] [CrossRef]
- Bertini, I.; Calabro, A.; De Carli, V.; Luchinat, C.; Nepi, S.; Porfirio, B.; Renzi, D.; Saccenti, E.; Tenori, L. The metabonomic signature of celiac disease. J. Proteome Res. 2009, 8, 170–177. [Google Scholar] [CrossRef]
- Karakula-Juchnowicz, H.; Rog, J.; Juchnowicz, D.; Loniewski, I.; Skonieczna-Zydecka, K.; Krukow, P.; Futyma-Jedrzejewska, M.; Kaczmarczyk, M. The study evaluating the effect of probiotic supplementation on the mental status, inflammation, and intestinal barrier in major depressive disorder patients using gluten-free or gluten-containing diet (SANGUT study): A 12-week, randomized, double-blind, and placebo-controlled clinical study protocol. Nutr. J. 2019, 18, 50. [Google Scholar] [CrossRef] [Green Version]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Russel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells. Gastroenterology 2017, 152, 1100–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, R. How do FODMAPs work? J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 36–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanayakkara, W.S.; Skidmore, P.M.; O’Brien, L.; Wilkinson, T.J.; Gearry, R.B. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: The evidence to date. Clin. Exp. Gastroenterol. 2016, 9, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, J.S.; Gearry, R.B.; Muir, J.G.; Irving, P.M.; Rose, R.; Rosella, O.; Haines, M.L.; Shepherd, S.J.; Gibson, P.R. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment. Pharmacol. Ther. 2010, 31, 874–882. [Google Scholar] [CrossRef]
- Magge, S.; Lembo, A. Low-FODMAP Diet for Treatment of Irritable Bowel Syndrome. Gastroenterol. Hepatol. 2012, 8, 739–745. [Google Scholar]
- de Punder, K.; Pruimboom, L. The dietary intake of wheat and other cereal grains and their role in inflammation. Nutrients 2013, 5, 771–787. [Google Scholar] [CrossRef] [Green Version]
- Dalla Pellegrina, C.; Perbellini, O.; Scupoli, M.T.; Tomelleri, C.; Zanetti, C.; Zoccatelli, G.; Fusi, M.; Peruffo, A.; Rizzi, C.; Chignola, R. Effects of wheat germ agglutinin on human gastrointestinal epithelium: Insights from an experimental model of immune/epithelial cell interaction. Toxicol. Appl. Pharmacol. 2009, 237, 146–153. [Google Scholar] [CrossRef]
- Haas, H.; Falcone, F.H.; Schramm, G.; Haisch, K.; Gibbs, B.F.; Klaucke, J.; Poppelmann, M.; Becker, W.M.; Gabius, H.J.; Schlaak, M. Dietary lectins can induce in vitro release of IL-4 and IL-13 from human basophils. Eur. J. Immunol. 1999, 29, 918–927. [Google Scholar] [CrossRef]
- Muraille, E.; Pajak, B.; Urbain, J.; Leo, O. Carbohydrate-bearing cell surface receptors involved in innate immunity: Interleukin-12 induction by mitogenic and nonmitogenic lectins. Cell Immunol. 1999, 191, 1–9. [Google Scholar] [CrossRef]
- Sodhi, A.; Kesherwani, V. Production of TNF-alpha, IL-1beta, IL-12 and IFN-gamma in murine peritoneal macrophages on treatment with wheat germ agglutinin in vitro: Involvement of tyrosine kinase pathways. Glycoconj. J. 2007, 24, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Capannolo, A.; Viscido, A.; Barkad, M.A.; Valerii, G.; Ciccone, F.; Melideo, D.; Frieri, G.; Latella, G. Non-Celiac Gluten Sensitivity among Patients Perceiving Gluten-Related Symptoms. Digestion 2015, 92, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, R.; Cristofori, F.; Castellaneta, S.; Polloni, C.; Albano, V.; Dellatte, S.; Indrio, F.; Cavallo, L.; Catassi, C. Clinical, serologic, and histologic features of gluten sensitivity in children. J. Pediatr. 2014, 164, 463–467.e1. [Google Scholar] [CrossRef]
