Effects of Intermittent Fasting on Hypothalamus–Pituitary–Thyroid Axis, Palatable Food Intake, and Body Weight in Stressed Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Brain and Adipose Tissue mRNA Quantification by Real-Time PCR Analysis
2.3. Determination of Serum Hormones Content
2.4. Immunoblotting
2.5. Adipose Tissue Histologic Analysis and Immunohistochemistry (IHC)
2.6. Statistical Analysis
3. Results
3.1. Changes after 2 Weeks in Stressed and Non-Stressed Rats Eating Chow or Palatable Food
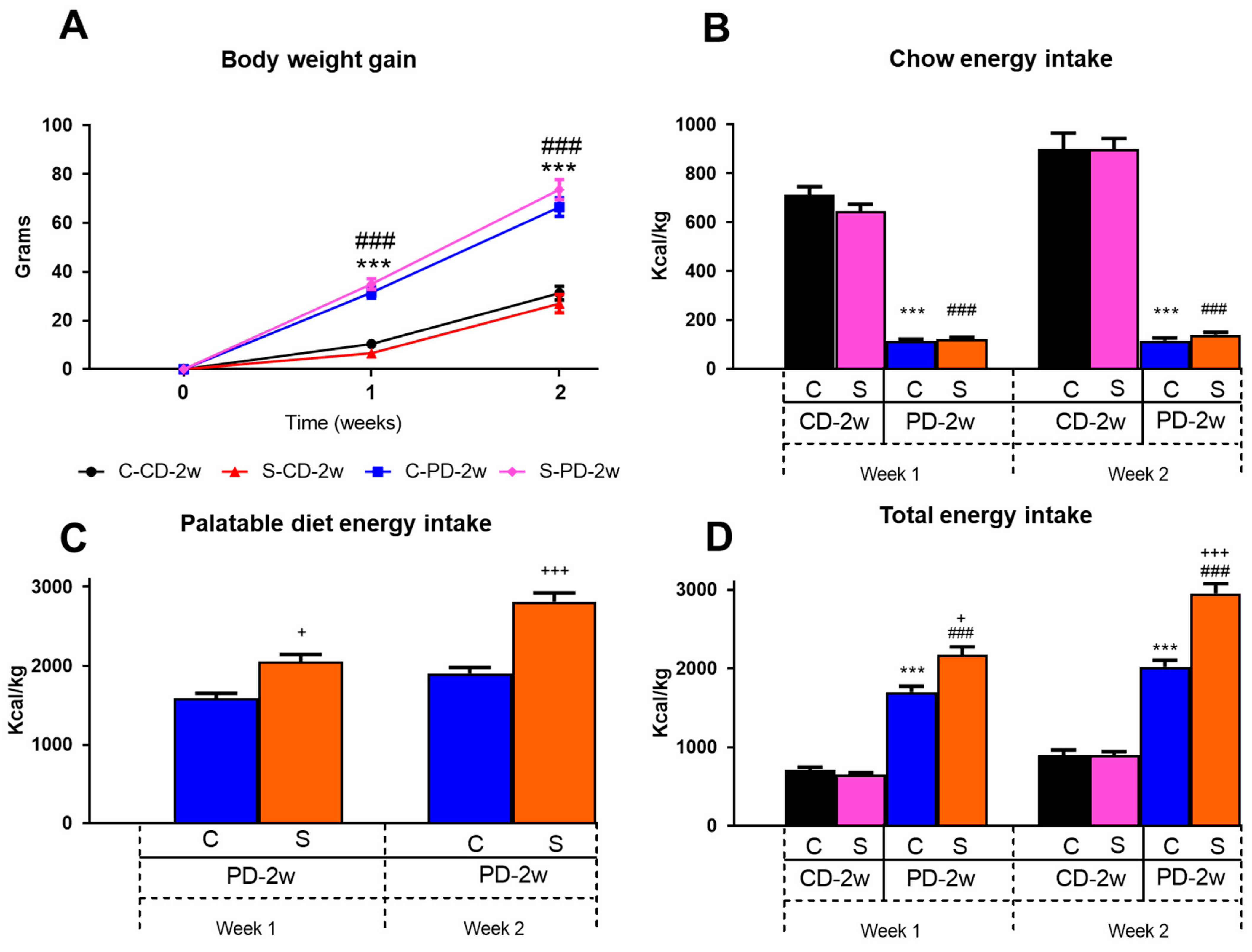
3.1.1. Body Weight Gain
3.1.2. Energy Intake
3.1.3. Adipose Tissue Morphology and Thermogenesis
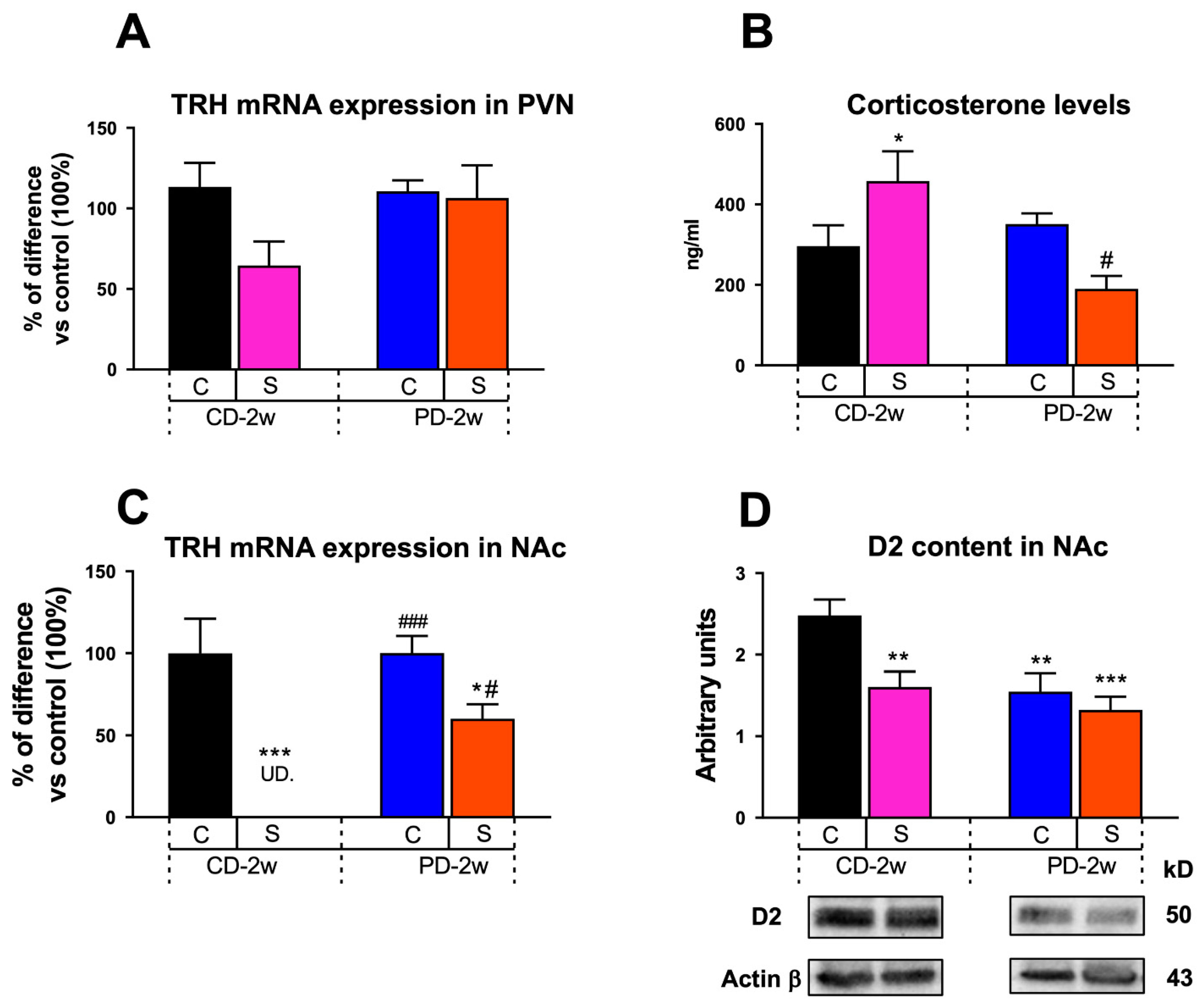
3.1.4. Neuroendocrine Changes
3.1.5. Limbic Changes
3.2. Changes Induced by Three Weeks of Intermittent Fasting in Stressed and Non-Stressed Rats Eating Palatable Food
3.2.1. Body Weight
3.2.2. Energy Intake
3.2.3. Adipose Tissue Morphology and Thermogenesis
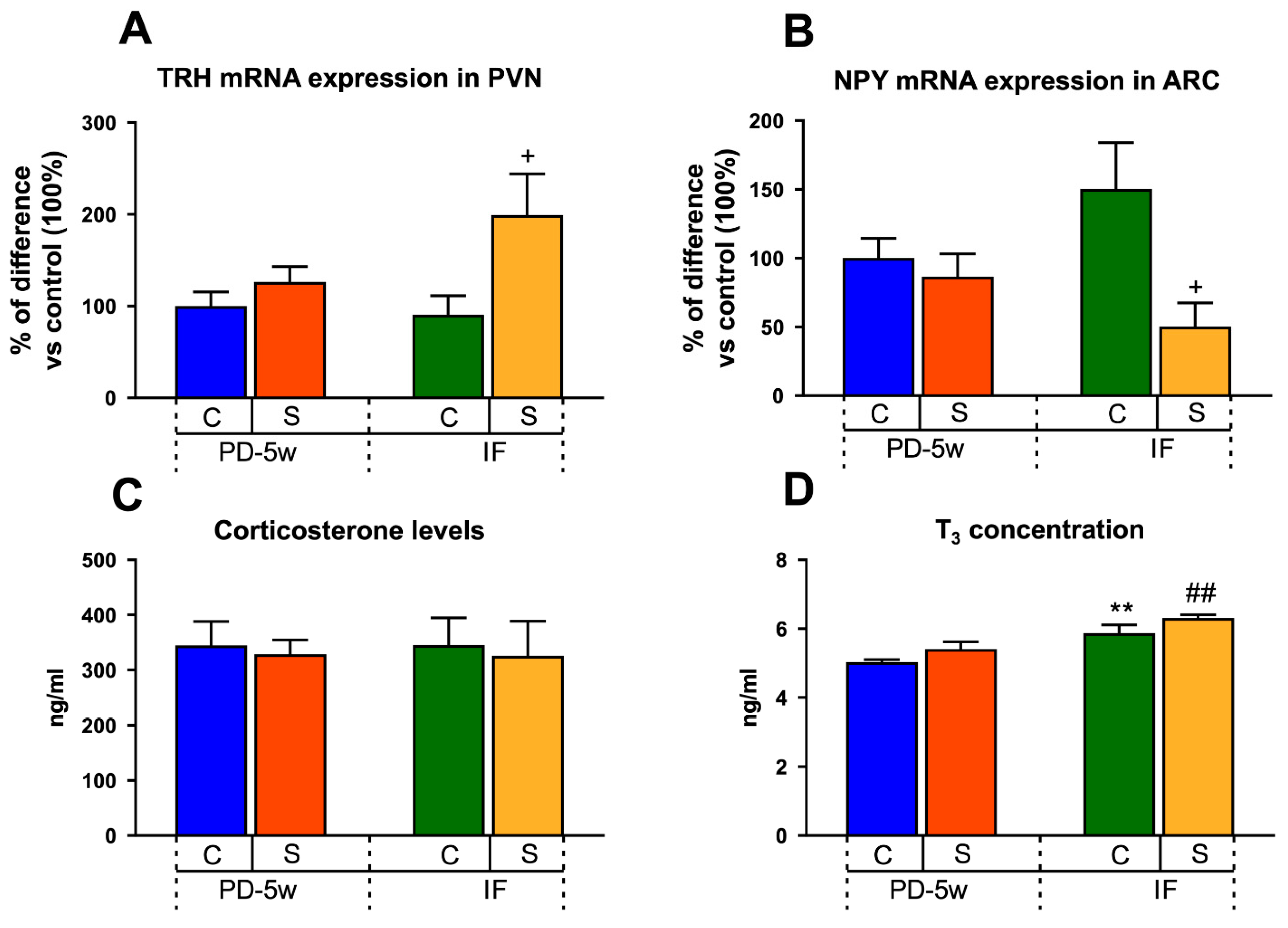
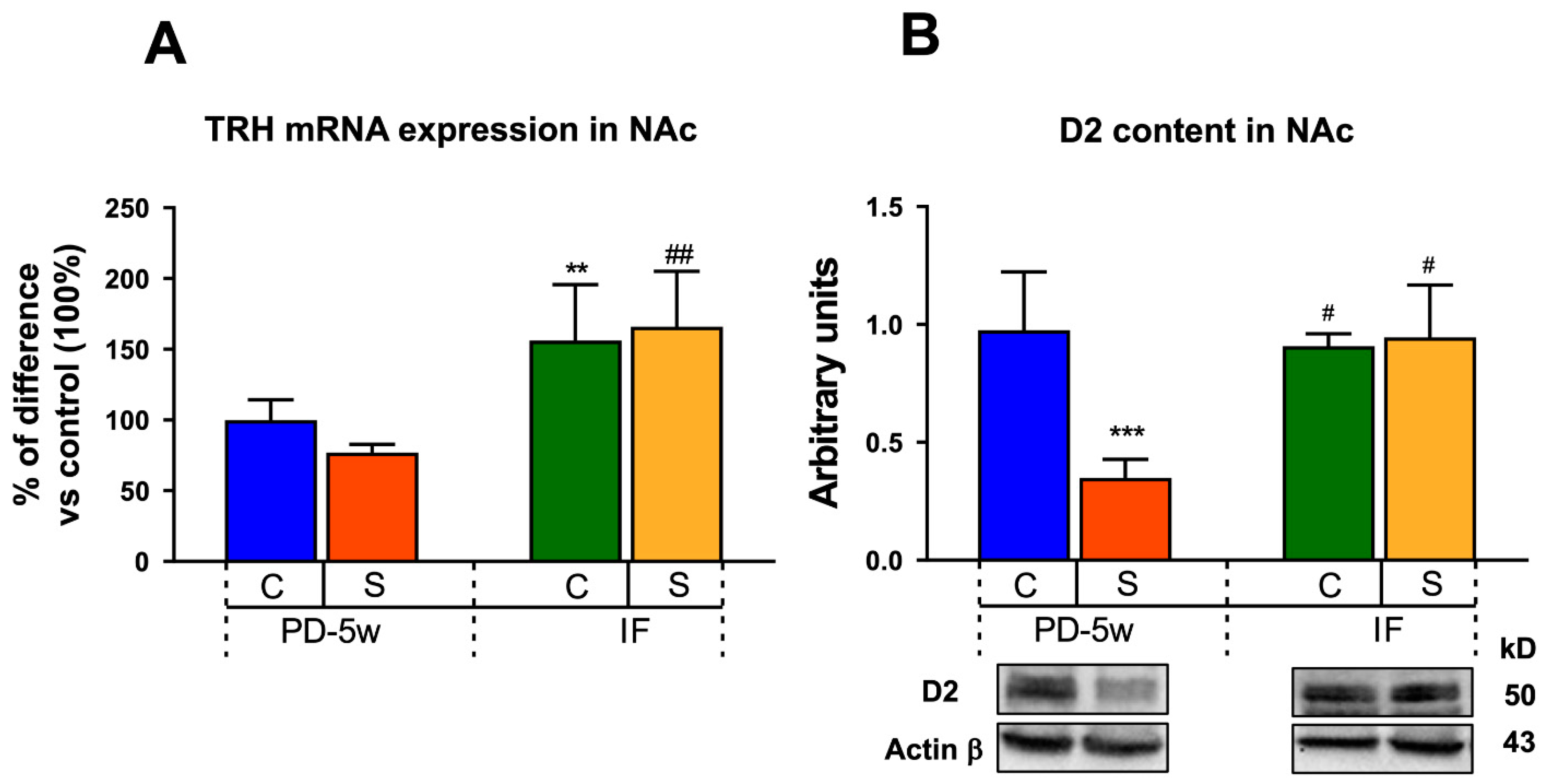
3.2.4. Neuroendocrine Changes
3.2.5. Limbic Changes
4. Discussion
4.1. Two-Week Stressed and Non-Stressed Rats Eating Chow or Palatable Food
4.1.1. Neuroendocrine Changes
4.1.2. Limbic Changes
4.2. Effects of 3 Weeks of Intermittent Fasting Schedule in Stressed and Non-Stressed Rats Fed with Palatable Food
4.2.1. Neuroendocrine Changes
4.2.2. Limbic Changes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falcón, E.; Valdés-Moreno, M.; Rodríguez, C.; Sanabrais-Jiménez, M.; Hernández-Muñoz, S.; Camarena, B.; de Gortari, P. Interaction between three stress-related gene polymorphisms and food addiction increases the risk to develop obesity in a sample of Mexican people attending a nutrition clinic. Psychoneuroendocrinology 2021, 125, 105099. [Google Scholar] [CrossRef]
- Alvarez-Salas, E.; González, A.; Amaya, M.I.; de Gortari, P. Accumbal TRH is downstream of the effects of isolation stress on hedonic food intake in rats. Nutr. Neurosci. 2021, 7, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Hewagalamulage, S.D.; Lee, T.K.; Clarke, I.J.; Henry, B.A. Stress, cortisol, and obesity: A role for cortisol responsiveness in identifying individuals prone to obesity. Domest. Anim. Endocrinol. 2016, 56, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Yau, Y.H.C.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- van Rossum, E.F.C. Obesity and cortisol: New perspectives on an old theme. Obesity 2017, 25, 500–501. [Google Scholar] [CrossRef]
