Microbiome as Mediator of Diet on Colorectal Cancer Risk: The Role of Vitamin D, Markers of Inflammation and Adipokines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Circulating Biomarkers
2.3. Single Nucleotide Polymorphism (SNPs) Analysis
2.4. Microbiota Analysis
2.5. Dietary Assessment
2.6. Statistical Analysis
3. Results
3.1. Risk Factors and Serum Biomarkers
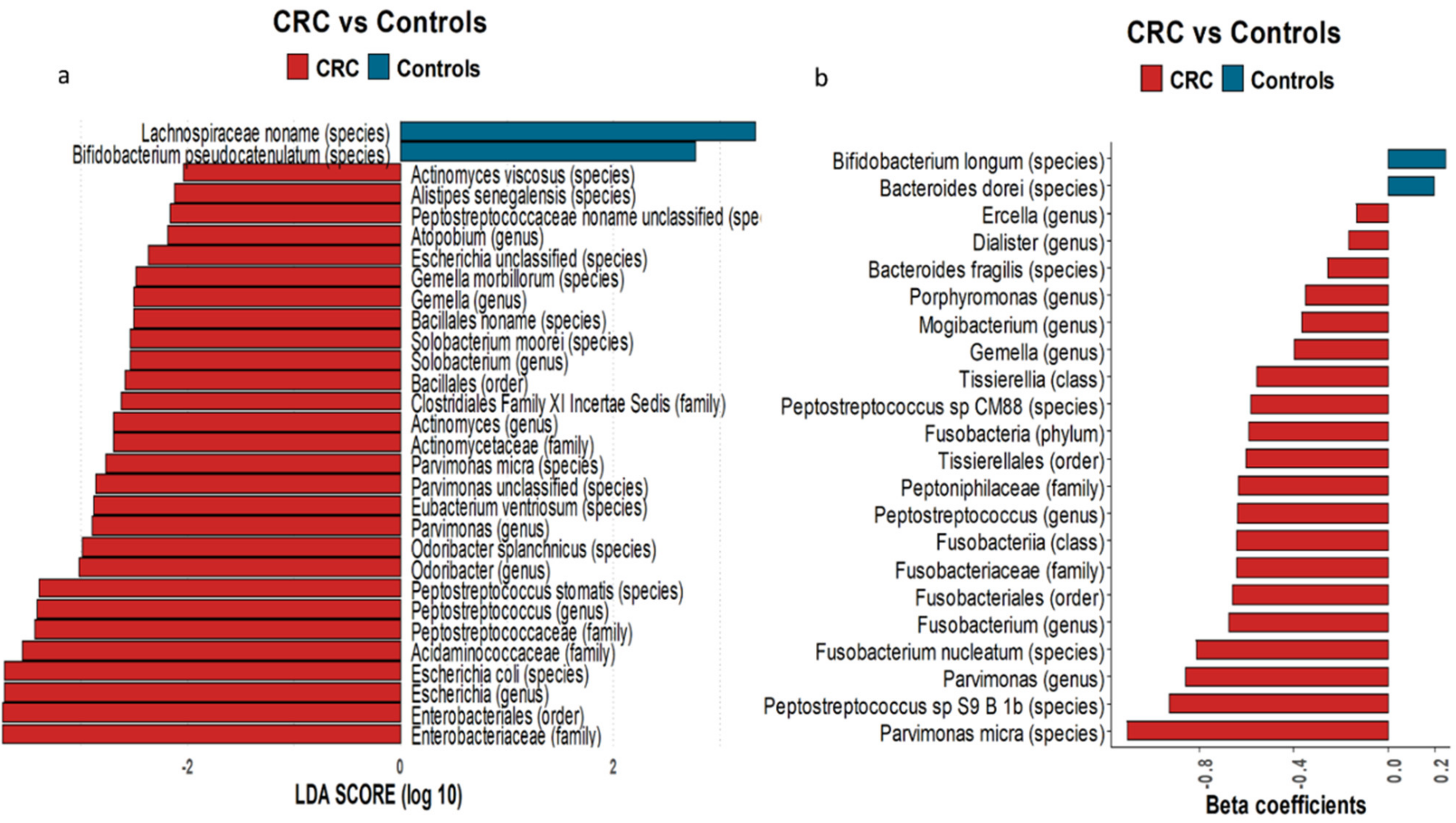
3.2. Microbiome Biomarkers and Functional Profiles
3.3. Interplay between Vitamin D, Dietary Habits, and Microbiota in CRC
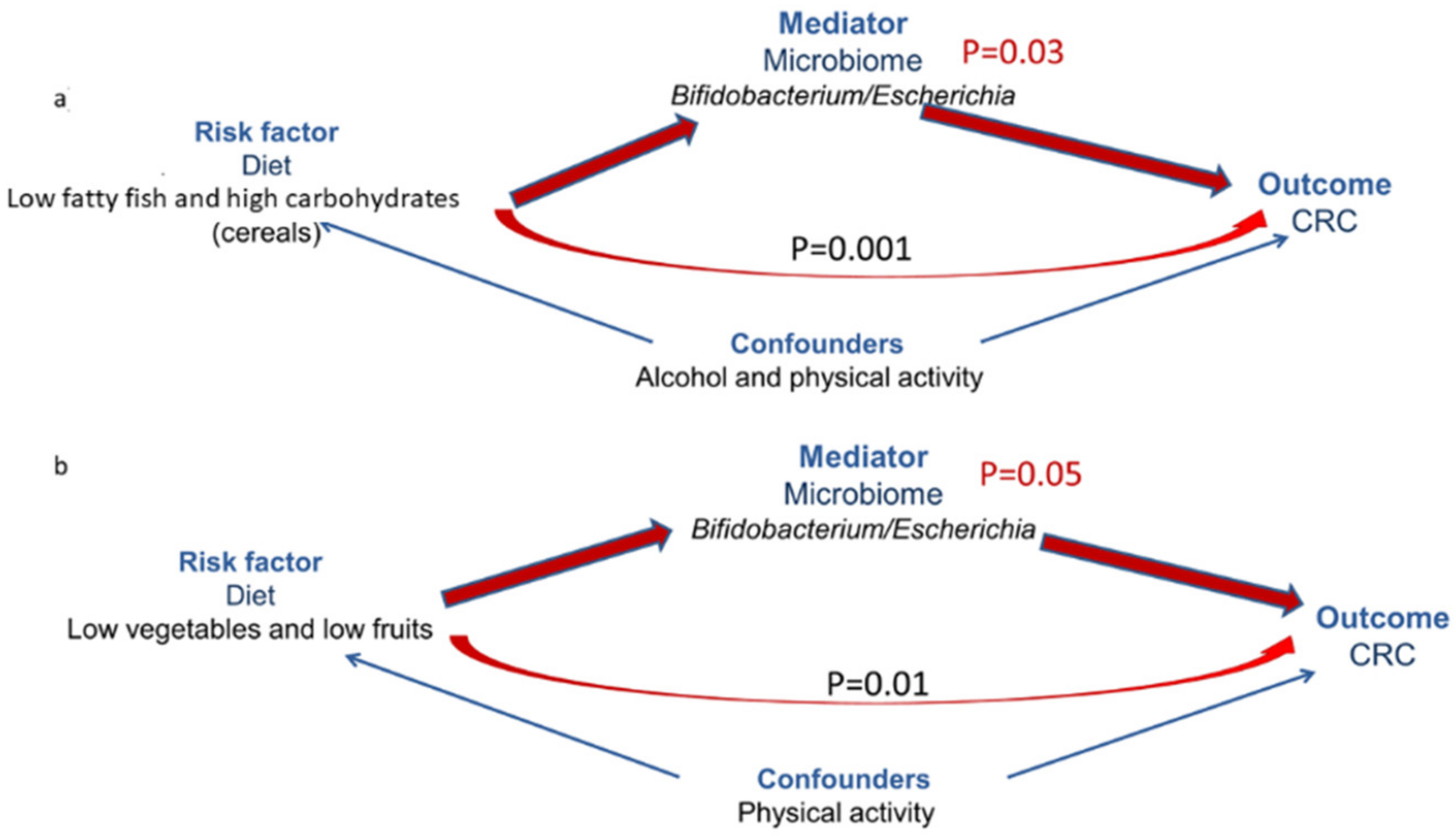
3.4. Microbiome-Mediated Diet Effect on CRC Risk
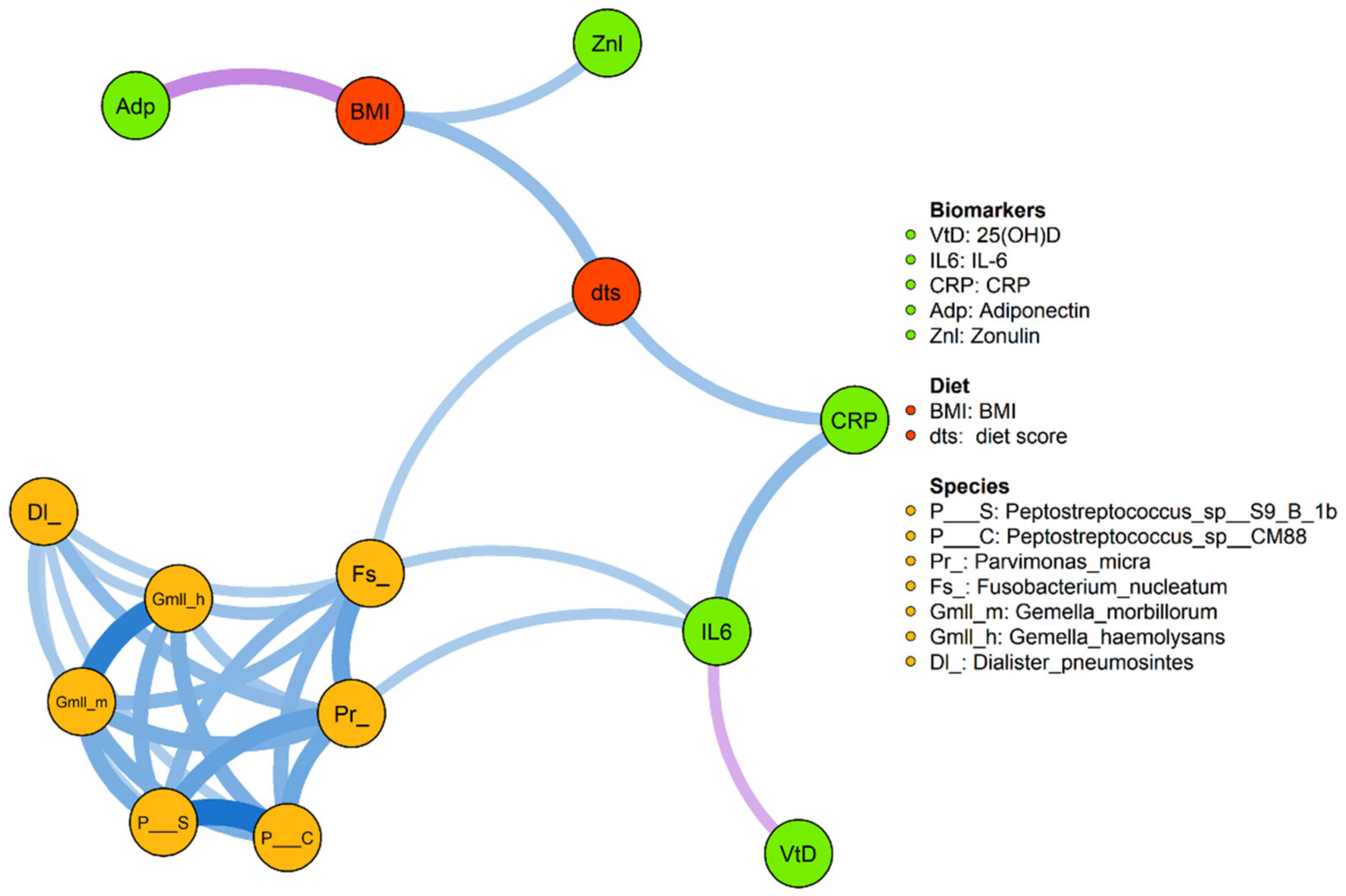
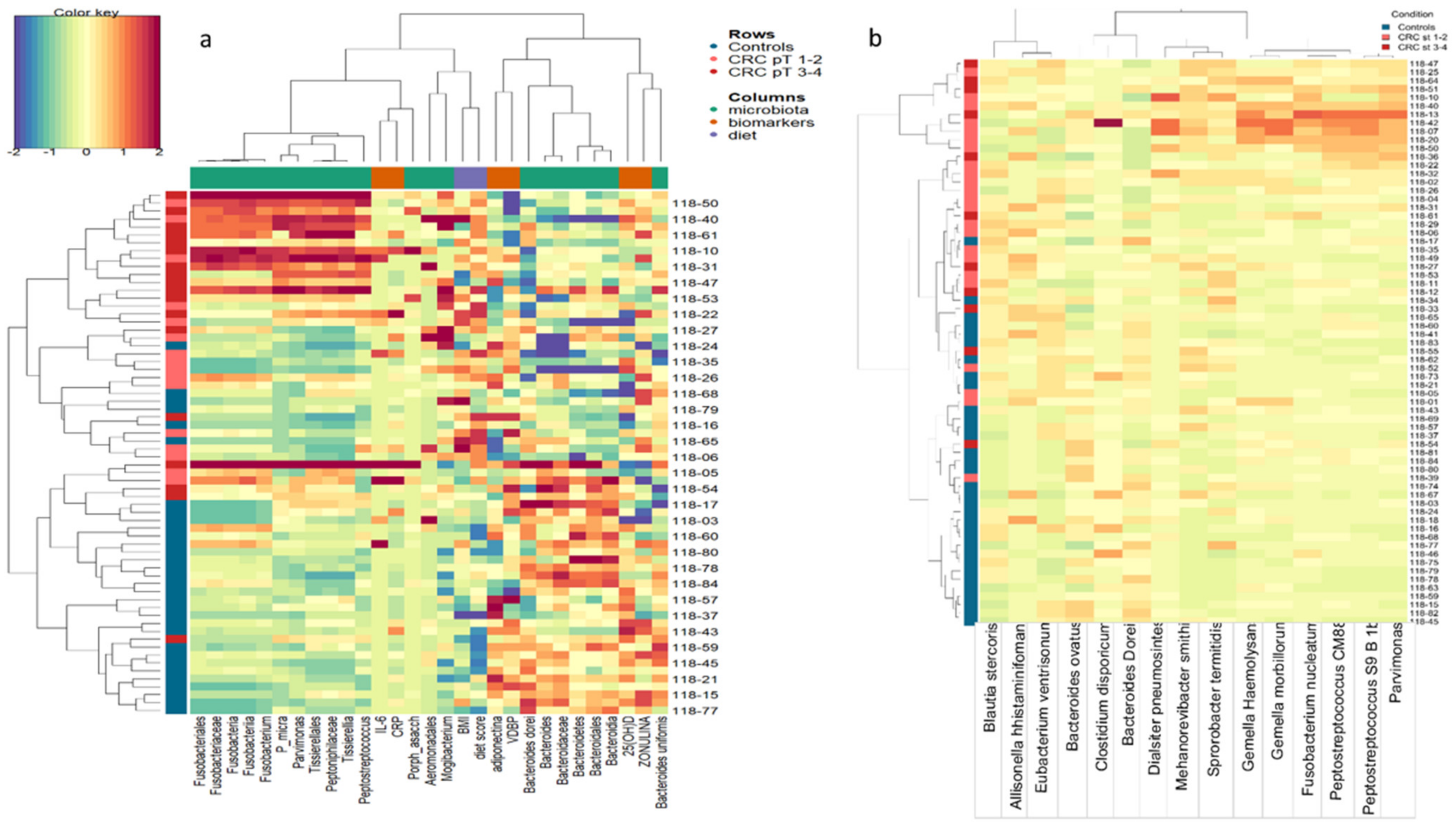
3.5. Integrative Data Analysis
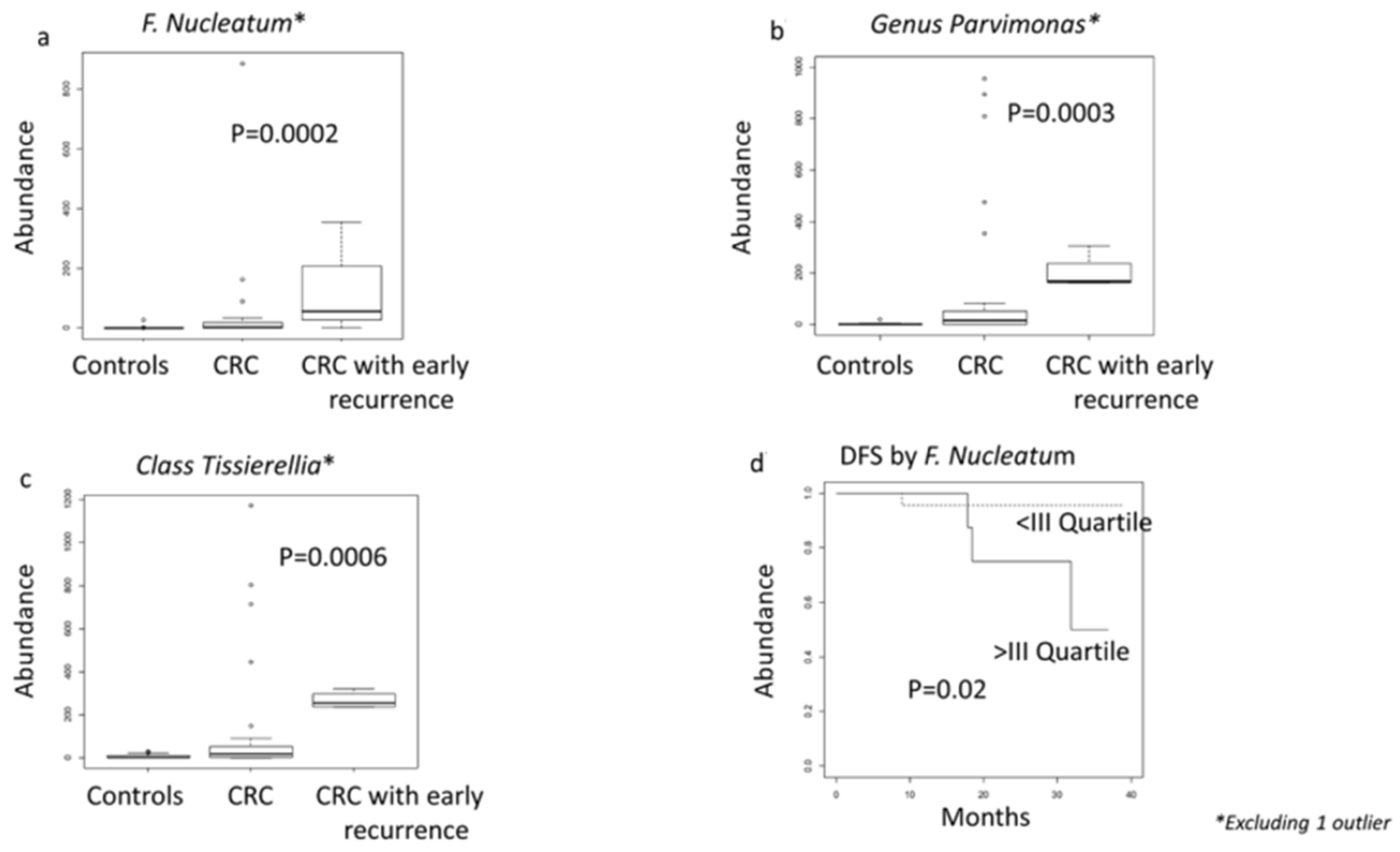
3.6. Microbiome Associated with CRC Prognostic Factors and Relapse
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
Availability of Data and Material
Abbreviations
| 25(OH)D | 25-Hydroxyvitamin D |
| AUC | Area under the curve |
| BMI | Body mass index |
| CRC | Colorectal cancer |
| F. | Fusobacterium |
| hs-CRP | High-sensitivity C-reactive protein |
| IBD | Inflammatory bowel diseases |
| IL-6 | Interleukin-6 |
| LDA | Linear discriminant analysis |
| LEfSe | Linear discriminant analysis effect size |
| OTUs | Operational taxonomic units |
| ROC | Receiver operating characteristics |
| ORs | Odds ratios |
| AUC | Area under the ROC curve |
| VDBP | Vitamin D-binding protein |
| CCA | Canonical correspondence analysis |
| WCRF | World Cancer Research Fund International |
| FDR | False discovery rate |
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhaes, B.; Peleteiro, B.; Lunet, N. Dietary patterns and colorectal cancer: Systematic review and meta-analysis. Eur. J. Cancer Prev. 2012, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Shams-White, M.M.; Brockton, N.T.; Mitrou, P. Operationalizing the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Cancer Prevention Recommendations: A Standardized Scoring System. Nutrients 2019, 11, 1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnagnarella, P.; Gandini, S.; La, V.C.; Maisonneuve, P. Glycemic index, glycemic load, and cancer risk: A meta-analysis. Am. J. Clin. Nutr. 2008, 87, 1793–1801. [Google Scholar] [CrossRef] [Green Version]
