Optimal Pair Matching Combined with Machine Learning Predicts a Significant Reduction in Myocardial Infarction Risk in African Americans Following Omega-3 Fatty Acid Supplementation
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Utilization of Optimal Pair Matching to Balance Potential Confounding Variables
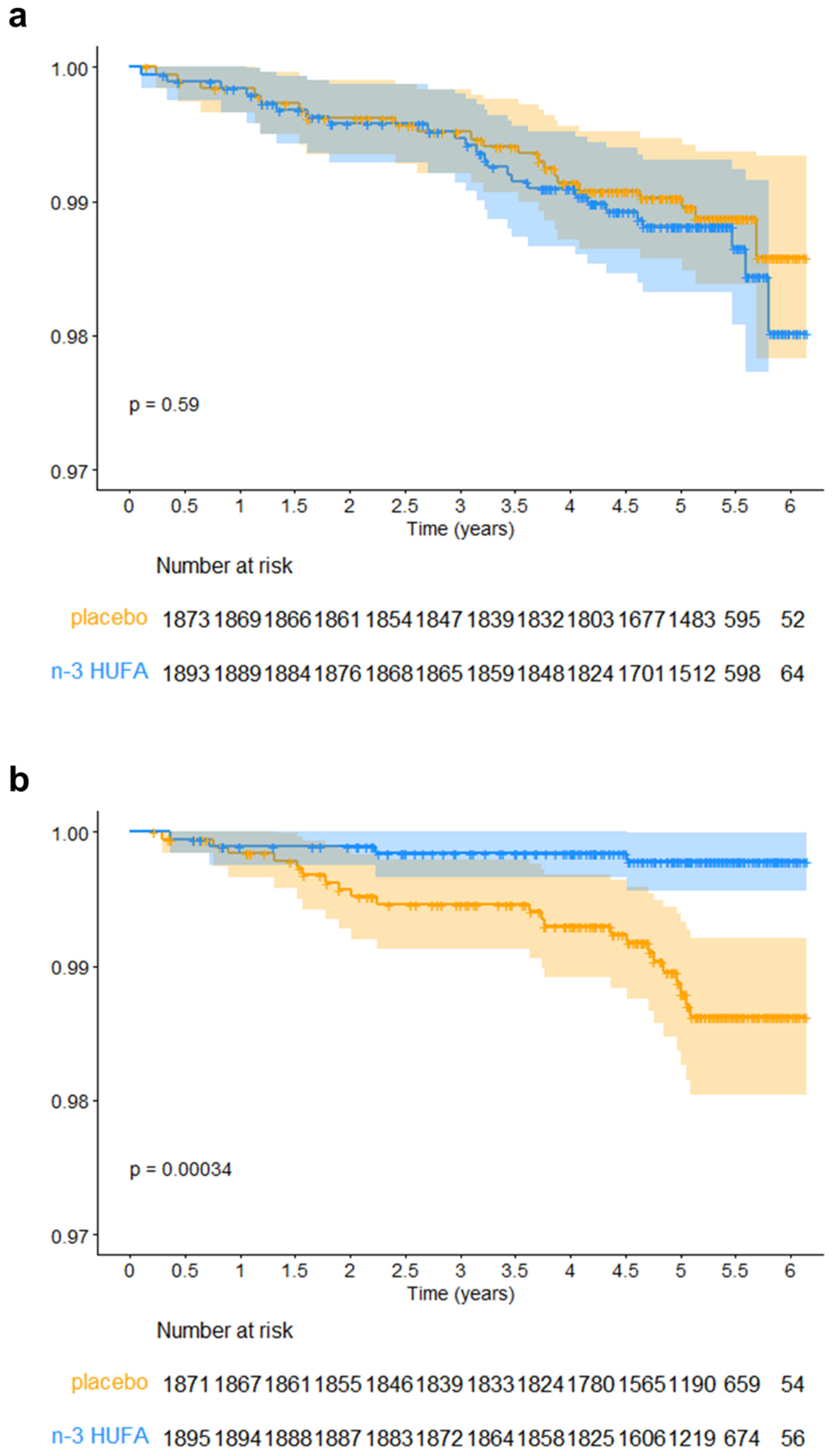
3.2. Effect of n-3 HUFA Supplementation on MI in AfAm and NHW Participants
3.3. Logistic Regression Analysis with LASSO to Select Important Variables and Bootstrap to Estimate the Standard Errors
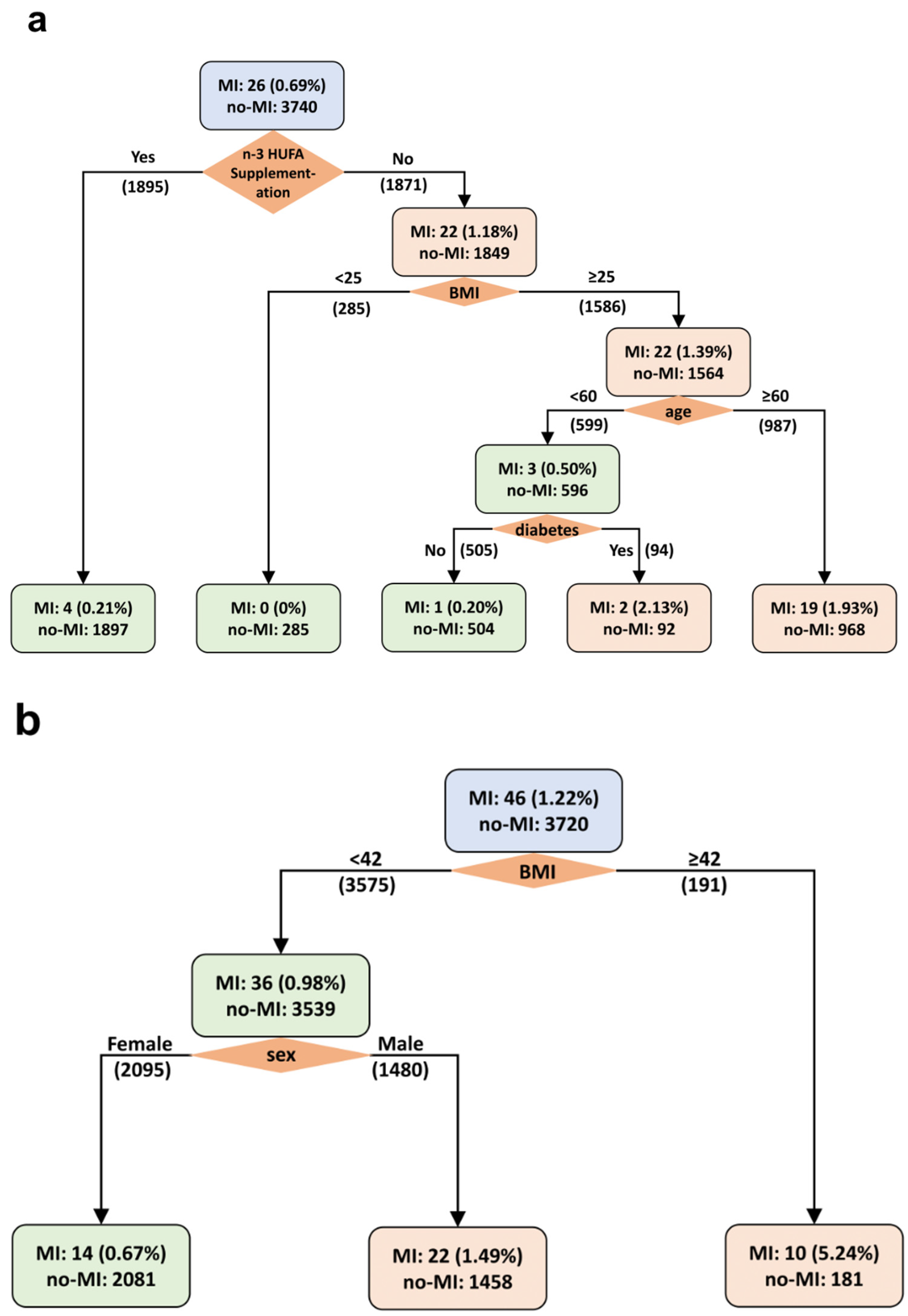
3.4. Weighted Decision Tree Analysis of MI in AfAm and NHW Participants
3.5. LASSO Regression Analysis and Weighted Decision Tree of Stroke or CVD Mortality in AfAm and NHW Participants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Kromhout, D.; Giltay, E.J.; Geleijnse, J.M. n-3 Fatty acids and cardiovascular events after myocardial infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar] [CrossRef]
- Rauch, B.; Schiele, R.; Schneider, S.; Diller, F.; Victor, N.; Gohlke, H.; Gottwik, M.; Steinbeck, G.; Del Castillo, U.; Sack, R.; et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010, 122, 2152–2159. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Hooper, L. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 11. [Google Scholar]
- ASCEND Study Collaborative Group. Effects of n-3 fatty acid supplements in diabetes mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n−3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Shen, S.; Gong, C.; Jin, K.; Zhou, L.; Xiao, Y.; Ma, L. Omega-3 fatty acid supplementation and coronary heart disease risks: A meta-analysis of randomized controlled clinical trials. Front. Nutr. 2022, 9, 809311. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, F.B.; Manson, J.E. Marine omega-3 supplementation and cardiovascular disease: An updated meta-analysis of 13 randomized controlled trials involving 127,477 participants. J. Am. Heart Assoc. 2019, 8, e013543. [Google Scholar] [CrossRef]