- Wester, V.L.; Staufenbiel, S.M.; Veldhorst, M.A.B.; Visser, J.A.; Manenschijn, L.; Koper, J.W.; Klessens-Godfroy, F.J.M.; Van Den Akker, E.L.T.; Van Rossum, E.F.C. Long-term cortisol levels measured in scalp hair of obese patients. Obesity 2014, 22, 1956–1958. [Google Scholar] [CrossRef]
- van Haasteren, G.A.; Rondeel, J.M.M.; Themmen, A.P.N.; De Jong, F.H.; Valentijn, K.; Vaudry, H.; Bauer, K.; Visser, T.J.; de Greef, W.J. Starvation-induced changes in the hypothalamic content of prothyrotrophin-releasing hormone (proTRH) mRNA and the hypothalamic release of proTRH-derived peptides: Role of the adrenal gland. J. Endocrinol. 1995, 145, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Nillni, E.A.; Vaslet, C.; Harris, M.; Hollenberg, A.; Bjorbaek, C.; Flier, J.S. Leptin Regulates Prothyrotropin-releasing Hormone Biosynthesis: Evidence for direct and indirect pathways. J. Biol. Chem. 2000, 275, 36124–36133. [Google Scholar] [CrossRef]
- Alkemade, A.; Unmehopa, U.A.; Wiersinga, W.M.; Swaab, D.F.; Fliers, E. Glucocorticoids Decrease Thyrotropin-Releasing Hormone Messenger Ribonucleic Acid Expression in the Paraventricular Nucleus of the Human Hypothalamus. J. Clin. Endocrinol. Metab. 2005, 90, 323–327. [Google Scholar] [CrossRef]
- Kakucska, I.; Qi, Y.; Lechan, R.M. Changes in adrenal status affect hypothalamic thyrotropin-releasing hormone gene expression in parallel with corticotropin-releasing hormone. Endocrinology 1995, 136, 2795–2802. [Google Scholar] [CrossRef]
- Adafer, R.; Messaadi, W.; Meddahi, M.; Patey, A.; Haderbache, A.; Bayen, S.; Messaadi, N. Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating’ s Effects on Human Health. Nutrients 2020, 12, 3770. [Google Scholar] [CrossRef]
