Dietary Branched-Chain Amino Acids (BCAAs) and Risk of Dyslipidemia in a Chinese Population
Abstract
:1. Instruction
2. Research Design and Methods
2.1. Study Population and Data Collection
2.2. Dietary BCAAs Assessment
2.3. Laboratory Measurement
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.H.; Li, J.J. Aging and dyslipidemia: A review of potential mechanisms. Ageing Res. Rev. 2015, 19, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yang, Z.; Wu, Y.; Yin, R.X.; Liao, Y.; Wang, J.; Gao, B.; Zhang, L. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis 2016, 248, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Deng, Q.; Wang, L.; Huang, Z.; Zhou, M.; Li, Y.; Zhao, Z.; Zhang, Y.; Wang, L. Prevalence of dyslipidemia and achievement of low-density lipoprotein cholesterol targets in Chinese adults: A nationally representative survey of 163,641 adults. Int. J. Cardiol. 2018, 260, 196–203. [Google Scholar] [CrossRef]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; Wareham, N.; Bingham, S.; Boekholdt, S.M.; Khaw, K.T.; Gudnason, V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007, 115, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.S.; Welsh, C.E.; Celis-Morales, C.A.; Mackay, D.; Lewsey, J.; Gray, S.R.; Lyall, D.M.; Cleland, J.G.; Gill, J.; Jhund, P.S.; et al. Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat. Med. 2019, 25, 1753–1760. [Google Scholar] [CrossRef]
- Farzadfar, F.; Finucane, M.M.; Danaei, G.; Pelizzari, P.M.; Cowan, M.J.; Paciorek, C.J.; Singh, G.M.; Lin, J.K.; Stevens, G.A.; Riley, L.M.; et al. National, regional, and global trends in serum total cholesterol since 1980: Systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3·0 million participants. Lancet 2011, 377, 578–586. [Google Scholar] [CrossRef]
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Yan, Y.; Bazzano, L.A.; Juonala, M.; Raitakari, O.T.; Viikari, J.; Prineas, R.; Dwyer, T.; Sinaiko, A.; Burns, T.L.; Daniels, S.R.; et al. Long-Term Burden of Increased Body Mass Index from Childhood on Adult Dyslipidemia: The i3C Consortium Study. J. Clin. Med. 2019, 8, 1725. [Google Scholar] [CrossRef]
- Stevens, W.; Peneva, D.; Li, J.Z.; Liu, L.Z.; Liu, G.; Gao, R.; Lakdawalla, D.N. Estimating the future burden of cardiovascular disease and the value of lipid and blood pressure control therapies in China. BMC Health Serv. Res. 2016, 16, 175. [Google Scholar] [CrossRef] [Green Version]
- Bloomgarden, Z.T. Insulin resistance, dyslipidemia, and cardiovascular disease. Diabetes Care. 2007, 30, 2164–2170. [Google Scholar] [CrossRef] [Green Version]
