Effects of a Self-Prepared Carbohydrate-Reduced High-Protein Diet on Cardiovascular Disease Risk Markers in Patients with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
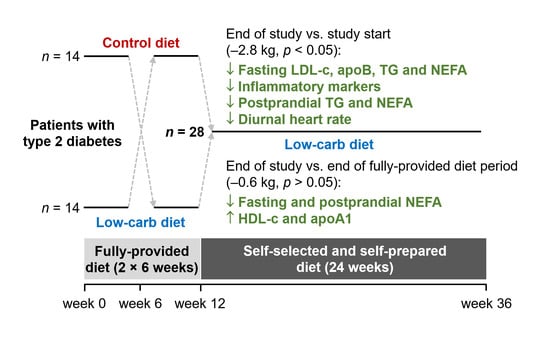
2.2. Study Design
2.3. Outcome Assessment
2.3.1. Fasting CVD Risk Factor Profile
2.3.2. Postprandial Lipid Responses
2.3.3. Diurnal Blood Pressure and Heart Rate
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Self-Prepared Diet
3.2. Changes in Body Weight and Medications
3.3. Lipid and Inflammatory Marker Responses
3.4. Diurnal Blood Pressure and Heart Rate Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- International Diabetes Federation. Key Figures from the IDF Diabetes Atlas 9th Edition. Available online: https://www.diabetesatlas.org/en/ (accessed on 23 April 2021).
- Pearson-Stuttard, J.; Bennett, J.; Cheng, Y.J.; Vamos, E.P.; Cross, A.J.; Ezzati, M.; Gregg, E.W. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: An epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021, 9, 165–173. [Google Scholar] [CrossRef]
- Martin-Timon, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Canizo-Gomez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef]
- Saely, C.H.; Sternbauer, S.; Vonbank, A.; Heinzle, C.; Zanolin-Purin, D.; Larcher, B.; Mader, A.; Leiherer, A.; Muendlein, A.; Drexel, H. Type 2 diabetes mellitus is a strong predictor of LDL cholesterol target achievement in patients with peripheral artery disease. J. Diabetes Complicat. 2020, 34, 5. [Google Scholar] [CrossRef]
- Verges, B. Pathophysiology of diabetic dyslipidaemia: Where are we? Diabetologia 2015, 58, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty acids, obesity, and insulin resistance: Time for a reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Libianto, R.; Batu, D.; MacIsaac, R.J.; Cooper, M.E.; Ekinci, E.I. Pathophysiological links between diabetes and blood pressure. Can. J. Cardiol. 2018, 34, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sidell, M.A.; Arterburn, D.; Daley, M.F.; Desai, J.; Fitzpatrick, S.L.; Horberg, M.A.; Koebnick, C.; McCormick, E.; Oshiro, C.; et al. Racial/ethnic disparities in the prevalence of diabetes and prediabetes by bmi: Patient outcomes research to advance learning (PORTAL) multisite cohort of adults in the U.S. Diabetes Care 2019, 42, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jönsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.F.; Bolick, J.P.; Kris-Etherton, P.M.; Sikand, G.; Aspry, K.E.; Soffer, D.E.; Willard, K.E.; Maki, K.C. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: A scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J. Clin. Lipidol. 2019, 13, 689–711. [Google Scholar] [CrossRef] [PubMed]
- Hyde, P.N.; Sapper, T.N.; Crabtree, C.D.; LaFountain, R.A.; Bowling, M.L.; Buga, A.; Fell, B.; McSwiney, F.T.; Dickerson, R.M.; Miller, V.J.; et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Samkani, A.; Skytte, M.J.; Kandel, D.; Kjaer, S.; Astrup, A.; Deacon, C.F.; Holst, J.J.; Madsbad, S.; Rehfeld, J.F.; Haugaard, S.B.; et al. A carbohydrate-reduced high-protein diet acutely decreases postprandial and diurnal glucose excursions in type 2 diabetes patients. Br. J. Nutr. 2018, 119, 910–917. [Google Scholar] [CrossRef]
- Snorgaard, O.; Poulsen, G.M.; Andersen, H.K.; Astrup, A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000354. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes 2004, 53, 2375–2382. [Google Scholar] [CrossRef]
- Skytte, M.J.; Samkani, A.; Astrup, A.; Larsen, T.M.; Frystyk, J.; Poulsen, H.E.; Henriksen, T.; Holst, J.J.; Andersen, O.; Madsbad, S.; et al. Effects of a highly controlled carbohydrate-reduced high-protein diet on markers of oxidatively generated nucleic acid modifications and inflammation in weight stable participants with type 2 diabetes; a randomized controlled trial. Scand J. Clin. Lab. Investig. 2020, 80, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Skytte, M.J.; Samkani, A.; Petersen, A.D.; Thomsen, M.N.; Astrup, A.; Chabanova, E.; Frystyk, J.; Holst, J.J.; Thomsen, H.S.; Madsbad, S.; et al. A carbohydrate-reduced high-protein diet improves HbA1c and liver fat content in weight stable participants with type 2 diabetes: A randomised controlled trial. Diabetologia 2019, 62, 2066–2078. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes—2018: Classification and diagnosis of diabetes. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.I.; De Leeuw, I.; Hermansen, K.; Karamanos, B.; Karlstrom, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.A.; Rizkalla, S.; Slama, G.; et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- MADLOG. Available online: https://www.madlog.dk/en/ (accessed on 23 April 2021).