- Morales-Suarez-Varela, M.; Collado Sánchez, E.; Peraita-Costa, I.; Llopis-Morales, A.; Soriano, J.M.; Fiorentino, V. Intermittent Fasting and the Possible Benefits in Obesity, Diabetes, and Multiple Sclerosis: A Systematic Review of Randomized Clinical Trials. Nutrients 2021, 13, 3179. [Google Scholar] [CrossRef] [PubMed]
- Zubrzycki, A.; Cierpka-Kmiec, K.; Kmiec, Z.; Wronska, A. The role of low-calorie diets and intermittent fasting in the treatment of obesity and type-2 diabetes. J. Physiol. Pharmacol. 2018, 69, 663–683. [Google Scholar] [CrossRef]
- Kim, B.H.; Joo, Y.; Kim, M.S.; Choe, H.K.; Tong, Q.; Kwon, O. Effects of Intermittent Fasting on the Circulating Levels and Circadian Rhythms of Hormones. Endocrinol. Metab. 2021, 36, 745–756. [Google Scholar] [CrossRef] [PubMed]
- García-Luna, C.; Soberanes-Chávez, P.; de Gortari, P. Prepuberal light phase feeding induces neuroendocrine alterations in adult rats. Mol. Neurophysiol. Lab. 2017, 232, 15–28. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S.; Adamello, V.; Jolla, L. Fasting, circadian rhythms, and time restricted feeding in healthy lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; Ditacchio, L.; Eric, A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; James, A.; et al. Time restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high fat diet. Cell Metab. 2013, 15, 848–860. [Google Scholar] [CrossRef]
- Nakhate, K.; Subhedar, N. Central regulation of feeding behavior during social isolation of rat: Evidence for the role of endogenous CART system Drug design and its Pharmacological Screening View project Ventral tegmental area View project. Artic. Int. J. Obes. 2010, 35, 773–784. [Google Scholar] [CrossRef]
- Krolow, R.; Noschang, C.; Arcego, D.M.; Huffell, A.P.; Marcolin, M.L.; Benitz, A.N.; Lampert, C.; Fitarelli, R.D.; Dalmaz, C. Sex-specific effects of isolation stress and consumption of palatable diet during the prepubertal period on metabolic parameters. Metabolism 2013, 62, 1268–1278. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Galarraga, M.; Campión, J.; Munõz-Barrutia, A.; Boqué, N.; Moreno, H.; Martínez, J.A.; Milagro, F.; Ortiz-de-Solórzano, C. Adiposoft: Automated software for the analysis of white adipose tissue cellularity in histological sections. J. Lipid Res. 2012, 53, 2791–2796. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Noriega, L.G.; Morales-Romero, A.M.; Nieto-Camacho, A.; Granados-Portillo, O.; Rodríguez-López, L.A.; Alemán, G.; Furuzawa-Carballeda, J.; Tovar, A.R.; Cisneros-Zevallos, L.; et al. Goat’s Milk Intake Prevents Obesity, Hepatic Steatosis and Insulin Resistance in Mice Fed A High-Fat Diet by Reducing Inflammatory Markers and Increasing Energy Expenditure and Mitochondrial Content in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 5530. [Google Scholar] [CrossRef] [PubMed]
- Kakucska, I.; Romero, L.I.; Clark, B.D.; Rondeel, J.M.M.; Qi, Y.; Alex, S.; Emerson, C.H.; Lechan, R.M. Suppression of thyrotropin-releasing hormone gene expression by interleukin-1-beta in the rat: Implications for nonthyroidal illness. Neuroendocrinology 1994, 59, 129–137. [Google Scholar] [CrossRef]
- Alsio, J.; Olszewski, P.K.; Norbäck, A.H.; Gunnarsson, Z.E.A.; Levine, A.S.; Pickering, C.; Schiöth, H.B. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience 2010, 171, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, P.; Broadbent, S.; Vaishali, K.; Nayak, B.; Bhat, H.V. Prevention of adolescent obesity: The global picture and an indian perspective. Diabetes Metab. Syndr. 2020, 14, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Porro, S.; Genchi, V.A.; Cignarelli, A.; Natalicchio, A.; Laviola, L.; Giorgino, F.; Perrini, S. Dysmetabolic adipose tissue in obesity: Morphological and functional characteristics of adipose stem cells and mature adipocytes in healthy and unhealthy obese subjects. J. Endocrinol. Investig. 2020, 44, 921–941. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, A. Beige Fat Maintenance; Toward a Sustained Metabolic Health. Front. Endocrinol. 2020, 11, 634. [Google Scholar] [CrossRef]
- Zubiría, M.G.; Alzamendi, A.; Moreno, G.; Portales, A.; Castrogiovanni, D.; Spinedi, E.; Giovambattista, A. Relationship between the Balance of Hypertrophic/Hyperplastic Adipose Tissue Expansion and the Metabolic Profile in a High Glucocorticoids Model. Nutrients 2016, 8, 410. [Google Scholar] [CrossRef]