- Carroccio, A.; Mansueto, P.; Iacono, G.; Soresi, M.; D’Alcamo, A.; Cavataio, F.; Brusca, I.; Florena, A.M.; Ambrosiano, G.; Seidita, A.; et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: Exploring a new clinical entity. Am. J. Gastroenterol. 2012, 107, 1898–1906; quiz 1907. [Google Scholar] [CrossRef] [Green Version]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Lionetti, E.; Pulvirenti, A.; Vallorani, M.; Catassi, G.; Verma, A.K.; Gatti, S.; Catassi, C. Re-challenge Studies in Non-celiac Gluten Sensitivity: A Systematic Review and Meta-Analysis. Front. Physiol. 2017, 8, 621. [Google Scholar] [CrossRef] [Green Version]
- Molina-Infante, J.; Carroccio, A. Suspected Nonceliac Gluten Sensitivity Confirmed in Few Patients After Gluten Challenge in Double-Blind, Placebo-Controlled Trials. Clin. Gastroenterol. Hepatol. 2017, 15, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Volta, U.; Bardella, M.T.; Calabro, A.; Troncone, R.; Corazza, G.R.; The Study Group for Non-Celiac Gluten Sensitivity. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef] [Green Version]
- Volta, U.; Tovoli, F.; Cicola, R.; Parisi, C.; Fabbri, A.; Piscaglia, M.; Fiorini, E.; Caio, G. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J. Clin. Gastroenterol. 2012, 46, 680–685. [Google Scholar] [CrossRef]
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollon, J.; Puppa, E.L.; Greenwald, B.; Goldberg, E.; Guerrerio, A.; Fasano, A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients 2015, 7, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Lammers, K.M.; Casolaro, V.; Cammarota, M.; Giuliano, M.T.; De Rosa, M.; Stefanile, R.; Mazzarella, G.; Tolone, C.; Russo, M.I.; et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: Celiac disease and gluten sensitivity. BMC Med. 2011, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbaro, M.R.; Cremon, C.; Morselli-Labate, A.M.; Di Sabatino, A.; Giuffrida, P.; Corazza, G.R.; Di Stefano, M.; Caio, G.; Latella, G.; Ciacci, C.; et al. Serum zonulin and its diagnostic performance in non-coeliac gluten sensitivity. Gut 2020, 69, 1966–1974. [Google Scholar] [CrossRef]
- Ajamian, M.; Steer, D.; Rosella, G.; Gibson, P.R. Serum zonulin as a marker of intestinal mucosal barrier function: May not be what it seems. PLoS ONE 2019, 14, e0210728. [Google Scholar] [CrossRef]
- Scheffler, L.; Crane, A.; Heyne, H.; Tonjes, A.; Schleinitz, D.; Ihling, C.H.; Stumvoll, M.; Freire, R.; Fiorentino, M.; Fasano, A.; et al. Widely Used Commercial ELISA Does Not Detect Precursor of Haptoglobin2, but Recognizes Properdin as a Potential Second Member of the Zonulin Family. Front. Endocrinol. 2018, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A. Zonulin measurement conundrum: Add confusion to confusion does not lead to clarity. Gut 2020. [Google Scholar] [CrossRef]
- Brottveit, M.; Beitnes, A.C.; Tollefsen, S.; Bratlie, J.E.; Jahnsen, F.L.; Johansen, F.E.; Sollid, L.M.; Lundin, K.E. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am. J. Gastroenterol. 2013, 108, 842–850. [Google Scholar] [CrossRef]
- Barbaro, M.R.; Di Sabatino, A.; Cremon, C.; Giuffrida, P.; Fiorentino, M.; Altimari, A.; Bellacosa, L.; Stanghellini, V.; Barbara, G. Interferon-gamma is increased in the gut of patients with irritable bowel syndrome and modulates serotonin metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G439–G447. [Google Scholar] [CrossRef] [Green Version]
- Carroccio, A.; Giannone, G.; Mansueto, P.; Soresi, M.; La Blasca, F.; Fayer, F.; Iacobucci, R.; Porcasi, R.; Catalano, T.; Geraci, G.; et al. Duodenal and Rectal Mucosa Inflammation in Patients with Non-celiac Wheat Sensitivity. Clin. Gastroenterol. Hepatol. 2019, 17, 682–690. [Google Scholar] [CrossRef]