- Uzhova, I.; Fuster, V.; Fernández-Ortiz, A.; Ordovás, J.M.; Sanz, J.; Fernández-Friera, L.; López-Melgar, B.; Mendiguren, J.M.; Ibáñez, B.; Bueno, H.; et al. The Importance of Breakfast in Atherosclerosis Disease: Insights From the PESA Study. J. Am. Coll. Cardiol. 2017, 70, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, J.; Daniels, S.R.; Hagberg, N.; Isasi, C.R.; Kelly, A.S.; Lloyd-Jones, D.; Pate, R.R.; Pratt, C.; Shay, C.M.; Towbin, J.A.; et al. Cardiovascular Health Promotion in Children: Challenges and Opportunities for 2020 and Beyond: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e236–e255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, G.A.; Wilson, J.F. In the clinic. Obesity. Ann. Intern. Med. 2008, 149, ITC4-1–ITC4-15; quiz ITC4-16. [Google Scholar]
- Li, T.; Zhang, Z.; Kolwicz, S.J.; Abell, L.; Roe, N.D.; Kim, M.; Zhou, B.; Cao, Y.; Ritterhoff, J.; Gu, H.; et al. Defective Branched-Chain Amino Acid Catabolism Disrupts Glucose Metabolism and Sensitizes the Heart to Ischemia-Reperfusion Injury. Cell Metab. 2017, 25, 374–385. [Google Scholar] [CrossRef] [Green Version]
- Hilt, Z.T.; Morrell, C.N. Essential Amino Acids-Essential in Arterial Thrombosis. Circulation. 2020, 142, 65–67. [Google Scholar] [CrossRef]
- Rahimi, M.H.; Shab-Bidar, S.; Mollahosseini, M.; Djafarian, K. Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: A meta-analysis of randomized clinical trials. Nutrition 2017, 42, 30–36. [Google Scholar] [CrossRef]
- Bai, G.H.; Tsai, M.C.; Tsai, H.W.; Chang, C.C.; Hou, W.H. Effects of branched-chain amino acid-rich supplementation on EWGSOP2 criteria for sarcopenia in older adults: A systematic review and meta-analysis. Eur. J. Nutr. 2022, 61, 637–651. [Google Scholar] [CrossRef]
- Neinast, M.D.; Jang, C.; Hui, S.; Murashige, D.S.; Chu, Q.; Morscher, R.J.; Li, X.; Zhan, L.; White, E.; Anthony, T.G.; et al. Quantitative Analysis of the Whole-Body Metabolic Fate of Branched-Chain Amino Acids. Cell Metab. 2019, 29, 417–429. [Google Scholar] [CrossRef] [Green Version]
- Okekunle, A.P.; Wu, X.; Duan, W.; Feng, R.; Li, Y.; Sun, C. Dietary Intakes of Branched-Chained Amino Acid and Risk for Type 2 Diabetes in Adults: The Harbin Cohort Study on Diet, Nutrition and Chronic Non-Communicable Diseases Study. Can. J. Diabetes. 2018, 42, 484–492. [Google Scholar] [CrossRef]
- McCormack, S.E.; Shaham, O.; McCarthy, M.A.; Deik, A.A.; Wang, T.J.; Gerszten, R.E.; Clish, C.B.; Mootha, V.K.; Grinspoon, S.K.; Fleischman, A. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr. Obes. 2013, 8, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Le Couteur, D.G.; Solon-Biet, S.M.; Cogger, V.C.; Ribeiro, R.; de Cabo, R.; Raubenheimer, D.; Cooney, G.J.; Simpson, S.J. Branched chain amino acids, aging and age-related health. Ageing Res. Rev. 2020, 64, 101198. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Dong, J.; Zhao, H.; Li, H.; Guo, H.; Wang, S.; Zhang, C.; Wang, S.; Wang, M.; Yu, S.; et al. Association of branched-chain amino acids with carotid intima-media thickness and coronary artery disease risk factors. PLoS ONE 2014, 9, e99598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Dong, J.; Guo, H.; Li, H.; Wang, S.; Zhao, H.; Zhou, W.; Yu, S.; Wang, M.; Chen, W. Rapid and precise measurement of serum branched-chain and aromatic amino acids by isotope dilution liquid chromatography tandem mass spectrometry. PLoS ONE 2013, 8, e81144. [Google Scholar]