- Montalban-Arques, A.; Scharl, M. Intestinal microbiota and colorectal carcinoma: Implications for pathogenesis, diagnosis, and therapy. EBioMedicine 2019, 48, 648–655. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.M.; Manghi, P.; Asnicar, F. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 2019, 24, 667–678. [Google Scholar] [CrossRef] [Green Version]
- Wirbel, J.; Pyl, P.T.; Kartal, E. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for col-orectal cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Belcheva, A.; Irrazabal, T.; Robertson, S.J. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 2014, 17, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Pohl, C.; Hombach, A.; Kruis, W. Chronic inflammatory bowel disease and cancer. Hepatogastroenterology 2000, 47, 57–70. [Google Scholar]
- Tsilidis, K.K.; Branchini, C.; Guallar, E.; Helzlsouer, K.J.; Erlinger, T.P.; Platz, E.A. C-reactive protein and colorectal cancer risk: A systematic review of prospective studies. Int. J. Cancer 2008, 123, 1133–1140. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Inflammatory cytokines in cancer: Tumour necrosis factor and interleukin 6 take the stage. Ann. Rheum. Dis. 2011, 70, i104–i108. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 23, 421–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, S.L.; Donohoe, C.L.; Lysaght, J.; Reynolds, J.V. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc. Nutr. Soc. 2012, 71, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Zhang, L.; Xu, H.-J.; Li, Y.; Hu, C.-M.; Yang, J.-Y.; Sun, M. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef] [Green Version]
- Meeker, S.; Seamons, A.; Paik, J.; Treuting, P.M.; Brabb, T.; Grady, W.M.; Maggio-Price, L. Increased Dietary Vitamin D Suppresses MAPK Signaling, Colitis, and Colon Cancer. Cancer Res. 2014, 74, 4398–4408. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, M.H.; Owen, J.; Ahearn, T. Effects of supplemental vitamin D and calcium on biomarkers of inflamma-tion in colorectal adenoma patients: A randomized, controlled clinical trial. Cancer Prev. Res. 2011, 4, 1645–1654. [Google Scholar] [CrossRef] [Green Version]