- Lv, Y.F.; Yu, J.; Sheng, Y.L.; Huang, M.; Kong, X.C.; Di, W.J.; Liu, J.; Zhou, H.; Liang, H.; Ding, G.X. Glucocorticoids Suppress the Browning of Adipose Tissue via miR-19b in Male Mice. Endocrinology 2018, 159, 310–322. [Google Scholar] [CrossRef]
- Rosmond, R.; Dallman, M.F.; Björntorp, P. Stress-Related Cortisol Secretion in Men: Relationships with Abdominal Obesity and Endocrine, Metabolic and Hemodynamic Abnormalities. J. Clin. Endocrinol. Metab. 1998, 83, 1853–1859. [Google Scholar] [CrossRef]
- Cavalieri, R.R.; Castle, J.N.; McMahon, F.A. Effects of Dexamethasone on Kinetics and Distribution of Triiodothyronine in the Rat. Endocrinology 1984, 114, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Frontini, A.; Giordano, A.; Cinti, S. Endothelial cells of adipose tissues: A niche of adipogenesis. Cell Cycle 2012, 11, 2765–2766. [Google Scholar] [CrossRef] [PubMed]
- Sidossis, L.; Kajimura, S. Brown and beige fat in humans: Thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Investig. 2015, 125, 478–486. [Google Scholar] [CrossRef]
- Rahnert, J.A.; Zheng, B.; Hudson, M.B.; Woodworth-Hobbs, M.E.; Price, S.R. Glucocorticoids Alter CRTC-CREB Signaling in Muscle Cells: Impact on PGC-1α Expression and Atrophy Markers. PLoS ONE 2016. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Stewart, K.; Goodman, R.H. Structure of the gene encoding rat thyrotropin releasing hormone. J. Biol. Chem. 1988, 263, 16604–16609. [Google Scholar] [CrossRef]
- Pecoraro, N.; Reyes, F.; Gomez, F.; Bhargava, A.; Dallman, M.F. Chronic Stress Promotes Palatable Feeding, which Reduces Signs of Stress: Feedforward and Feedback Effects of Chronic Stress. Endocrinology 2004, 145, 3754–3762. [Google Scholar] [CrossRef]
- Ortolani, D.; Oyama, L.M.; Ferrari, E.M.; Melo, L.L.; Spadari-Bratfisch, R.C. Effects of comfort food on food intake, anxiety-like behavior and the stress response in rats. Physiol. Behav. 2011, 103, 487–492. [Google Scholar] [CrossRef]
- Johnson, P.M.; Kenny, P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010, 13, 635–641. [Google Scholar] [CrossRef]