- Kujala, U.M.; Peltonen, M.; Laine, M.K.; Kaprio, J.; Heinonen, O.J.; Sundvall, J.; Eriksson, J.G.; Jula, A.; Sarna, S.; Kainulainen, H. Branched-Chain Amino Acid Levels Are Related with Surrogates of Disturbed Lipid Metabolism among Older Men. Front. Med. 2016, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhao, L.; Zhang, J.; Yang, Z.; Yang, L.; Huang, J.; Fang, H.; Guo, Q.; Xu, X.; Ju, L.; et al. China Nutrition and Health Surveys (1982−2017). China CDC Wkly. 2021, 3, 193–195. [Google Scholar] [CrossRef]
- JCfDCGoPaToDi, A. Guidelines for the Prevention and Treatment of Dyslipids in Chinese Adults (Revised 2016). Zhongguo Xun Huan Za Zhi 2016, 31, 937–953. [Google Scholar]
- Yang, Y. Institute for Nutrition and Food Safety of the Chinese Center for Disease Control and Prevention. In China Food Compositiontable 2018; Peking University Medical Press: Beijing, China, 2018. [Google Scholar]
- Yang, Y. Institute for Nutrition and Food Safety of the Chinese Center for Disease Control and Prevention. In China Food Compositiontable 2019; Peking University Medical Press: Beijing, China, 2019. [Google Scholar]
- McGarrah, R.W.; Zhang, G.F.; Christopher, B.A.; Deleye, Y.; Walejko, J.M.; Page, S.; Ilkayeva, O.; White, P.J.; Newgard, C.B. Dietary branched-chain amino acid restriction alters fuel selection and reduces triglyceride stores in hearts of Zucker fatty rats. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E216–E223. [Google Scholar] [CrossRef]
- Wang, F.H.; Liu, J.; Deng, Q.J.; Qi, Y.; Wang, M.; Wang, Y.; Zhang, X.G.; Zhao, D. Association between plasma essential amino acids and atherogenic lipid profile in a Chinese population: A cross-sectional study. Atherosclerosis 2019, 286, 7–13. [Google Scholar] [CrossRef]
- Hamaya, R.; Mora, S.; Lawler, P.R.; Cook, N.R.; Ridker, P.M.; Buring, J.E.; Lee, I.M.; Manson, J.E.; Tobias, D.K. Association of Plasma Branched-Chain Amino Acid With Biomarkers of Inflammation and Lipid Metabolism in Women. Circ. Genom Precis Med. 2021, 14, e3330. [Google Scholar] [CrossRef]
- Yamakado, M.; Tanaka, T.; Nagao, K.; Ishizaka, Y.; Mitushima, T.; Tani, M.; Toda, A.; Toda, E.; Okada, M.; Miyano, H.; et al. Plasma amino acid profile is associated with visceral fat accumulation in obese Japanese subjects. Clin. Obes. 2012, 2, 29–40. [Google Scholar] [CrossRef]