- Gandini, S.; Boniol, M.; Haukka, J.; Byrnes, G.; Cox, B.; Sneyd, M.J.; Mullie, P.; Autier, P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int. J. Cancer 2011, 128, 1414–1424. [Google Scholar] [CrossRef]
- Gandini, S.; Gnagnarella, P.; Serrano, D.; Pasquali, E.; Raimondi, S. Vitamin D Receptor Polymorphisms and Cancer. Neurotransm. Interact. Cogn. Funct. 2014, 810, 69–105. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer inci-dence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Adorini, L.; Penna, G.; Giarratana, N.; Roncari, A.; Amuchastegui, S.; Daniel, K.C.; Uskokovic, M. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J. Steroid Biochem. Mol. Biol. 2004, 89, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.; Prietl, B.; Tauschmann, M.; Mautner, S.I.; Kump, P.K.; Treiber, G.; Wurm, P.; Gorkiewicz, G.; Högenauer, C.; Pieber, T.R. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur. J. Nutr. 2016, 55, 1479–1489. [Google Scholar] [CrossRef] [Green Version]
- Manzari, C.; Fosso, B.; Marzano, M.; Annese, A.; Caprioli, R.; D’Erchia, A.M.; Gissi, C.; Intranuovo, M.; Picardi, E.; Santamaria, M.; et al. The influence of invasive jellyfish blooms on the aquatic microbiome in a coastal lagoon (Varano, SE Italy) detected by an Illumina-based deep sequencing strategy. Biol. Invasions 2015, 17, 923–940. [Google Scholar] [CrossRef] [Green Version]
- Manzari, C.; Chiara, M.; Costanza, A.; Leoni, C.; Volpicella, M.; Picardi, E.; D’Erchia, A.M.; Placido, A.; Trotta, M.; Horner, D.; et al. Draft genome sequence of Sphingobium sp. strain ba1, resistant to kanamycin and nickel ions. FEMS Microbiol. Lett. 2014, 361, 8–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quince, C.; Walker, A.W.; Simpson, J.T.; Loman, N.J.; Segata, N. Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol. 2017, 35, 833–844. [Google Scholar] [CrossRef] [Green Version]
