Dietary Adherence to Recommendations among a Cohort of Adults and Teens with Celiac Disease Maintaining a Gluten-Free Diet Compared to a Nationally Representative Sample: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
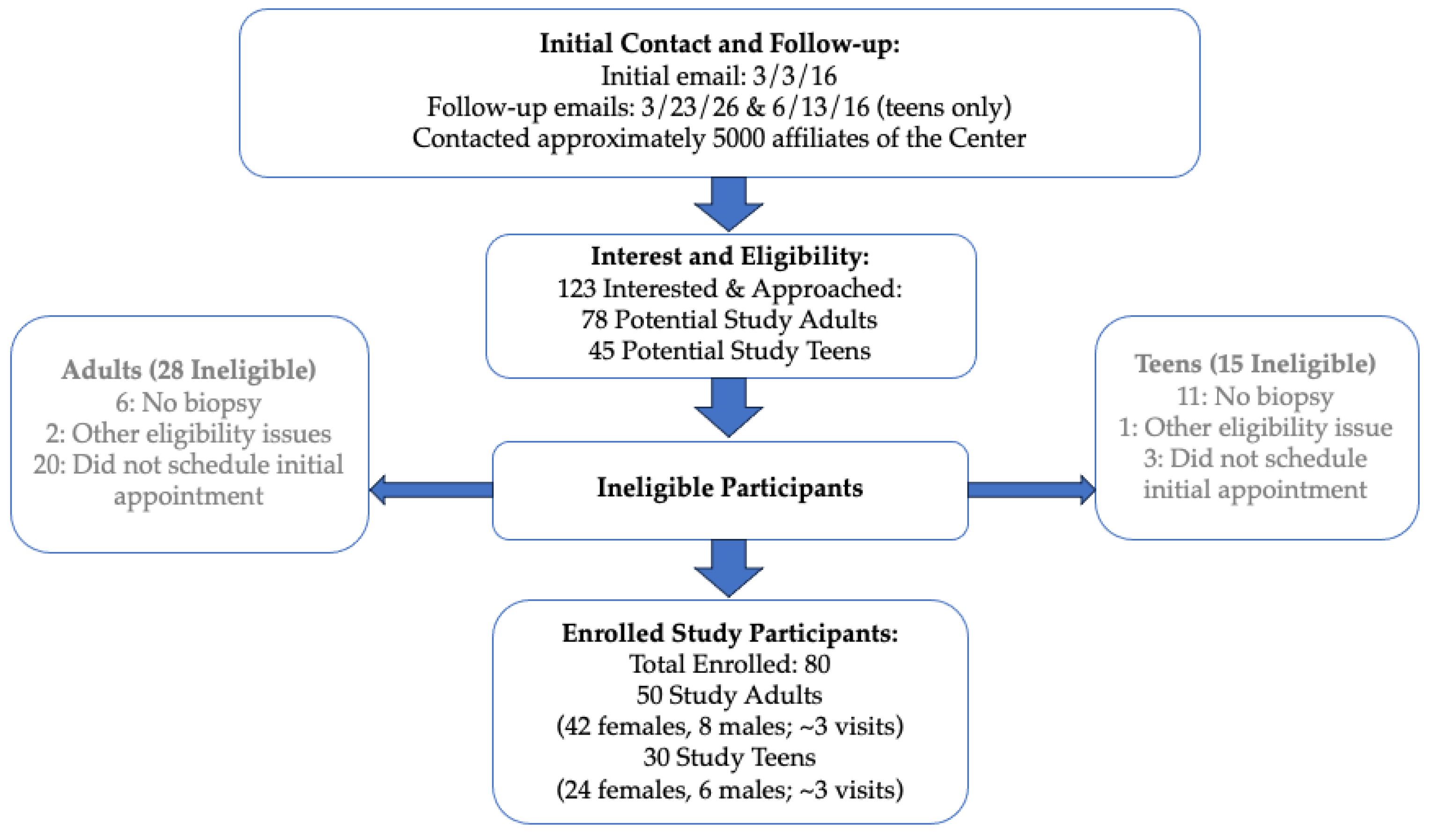
2.1. Methods and Recruitment
2.2. Demographics and Medical History
2.3. Diet Assessment
2.3.1. Twenty-Four Hour (24 h) Recalls
2.3.2. Nutrient Assessment
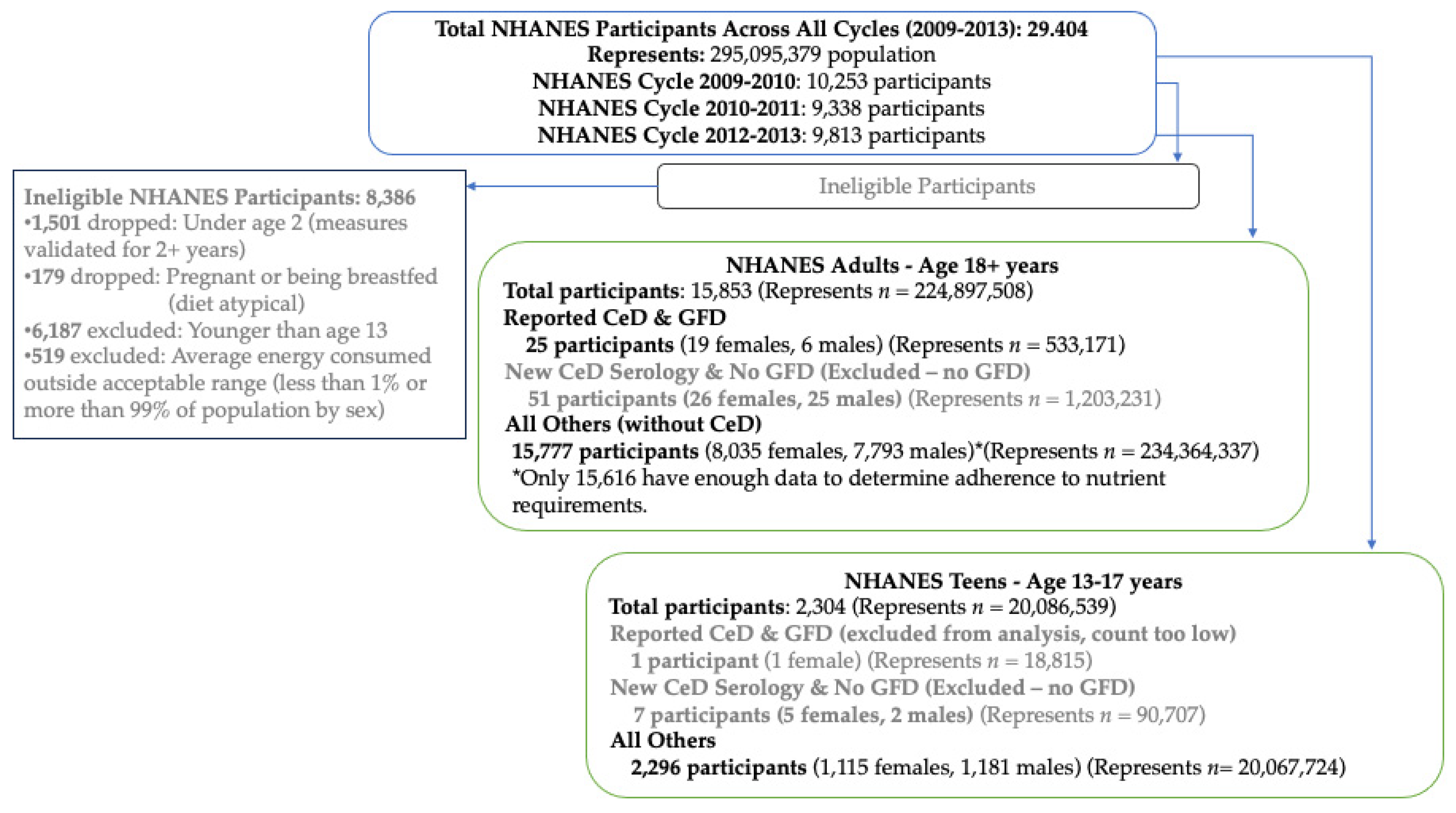
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Nutrient Status
3.2.1. Nutrients Consumed Below Recommendations
3.2.2. Nutrients Consumed Exceeding Recommendations
3.2.3. Nutrients of Concern Encouraged to Be Limited
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Study Adults n = 50 | NHANES CeD and GFD n = 25 | |
|---|---|---|
| Gender n (%) | ||
| Female | 42 (84.0) | 19 (77.2) |
| Age in Years | * | |
| Mean (SD) | 50.7 (17.8) | 57.7 (4.0) |
| Race/Ethnicity n (%) | ||
| Non-Hispanic White | 47 (94.0) | 19 (93.5) |
| All Other Races/Ethnicities | 3 (6.0) | 6 (6.5) |
| Body Mass Index Status n (%) | ||
| Underweight | 2 (4.0) | 1 (1.5) |
| Normal | 35 (70.0) | 14 (61.5) |
| Overweight | 10 (20.0) | 3 (11.5) |
| Obesity | 3 (6.0) | 7 (25.4) |
| Education n (%) | * | |
| <High School | 4 (8.0) | 2 (3.5) |
| HS Graduate/Some College | 12 (24.0) | 13 (40.3) |
| College Graduate or Higher | 34 (68.0) | 10 (56.2) |
References
- Rubio-Tapia, A.; Reilly, N.R.; Green, P.H.R. The prevalence of celiac disease in the United States. Am. J. Gastroenterol. 2012, 107, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Theethira, T.G.; Dennis, M.; Leffler, D.A. Nutritional consequences of celiac disease and the gluten-free diet. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, I.A.; Reilly, N.R.; Rubio-Tapia, A. Celiac disease: Clinical features and diagnosis. Gastroenterol. Clin. N. Am. 2019, 48, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, presentation, and diagnosis of celiac disease. Gastroenterology 2021, 160, 63–75. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Mortality and malignancy in celiac disease. Gastrointest. Endosc. Clin. N. Am. 2012, 22, 705–722. [Google Scholar] [CrossRef]