- Green, C.R.; Wallace, M.; Divakaruni, A.S.; Phillips, S.A.; Murphy, A.N.; Ciaraldi, T.P.; Metallo, C.M. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 2016, 12, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, L.; Budde, K.; Kastenmüller, G.; Kaul, A.; Völker, U.; Völzke, H.; Adamski, J.; Kühn, J.P.; Krumsiek, J.; Artati, A.; et al. Associations between adipose tissue volume and small molecules in plasma and urine among asymptomatic subjects from the general population. Sci Rep. 2020, 10, 1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajuthi, S.P.; Sharma, N.K.; Comeau, M.E.; Chou, J.W.; Bowden, D.W.; Freedman, B.I.; Langefeld, C.D.; Parks, J.S.; Das, S.K. Genetic regulation of adipose tissue transcript expression is involved in modulating serum triglyceride and HDL-cholesterol. Gene 2017, 632, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Cholesterol Education Program (NCEP) Expert Panel. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, C.H.; Kim, E.H.; Bae, S.J.; Choe, J.; Park, J.Y.; Park, S.W.; Yun, Y.D.; Baek, S.J.; Mok, Y.; et al. Impaired fasting glucose and risk of cardiovascular disease in Korean men and women: The Korean Heart Study. Diabetes Care 2013, 36, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Verbeek, R.; Hoogeveen, R.M.; Langsted, A.; Stiekema, L.; Verweij, S.L.; Hovingh, G.K.; Wareham, N.J.; Khaw, K.T.; Boekholdt, S.M.; Nordestgaard, B.G.; et al. Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting. Eur. Heart J. 2018, 39, 2589–2596. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Granger, C.B.; Craig, D.; Haynes, C.; Bain, J.; Stevens, R.D.; Hauser, E.R.; Newgard, C.B.; Kraus, W.E.; Newby, L.K.; et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis 2014, 232, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Sun, Y.; Sun, L.; Liu, X.; Zeng, R.; Lin, X.; Li, Y. Effects of gut microbiota and fatty acid metabolism on dyslipidemia following weight-loss diets in women: Results from a randomized controlled trial. Clin. Nutr. 2021, 40, 5511–5520. [Google Scholar] [CrossRef]
- Mente, A.; Dehghan, M.; Rangarajan, S.; McQueen, M.; Dagenais, G.; Wielgosz, A.; Lear, S.; Li, W.; Chen, H.; Yi, S.; et al. Association of dietary nutrients with blood lipids and blood pressure in 18 countries: A cross-sectional analysis from the PURE study. Lancet Diabetes Endocrinol. 2017, 5, 774–787. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, M.; Kuwabara, R.; Niwa, K.; Hisatome, I.; Smits, G.; Roncal-Jimenez, C.A.; MacLean, P.S.; Yracheta, J.M.; Ohno, M.; Lanaspa, M.A.; et al. Different Risk for Hypertension, Diabetes, Dyslipidemia, and Hyperuricemia According to Level of Body Mass Index in Japanese and American Subjects. Nutrients 2018, 10, 1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hemelrijck, M.; Ulmer, H.; Nagel, G.; Peter, R.S.; Fritz, J.; Myte, R.; van Guelpen, B.; Föger, B.; Concin, H.; Häggström, C.; et al. Longitudinal study of body mass index, dyslipidemia, hyperglycemia, and hypertension in 60,000 men and women in Sweden and Austria. PLoS ONE 2018, 13, e197830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