- Picarelli, A.; Borghini, R.; Di Tola, M.; Marino, M.; Urciuoli, C.; Isonne, C.; Puzzono, M.; Porowska, B.; Rumi, G.; Lonardi, S.; et al. Intestinal, Systemic, and Oral Gluten-related Alterations in Patients with Nonceliac Gluten Sensitivity. J. Clin. Gastroenterol. 2016, 50, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Uhde, M.; Caio, G.; De Giorgio, R.; Green, P.H.; Volta, U.; Alaedini, A. Subclass Profile of IgG Antibody Response to Gluten Differentiates Nonceliac Gluten Sensitivity From Celiac Disease. Gastroenterology 2020. [Google Scholar] [CrossRef] [PubMed]
- Giancola, F.; Volta, U.; Repossi, R.; Latorre, R.; Beeckmans, D.; Carbone, F.; Van den Houte, K.; Bianco, F.; Bonora, E.; Gori, A.; et al. Mast cell-nerve interactions correlate with bloating and abdominal pain severity in patients with non-celiac gluten/wheat sensitivity. Neurogastroenterol. Motil. 2020, 32, e13814. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Rivera-Gutierrez, X.; Cobos-Quevedo, O.J.; Grube-Pagola, P.; Meixueiro-Daza, A.; Hernandez-Flores, K.; Cabrera-Jorge, F.J.; Vivanco-Cid, H.; Dowd, S.E.; Remes-Troche, J.M. First Insights into the Gut Microbiota of Mexican Patients with Celiac Disease and Non-Celiac Gluten Sensitivity. Nutrients 2018, 10, 1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efthymakis, K.; Clemente, E.; Marchioni, M.; Di Nicola, M.; Neri, M.; Sallese, M. An Exploratory Gene Expression Study of the Intestinal Mucosa of Patients with Non-Celiac Wheat Sensitivity. Int. J. Mol. Sci. 2020, 21, 1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Sabatino, A.; Volta, U.; Salvatore, C.; Biancheri, P.; Caio, G.; De Giorgio, R.; Di Stefano, M.; Corazza, G.R. Small Amounts of Gluten in Subjects With Suspected Nonceliac Gluten Sensitivity: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1604–1612. [Google Scholar] [CrossRef]
- Elli, L.; Tomba, C.; Branchi, F.; Roncoroni, L.; Lombardo, V.; Bardella, M.T.; Ferretti, F.; Conte, D.; Valiante, F.; Fini, L.; et al. Evidence for the Presence of Non-Celiac Gluten Sensitivity in Patients with Functional Gastrointestinal Symptoms: Results from a Multicenter Randomized Double-Blind Placebo-Controlled Gluten Challenge. Nutrients 2016, 8, 84. [Google Scholar] [CrossRef]
- Francavilla, R.; Cristofori, F.; Verzillo, L.; Gentile, A.; Castellaneta, S.; Polloni, C.; Giorgio, V.; Verduci, E.; D’Angelo, E.; Dellatte, S.; et al. Randomized Double-Blind Placebo-Controlled Crossover Trial for the Diagnosis of Non-Celiac Gluten Sensitivity in Children. Am. J. Gastroenterol. 2018, 113, 421–430. [Google Scholar] [CrossRef]
- Peters, S.L.; Biesiekierski, J.R.; Yelland, G.W.; Muir, J.G.; Gibson, P.R. Randomised clinical trial: Gluten may cause depression in subjects with non-coeliac gluten sensitivity—An exploratory clinical study. Aliment. Pharmacol. Ther. 2014, 39, 1104–1112. [Google Scholar] [CrossRef] [Green Version]
- Gibson, P.R.; Lundin, K.E.A.; Guandalini, S. Is Non-Celiac Rice-Starch Sensitivity as Common in Children as Non-Celiac Gluten Sensitivity? Am. J. Gastroenterol. 2018, 113, 1254. [Google Scholar] [CrossRef]
- Zanini, B.; Basche, R.; Ferraresi, A.; Ricci, C.; Lanzarotto, F.; Marullo, M.; Villanacci, V.; Hidalgo, A.; Lanzini, A. Randomised clinical study: Gluten challenge induces symptom recurrence in only a minority of patients who meet clinical criteria for non-coeliac gluten sensitivity. Aliment. Pharmacol. Ther. 2015, 42, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierod, M.B.; Henriksen, C.; Lundin, K.E.A. Fructan, Rather Than Gluten, Induces Symptoms in Patients With Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018, 154, 529–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haro, C.; Villatoro, M.; Vaquero, L.; Pastor, J.; Gimenez, M.J.; Ozuna, C.V.; Sanchez-Leon, S.; Garcia-Molina, M.D.; Segura, V.; Comino, I.; et al. The Dietary Intervention of Transgenic Low-Gliadin Wheat Bread in Patients with Non-Celiac Gluten Sensitivity (NCGS) Showed No Differences with Gluten Free Diet (GFD) but Provides Better Gut Microbiota Profile. Nutrients 2018, 