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.B.; Paultre, F.; Maggio, C.; Mezzitis, N.; Pi-Sunyer, F.X. The use of areas under curves in diabetes research. Diabetes Care 1995, 18, 245–250. [Google Scholar] [CrossRef]
- Athinarayanan, S.J.; Hallberg, S.J.; McKenzie, A.L.; Lechner, K.; King, S.; McCarter, J.P.; Volek, J.S.; Phinney, S.D.; Krauss, R.M. Impact of a 2-year trial of nutritional ketosis on indices of cardiovascular disease risk in patients with type 2 diabetes. Cardiovasc. Diabetol. 2020, 19, 208. [Google Scholar] [CrossRef]
- Yu, Z.; Nan, F.; Wang, L.Y.; Jiang, H.; Chen, W.; Jiang, Y. Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2020, 39, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Sato, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Influence of fat and carbohydrate proportions on the metabolic profile in patients with type 2 diabetes: A meta-analysis. Diabetes Care 2009, 32, 959–965. [Google Scholar] [CrossRef]
- Lu, M.; Lu, Q.; Zhang, Y.; Tian, G. ApoB/apoA1 is an effective predictor of coronary heart disease risk in overweight and obesity. J. Biomed. Res. 2011, 25, 266–273. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I. The apoB/apoA-I ratio: A strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy—A review of the evidence. J. Intern. Med. 2006, 259, 493–519. [Google Scholar] [CrossRef]
- Johansen, C.D.; Olsen, R.H.; Pedersen, L.R.; Kumarathurai, P.; Mouridsen, M.R.; Binici, Z.; Intzilakis, T.; Køber, L.; Sajadieh, A. Resting, night-time, and 24 h heart rate as markers of cardiovascular risk in middle-aged and elderly men and women with no apparent heart disease. Eur. Heart J. 2013, 34, 1732–1739. [Google Scholar] [CrossRef]
- Qu, D.; Liu, J.; Lau, C.W.; Huang, Y. IL-6 in diabetes and cardiovascular complications. Br. J. Pharmacol. 2014, 171, 3595–3603. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value |
|---|---|
| Sex, male/female (n (%)) | 20/8 (71/29) |
| Age (years) | 64 ± 7.7 |
| Duration of T2DM (years) | 7.0 ± 5.4 |
| Body mass index (kg/m2) | 30.1 ± 5.2 |
| Fasting plasma glucose (mmol/L) | 9.4 ± 1.4 |
| HbA1c (mmol/mol) | 59.6 ± 8.4 |
| HbA1c (%) | 7.6 ± 0.8 |
| Medication use (n (%)) | |
| No hypoglycemic agents | 4 (14) |
| Using hypoglycemic medication | 24 (86) |
| 1 hypoglycemic agent | 15 (54) |
| 2 hypoglycemic agents | 6 (21) |
| 3 hypoglycemic agents | 3 (11) |
| >3 hypoglycemic agents | 0 (0) |
| Biguanide | 22 (79) |
| DPP-4 inhibitors | 9 (32) |
| SGLT2 inhibitors | 5 (18) |
| Using lipid-lowering medication | 20 (71) |
| Using antihypertensive medication | 16 (57) |
| Baseline (Week 0) | End of Fully-Provided CRHP Diet (Week 6 or 12) | End of Self-Prepared CRHP Diet (Week 36) | P1 | P2 | |
|---|---|---|---|---|---|
| Total cholesterol (mmol/L) | 3.9 ± 0.9 | 3.4 ± 0.8 | 3.7 ± 0.9 | 0.003 | 0.045 |
| HDL-cholesterol (mmol/L) | 1.11 ± 0.24 | 1.07 ± 0.22 | 1.14 ± 0.25 | 0.196 | 0.003 |
| LDL-cholesterol (mmol/L) | 2.44 ± 0.85 | 2.04 ± 0.73 | 2.26 ± 0.85 | 0.009 | 0.062 |
| VLDL-cholesterol (mmol/L) | 0.34 (0.30, 0.39) | 0.24 (0.21, 0.27) | 0.24 (0.22, 0.28) | <0.001 | 0.247 |
| NEFA, fasting (mmol/L) | 0.77 (0.69, 0.85) | 0.64 (0.58, 0.71) | 0.46 (0.41, 0.51) | <0.001 | <0.001 |
| NEFA, 4-h AUC (pmol/L) | 102 (92, 114) | 81 (75, 88) | 67 (60, 74) | <0.001 | <0.001 |