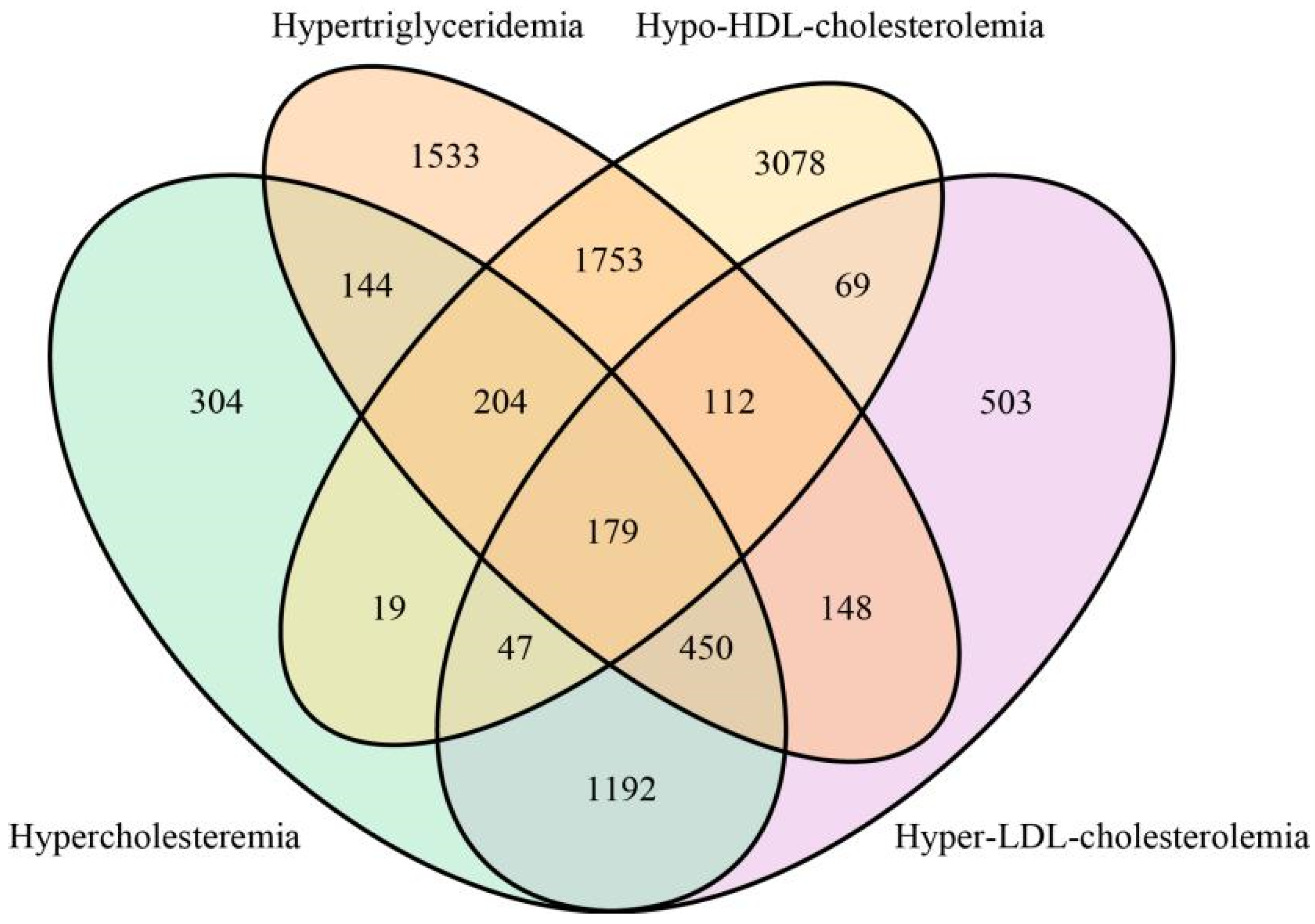


| Hypercholesteremia (n = 2539) | Hypertriglyceridemia (n = 4523) | Hypo-HDL-Cholesterolemia (n = 5461) | Hyper-LDL-Cholesterolemia (n = 2700) | Dyslipidemia (n = 9792) | Normolipemic (n = 9541) | p Value | |
|---|---|---|---|---|---|---|---|
| Dyslipidemia vs. Normolipemic | |||||||
| Age (years) | 55.51 (11.78) | 51.61 (11.69) | 51.01 (12.56) | 55.24 (11.89) | 52.45 (12.35) | 52.69 (12.14) | 0.181 |
| Male, n (%) | 1239 (48.8) | 2700 (59.69) | 3314 (60.68) | 1352 (50.07) | 5522 (56.39) | 5284 (55.38) | 0.157 |
| BMI (kg/m2) | 24.36 (22.87) | 25.16 (29.53) | 24.97 (26.93) | 24.64 (22.18) | 24.73 (25.96) | 24.6 (12.04) | 0.646 |
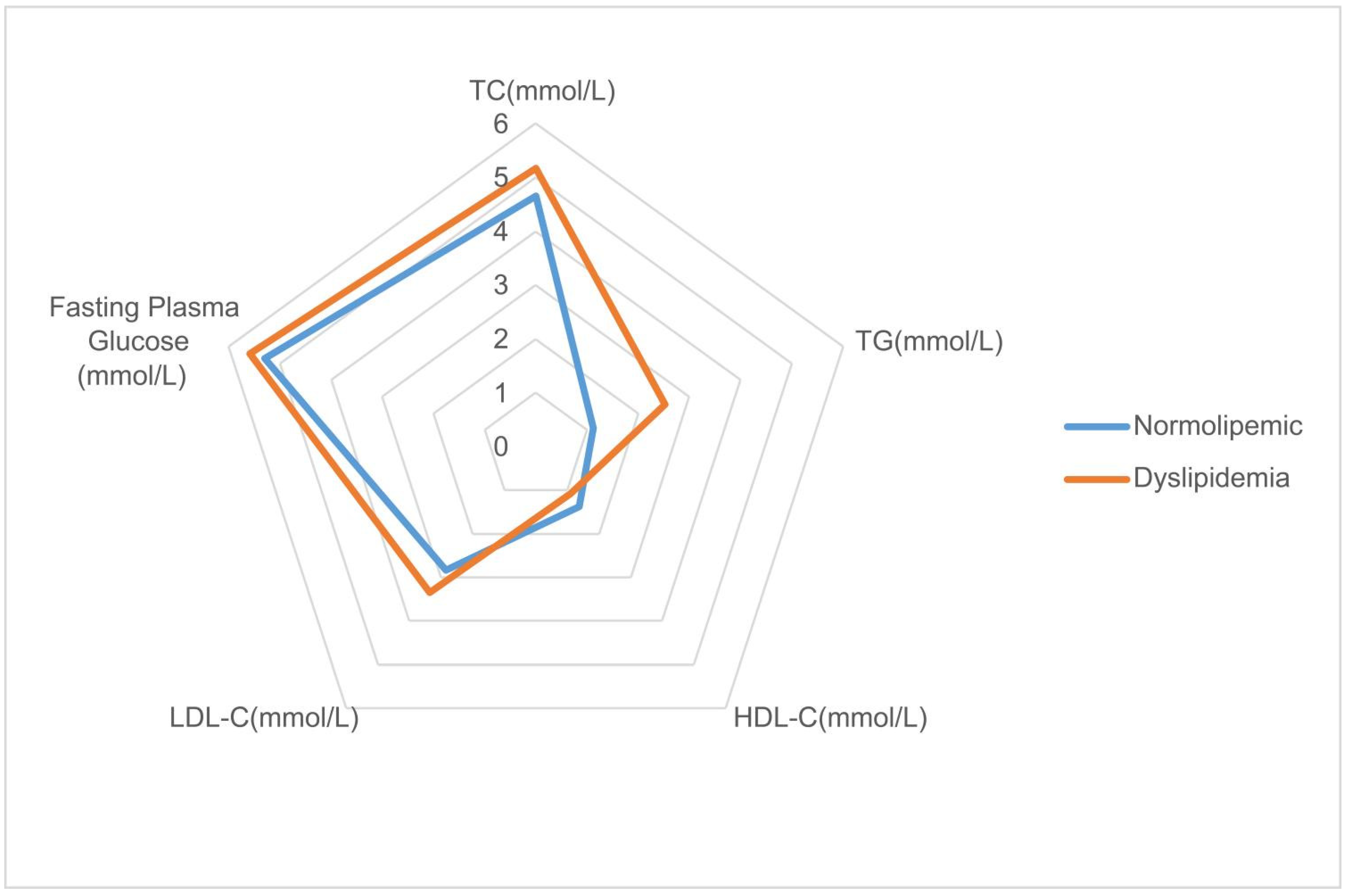
| Fasting plasma glucose (mmol/L) | 5.8 (1.98) | 5.83 (2.04) | 5.53 (1.67) | 5.71 (1.83) | 5.59 (1.73) | 5.3 (1.19) | <0.001 |
| Serum uric acid (μmol/L) | 334.31 (95.36) | 349.84 (95.59) | 329.71 (91.61) | 335.51 (93.13) | 330.86 (92.72) | 303.15 (81.01) | <0.001 |
| Total cholesterol (mmol/L) | 6.7 (3.43) | 5.34 (2.7) | 4.5 (1.07) | 6.52 (0.8) | 5.17 (2.12) | 4.66 (0.69) | <0.001 |
| Triglyceride (mmol/L) | 2.84 (3.85) | 3.89 (3.05) | 2.71 (2.93) | 2.05 (1.27) | 2.53 (2.45) | 1.12 (0.46) | <0.001 |
| HDL cholesterol (mmol/L) | 1.4 (0.43) | 1.03 (0.28) | 0.87 (0.14) | 1.34 (0.38) | 1.09 (0.36) | 1.38 (0.28) | <0.001 |
| LDL cholesterol (mmol/L) | 4.34 (1.03) | 3.32 (0.91) | 2.85 (0.82) | 4.6 (0.63) | 3.35 (1.03) | 2.84 (0.63) | <0.001 |
| Systolic pressure (mmHg) | 140.11 (31.36) | 139.29 (35.98) | 134.3 (28.32) | 139.51 (30.84) | 136.81 (31.79) | 135.15 (30.21) | <0.001 |
| Diastolic pressure (mmHg) | 83.27 (26.78) | 84.87 (33.84) | 81.21 (24.31) | 82.8 (26.19) | 82.38 (28.29) | 80.69 (26.42) | <0.001 |
| Current smoker, n (%) | 719 (28.32) | 1530 (33.83) | 1805 (33.05) | 789 (29.22) | 3071 (31.36) | 2818 (29.54) | 0.006 |
| Current drinker, n (%) | 349 (13.75) | 680 (15.03) | 662 (12.12) | 355 (13.15) | 1268 (12.95) | 1117 (11.71) | 0.009 |
| Educational level | |||||||
| None or elementary school | 1254 (49.39) | 1891 (41.81) | 2162 (39.59) | 1360 (50.37) | 4300 (43.91) | 4630 (48.53) | <0.001 |
| Middle school | 747 (29.42) | 1578 (34.89) | 1936 (35.45) | 825 (30.56) | 3282 (33.52) | 3102 (32.51) | |
| High school | 356 (14.02) | 655 (14.48) | 822 (15.05) | 358 (13.26) | 1394 (14.24) | 1206 (12.64) | |
| College | 182 (7.17) | 399 (8.82) | 541 (9.91) | 157 (5.81) | 816 (8.33) | 603 (6.32) | |
| Energy intake (kcal/day) | 1764.41 (614.1) | 1822.73 (636) | 1808.29 (612.29) | 1757.15 (611.38) | 1798.21 (621.87) | 1822.79 (630.62) | 0.006 |