- Gnagnarella, P.; Draga, D.; Misotti, A.M. Validation of a short questionnaire to record adherence to the Mediterra-nean diet: An Italian experience. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1140–1147. [Google Scholar] [CrossRef]
- Pisani, P.; Faggiano, F.; Krogh, V.; Palli, D.; Vineis, P.; Berrino, F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int. J. Epidemiol. 1997, 26, 152S–160S. [Google Scholar] [CrossRef] [Green Version]
- Tavani, A.; Giordano, L.; Gallus, S.; Talamini, R.; Franceschi, S.; Giacosa, A.; Montella, M.; La Vecchia, C. Consumption of sweet foods and breast cancer risk in Italy. Ann. Oncol. 2005, 17, 341–345. [Google Scholar] [CrossRef]
- World Cancer Research Fund; American Institute of Cancer Research. Continuous Update Project Expert Report 2018: Meat, Fish and Dairy Products and Risk of Cancer; American Institute of Cancer Research: Washington, DC, USA, 2018. [Google Scholar]
- World Cancer Research Fund; American Institute of Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Prospective: Continuous Update Project Expert Report 2018; American Institute of Cancer Research: Washington, DC, USA, 2018. [Google Scholar]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O‘Riordain, M.; Shanahan, F.; O’Toole, P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2017, 67, 1454–1463. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 27, 121–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, J.C.; Jobin, C. The complex interplay between inflammation, the microbiota and colorectal cancer. Gut Microbes 2013, 4, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woting, A.; Pfeiffer, N.; Loh, G.; Klaus, S.; Blaut, M. Clostridium ramosum Promotes High-Fat Diet-Induced Obesity in Gnotobiotic Mouse Models. mBio 2014, 5, e01530–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- len-Vercoe, E.; Daigneault, M.; White, A. Anaerostipes hadrus comb. nov.; a dominant species within the human colonic microbiota; reclassification of Eubacterium hadrum Moore et al. 1976. Anaerobe 2012, 18, 523–529. [Google Scholar] [CrossRef]