10, 1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncoroni, L.; Bascunan, K.A.; Vecchi, M.; Doneda, L.; Bardella, M.T.; Lombardo, V.; Scricciolo, A.; Branchi, F.; Elli, L. Exposure to Different Amounts of Dietary Gluten in Patients with Non-Celiac Gluten Sensitivity (NCGS): An Exploratory Study. Nutrients 2019, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Carroccio, A.; Rini, G.; Mansueto, P. Non-celiac wheat sensitivity is a more appropriate label than non-celiac gluten sensitivity. Gastroenterology 2014, 146, 320–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakkoli, A.; Lewis, S.K.; Tennyson, C.A.; Lebwohl, B.; Green, P.H. Characteristics of patients who avoid wheat and/or gluten in the absence of Celiac disease. Dig. Dis. Sci. 2014, 59, 1255–1261. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328. [Google Scholar] [CrossRef]
- Rej, A.; Trott, N.; Aziz, I.; Sanders, D.S. A Gluten-Free Diet: The Express Route to Fructan Reduction. Am. J. Gastroenterol. 2019, 114, 1553. [Google Scholar] [CrossRef]
- Roncoroni, L.; Bascunan, K.A.; Doneda, L.; Scricciolo, A.; Lombardo, V.; Branchi, F.; Ferretti, F.; Dell’Osso, B.; Montanari, V.; Bardella, M.T.; et al. A Low FODMAP Gluten-Free Diet Improves Functional Gastrointestinal Disorders and Overall Mental Health of Celiac Disease Patients: A Randomized Controlled Trial. Nutrients 2018, 10, 1023. [Google Scholar] [CrossRef] [Green Version]
- Dale, H.F.; Hatlebakk, J.G.; Hovdenak, N.; Ystad, S.O.; Lied, G.A. The effect of a controlled gluten challenge in a group of patients with suspected non-coeliac gluten sensitivity: A randomized, double-blind placebo-controlled challenge. Neurogastroenterol. Motil. 2018, 30, e13332. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Newnham, E.D.; Irving, P.M.; Barrett, J.S.; Haines, M.; Doecke, J.D.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Gluten causes gastrointestinal symptoms in subjects without celiac disease: A double-blind randomized placebo-controlled trial. Am. J. Gastroenterol. 2011, 106, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Shahbazkhani, B.; Sadeghi, A.; Malekzadeh, R.; Khatavi, F.; Etemadi, M.; Kalantri, E.; Rostami-Nejad, M.; Rostami, K. Non-Celiac Gluten Sensitivity Has Narrowed the Spectrum of Irritable Bowel Syndrome: A Double-Blind Randomized Placebo-Controlled Trial. Nutrients 2015, 7, 4542–4554. [Google Scholar] [CrossRef] [Green Version]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Cargiolli, M.; Cassarano, S.; Polese, B.; De Conno, B.; Aurino, L.; Mancino, N.; Sarnelli, G. Diet and functional dyspepsia: Clinical correlates and therapeutic perspectives. World J. Gastroenterol. 2020, 26, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.P. The low-FODMAP diet in the management of functional dyspepsia in East and Southeast Asia. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbazkhani, B.; Fanaeian, M.M.; Farahvash, M.J.; Aletaha, N.; Alborzi, F.; Elli, L.; Shahbazkhani, A.; Zebardast, J.; Rostami-Nejad, M. Prevalence of Non-Celiac Gluten Sensitivity in Patients with Refractory Functional Dyspepsia: A Randomized Double-blind Placebo Controlled Trial. Sci. Rep. 2020, 10, 2401. [Google Scholar] [CrossRef] [Green Version]
- Monsbakken, K.W.; Vandvik, P.O.; Farup, P.G. Perceived food intolerance in subjects with irritable bowel syndrome—Etiology, prevalence and consequences. Eur. J. Clin. Nutr. 2006, 60, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Aziz, I.; Trott, N.; Briggs, R.; North, J.R.; Hadjivassiliou, M.; Sanders, D.S. Efficacy of a Gluten-Free Diet in Subjects With Irritable Bowel Syndrome-Diarrhea Unaware of Their HLA-DQ2/8 Genotype. Clin. Gastroenterol. Hepatol. 2016, 14, 696–703. [Google Scholar] [CrossRef] [Green Version]