- Wang, G.; Volkow, N.D.; Logan, J.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusil, N. Brain dopamine and obesity. Lancet 2001, 357, 354–357. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Maynard, L.; Jayne, M.; Fowler, J.S.; Zhu, W.; Logan, J.; John Gatley, S.; Ding, Y.S.; Wong, C.; et al. Brain dopamine is associated with eating behaviors in humans. Int. J. Eat. Disord. 2003, 33, 136–142. [Google Scholar] [CrossRef]
- Hamdi, A.; Porter, J.; Chandan, P. Decreased striatal D2 dopamine receptors in obese Zucker rats: Changes during aging. Brain Res. 1992, 589, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Becker-Krail, D.D.; Walker, W.H.; Nelson, R.J. The Ventral Tegmental Area and Nucleus Accumbens as Circadian Oscillators: Implications for Drug Abuse and Substance Use Disorders. Front. Physiol. 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Tamura, E.K.; Oliveira-Silva, K.S.; Ferreira-Moraes, F.A.; Marinho, E.A.V.; Guerrero-Vargas, N.N. Circadian rhythms and substance use disorders: A bidirectional relationship. Pharmacol. Biochem. Behav. 2021, 201, 173105. [Google Scholar] [CrossRef] [PubMed]
- Aouichat, S.; Chayah, M.; Bouguerra-Aouichat, S.; Agil, A. Time-Restricted Feeding Improves Body Weight Gain, Lipid Profiles, and Atherogenic Indices in Cafeteria-Diet-Fed Rats: Role of Browning of Inguinal White Adipose Tissue. Nutrients 2020, 12, 2185. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Page, A.J.; Hutchison, A.T.; Wittert, G.A.; Heilbronn, L.K. Intermittent fasting increases energy expenditure and promotes adipose tissue browning in mice. Nutrition 2019, 66, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Lombardi, A.; Goglia, F. Thyroid hormone and uncoupling proteins. FEBS Lett. 2003, 543, 5–10. [Google Scholar] [CrossRef]
- Wulf, A.; Harneit, A.; Kröger, M.; Kebenko, M.; Wetzel, M.G.; Weitzel, J.M. T3-mediated expression of PGC-1α via a far upstream located thyroid hormone response element. Mol. Cell. Endocrinol. 2008, 287, 90–95. [Google Scholar] [CrossRef]
- Sugden, M.C.; Grimshaw, R.M.; Lall, H.; Holness, M.J. Regional variations in metabolic responses of white adipose tissue to food restriction. Am. J. Physiol. Endocrinol. Metab. 1994, 267, E892–E899. [Google Scholar] [CrossRef]