| Triacylglycerol, fasting (mmol/L) | 1.71 (1.49, 1.97) | 1.20 (1.07, 1.35) | 1.22 (1.09, 1.38) | <0.001 | 0.247 |
| Triacylglycerol, 4-h AUC (mmol/L) | 441 (388, 501) | 335 (298, 376) | 344 (307, 386) | <0.001 | 0.188 |
| ApoA1 (µmol/L) | 49.1 ± 6.5 | 45.4 ± 6.3 | 48.5 ± 7.4 | 0.489 | 0.004 |
| ApoB (µmol/L) | 1.71 ± 0.43 | 1.45 ± 0.37 | 1.56 ± 0.41 | <0.001 | 0.196 |
| ApoB/ApoA1 | 0.036 ± 0.010 | 0.033 ± 0.009 | 0.033 ± 0.010 | 0.001 | 0.595 |
| C-reactive protein (mg/L) | 2.3 (1.5, 3.3) | 1.6 (0.9, 2.5) | 1.5 (0.9, 2.3) | 0.016 | 0.771 |
| Interleukin-6 (pg/mL) | 0.8 (0.7, 1.1) | 0.7 (0.6, 0.8) | 0.8 (0.6, 0.9) | 0.322 | 0.549 |
| Tumor necrosis factor-α (pg/mL) | 2.41 (2.08, 2.79) | 2.22 (1.95, 2.53) | 2.18 (1.91, 2.49) | <0.001 | 0.672 |
| Baseline (Week 0) | End of Fully-Provided CRHP Diet (Week 6 or 12) | End of Self-Prepared CRHP Diet (Week 36) | P1 | P2 | |
|---|---|---|---|---|---|
| Systolic BP, daily average (mmHg) | 126 (122, 131) | 120 (116, 125) | 126 (122, 130) | 0.811 | 0.144 |
| Systolic BP, 24-h AUC (mmHg·h) | 2646 (2553, 2743) | 2511 (2417, 2608) | 2636 (2556, 2719) | 0.785 | 0.089 |
| Diastolic BP, daily average (mmHg) | 77 (73, 81) | 74 (70, 79) | 75 (72, 79) | 0.262 | 0.418 |
| Diastolic BP, 24-h AUC (mmHg·h) | 1627 ± 199 | 1534 ± 181 | 1588 ± 159 | 0.082 | 0.344 |
| Mean arterial pressure, daily average (mmHg) | 94 ± 9 | 90 ± 9 | 93 ± 7 | 0.221 | 0.296 |
| Mean arterial pressure, 24-h AUC (mmHg·h) | 1972 ± 197 | 1879 ± 181 | 1947 ± 153 | 0.316 | 0.263 |
| Pulse pressure, daily average (mmHg) | 49 ± 7 | 47 ± 8 | 50 ± 8 | 0.236 | 0.231 |
| Pulse pressure, 24-h AUC (mmHg·h) | 1030 ± 154 | 987 ± 175 | 1056 ± 166 | 0.231 | 0.224 |
| Heart rate, daily average (bpm) | 78 ± 10 | 75 ± 10 | 73 ± 8 | <0.001 | 0.303 |
| Heart rate, 24-h AUC (bpm·h) | 1634 ± 201 | 1580 ± 206 | 1528 ± 171 | <0.001 | 0.316 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, A.H.; Skytte, M.J.; Samkani, A.; Thomsen, M.N.; Astrup, A.; Ritz, C.; Frystyk, J.; Holst, J.J.; Madsbad, S.; Haugaard, S.B.; et al. Effects of a Self-Prepared Carbohydrate-Reduced High-Protein Diet on Cardiovascular Disease Risk Markers in Patients with Type 2 Diabetes. Nutrients 2021, 13, 1694. https://doi.org/10.3390/nu13051694
Alzahrani AH, Skytte MJ, Samkani A, Thomsen MN, Astrup A, Ritz C, Frystyk J, Holst JJ, Madsbad S, Haugaard SB, et al. Effects of a Self-Prepared Carbohydrate-Reduced High-Protein Diet on Cardiovascular Disease Risk Markers in Patients with Type 2 Diabetes. Nutrients. 2021; 13(5):1694. https://doi.org/10.3390/nu13051694
Chicago/Turabian StyleAlzahrani, Ahmad H., Mads J. Skytte, Amirsalar Samkani, Mads N. Thomsen, Arne Astrup, Christian Ritz, Jan Frystyk, Jens J. Holst, Sten Madsbad, Steen B. Haugaard, and et al. 2021. "Effects of a Self-Prepared Carbohydrate-Reduced High-Protein Diet on Cardiovascular Disease Risk Markers in Patients with Type 2 Diabetes" Nutrients 13, no. 5: 1694. https://doi.org/10.3390/nu13051694
APA StyleAlzahrani, A. H., Skytte, M. J., Samkani, A., Thomsen, M. N., Astrup, A., Ritz, C., Frystyk, J., Holst, J. J., Madsbad, S., Haugaard, S. B., Krarup, T., Larsen, T. M., & Magkos, F. (2021). Effects of a Self-Prepared Carbohydrate-Reduced High-Protein Diet on Cardiovascular Disease Risk Markers in Patients with Type 2 Diabetes. Nutrients, 13(5), 1694. https://doi.org/10.3390/nu13051694