| Carbohydrate intake (g/day) | 220.68 (88.74) | 233.96 (94.11) | 241.04 (98.02) | 221.8 (89.11) | 233.85 (95.25) | 235.05 (96.56) | 0.387 |
| Protein intake (g/day) | 55.59 (24.44) | 56.36 (24.75) | 56.12 (24.21) | 55.65 (24.58) | 55.77 (24.43) | 54.71 (23.5) | 0.002 |
| Fat intake (g/day) | 74.36 (40.24) | 74.83 (40.89) | 72.07 (38.74) | 73.92 (40) | 73.3 (39.74) | 75.58 (40.77) | <0.001 |
| MET-h/d | 22.61 (17.97) | 22.07 (16.96) | 21.42 (16.74) | 22.71 (17.70) | 21.95 (17.24) | 23.62 (19.09) | <0.001 |
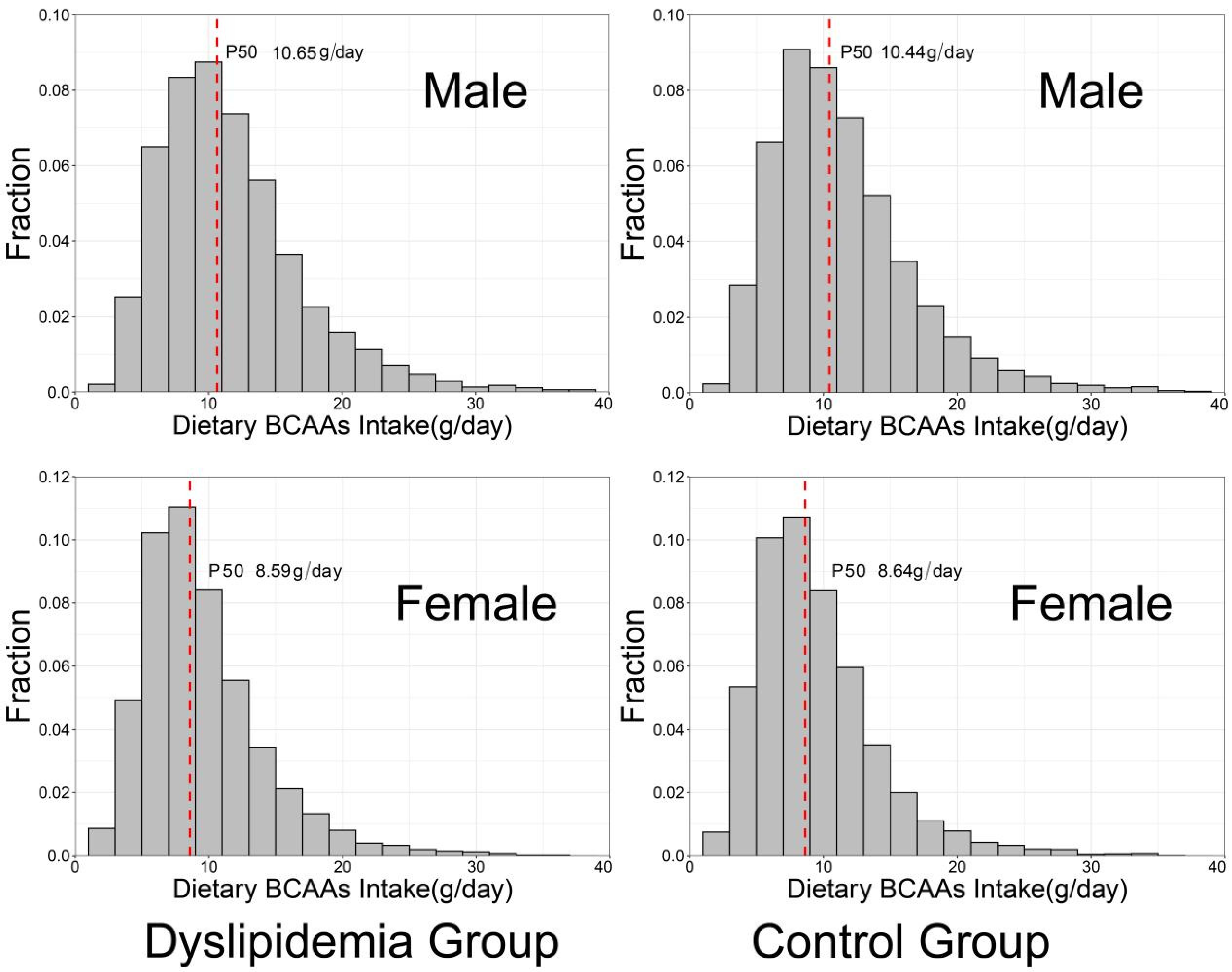
| BCAAs intake (g/day) | 10.83 (5.23) | 10.8 (5.21) | 10.74 (5.17) | 10.76 (5.13) | 10.73 (5.19) | 10.57 (5.15) | 0.029 |
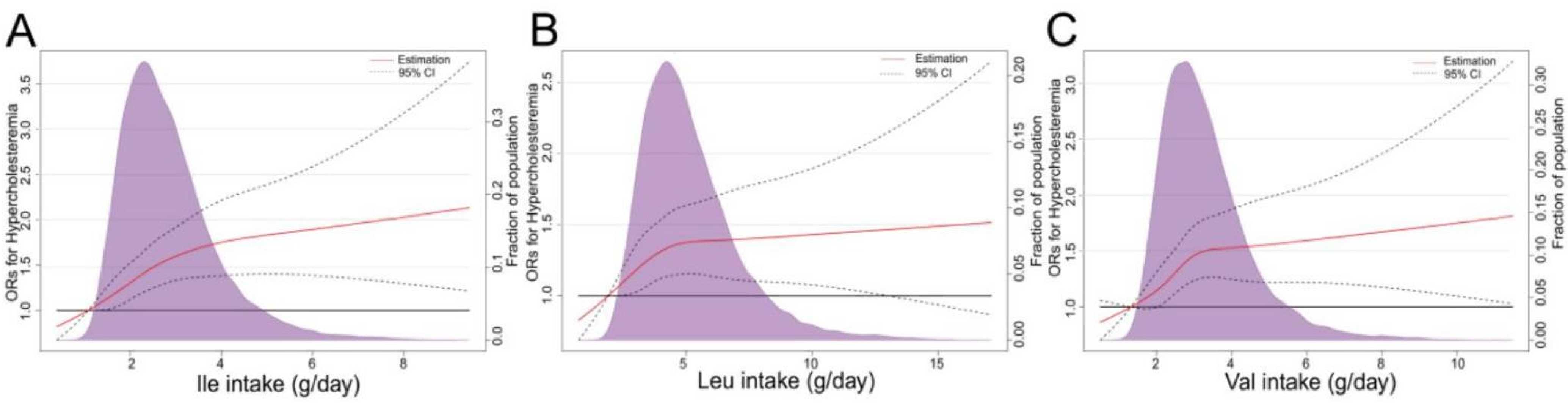
| Ile intake (g/day) | 2.72 (1.31) | 2.71 (1.31) | 2.69 (1.29) | 2.70 (1.29) | 2.69 (1.30) | 2.65 (1.31) | 0.0700 |
| Leu intake (g/day) | 4.89 (2.40) | 4.88 (2.40) | 4.87 (2.38) | 4.86 (2.34) | 4.86 (2.39) | 4.79 (2.39) | 0.0706 |
| Val intake (g/day) | 3.22 (1.53) | 3.20 (1.52) | 3.19 (1.52) | 3.20 (1.50) | 3.18 (1.52) | 3.15 (1.53) | 0.0836 |
| Quartile of Dietary BCAAs Intake (g/Day) | |||||
|---|---|---|---|---|---|
| Q1 (Referent), <7.03 | Q2, 7.03 to <9.64 | Q3, 9.64 to <13.09 | Q4, ≥13.09 | p for Trend | |
| Total | |||||
| n (%) | 4833 (25.0) | 4833 (25.0) | 4833 (25.0) | 4834 (25.0) | |
| Total cholesterol (mmol/L) | 4.86 (1.05) | 4.93 (2.62) | 4.93 (1.09) | 4.95 (1.06) | <0.0001 |
| Triglyceride (mmol/L) | 1.78 (1.6) | 1.82 (1.9) | 1.86 (2.15) | 1.88 (1.93) | 0.265 |
| HDL cholesterol (mmol/L) | 1.24 (0.34) | 1.23 (0.35) | 1.23 (0.36) | 1.22 (0.36) | <0.0001 |
| LDL cholesterol (mmol/L) | 3.05 (0.87) | 3.08 (0.89) | 3.10 (0.91) | 3.15 (0.9) | <0.0001 |
| Dyslipidemia Group | |||||
| n (%) | 2403 (24.54) | 2431 (24.83) | 2445 (24.97) | 2513 (25.66) | |
| Total cholesterol (mmol/L) | 5.09 (1.28) | 5.22 (3.61) | 5.17 (1.32) | 5.19 (1.26) | 0.0007 |
| Triglyceride (mmol/L) | 2.43 (2.03) | 2.5 (2.46) | 2.59 (2.8) | 2.6 (2.43) | 0.9536 |
| HDL cholesterol (mmol/L) | 1.10 (0.35) | 1.10 (0.36) | 1.08 (0.35) | 1.09 (0.37) | 0.0014 |
| LDL cholesterol (mmol/L) | 3.31 (1.01) | 3.34 (1.03) | 3.34 (1.06) | 3.39 (1.02) | <0.0001 |
| Control Group | |||||
| n (%) | 2430 (25.47) | 2402 (25.18) | 2388 (25.03) | 2321 (24.33) | |
| Total cholesterol (mmol/L) | 4.63 (0.69) | 4.65 (0.69) | 4.68 (0.7) | 4.69 (0.69) | <0.0001 |
| Triglyceride (mmol/L) | 1.14 (0.45) | 1.13 (0.46) | 1.11 (0.46) | 1.11 (0.46) | 0.0029 |
| HDL cholesterol (mmol/L) | 1.37 (0.27) | 1.37 (0.28) | 1.39 (0.3) | 1.37 (0.29) | <0.0001 |
| LDL cholesterol (mmol/L) | 2.80 (0.62) | 2.83 (0.62) | 2.85 (0.63) | 2.88 (0.64) | 0.0026 |
| Quartile of Dietary BCAAs Consumption (g/Day) | p for Trend | ||||
|---|---|---|---|---|---|
| Q1 (Referent), <7.03 | Q2, 7.03 to <9.64 | Q3, 9.64 to <13.09 | Q4, ≥13.09 | ||
| Hypercholesteremia vs. Control group | |||||