- Reilly, N.R.; Green, P.H.R. Presentation of celiac disease in children and adults. In Celiac Disease; Springer: New York, NY, USA, 2013; pp. 95–105. [Google Scholar]
- Sharma, G.M.; Pereira, M.; Williams, K.M. Gluten detection in foods available in the United States–A market survey. Food Chem. 2015, 169, 120–126. [Google Scholar] [CrossRef]
- Thompson, T.; Lee, A.R.; Grace, T. Gluten contamination of grains, seeds, and flours in the United States: A pilot study. J. Am. Diet. Assoc. 2010, 110, 937–940. [Google Scholar] [CrossRef]
- Thompson, T.; Simpson, S. A comparison of gluten levels in labeled gluten-free and certified gluten-free foods sold in the United States. Eur. J. Clin. Nutr. 2015, 69, 143–146. [Google Scholar] [CrossRef]
- Lee, A.R.; Ng, D.L.; Dave, E.; Ciaccio, E.J.; Green, P.H. The effect of substituting alternative grains in the diet on the nutritional profile of the gluten-free diet. J. Hum. Nutr. Diet. 2009, 22, 359–363. [Google Scholar] [CrossRef]
- Simón, E.; Larretxi, I.; Churruca, I.; Lasa, A.; Bustamante, M.Á.; Navarro, V.; Fernández-Gil, M.D.P.; Miranda, J.; Lasa, A.; del Pilar Fernández-Gil, M.; et al. Nutritional and sensorial aspects of gluten-free products. In Nutritional and Analytical Approaches of Gluten-Free Diet in Celiac Disease; Springer: Cham, Switzerland, 2017; pp. 59–78. [Google Scholar]
- Cadenhead, J.W.; Martínez-Steele, E.; Contento, I.; Kushi, L.H.; Lee, A.R.; Nguyen, T.T.T.; Lebwohl, B.; Green, P.H.; Wolf, R.L. Diet quality, ultra-processed food consumption, and quality of life in a cross-sectional cohort of adults and teens with celiac disease. J. Hum. Nutr. Diet. 2023, 36, 1144–1158. [Google Scholar] [CrossRef]