- Carroccio, A.; Soresi, M.; D’Alcamo, A.; Sciume, C.; Iacono, G.; Geraci, G.; Brusca, I.; Seidita, A.; Adragna, F.; Carta, M.; et al. Risk of low bone mineral density and low body mass index in patients with non-celiac wheat-sensitivity: A prospective observation study. BMC Med. 2014, 12, 230. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Nardelli, A.; Borojevic, R.; De Palma, G.; Causada Calo, N.; McCarville, J.; Caminero, A.; Basra, D.; Mordhorst, A.; Ignatova, E.; et al. Gluten-free Diet Reduces Symptoms, Particularly Diarrhea, in Patients with Irritable Bowel Syndrome and Anti-gliadin IgG. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Vazquez-Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; Marietta, E.; O’Neill, J.; Carlson, P.; Lamsam, J.; Janzow, D.; Eckert, D.; et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterology 2013, 144, 903–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritscher-Ravens, A.; Schuppan, D.; Ellrichmann, M.; Schoch, S.; Rocken, C.; Brasch, J.; Bethge, J.; Bottner, M.; Klose, J.; Milla, P.J. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014, 147, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Calasso, M.; Francavilla, R.; Cristofori, F.; De Angelis, M.; Gobbetti, M. New Protocol for Production of Reduced-Gluten Wheat Bread and Pasta and Clinical Effect in Patients with Irritable Bowel Syndrome: A randomised, Double-Blind, Cross-Over Study. Nutrients 2018, 10, 1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dionne, J.; Ford, A.C.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPs Diet in Treating Symptoms of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1290–1300. [Google Scholar] [CrossRef] [Green Version]
- Barone, M.; Gemello, E.; Viggiani, M.T.; Cristofori, F.; Renna, C.; Iannone, A.; Di Leo, A.; Francavilla, R. Evaluation of Non-Celiac Gluten Sensitivity in Patients with Previous Diagnosis of Irritable Bowel Syndrome: A Randomized Double-Blind Placebo-Controlled Crossover Trial. Nutrients 2020, 12, 705. [Google Scholar] [CrossRef] [Green Version]
- Cangemi, D.J.; Lacy, B.E. Management of irritable bowel syndrome with diarrhea: A review of nonpharmacological and pharmacological interventions. Therap. Adv. Gastroenterol. 2019, 12, 1756284819878950. [Google Scholar] [CrossRef]
- Pimentel, M. Review article: Potential mechanisms of action of rifaximin in the management of irritable bowel syndrome with diarrhoea. Aliment. Pharmacol. Ther. 2016, 43 (Suppl. 1), 37–49. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Braun, C.; Enck, P. Effects of Rifaximin on Central Responses to Social Stress-a Pilot Experiment. Neurotherapeutics 2018, 15, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Kuti, D.; Winkler, Z.; Horvath, K.; Juhasz, B.; Paholcsek, M.; Stagel, A.; Gulyas, G.; Czegledi, L.; Ferenczi, S.; Kovacs, K.J. Gastrointestinal (non-systemic) antibiotic rifaximin differentially affects chronic stress-induced changes in colon microbiome and gut permeability without effect on behavior. Brain Behav. Immun. 2019. [Google Scholar] [CrossRef]
- Jackson, J.; Eaton, W.; Cascella, N.; Fasano, A.; Santora, D.; Sullivan, K.; Feldman, S.; Raley, H.; McMahon, R.P.; Carpenter, W.T., Jr.; et al. Gluten sensitivity and relationship to psychiatric symptoms in people with schizophrenia. Schizophr. Res. 2014, 159, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Busby, E.; Bold, J.; Fellows, L.; Rostami, K. Mood Disorders and Gluten: It’s Not All in Your Mind! A Systematic Review with Meta-Analysis. Nutrients 2018, 10, 1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doenyas, C. Dietary interventions for autism spectrum disorder: New perspectives from the gut-brain axis. Physiol. Behav. 2018, 194, 577–582. [Google Scholar] [CrossRef] [PubMed]