| Case/control subjects, n | 593/2430 | 623/2402 | 663/2388 | 660/2321 | |
| Crude OR (95% CI) | 1 | 1.06 (0.94, 1.20) | 1.14 (1.01, 1.29) | 1.17 (1.03, 1.33) | 0.0565 |
| Adjusted OR * (95% CI) | 1 | 1.14 (1.01, 1.30) | 1.28 (1.13, 1.45) | 1.39 (1.22, 1.59) | <0.0001 |
| Adjusted OR † (95% CI) | 1 | 1.15 (1.00, 1.32) | 1.25 (1.08, 1.46) | 1.29 (1.05, 1.58) | 0.034 |
| Hypertriglyceridemia vs. Control group | |||||
| Case/control subjects, n | 1097/2430 | 1107/2402 | 1128/2388 | 1191/2321 | |
| Crude OR (95% CI) | 1 | 1.02 (0.92, 1.13) | 1.05 (0.95, 1.16) | 1.14 (1.03, 1.26) | 0.0646 |
| Adjusted OR * (95% CI) | 1 | 0.99 (0.89, 1.10) | 0.99 (0.889, 1.09) | 1.02 (0.92, 1.14) | 0.8894 |
| Adjusted OR † (95% CI) | 1 | 0.95 (0.85, 1.05) | 0.92 (0.81, 1.04) | 0.90 (0.76, 1.06) | 0.5309 |
| Hypo-HDL-cholesterolemia vs. Control group | |||||
| Case/control subjects, n | 1331/2430 | 1351/2402 | 1369/2388 | 1410/2321 | |
| Crude OR (95% CI) | 1 | 1.02 (0.93, 1.12) | 1.05 (0.95, 1.15) | 1.11 (1.01, 1.22) | 0.1528 |
| Adjusted OR * (95% CI) | 1 | 0.98 (0.89, 1.08) | 0.97 (0.88, 1.06) | 0.97 (0.88, 1.07) | 0.9034 |
| Adjusted OR † (95% CI) | 1 | 0.93 (0.84, 1.02) | 0.89 (0.79, 1.00) | 0.87 (0.74, 1.01) | 0.2402 |
| Hyper-LDL-cholesterolemia vs. Control group | |||||
| Case/control subjects, n | 633/2430 | 677/2402 | 698/2388 | 692/2321 | |
| Crude OR (95% CI) | 1 | 1.09 (0.96, 1.23) | 1.13 (1.00, 1.28) | 1.15 (1.02, 1.30) | 0.1145 |
| Adjusted OR * (95% CI) | 1 | 1.16 (1.02, 1.31) | 1.24 (1.09, 1.40) | 1.33 (1.17, 1.51) | 0.0001 |
| Adjusted OR † (95% CI) | 1 | 1.15 (1.01, 1.31) | 1.20 (1.03, 1.39) | 1.18 (0.97, 1.45) | 0.1013 |
| Dyslipidemia vs. Control group | |||||
| Case/control subjects, n | 2403/2430 | 2431/2402 | 2445/2388 | 2513/2321 | |
| Crude OR (95% CI) | 1 | 1.02 (0.94, 1.11) | 1.04 (0.957, 1.12) | 1.10 (1.01, 1.19) | 0.1388 |
| Adjusted OR * (95% CI) | 1 | 1.02 (0.94, 1.10) | 1.02 (0.939, 1.10) | 1.06 (0.98, 1.16) | 0.4982 |
| Adjusted OR † (95% CI) | 1 | 0.98 (0.90, 1.06) | 0.96 (0.87, 1.06) | 0.95 (0.828, 1.08) | 0.8417 |
| Quartile of Dietary BCAAs Consumption (g/Day) | p Value for Interaction | ||||
|---|---|---|---|---|---|
| Q1 (Referent), <7.03 | Q2, 7.03 to <9.64 | Q3, 9.64 to <13.09 | Q4, ≥13.09 | ||
| Sex | 0.8826 | ||||
| Male (6523) | 1 | 1.17 (0.95, 1.42) | 1.29 (1.06, 1.56) | 1.35 (1.12, 1.63) | |
| Female (5557) | 1 | 1.14 (0.96, 1.35) | 1.29 (1.08, 1.53) | 1.46 (1.21, 1.76) | |
| Age, years | 0.0047 | ||||
| <55 (6845) | 1 | 1.24 (1.02, 1.51) | 1.50 (1.25, 1.82) | 1.64 (1.36, 1.98) | |
| ≥55 (5235) | 1 | 1.10 (0.93, 1.30) | 1.11 (0.93, 1.32) | 1.18 (0.98, 1.42) | |
| BMI, kg/m2 | 0.0739 | ||||
| <24 (5215) | 1 | 1.33 (1.10, 1.61) | 1.43 (1.18, 1.74) | 1.47 (1.20, 1.81) | |
| ≥24 (6865) | 1 | 1.03 (0.87, 1.22) | 1.20 (1.02, 1.42) | 1.34 (1.13, 1.58) | |
| Current smoking | 0.7721 | ||||