- Fulgoni III, V.L.; Keast, D.R.; Bailey, R.L.; Dwyer, J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J. Nutr. 2011, 141, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Taetzsch, A.; Das, S.K.; Brown, C.; Krauss, A.; Silver, R.E.; Roberts, S.B. Are gluten-free diets more nutritious? An evaluation of self-selected and recommended gluten-free and gluten-containing dietary patterns. Nutrients 2018, 10, 1881. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration (USDA). Code of Federal Regulations (CFR); Title 21-FR-21; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2024.
- Matthias, T.; Jeremias, P.; Neidhöfer, S.; Lerner, A. The industrial food additive, microbial transglutaminase, mimics tissue transglutaminase and is immunogenic in celiac disease patients. Autoimmun. Rev. 2016, 15, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of gluten-free diet on gut microbiota composition in patients with celiac disease and non-celiac gluten/wheat sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Mager, D.R.; Liu, A.; Marcon, M.; Harms, K.; Brill, H.; Mileski, H.; Dowhaniuk, J.; Nasser, R.; Carroll, M.W.; Persad, R.; et al. Diet patterns in an ethnically diverse pediatric population with celiac disease and chronic gastrointestinal complaints. Clin. Nutr. ESPEN 2019, 30, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Melini, F. Gluten-free diet: Gaps and needs for a healthier diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef]
- Penagini, F.; Dilillo, D.; Meneghin, F.; Mameli, C.; Fabiano, V.; Zuccotti, G.V. Gluten-free diet in children: An approach to a nutritionally adequate and balanced diet. Nutrients 2013, 5, 4553–4565. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Thompson, T.; Dennis, M.; Higgins, L.A.; Lee, A.R.; Sharrett, M.K. Gluten-free diet survey: Are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J. Hum. Nutr. Diet. 2005, 18, 163–169. [Google Scholar] [CrossRef]
- Unalp-Arida, A.; Liu, R.; Ruhl, C.E. Nutrient intake differs among persons with celiac disease and gluten-related disorders in the United States. Sci. Rep. 2022, 12, 5566. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics. National Health and Nutrition Examination Survey, 2013–2014: Overview. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overview.aspx?BeginYear=2013 (accessed on 15 December 2021).