- Muñoz-Tamayo, R.; Laroche, B.; Walter, É.; Doré, J.; Duncan, S.H.; Flint, H.J.; Leclerc, M. Kinetic modelling of lactate utilization and butyrate production by key human colonic bacterial species. FEMS Microbiol. Ecol. 2011, 76, 615–624. [Google Scholar] [CrossRef] [Green Version]
- Murray-Stewart, T.R.; Woster, P.M.; Casero, J.R.A. Targeting polyamine metabolism for cancer therapy and prevention. Biochem. J. 2016, 473, 2937–2953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinelli, J.B.; Yoon, H.; Ringel, A.E.; Jeanfavre, S.; Clish, C.B.; Haigis, M.C. Metabolic recycling of ammonia via glutamate de-hydrogenase supports breast cancer biomass. Science 2017, 6365, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.-F.; Zou, J.; Dong, J. Fish consumption and risk of gastrointestinal cancers: A meta-analysis of cohort studies. World J. Gastroenterol. 2014, 20, 15398–15412. [Google Scholar] [CrossRef]
- Wu, S.; Feng, B.; Li, K. Fish consumption and colorectal cancer risk in humans: A systematic review and me-ta-analysis. Am. J. Med. 2012, 125, 551–559. [Google Scholar] [CrossRef]
- Habermann, N.; Schon, A.; Lund, E.K.; Glei, M. Fish fatty acids alter markers of apoptosis in colorectal adenoma and ad-enocarcinoma cell lines but fish consumption has no impact on apoptosis-induction ex vivo. Apoptosis 2010, 15, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: Results from the EPIC–Oxford study. Public Health Nutr. 2010, 14, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Greathouse, K.L.; White, J.R.; Padgett, R.N. Gut microbiome meta-analysis reveals dysbiosis is independent of body mass index in predicting risk of obesity-associated CRC. BMJ Open Gastroenterol. 2019, 6, e000247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, S.; Fang, L.; Lee, M.-H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol. Rep. 2018, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Helsingen, L.M.; Vandvik, P.O.; Jodal, H.C. Colorectal cancer screening with faecal immunochemical testing, sig-moidoscopy or colonoscopy: A clinical practice guideline. BMJ 2019, 367, l5515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kipnis, V.; Midthune, D.; Freedman, L. Bias in dietary-report instruments and its implications for nutritional epi-demiology. Public Health Nutr. 2002, 5, 915–923. [Google Scholar] [CrossRef] [Green Version]