| Yes (3537) | 1 | 1.23 (0.95, 1.59) | 1.21 (0.94, 1.57) | 1.328 (1.04, 1.70) | |
| No (8543) | 1 | 1.12 (0.97, 1.30) | 1.32 (1.14, 1.53) | 1.43 (1.22, 1.67) | |
| Current drinking | 0.4331 | ||||
| Yes (1466) | 1 | 0.89 (0.60, 1.32) | 1.16 (0.80, 1.67) | 1.17 (0.82, 1.66) | |
| No (10,614) | 1 | 1.18 (1.03, 1.35) | 1.29 (1.13, 1.48) | 1.41 (1.23, 1.63) | |
| METs-h/day | 0.3213 | ||||
| <22.77 (7439) | 1 | 1.06 (0.90, 1.24) | 1.10 (0.94, 1.29) | 1.28 (1.09, 1.51) | |
| ≥22.77 (4641) | 1 | 1.35 (1.09, 1.69) | 1.71 (1.37, 2.12) | 1.67 (1.34, 2.08) | |
| Region | 0.4076 | ||||
| urban | 1 | 1.06 (0.86, 1.31) | 1.32 (1.08, 1.62) | 1.44 (1.17, 1.77) | |
| rural | 1 | 1.20 (1.02, 1.41) | 1.24 (1.06, 1.47) | 1.36 (1.15, 1.61) | |
| Energy intake (kcal/day) | 0.2401 | ||||
| <2200 (9325) | 1 | 1.15 (1.01, 1.32) | 1.34 (1.17, 1.54) | 1.47 (1.26, 1.73) | |
| ≥2200 (2755) | 1 | 1.22 (0.62, 2.39) | 1.34 (0.72, 2.48) | 1.62 (0.89, 2.96) | |
| Normolipemic (n = 9541) | Dyslipidemia (n = 9792) | Total (n = 19,333) | |
|---|---|---|---|
| Cereals (g/day) | 3.88 (2.25) | 3.82 (2.23) | 3.85 (2.24) |
| Beans (g/day) | 0.78 (1.36) | 0.75 (1.28) | 0.77 (1.32) |
| Vegetables (g/day) | 0.93 (1.13) | 0.97 (1.15) | 0.95 (1.14) |
| Fruits (g/day) | 0.04 (0.26) | 0.04 (0.25) | 0.04 (0.26) |
| Nuts (g/day) | 0.12 (0.44) | 0.12 (0.46) | 0.12 (0.45) |
| Red meat (g/day) | 1.82 (2.09) | 1.92 (2.20) | 1.87 (2.14) |
| Poultry (g/day) | 0.43 (1.08) | 0.45 (1.12) | 0.44 (1.10) |
| Dairy products (g/day) | 0.13 (0.62) | 0.12 (0.51) | 0.12 (0.56) |
| Eggs (g/day) | 0.55 (0.76) | 0.57 (0.76) | 0.56 (0.76) |
| Fish and seafoods (g/day) | 0.85 (1.85) | 0.98 (1.98) | 0.92 (1.92) |
| Snacks (g/day) | 0.24 (1.25) | 0.2 (1.06) | 0.22 (1.16) |
| Beverage (g/day) | 0.18 (1.64) | 0.18 (1.58) | 0.18 (1.61) |
| Condiments (g/day) | 0.13 (0.36) | 0.14 (0.50) | 0.13 (0.43) |
| Others (g/day) | 0.47 (1.18) | 0.47 (1.14) | 0.47 (1.16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Zhu, Q.; Li, Y.; Song, P.; Zhang, J. Dietary Branched-Chain Amino Acids (BCAAs) and Risk of Dyslipidemia in a Chinese Population. Nutrients 2022, 14, 1824. https://doi.org/10.3390/nu14091824
Yu L, Zhu Q, Li Y, Song P, Zhang J. Dietary Branched-Chain Amino Acids (BCAAs) and Risk of Dyslipidemia in a Chinese Population. Nutrients. 2022; 14(9):1824. https://doi.org/10.3390/nu14091824
Chicago/Turabian StyleYu, Lianlong, Qianrang Zhu, Yuqian Li, Pengkun Song, and Jian Zhang. 2022. "Dietary Branched-Chain Amino Acids (BCAAs) and Risk of Dyslipidemia in a Chinese Population" Nutrients 14, no. 9: 1824. https://doi.org/10.3390/nu14091824
APA StyleYu, L., Zhu, Q., Li, Y., Song, P., & Zhang, J. (2022). Dietary Branched-Chain Amino Acids (BCAAs) and Risk of Dyslipidemia in a Chinese Population. Nutrients, 14(9), 1824. https://doi.org/10.3390/nu14091824