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L.; Carroll, M.D.; Kruszan-Moran, D.; Dohrmann, S.M.; Curtin, L.R. National Health and Nutrition Examination Survey: Analytic guidelines, 1999–2010. National Center for Health Statistics Vital Health Stat. 2013, 2, 1–24. [Google Scholar]
- National Center for Health Statistics. National Health and Nutrition Examination Survey: Analytic Guidelines, 2011–2016. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/analyticguidelines/11-16-analytic-guidelines.pdf (accessed on 15 December 2021).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: 2009–2010: Demographics Data. Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Demographics&Cycle=2009-2010 (accessed on 15 December 2021).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: 2011–2012: Demographics Data. Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Demographics&Cycle=2011-2012 (accessed on 15 December 2021).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: 2013–2014: Demographics Data. Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Demographics&Cycle=2013-2014 (accessed on 15 December 2021).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: 2009–2010 Examination Data: Body Measures. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/BMX_F.XPT (accessed on 15 December 2021).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: 2011–2012 Examination Data: Body Measures. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/BMX_G.XPT (accessed on 15 December 2021).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: 2013–2014 Examination Data: Body Measures. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/BMX_H.XPT (accessed on 15 December 2021).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: 2009–2010 Laboratory Data: Tissue Transglutaminase Assay (IgA-TTG) & IgA Endomyseal Antibody Assay (IgA EMA). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/TGEMA_F.XPT (accessed on 15 December 2021).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: 2011–2012 Laboratory Data: Tissue Transglutaminase Assay (IgA-TTG) & IgA Endomyseal Antibody Assay (IgA EMA). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/TGEMA_G.XPT (accessed on 15 December 2021).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: 2013–2014 Laboratory Data: Tissue Transglutaminase Assay (IgA-TTG) & IgA Endomyseal Antibody Assay (IgA EMA). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/TGEMA_H.XPT (accessed on 15 December 2021).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: MEC in-Person Dietary Interviewers Procedures Manual (2010). Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/manuals/mec_in_person_dietary_procedures_manual_mar_2010.pdf (accessed on 15 June 2019).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: Phone Follow-Up Dietary Interviewers Procedures Manual (2010). Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/manuals/phone_follow_up_dietary_procedures_manual_mar_2010.pdf (accessed on 15 June 2019).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: MEC in-Person Dietary Interviewers Procedures Manual (2012). Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/manuals/mec_in_person_dietary_procedures_manual_jan_2012.pdf (accessed on 15 June 2019).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: Phone Follow-Up Dietary Interviewers Procedures Manual (2012). Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/manuals/phone_follow-up_dietary_procedures.pdf (accessed on 15 June 2019).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: MEC in-Person Dietary Interviewers Procedures Manual (2014). Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/mec_in_person_dietary_procedures_manual_jan_2014.pdf (accessed on 15 June 2019).
- National Center for Health Statistics. National Health and Nutrition Examination Survey: Phone Follow-Up Dietary Interviewers Procedures Manual (2013). Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/phone_follow-up_dietary_interviewers_manual.pdf (accessed on 15 June 2019).
- United States Department of Agriculture, Agriculture Research Service Food Surveys Research Group. What We Eat in America: Individual Food File, Day 1. 2009–2010. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/DR1TOT_F.htm (accessed on 15 June 2019).
- United States Department of Agriculture, Agriculture Research Service Food Surveys Research Group. What We Eat in America: Individual Food File, Day 2. 2009–2010. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/DR2TOT_F.htm (accessed on 15 June 2019).
- United States Department of Agriculture, Agriculture Research Service Food Surveys Research Group. What We Eat in America: Individual Food File, Day 1. 2011–2012. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/DR1TOT_G.htm (accessed on 15 June 2019).
- United States Department of Agriculture, Agriculture Research Service Food Surveys Research Group. What We Eat in America: Individual Food File, Day 2. 2011–2012. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/DR2TOT_G.htm (accessed on 15 June 2019).
- United States Department of Agriculture, Agriculture Research Service Food Surveys Research Group. What We Eat in America: Individual Food File, Day 1. 2013–2014. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/DR1TOT_H.htm (accessed on 15 June 2019).
- United States Department of Agriculture, Agriculture Research Service Food Surveys Research Group. What We Eat in America: Individual Food File, Day 2. 2013–2014. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/DR2TOT_H.htm (accessed on 15 June 2019).