- Hoang, T.; Kim, J. Comparative effect of statins and omega-3 supplementation on cardiovascular events: Meta-analysis and network meta-analysis of 63 randomized controlled trials including 264,516 participants. Nutrients 2020, 12, 2218. [Google Scholar] [CrossRef]
- Elagizi, A.; Lavie, C.J.; O’Keefe, E.; Marshall, K.; O’Keefe, J.H.; Milani, R.V. An update on omega-3 polyunsaturated fatty acids and cardiovascular health. Nutrients 2021, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Khan, H.; Xiao, J.; Cheang, W.S. Effects of arachidonic acid metabolites on cardiovascular health and disease. Int. J. Mol. Sci. 2021, 22, 12029. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.A.; Sergeant, S.; Ruczinski, I.; Torgerson, D.G.; Hugenschmidt, C.E.; Kubala, M.; Vaidya, D.; Suktitipat, B.; Ziegler, J.T.; Ivester, P.; et al. The impact of FADS genetic variants on ω-6 polyunsaturated fatty acid metabolism in African Americans. BMC Genet. 2011, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, S.; Hugenschmidt, C.E.; Rudock, M.E.; Ziegler, J.T.; Ivester, P.; Ainsworth, H.C.; Vaidya, D.; Case, L.D.; Langefeld, C.D.; Freedman, B.I.; et al. Differences in arachidonic acid levels and fatty acid desaturase (FADS) gene variants in African Americans and European Americans with diabetes or the metabolic syndrome. Br. J. Nutr. 2012, 107, 547–555. [Google Scholar] [CrossRef]
- Hester, A.G.; Murphy, R.C.; Uhlson, C.J.; Ivester, P.; Lee, T.C.; Sergeant, S.; Miller, L.R.; Howard, T.D.; Mathias, R.A.; Chilton, F.H. Relationship between a common variant in the fatty acid desaturase (FADS) cluster and eicosanoid generation in humans. J. Biol. Chem. 2014, 289, 22482–22489. [Google Scholar] [CrossRef]