| CRC (N, %) | Controls (N, %) | Total (N, %) | p-Value | ||
|---|---|---|---|---|---|
| Sex | Females | 10 (29.4) | 14 (43.7) | 24 (36.4) | 0.23 |
| Males | 24 (70.6) | 18 (56.3) | 42 (63.6) | ||
| Age | ≤60 years | 18 (52.9) | 20 (62.5) | 38 (57.6) | 0.43 |
| >60 years | 16 (47.1) | 12 (36.5) | 28 (42.4) | ||
| BMI | ≤25 | 11 (33.3) | 20 (62.5) | 31 (47.7) | 0.02 |
| >25 | 22 (66.7) | 12 (37.5) | 34 (52.3) | ||
| Regular physical activity | No | 20 (58.8) | 8 (25.0) | 28 (42.4) | 0.006 |
| Yes | 14 (42.2) | 24 (75.0) | 38 (57.6) | ||
| Regular alcohol consumption | No | 5 (14.7) | 15 (46.9) | 20 (30.3) | 0.005 |
| Yes | 29 (85.3) | 17 (53.1) | 46 (69.7) | ||
| Colon cancer family history | No | 25 (73.5) | 16 (50.0) | 41 (62.1) | 0.05 |
| Yes | 9 (26.5) | 16 (50.0) | 25 (37.9) | ||
| Smoking | Never | 12 (35.3) | 14 (75.0) | 36 (54.5) | 0.005 |
| Current | 9 (26.5) | 3 (9.4) | 12 (18.2) | ||
| Former | 13 (38.2) | 5 (15.6) | 18 (27.3) |
| CRC | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Median | Lower Quartile | Upper Quartile | Median | Lower Quartile | Upper Quartile | p-Values | ||
| 25-OHD (ng/mL) 1 | 19.8 | 11.2 | 25.1 | 23.4 | 16.1 | 31.4 | 0.12 | |
| VDBP (µg/mL) | 235 | 166 | 295 | 249 | 209 | 309.5 | 0.58 | |
| Zonulin (ng/mL) | 119 | 74 | 178 | 109 | 54 | 315 | 0.94 | |
| IGFII (ng/mL) | 671 | 578 | 769 | 695 | 614 | 806 | 0.41 | |
| IGFBP3 (µg/mL) | 2.17 | 1.95 | 2.59 | 2.36 | 2.16 | 2.64 | 0.09 | |
| CRP (mg/dL) | 0.23 | 0.12 | 0.39 | 0.10 | 0.05 | 0.20 | 0.01 | |
| Adiponectin (µg/mL) | 4.87 | 3.41 | 9.48 | 7.77 | 6.23 | 12.39 | 0.03 | |
| Leptin (ng/mL) | 6.56 | 4.25 | 14.15 | 6.71 | 5.19 | 15.43 | 0.67 | |
| N. (%) | N. (%) | |||||||
| Vitamin D (ng/mL) 1 | Sufficient | 24 (70.6) | 29 (90.6) | 0.04 | ||||
| Deficient | 10 (29.4) | 3 (9.4) | ||||||
| hs-CRP (mg/dL) 2 | ≤0.1 | 7 (20.6) | 16 (50) | 0.012 | ||||