| Study (Reference) | Patients | Study Design | No. of Patients | Methods | Main Findings | Limitations |
|---|---|---|---|---|---|---|
| Biesiekierski et al. 2011 [93] | NCGS w/IBS | Randomized DBPC trial | 34 patients | Two bread slices plus one muffin per day with a GFD for up to 6 weeks | 13/19 patients (68%) in the gluten group reported inadequate control of symptoms compared with 6/15 (40%) under placebo | Nocebo effect |
| Carroccio et al. 2012 [57] | NCGS | Randomized DBPC Crossover trial | 920 patients | Capsules with wheat (20 g) vs. placebo for 2 consecutive weeks. After 1 week of wash out, patients received the other treatments for another 2 weeks | 276 (30%) of patients diagnosed as non-celiac wheat sensitivity. DBPC challenge induced >30% increase in symptoms | This is a retrospective study and the data were not recorded according to a predesigned protocol |
| Biesiekierski et al. 2013 [89] | NCGS w/IBS symptoms | Randomized DBPC Crossover trial | 37 patients | GFD for 6 weeks. Patients were randomly assigned to one of three diet treatments: high-gluten (16 g gluten/d), low-gluten (2 g gluten/d and 14 g whey protein/d), or control (16 g whey protein/d) diets for 1 week. After a wash out period of at least 2 weeks, participants crossed over to the next diet. Challenge duration: 5 weeks | Gluten-specific responses in 8% (three) of patients; 16% (six) of patients had an increase in overall GI symptoms in high gluten diet | High nocebo effect. Lack of association known from literature (i.e., between fatigue and gluten) |
| Peters et al. 2014 [81] | NCGS w/IBS symptoms | Randomized DBPC Crossover trial | 22 patients | Low FODMAPs-GFD for 3 days. Challenge gluten-free food was supplemented with gluten (16 g/day), whey (16 g/day), or not supplemented (placebo) and administrated for 3 days. Wash out period between 3 and 14 days before crossover. | Gluten ingestion was associated with higher depression scores compared to placebo but not whey after gluten. Gastrointestinal symptoms were induced similarly by different dietary challenges | Small sample size. Restricted number of psychological end-points. Short dietary challenge to observe the maximum change in psychological states. Nocebo effect |
| Zanini et al. 2015 [83] | NCGS | Randomized DBPC Crossover | 35 non-CD patients | Participants randomly received gluten-containing flour or not-containing flour for 10 days, followed by a wash out period of 2 weeks, and then crossed over to receive the alternative treatment | 12 participants (34%) were classified as having NCGS | Some NCGS patients might be in an early “latent” stage of CD |
| Shahbazkhani et al. 2015 [94] | IBS | Randomized DBPC trial | 72 patients | 35/72 IBS patients were randomized in the gluten group, and 37/72 were in the placebo group. Patients previously following a strict GFD continued the gluten challenge for 6 weeks | Significant increase in GI symptoms after a gluten-containing meal challenge | Small sample size. Absence of crossover. High dose of gluten |
| Di Sabatino et al. 2015 [78] | Suspected NCGS | Randomized DBPC Crossover | 61 patients | Participants followed a strict GFD before randomization to gluten or placebo for 1 week, each via gastro-soluble capsules. After 1 week of wash out, participants crossed over to the other group, for another week. After the second treatment week, all patients continued with their wash out from gluten. Challenge duration: 5 weeks | Gluten significantly increased overall symptoms (intestinal symptoms: abdominal bloating and pain; extra-intestinal symptoms: foggy mind, depression, aphthous stomatitis) compared with placebo group | Relatively short period of wash-out from gluten; the lack of a control group of non–gluten-sensitive subjects |