- Manson, J.E.; Bassuk, S.S.; Cook, N.R.; Lee, I.M.; Mora, S.; Albert, C.M.; Buring, J.E.; for the VITAL Research Group. Vitamin D, marine n-3 fatty acids, and primary prevention of cardiovascular disease current evidence. Circ. Res. 2020, 126, 112–128. [Google Scholar] [CrossRef]
- Chilton, F.H.; Manichaikul, A.; Yang, C.; O’Connor, T.D.; Johnstone, L.M.; Blomquist, S.; Schembre, S.M.; Sergeant, S.; Zec, M.; Tsai, M.Y.; et al. Interpreting clinical trials with omega-3 supplements in the context of ancestry and FADS genetic variation. Front. Nutr. 2022, 8, 808054. [Google Scholar] [CrossRef]
- Bassuk, S.S.; Chandler, P.D.; Buring, J.E.; Manson, J.E.; VITAL Research Group. The VITamin D and OmegA-3 TriaL (VITAL): Do results differ by sex or race/ethnicity? Am. J. Lifestyle Med. 2021, 15, 372–391. [Google Scholar] [CrossRef]
- Ogata, S.; Manson, J.E.; Kang, J.H.; Buring, J.E.; Lee, I.M.; Nishimura, K.; Sakata, Y.; Danik, J.S.; D’Agostino, D.; Mora, S.; et al. Marine n-3 fatty acids and prevention of cardiovascular disease: A novel analysis of the VITAL trial using win ratio and hierarchical composite outcomes. Nutrients 2023, 15, 4235. [Google Scholar] [CrossRef]
- DeFranco, E.A.; Valentine, C.J.; Carlson, S.E.; Sands, S.A.; Gajewski, B.J. Racial disparity in efficacy of docosahexaenoic acid supplementation for prevention of preterm birth: Secondary analysis from a randomized, double-blind trial. Am. J. Obstet. Gynecol. MFM 2024, 6, 101358. [Google Scholar] [CrossRef]
- Thoemmes, F.J.; Kim, E.S. A systematic review of propensity score methods in the social sciences. Multivariate Behav. Res. 2011, 46, 90–118. [Google Scholar] [CrossRef] [PubMed]