| >0.1 | 27 (79.4) | 16 (50) | ||||||
| Adiponectin (µg/mL) 3 | ≤6 | 20 (58.8) | 7 (21.9) | 0.002 | ||||
| >6 | 14 (41.2) | 25 (78.1) | ||||||
| IL-6 (pg/mL) 4 | ≤4 | 25 (73.5) | 30 (93.8) | 0.03 | ||||
| >4 | 9 (26.5) | 2 (6.3) | ||||||
| VDR, GC, and CYP SNPs | CRC n = 34(%) | Controls n = 32 (%) | Total n = 66 (%) | p-Value | |
|---|---|---|---|---|---|
| Fok1 rs2228570 (A > G) FokI | GG (FF) or GA (Ff) | 27 (79.4) | 31 (96.9) | 58 (87.9) | 0.03 |
| (A = rare nucleotide) | AA (ff) | 7 (20.6) | 1 (3.1) | 8 (12.1) | |
| Bsm1 rs1544410 (C > T) BsmI | CC (bb) or CT (Bb) | 31 (91.2) | 27 (84.4) | 58 (87.9) | 0.39 |
| (T = rare nucleotide) | TT (BB) | 3 (8.8) | 5 (15.6) | 8 (12.1) | |
| Taq1 rs731236 (A > G) TaqI | AA (TT) or AG (Tt) | 32 (94) | 27 (84) | 58 (89) | 0.20 |
| (G = rare nucleotide) | GG (tt) | 2 (6) | 5 (16) | 7 (11) | |
| Apa1 rs7975232 (C > A) ApaI | AA (AA) or AC (Aa) | 27 (79.4) | 25 (78.1) | 52 (78.8) | 0.9 |
| (C = rare nucleotide) | CC (aa) | 7 (20.6) | 7 (21.9) | 14 (21.2) | |
| GC rs2282679 (T > G) | TT or TG | 31 (91.2) | 31 (96.9) | 62 (93.9) | 0.33 |
| (G = rare allele) | GG | 3 (8.8) | 1 (3.1) | 4 (6.1) | |
| GC rs4588 (G > T) | GG or GT | 31 (91.2) | 31 (96.9) | 62 (93.9) | 0.33 |
| (T = rare nucleotide) | TT | 3 (8.8) | 1 (3.1) | 4 (6.1) | |
| GC rs7041 (A > C) | CC or CA | 27 (79.4) | 28 (87.5) | 55 (83.3) | 0.38 |
| (A = rare nucleotide) | AA | 7 (20.6) | 4 (12.5) | 11 (16.7) | |
| CYP24A1 rs6013897 (T > A) | TT or TA | 29 (85.3) | 32 (100) | 61 (92.4) | 0.02 |
| (A = rare nucleotide) | AA | 5 (14.7) | 0 (0.0) | 5 (7.6) | |
| CYP27B1 rs10877012 (G > T) | GG or GT | 31 (91.2) | 29 (90.6) | 60 (90.9) | 0.93 |
| (T = rare nucleotide) | TT | 3 (8.8) | 3 (9.4) | 6 (9.1) | |
| CYP2R1 rs10741657 (A > G) | GG or GA | 31 (91.2) | 32 (100) | 63 (95.5) | 0.09 |
| (A = rare nucleotide) | AA | 3 (8.8) | 0 (0) | 3 (4.5) |
| Lifestyle Risk Score | OR | Lower 95% CI | Upper 95% CI | p-Values | |
|---|---|---|---|---|---|
| Risk factors | Regular physical activity | 0.28 | 0.08 | 0.99 | 0.049 |
| Ever smoking | 3.21 | 0.85 | 12.14 | 0.086 | |
| High alcohol | 6.20 | 1.27 | 30.20 | 0.024 | |