| Picarelli et al. 2016 [73] | NCGS | Randomized DBPC trial | 26 patients | A gluten-containing croissant (10 g of gluten per croissant) randomly assigned to 13 patients and a gluten-free croissant to the other 13 patients. Challenge duration: 1 day | No difference in the severity of GI or extraintestinal symptoms between gluten intake and placebo | Small sample size |
| Elli et al. 2016 [79] | NCGS w/functional gastrointestinal symptoms | Randomized DBPC Crossover trial | 98 patients | Patients were randomized to take gluten (5.6 g/day) or placebo for 7 days. Challenge duration: 21 days; 7 days on gluten or placebo, 7 days wash out, 7 days on gluten or placebo | 28 patients showed symptomatic relapse during blind gluten ingestion with worsening of quality of life; 14 patients reported symptomatic worsening after placebo ingestion | Arbitrary gluten dosage and choice of timing. Missing evaluation of possible influence by other food constituents. Symptomatic deterioration was also observed in placebo group |
| Skodje et al. 2018 [84] | Subjects with self-reported NCGS | Randomized DBPC Crossover trial | 59 subjects | Patients were randomized to follow diets containing gluten (5.7 g), fructans (2.1 g), or placebo, for 7 days. Following a minimum 7 days wash out, participants crossed over to next diet, until they completed all 3 challenges (gluten, fructan, and placebo) | Overall GSRS for IBS scores increased after fructans rather than gluten and placebo | High placebo response |
| Dale et al. 2018 [92] | Patients w/suspected NCGS | Randomized DBPC Crossover trial | 20 patients | Two muffins a day (11/0 g gluten or placebo) for 4 days and wash out for 3 days. (4 periods of 4 days, 2 w/gluten and 2 w/placebo) | Most severe symptoms reported after placebo. Only 4/20 patients (20%) correctly identified periods w/gluten | Short wash-out period. Small sample size. Lack of control of confounding dietary. Timing of symptoms evaluation (in the morning) could be confounding |
| Roncoroni et al. 2019 [86] | NCGS | Increasing gluten amount | 24 patients | GFD for 3 weeks, then patients received gradually increasing gluten diets: low-gluten diet (3.5–4 g gluten/day, week 1), mid-gluten diet (6.7–8 g gluten/day, week 2), and a high-gluten diet (10–13 g gluten/day, week 3). Patients w/o GI symptoms on a previous diet received more gluten-containing diet. Patients w/GI symptoms were shifted back to the previous-tolerated diet. Challenge duration: 6 weeks | Reintroduction of gluten in patients with NCGS who were on GFD induced different response: gluten at a low dosage induced a worsening of general well-being and the quality of life of a group of patients, whereas others tolerate even higher doses of dietary gluten | Small sample size. Absence of crossover |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbaro, M.R.; Cremon, C.; Wrona, D.; Fuschi, D.; Marasco, G.; Stanghellini, V.; Barbara, G. Non-Celiac Gluten Sensitivity in the Context of Functional Gastrointestinal Disorders. Nutrients 2020, 12, 3735. https://doi.org/10.3390/nu12123735
Barbaro MR, Cremon C, Wrona D, Fuschi D, Marasco G, Stanghellini V, Barbara G. Non-Celiac Gluten Sensitivity in the Context of Functional Gastrointestinal Disorders. Nutrients. 2020; 12(12):3735. https://doi.org/10.3390/nu12123735
Chicago/Turabian StyleBarbaro, Maria Raffaella, Cesare Cremon, Diana Wrona, Daniele Fuschi, Giovanni Marasco, Vincenzo Stanghellini, and Giovanni Barbara. 2020. "Non-Celiac Gluten Sensitivity in the Context of Functional Gastrointestinal Disorders" Nutrients 12, no. 12: 3735. https://doi.org/10.3390/nu12123735
APA StyleBarbaro, M. R., Cremon, C., Wrona, D., Fuschi, D., Marasco, G., Stanghellini, V., & Barbara, G. (2020). Non-Celiac Gluten Sensitivity in the Context of Functional Gastrointestinal Disorders. Nutrients, 12(12), 3735. https://doi.org/10.3390/nu12123735