- Zakrison, T.L.; Austin, P.C.; McCredie, V.A. A systematic review of propensity score methods in the acute care surgery literature: Avoiding the pitfalls and proposing a set of reporting guidelines. Eur. J. Trauma Emerg. Surg. 2018, 44, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Stuart, E.A.; King, G.; Imai, K.; Ho, D. MatchIt: Nonparametric preprocessing for parametric causal inference. J. Stat. Softw. 2011, 42, 1–28. [Google Scholar]
- Hansen, B.B.; Klopfer, S.O. Optimal full matching and related designs via network flows. J. Comput. Graph. Stat. 2006, 15, 609–627. [Google Scholar] [CrossRef]
- Gu, X.S.; Rosenbaum, P.R. Comparison of multivariate matching methods: Structures, distances, and algorithms. J. Comput. Graph. Stat. 1993, 2, 405–420. [Google Scholar] [CrossRef]
- Sekhon, J.S. Multivariate and propensity score matching software with automated balance optimization: The matching package for R. J. Stat. Softw. Forthcom. 2008, 42, 1–52. [Google Scholar]
- Therneau, T.; Lumley, T. Package Survival: A Package for Survival Analysis in R. R Package 2015, 2, 38. [Google Scholar]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B Stat. Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010, 33, 1. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall/CRC: Boca Raton, FL, USA, 1994. [Google Scholar]
- Breiman, L. Classification and Regression Trees; Routledge: Abingdon, UK, 2017. [Google Scholar]
- Therneau, T.; Atkinson, B.; Ripley, B.; Ripley, M.B. Package ‘Rpart’. 2015. Available online: https://cran.r-project.org/web/packages/rpart/rpart.pdf (accessed on 17 April 2024).
- Office of Minority Health. Heart Disease and African Americans. U.S. Department of Health & Human Services. Available online: https://minorityhealth.hhs.gov/heart-disease-and-african-americans (accessed on 17 April 2024).