| Diet | High sweets and cakes | 4.31 | 1.02 | 18.28 | 0.048 |
| Low fatty fish and highcereals/carbohydrates 2 | 5.88 | 1.49 | 25.0 | 0.011 | |
| WCRF score 1 | 0.23 | 0.08 | 0.67 | 0.007 |
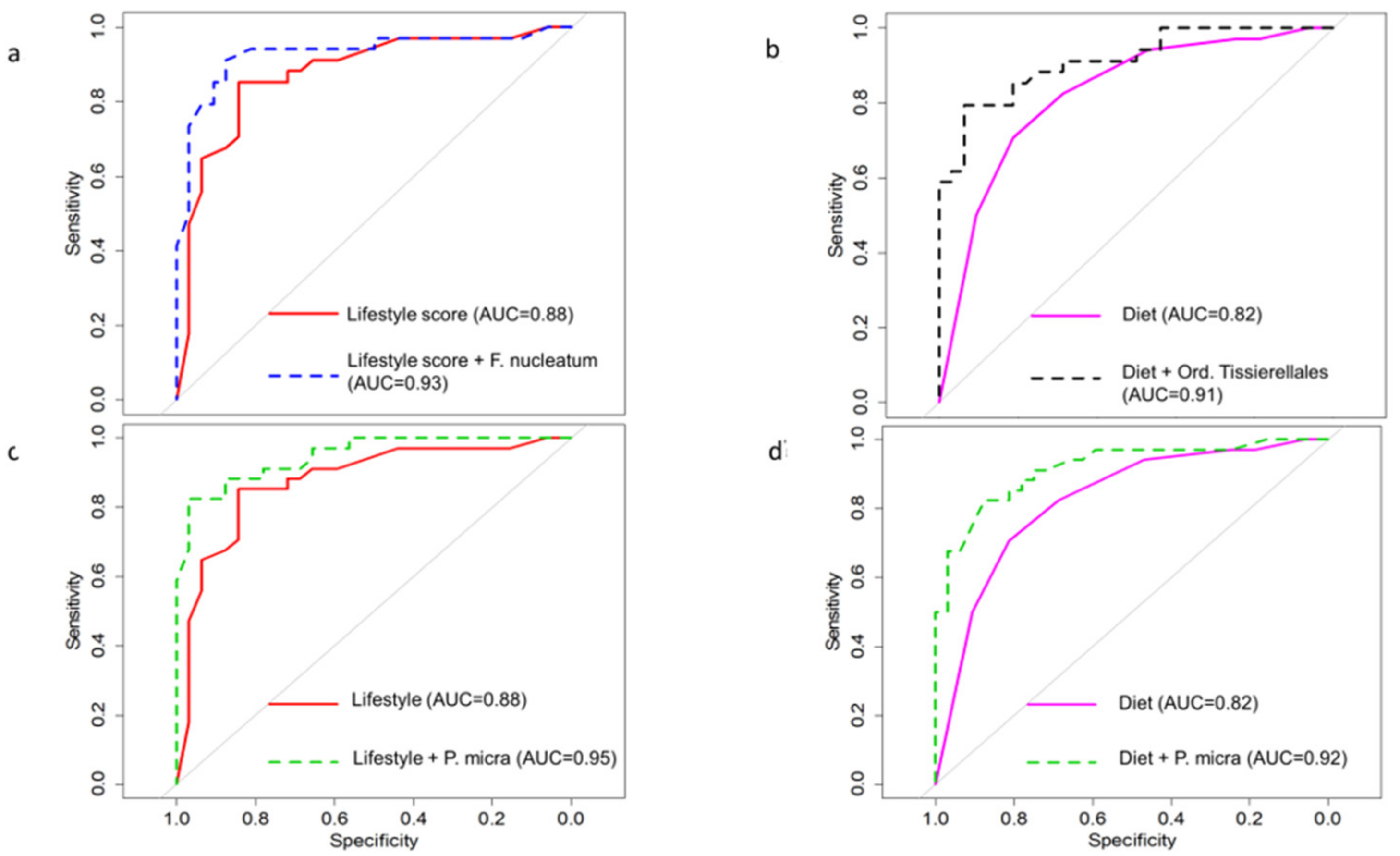
| Cross Validation | |||
|---|---|---|---|
| p-Value | AUC | AUC (95% CI) | |
| Lifestyle score | 0.0002 | 93% | 91% (83–99) |
| F. nucleatum | 0.006 | ||
| Lifestyle score | 0.0002 | 95% | 91% (86–98) |
| Parvimonas micra | 0.003 | ||
| Dietary score | 0.0004 | 92% | 88% (80–96) |
| Parvimonas micra | 0.003 | ||
| Dietary score | 0.0004 | 91% | 89% (81–96) |
| Tissierella | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, D.; Pozzi, C.; Guglietta, S.; Fosso, B.; Suppa, M.; Gnagnarella, P.; Corso, F.; Bellerba, F.; Macis, D.; Aristarco, V.; et al. Microbiome as Mediator of Diet on Colorectal Cancer Risk: The Role of Vitamin D, Markers of Inflammation and Adipokines. Nutrients 2021, 13, 363. https://doi.org/10.3390/nu13020363
Serrano D, Pozzi C, Guglietta S, Fosso B, Suppa M, Gnagnarella P, Corso F, Bellerba F, Macis D, Aristarco V, et al. Microbiome as Mediator of Diet on Colorectal Cancer Risk: The Role of Vitamin D, Markers of Inflammation and Adipokines. Nutrients. 2021; 13(2):363. https://doi.org/10.3390/nu13020363
Chicago/Turabian StyleSerrano, Davide, Chiara Pozzi, Silvia Guglietta, Bruno Fosso, Mariano Suppa, Patrizia Gnagnarella, Federica Corso, Federica Bellerba, Debora Macis, Valentina Aristarco, and et al. 2021. "Microbiome as Mediator of Diet on Colorectal Cancer Risk: The Role of Vitamin D, Markers of Inflammation and Adipokines" Nutrients 13, no. 2: 363. https://doi.org/10.3390/nu13020363
APA StyleSerrano, D., Pozzi, C., Guglietta, S., Fosso, B., Suppa, M., Gnagnarella, P., Corso, F., Bellerba, F., Macis, D., Aristarco, V., Manghi, P., Segata, N., Trovato, C., Zampino, M. G., Marzano, M., Bonanni, B., Rescigno, M., & Gandini, S. (2021). Microbiome as Mediator of Diet on Colorectal Cancer Risk: The Role of Vitamin D, Markers of Inflammation and Adipokines. Nutrients, 13(2), 363. https://doi.org/10.3390/nu13020363