- Puga, L.; Alcántara-Alonso, V.; Coffeen, U.; Jaimes, O.; de Gortari, P. TRH injected into the nucleus accumbens shell releases dopamine and reduces feeding motivation in rats. Behav. Brain Res. 2016, 306, 128–136. [Google Scholar] [CrossRef]
- Vijayan, E.; McCann, S.M. Suppression of feeding and drinking activity in rats following intraventricular injection of thyrotropin releasing hormone (TRH) 1. Endocrinology 1977, 100, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kohno, H.; Sakurada, T.; Tadano, T.; Kisara, K. Intracranial injection of thyrotropin releasing hormone (TRH) suppresses starvation-induced feeding and drinking in rats. Pharmacol. Biochem. Behav. 1982, 17, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Hartzell, D.; Azain, M.J.; Baile, C.A. TRH decreases food intake and increases water intake and body temperature in rats. Physiol. Behav. 2002, 77, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fekete, C.; Sarkar, S.; Rand, W.M.; Harney, J.W.; Emerson, C.H.; Bianco, A.C.; Beck-Sickinger, A.; Lechan, R.M. Neuropeptide Y1 and Y5 Receptors Mediate the Effects of Neuropeptide Y on the Hypothalamic-Pituitary-Thyroid Axis. Endocrinology 2002, 143, 4513–4519. [Google Scholar] [CrossRef]
- Fekete, C.; Kelly, J.; Mihály, E.; Sarkar, S.; Rand, W.M.; Légrádi, G.; Emerson, C.H.; Lechan, R.M. Neuropeptide Y Has a Central Inhibitory Action on the Hypothalamic-Pituitary-Thyroid Axis. Endocrinology 2001, 142, 2606–2613. [Google Scholar] [CrossRef]
- Bassareo, V.; Di Chiara, G. Differential Influence of Associative and Nonassociative Learning Mechanisms on the Responsiveness of Prefrontal and Accumbal Dopamine Transmission to Food Stimuli in Rats Fed Ad Libitum. J. Neurosci. 1997, 17, 851–861. [Google Scholar] [CrossRef]
- Hernandez, L.; Hoebel, B.G. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol. Behav. 1988, 44, 599–606. [Google Scholar] [CrossRef]
- Roitman, M.F.; Stuber, G.D.; Phillips, P.E.M.; Wightman, R.M.; Carelli, R.M. Dopamine Operates as a Subsecond Modulator of Food Seeking. J. Neurosci. 2004, 24, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Yokum, S.; Blum, K.; Bohon, C. Weight Gain Is Associated with Reduced Striatal Response to Palatable Food. J. Neurosci. 2010, 30, 13105–13109. [Google Scholar] [CrossRef]