- Chilton, F.H.; Dutta, R.; Reynolds, L.M.; Sergeant, S.; Mathias, R.A.; Seeds, M.C. Precision nutrition and omega-3 polyunsaturated fatty acids: A case for personalized supplementation approaches for the prevention and management of human diseases. Nutrients 2017, 9, 1165. [Google Scholar] [CrossRef]
- Fadason, T.; Schierding, W.; Lumley, T.; O’Sullivan, J.M. Chromatin interactions and expression quantitative trait loci reveal genetic drivers of multimorbidities. Nat. Commun. 2018, 9, 5198. [Google Scholar] [CrossRef]
- Lettre, G.; Palmer, C.D.; Young, T.; Ejebe, K.G.; Allayee, H.; Benjamin, E.J.; Bennett, F.; Bowden, D.W.; Chakravarti, A.; Dreisbach, A.; et al. Genome-wide association study of coronary heart disease and its risk factors in 8090 African Americans: The NHLBI CARe Project. PLoS Genet. 2011, 7, e1001300. [Google Scholar] [CrossRef]
- Harris, D.N.; Ruczinski, I.; Yanek, L.R.; Becker, L.C.; Becker, D.M.; Guio, H.; Cui, T.; Chilton, F.H.; A Mathias, R.; O’connor, T.D. Evolution of hominin polyunsaturated fatty acid metabolism: From Africa to the New World. Genome Biol. Evol. 2019, 11, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Chiusolo, S.; Bork, C.S.; Gentile, F.; Lundbye-Christensen, S.; Harris, W.S.; Schmidt, E.B.; De Caterina, R. Adipose tissue n-3/n-6 fatty acids ratios versus n-3 fatty acids fractions as predictors of myocardial infarction. Am. Heart J. 2023, 262, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.R.; Raskin, S. The eicosapentaenoic acid: Arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgrad. Med. 2019, 131, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Nayeem, M.A. Role of oxylipins in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1142–1154. [Google Scholar] [CrossRef]
- Caligiuri, S.P.; Parikh, M.; Stamenkovic, A.; Pierce, G.N.; Aukema, H.M. Dietary modulation of oxylipins in cardiovascular disease and aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H903–H918. [Google Scholar] [CrossRef]
- Yedgar, S.; Krimsky, M.; Cohen, Y.; Flower, R.J. Treatment of inflammatory diseases by selective eicosanoid inhibition: A double-edged sword? Trends Pharmacol. Sci. 2007, 28, 459–464. [Google Scholar] [CrossRef]
- Bing, R.J.; Lomnicka, M. Why do cyclo-oxygenase-2 inhibitors cause cardiovascular events? J. Am. Coll. Cardiol. 2002, 39, 521–522. [Google Scholar] [CrossRef]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Schmidt, S.; Kressel, G.; Willenberg, I.; Hammock, B.D.; Hahn, A.; Schebb, N.H. Modulation of blood oxylipin levels by long-chain omega-3 fatty acid supplementation in hyper-and normolipidemic men. Prostaglandins Leukot. Essent. Fatty Acids 2014, 90, 27–37. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Total (N = 7532) | AfAm (N = 3766) | NHW (N = 3766) | |
|---|---|---|---|---|
| Age | 62.8 ± 6.3 | 62.4 ± 6.6 | 63.1 ± 5.9 | |
| Female | 4587 (60.9) | 2347 (62.3) | 2240 (59.5) | |
| BMI | 30.3 ± 6.6 | 30.6 ± 6.5 | 29.9 ± 6.1 | |
| Current smoking | 979 (13.0) | 528 (14.0) | 451 (12.0) | |
| Hypertension medication | 4754 (63.1) | 2446 (64.9) | 2308 (61.3) | |
| Cholesterol medication | 2451 (32.5) | 1200 (31.9) | 1251 (33.2) | |
| Statin use | 2279 (30.3) | 1114 (29.6) | 1165 (30.9) | |
| Diabetes | 1617 (21.5) | 871 (23.1) | 746 (19.8) | |
| Diabetes medication | 1267 (16.8) | 675 (17.9) | 592 (15.7) | |