- Zylberberg, H.M.; Demmer, R.T.; Murray, J.A.; Green, P.H.R.; Lebwohl, B. Depression and insomnia among individuals with celiac disease or on a gluten-free diet in the United States: Results from a national survey. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1091. [Google Scholar] [CrossRef]
- Bliss, R.M. Researchers produced innovation in dietary recall. Agric. Res. 2004, 52, 10–12. [Google Scholar]
- Raper, N.; Perliff, B.; Ingwersen, L.; Steinfeldt, L.; Anand, J. An overview of USDA’s dietary intake system. J. Food Compos. Anal. 2004, 17, 545–555. [Google Scholar] [CrossRef]
- Nutrition Data System for Research (NDSR). Software Version 2018, Developed by the Nutrition Coordinating Center (NCC); University of Minnesota: Minneapolis, MN, USA, 2018. [Google Scholar]
- Schakel, S.F.; Buzzard, I.M.; Gebhardt, S.E. Procedures for estimating nutrient values for food composition databases. J. Food Compos. Anal. 1997, 10, 102–114. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration (USDA). Guidance for Industry: Guide for Developing and Using Data Bases for Nutrition Labeling. March 1998. Available online: www.fda.gov (accessed on 12 July 2024).
- National Institutes of Health (NIH). Nutrient Recommendations: Dietary Reference Intake. 2019. Available online: https://ods.od.nih.gov/ (accessed on 21 April 2019).
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. December 2020. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf (accessed on 31 August 2024).
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; van Heel, D.A.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef]
- James, S.P. National Institutes of Health consensus development conference statement on celiac disease, June 28–30, 2004. Gastroenterology 2005, 128, S1–S9. [Google Scholar] [CrossRef]
- Herman, M.L.; Rubio-Tapia, A.; Lahr, B.D.; Larson, M.F.; Van Dyke, C.T.; Murray, J.A. Patients with celiac disease are not followed up adequately. Clin. Gastroenterol. Hepatol. 2012, 10, 893–899. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). Folate: Fact Sheet for Health Professionals. 2022. Available online: https://ods.od.nih.gov (accessed on 12 July 2024).
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Infantino, V.; Naso, M.; Perna, S. Micronutrients dietary supplementation advices for celiac patients on long-term gluten-free diet with good compliance: A review. Medicina 2019, 55, 337. [Google Scholar] [CrossRef] [PubMed]
- Lebovits, J.; Lee, A.R. Micronutrient Considerations for Celiac Disease. Pract. Gastroenterol. 2023, 27, 26–42. [Google Scholar]
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W.; García, F.A.R.; Herzstein, J.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Folic acid supplementation for the prevention of neural tube defects: US preventive services task force recommendation statement. JAMA 2017, 317, 183–189. [Google Scholar] [PubMed]
- Kim, S.J.; Zhu, J.; Woodrow, J.; Allen, C.; Scholl, T.O.; Stein, E.M.; Ford, J.M.; Pharoah, P.D.; Dunning, A.M.; Shrubsole, M.J.; et al. Plasma folate, vitamin B-6, and vitamin B-12 and breast cancer risk in BRCA1-and BRCA2-mutation carriers: A prospective study. Am. J. Clin. Nutr. 2016, 104, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, J.J.; Cummings, J.L.; Parwani, A.V.; Nelson, J.B.; Zoltick, B.J.; Dhir, R. Increased cancer cell proliferation in prostate cancer patients with high levels of serum folate. Prostate 2011, 71, 1287–1293. [Google Scholar] [CrossRef]
- Van Wijngaarden, J.P.; Dhonukshe-Rutten, R.A.M.; van Schoor, N.M.; Swart, K.M.A.; Enneman, A.W.; Ham, A.C.; van Dijk, S.C.; Brouwer-Brolsma, E.M.; van der Velde, N.; Uitterlinden, A.G.; et al. Vitamin B12, folate, homocysteine, and bone health in adults and elderly people: A systematic review with meta-analyses. J. Nutr. Metab. 2013, 2013, 486186. [Google Scholar] [CrossRef]
- Viswanathan, M.; Treiman, K.A.; Kish-Doto, J.; Middleton, J.C.; Coker-Schwimmer, E.J.; Nicholson, W.K. Folic acid supplementation for the prevention of neural tube defects: An updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017, 317, 190–203. [Google Scholar] [CrossRef]
- Wien, T.N.; Pike, E.; Wisløff, T.; Staff, A.; Smeland, S.; Klemp, M. Cancer risk with folic acid supplements: A systematic review and meta-analysis. BMJ Open 2012, 2, e000653. [Google Scholar] [CrossRef]
- Institute of Medicine; Food and Nutrition Board; Panel on Macronutrients; Panel on the Definition of Dietary Fiber; Subcommittee on Upper Reference Levels of Nutrients; Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Reilly, N.R.; Lebwohl, B.; Hultcrantz, R.; Green, P.H.R. Increased risk of non-alcoholic fatty liver disease after diagnosis of celiac disease. J. Hepatol. 2015, 62, 1405–1411. [Google Scholar] [CrossRef]
- Capuzzi, D.M.; Morgan, J.M.; Brusco, O.A.; Intenzo, C.M. Niacin dosing: Relationship to benefits and adverse effects. Curr. Atheroscler. Rep. 2000, 2, 64–71. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). Vitamin A and Carotenoids: Fact Sheet for Health Professionals. 2023. Available online: https://ods.od.nih.gov (accessed on 14 July 2024).