- Hommel, J.D.; Trinko, R.; Sears, R.M.; Georgescu, D.; Liu, Z.W.; Gao, X.B.; Thurmon, J.J.; Marinelli, M.; DiLeone, R.J. Leptin Receptor Signaling in Midbrain Dopamine Neurons Regulates Feeding. Neuron 2006, 51, 801–810. [Google Scholar] [CrossRef]
- Fulton, S.; Pissios, P.; Manchon, R.P.; Stiles, L.; Frank, L.; Pothos, E.N.; Maratos-Flier, E.; Flier, J.S. Leptin Regulation of the Mesoaccumbens Dopamine Pathway. Neuron 2006, 51, 811–822. [Google Scholar] [CrossRef] [PubMed]


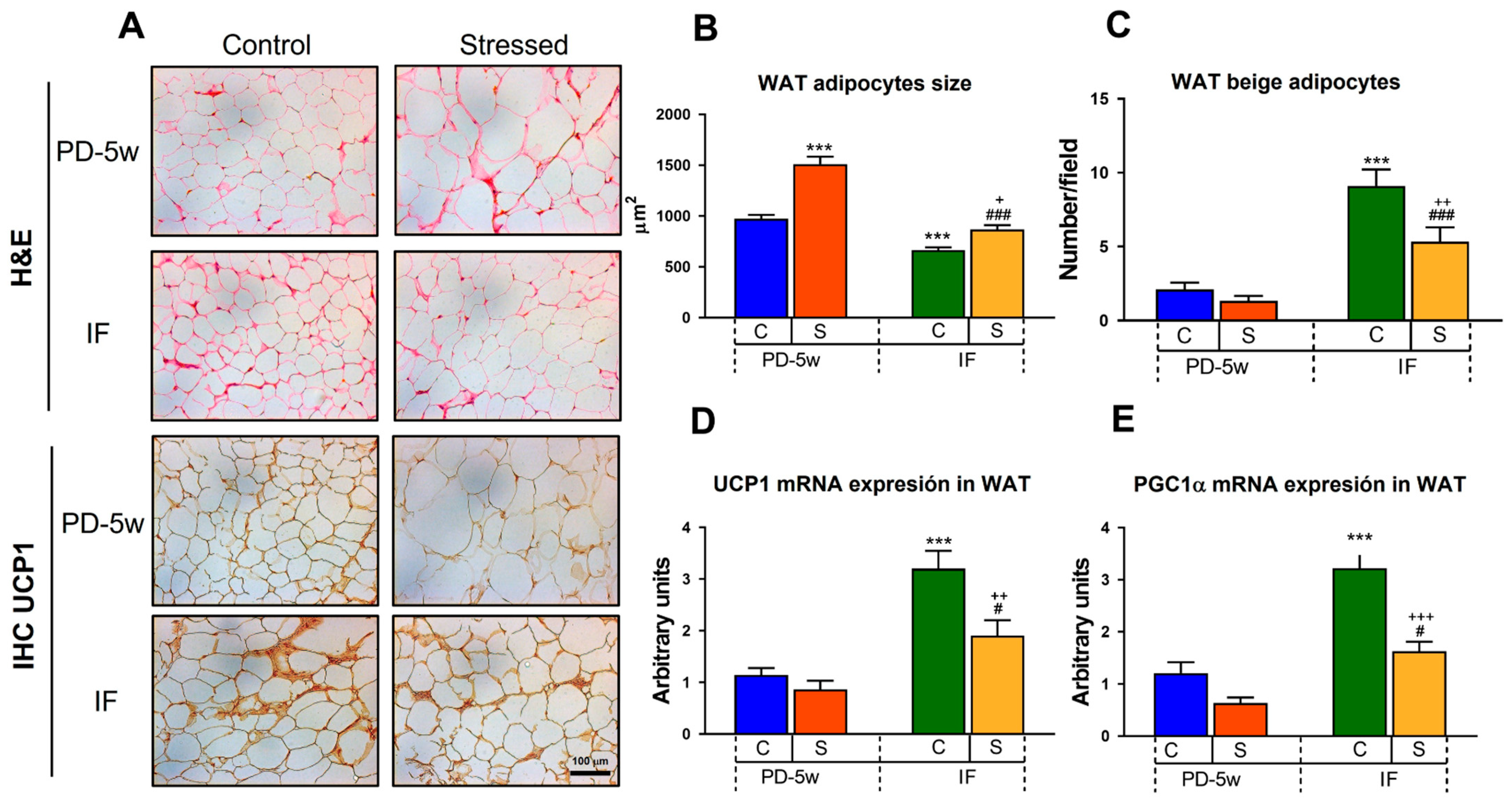
| C-PD-2w | S-PD-2w | |
|---|---|---|
| Adipocyte size (µm2) | 1351 ± 27 | 1855 ± 68 *** |
| Beige adipocytes (number/field) | 1.2 ± 0.3 | 0.4 ± 0.2 * |
| UCP1 mRNA (relative abundance) | 1.1 ± 0.2 | 0.5 ± 0.2 * |
| PGC1α mRNA (relative abundance) | 1.1 ± 0.2 | 0.3 ± 0.1 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Luna, C.; Prieto, I.; Soberanes-Chávez, P.; Alvarez-Salas, E.; Torre-Villalvazo, I.; Matamoros-Trejo, G.; de Gortari, P. Effects of Intermittent Fasting on Hypothalamus–Pituitary–Thyroid Axis, Palatable Food Intake, and Body Weight in Stressed Rats. Nutrients 2023, 15, 1164. https://doi.org/10.3390/nu15051164
García-Luna C, Prieto I, Soberanes-Chávez P, Alvarez-Salas E, Torre-Villalvazo I, Matamoros-Trejo G, de Gortari P. Effects of Intermittent Fasting on Hypothalamus–Pituitary–Thyroid Axis, Palatable Food Intake, and Body Weight in Stressed Rats. Nutrients. 2023; 15(5):1164. https://doi.org/10.3390/nu15051164
Chicago/Turabian StyleGarcía-Luna, Cinthia, Ixchel Prieto, Paulina Soberanes-Chávez, Elena Alvarez-Salas, Iván Torre-Villalvazo, Gilberto Matamoros-Trejo, and Patricia de Gortari. 2023. "Effects of Intermittent Fasting on Hypothalamus–Pituitary–Thyroid Axis, Palatable Food Intake, and Body Weight in Stressed Rats" Nutrients 15, no. 5: 1164. https://doi.org/10.3390/nu15051164
APA StyleGarcía-Luna, C., Prieto, I., Soberanes-Chávez, P., Alvarez-Salas, E., Torre-Villalvazo, I., Matamoros-Trejo, G., & de Gortari, P. (2023). Effects of Intermittent Fasting on Hypothalamus–Pituitary–Thyroid Axis, Palatable Food Intake, and Body Weight in Stressed Rats. Nutrients, 15(5), 1164. https://doi.org/10.3390/nu15051164