| Parental history of MI | 1161 (15.4) | 591 (15.7) | 570 (15.1) | |
| Fish consumption (≥1.5/wk) | 3750 (49.8) | 1890 (50.2) | 1860 (49.4) | |
| Aspirin use | 2885 (38.3) | 1431 (38.0) | 1454 (38.6) | |
| Vitamin D supplements | 2271 (30.2) | 1096 (29.1) | 1175 (31.2) | |
| CVD risk factors | 0 | 1661 (22.1) | 778 (20.7) | 883 (23.4) |
| 1 | 2634 (35.0) | 1315 (34.9) | 1319 (35.0) | |
| >1 | 3237 (43.0) | 1673 (44.4) | 1564 (41.5) | |
| Variables | Estimate | OR | Non-Parametric Bootstrap | Parametric Bootstrap | ||||
|---|---|---|---|---|---|---|---|---|
| Std. Error | p-Value | 95% CI for OR | Std. Error | p-Value | 95% CI for OR | |||
| (Intercept) | −3.7599 | 0.0233 | 1.9965 | 0.0597 | (0.0005, 1.1656) | 1.8195 | 0.0388 | (0.0007, 0.8239) |
| Female | −0.7184 | 0.4875 | 0.3501 | 0.0402 | (0.2455, 0.9683) | 0.3448 | 0.0372 | (0.2480, 0.9583) |
| Age | 0.0552 | 1.0568 | 0.0277 | 0.0463 | (1.0009, 1.1157) | 0.0251 | 0.0279 | (1.0060, 1.1100) |
| BMI | 0.0304 | 1.0309 | 0.0249 | 0.2221 | (0.9818, 1.0824) | 0.0234 | 0.1939 | (0.9847, 1.0792) |
| Current Smoker | 0.7167 | 2.0477 | 0.4155 | 0.0845 | (0.9069, 4.6232) | 0.4471 | 0.1089 | (0.8525, 4.9186) |
| Diabetes | 0.4599 | 1.5839 | 0.4255 | 0.2798 | (0.6879, 3.6469) | 0.3381 | 0.1738 | (0.8165, 3.0728) |
| Fish consumption (1.5/wk) | −0.7424 | 0.4760 | 0.3755 | 0.0480 | (0.2280, 0.9936) | 0.3274 | 0.0234 | (0.2505, 0.9042) |
| n-3 HUFA supplementation × AfAm | −1.7747 | 0.1695 | 0.6410 | 0.0056 | (0.0483, 0.5955) * | 0.6944 | 0.0106 | (0.0435, 0.6613) * |
| n-3 HUFA supplementation × NHW | 0.0000 | 1.0000 | 0.1508 | 1.0000 | (0.7442, 1.3438) * | 0.1566 | 1.0000 | (0.7357, 1.3593) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Hara, A.; Johnstone, L.; Hallmark, B.; Watkins, J.C.; Thomson, C.A.; Schembre, S.M.; Sergeant, S.; Umans, J.G.; Yao, G.; et al. Optimal Pair Matching Combined with Machine Learning Predicts a Significant Reduction in Myocardial Infarction Risk in African Americans Following Omega-3 Fatty Acid Supplementation. Nutrients 2024, 16, 2933. https://doi.org/10.3390/nu16172933
Sun S, Hara A, Johnstone L, Hallmark B, Watkins JC, Thomson CA, Schembre SM, Sergeant S, Umans JG, Yao G, et al. Optimal Pair Matching Combined with Machine Learning Predicts a Significant Reduction in Myocardial Infarction Risk in African Americans Following Omega-3 Fatty Acid Supplementation. Nutrients. 2024; 16(17):2933. https://doi.org/10.3390/nu16172933
Chicago/Turabian StyleSun, Shudong, Aki Hara, Laurel Johnstone, Brian Hallmark, Joseph C. Watkins, Cynthia A. Thomson, Susan M. Schembre, Susan Sergeant, Jason G. Umans, Guang Yao, and et al. 2024. "Optimal Pair Matching Combined with Machine Learning Predicts a Significant Reduction in Myocardial Infarction Risk in African Americans Following Omega-3 Fatty Acid Supplementation" Nutrients 16, no. 17: 2933. https://doi.org/10.3390/nu16172933
APA StyleSun, S., Hara, A., Johnstone, L., Hallmark, B., Watkins, J. C., Thomson, C. A., Schembre, S. M., Sergeant, S., Umans, J. G., Yao, G., Zhang, H. H., & Chilton, F. H. (2024). Optimal Pair Matching Combined with Machine Learning Predicts a Significant Reduction in Myocardial Infarction Risk in African Americans Following Omega-3 Fatty Acid Supplementation. Nutrients, 16(17), 2933. https://doi.org/10.3390/nu16172933