- Melhus, H.; Michaëlsson, K.; Kindmark, A.; Bergström, R.; Holmberg, L.; Mallmin, H.; Wolk, A.; Ljunghall, S. Excessive dietary intake of vitamin A is associated with reduced bone mineral density and increased risk for hip fracture. Ann. Intern. Med. 1998, 129, 770–778. [Google Scholar] [CrossRef] [PubMed]
| Study Adults n = 50 | NHANES Adults n = 15,777 | |
|---|---|---|
| Gender n (%) | *** | |
| Female | 42 (84.0) | 8009 (50.9) |
| Age in Years | ||
| Mean (SD) | 50.7 (17.8) | 46.5 (0.4) |
| Race/Ethnicity n (%) | *** | |
| Non-Hispanic White | 47 (94.0) | 6818 (66.7) |
| All Other Races/Ethnicities | 3 (6.0) | 8957 (33.3) |
| Body Mass Index Status n (%) | *** | |
| Underweight | 2 (4.0) | 285 (1.7) |
| Normal | 35 (70.0) | 4612 (30.2) |
| Overweight | 10 (20.0) | 4989 (32.3) |
| Obesity | 3 (6.0) | 5730 (35.8) |
| Education n (%) | *** | |
| <High School | 4 (8.0) | 3554 (16.4) |
| HS Graduate/Some College | 12 (24.0) | 7794 (53.9) |
| College Graduate or Higher | 34 (68.0) | 3566 (29.7) |
| Study Teens n = 30 | NHANES Teens n = 2296 | |
|---|---|---|
| Gender n (%) | ** | |
| Female | 24 (80.0) | 1115 (51.7) |
| Age in Years | * | |
| Mean (SD) | 15.7 (1.5) | 15.0 (0.1) |
| Race/Ethnicity n (%) | *** | |
| Non-Hispanic White | 29 (96.7) | 623 (56.4) |
| All Other Races/Ethnicities | 1 (3.3) | 1673 (43.6) |
| Body Mass Index Status n (%) | *** | |
| Underweight | 2 (6.7) | 58 (2.2) |
| Normal | 28 (93.3) | 1390 (63.3) |
| Overweight | 0 (0.0) | 369 (14.5) |
| Obesity | 0 (0.0) | 460 (20.0) |
| Education n (%) | * | |
| <Middle School | 0 (0.0) | 13 (0.6) |
| Middle School (6th–8th Grades) | 7 (23.3) | 1054 (44.8) |
| High School (9th–12th Grades) | 22 (73.3) | 1204 (53.7) |
| Some College | 1 (3.3) | 25 (1.0) |
| Study Adults n = 50 n (%) | NHANES Adults n = 15,616 n (Weighted %) | ||
|---|---|---|---|
| Macronutrients, Fiber, and Fatty Acids | |||
| Energy < TEE | 0 (0.0%) | 5247.1 (33.6%) | *** |
| Fiber < AI | 30 (60.0%) | 13,959.6 (88.5%) | *** |
| Protein < EAR | 1 (2.0%) | 3069.6 (19.6%) | *** |
| Carbohydrate < %AMDR | 36 (72.0%) | 5199.1 (33.0%) | *** |
| Linoleic Acid < AI | 12 (24.0%) | 6934.5 (44.0%) | |
| Linoleic Acid < %AMDR | 10 (20.0%) | 3522.0 (22.3%) | |
| Linolenic Acid < AI | 13 (26.0%) | 6688.1 (42.4%) | |
| Linolenic Acid < %AMDR | 16 (32.0%) | 6334.5 (40.2%) | |
| Minerals and Vitamins | |||
| Calcium < EAR | 22 (44.0%) | 7570.9 (48.0%) | |
| Iron < EAR | 5 (10.0%) | 1053.9 (6.7%) | |
| Magnesium < EAR | 7 (14.0%) | 8999.6 (57.0%) | *** |
| Potassium < AI | 17 (34.0%) | 10,435.1 (66.1%) | *** |
| Zinc < EAR | 4 (8.0%) | 4238.7 (28.9%) | *** |
| Vitamin A < EAR | 3 (6.0%) | 8096.7 (51.3%) | *** |
| Vitamin E < EAR | 26 (52.0%) | 15,550.2 (98.6%) | *** |
| Vitamin D < EAR | 37 (74.0%) | 14,205.2 (90.0%) | * |
| Vitamin C < EAR | 12 (24.0%) | 8292.9 (52.6%) | *** |
| Thiamin < EAR | 11 (22.0%) | 2246.8 (14.2%) | |
| Niacin < EAR | 0 (0.0%) | 2168.1 (13.7%) | * |
| Vitamin B6 < EAR | 2 (4.0%) | 2836.1 (18.0%) | |
| Vitamin B12 < EAR | 3 (6.0%) | 2213.9 (14.0%) | |
| Choline < AI | 38 (76.0%) | 13,689.9 (86.8%) | |
| Vitamin K < AI | 12 (24.0%) | 10,274.3 (65.1%) | *** |
| Folate < EAR | 23 (46.0%) | 3466.3 (22.0%) | *** |
| Study Teens n = 30 n (%) | NHANES Teens n = 2296 n (Weighted %) | ||
|---|---|---|---|
| Macronutrients, Fiber, and Fatty Acids | |||
| Energy < TEE | 12 (40.0%) | 1361.3 (59.8%) | |
| Fiber < AI | 24 (80.0%) | 2251.9 (98.1%) | *** |
| Protein < EAR | 0 (0.0%) | 449.1 (19.7%) | |
| Carbohydrate < %AMDR | 11 (36.7%) | 359.7 (15.7%) | |
| Linoleic Acid < AI | 9 (30.0%) | 1057.3 (46.0%) | |
| Linoleic Acid < %AMDR | 5 (16.7%) | 627.2 (27.3%) | |
| Linolenic Acid < AI | 6 (20.0%) | 1173.5 (51.1%) | * |
| Linolenic Acid < %AMDR | 12 (40.0%) | 1204.4 (52.5%) | |
| Minerals and Vitamins | |||
| Calcium < EAR | 17 (56.7%) | 1413.9 (61.6%) | |
| Iron < EAR | 9 (30.0%) | 265.3 (11.6%) | |
| Magnesium < EAR | 14 (46.7%) | 1668.8 (72.7%) | |
| Phosphorus < EAR | 9 (30.0%) | 713.3 (31.1%) | |
| Potassium < AI | 14 (46.7%) | 1558.6 (67.9%) | |
| Zinc < EAR | 3 (10.0%) | 676.2 (29.4%) | * |
| Vitamin A < EAR | 5 (16.7%) | 1210.5 (52.7%) | *** |
| Vitamin E < EAR | 18 (60.0%) | 2290.4 (99.8%) | *** |
| Vitamin D < EAR | 27 (90.0%) | 2025.0 (88.2%) | *** |
| Vitamin C < EAR | 8 (26.7%) | 1220.7 (53.2%) | |
| Thiamin < EAR | 5 (16.7%) | 310.5 (13.5%) | |
| Riboflavin < EAR | 3 (10.0%) | 203.6 (8.9%) | |
| Niacin < EAR | 0 (0.0%) | 412.9 (18.0%) | |
| Vitamin B6 < EAR | 2 (6.7%) | 360.1 (15.7%) | |
| Vitamin B12 < EAR | 4 (13.3%) | 282.6 (12.3%) | |
| Choline < AI | 25 (83.3%) | 2086.1 (90.9%) | |
| Vitamin K < AI | 12 (40.0%) | 1637.1 (71.3%) | *** |
| Folate < EAR | 15 (50.0%) | 514.1 (22.4%) | * |
| Study Adults n = 50 n (%) | NHANES CeD & GFD n = 25 n (Weighted %) | ||
|---|---|---|---|
| Macronutrients, Fiber, and Fatty Acids | |||
| Protein < EAR | 1 (2.0%) | 4.2 (16.6%) | |
| Carbohydrate < %AMDR | 36 (72.0%) | 8.1 (32.6%) | * |
| Fiber < AI | 30 (60.0%) | 20.1 (80.4%) | |
| Linoleic Acid < AI | 12 (24.0%) | 11.1 (44.4%) | |
| Linoleic Acid < %AMDR | 10 (20.0%) | 4.1 (16.5%) | |
| Linolenic Acid < AI | 13 (26.0%) | 10.1 (40.3%) | |
| Linolenic Acid < %AMDR | 16 (32.0%) | 10.7 (42.7%) | |
| Minerals and Vitamins | |||
| Calcium < EAR | 22 (44.0%) | 12.2 (48.8%) | |
| Iron < EAR | 5 (10.0%) | 5.0 (20.1%) | |
| Magnesium < EAR | 7 (14.0%) | 9.8 (39.3%) | |
| Potassium < AI | 17 (34.0%) | 14.1 (56.4%) | |
| Vitamin A < EAR | 3 (6.0%) | 5.0 (20.0%) | |
| Vitamin E < EAR | 26 (52.0%) | 23.2 (92.6%) | * |
| Vitamin D < EAR | 37 (74.0%) | 23.1 (92.4%) | |
| Vitamin C < EAR | 12 (24.0%) | 8.5 (34.1%) | |
| Thiamin < EAR | 11 (22.0%) | 4.2 (17.0%) | |
| Niacin < EAR | 0 (0.0%) | 6.3 (25.1%) | * |
| Vitamin B6 < EAR | 2 (4.0%) | 4.8 (19.2%) | |
| Vitamin B12 < EAR | 3 (6.0%) | 3.2 (12.8%) | |
| Choline < AI | 38 (76.0%) | 20.2 (80.9%) | |
| Vitamin K < AI | 12 (24.0%) | 9.2 (36.7%) | |
| Folate < EAR | 23 (46.0%) | 4.1 (16.3%) | |
| Nutrient Consumption Above Recommendations | Study Adults n = 50 n (%) | NHANES Adults n = 15,616 n (Weighted %) | |
|---|---|---|---|
| Total Fat > %AMDR | 40 (80.0%) | 6726.3 (42.6%) | *** |
| Linoleic Acid > %AMDR | 4 (8.0%) | 1731.4 (11.0%) | |
| Linolenic Acid > %AMDR | 5 (10.0%) | 1201.6 (7.6%) | |
| Niacin (Vitamin B3) > UL | 28 (56.0%) | 2842.2 (18.0%) | *** |
| Nutrient Consumption Above Recommendations | Study Teens n = 30 n (%) | NHANES Teens n = 2296 n (Weighted %) | |
|---|---|---|---|
| Total Fat > %AMDR | 20 (66.7%) | 862.9 (37.6%) | |
| Niacin > UL | 24 (80.0%) | 720.9 (31.4%) | *** |
| Vitamin A > UL | 3 (10.0%) | 2.0 (0.1%) | *** |
| Folate > UL | 6 (20.0%) | 470.0 (20.5%) | |
| Nutrient Consumption Above Recommendations | Study Adults n = 50 n (%) | NHANES CeD & GFD n = 25 n (Weighted %) |
|---|---|---|
| Total Fat > %AMDR | 40 (80.0%) | 11.0 (43.9%) |
| Linoleic Acid > %AMDR | 4 (8.0%) | 5.2 (20.9%) |
| Linolenic Acid > %AMDR | 5 (10.0%) | 2.0 (8.0%) |
| Niacin (Vitamin B3) > UL | 28 (56.0%) | 4.6 (18.5%) |
| Folate > UL | 0 (0.0%) | 2.5 (10.0%) |
| Nutrients to Limit | Study Adults n = 50 | NHANES Adults n = 15,777 | |
|---|---|---|---|
| Sodium 2 [Mean mg (SD)] | 3359 (940) | 6667 (2915) | *** |
| Saturated Fat [Mean % Energy (SD)] | 12.8 (3.1) | 10.9 (3.2) | ** |
| Added Sugar [Mean % Energy (SD)] | 9.5 (5.4) | 12.7 (8.4) | ** |
| Nutrients to Limit | Study Adults n = 50 | NHANES CeD and GFD n = 25 | |
|---|---|---|---|
| Sodium 2 [Mean mg (SD)] | 3359 (940) | 5805 (2522) | ** |
| Saturated Fat [Mean % Energy (SD)] | 12.8 (3.1) | 10.1 (2.7) | * |
| Added Sugar [Mean % Energy (SD)] | 9.5 (5.4) | 9.6 (4.4) | |
| Nutrient Consumption | Study Teens n = 30 | NHANES Teens n = 2296 | |
|---|---|---|---|
| Sodium 2 [Mean mg (SD)] | 3394 (1220) | 6393 (2837) | *** |
| Saturated Fat 2 [Mean % Energy (SD)] | 13.6 (4.2) | 11.2 (2.9) | |
| Added Sugar [Mean % Energy (SD)] | 11.2 (6.8) | 15.6 (7.5) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadenhead, J.W.; Lee, A.R.; Nguyen, T.T.T.; Lebwohl, B.; Green, P.H.R.; Wolf, R.L. Dietary Adherence to Recommendations among a Cohort of Adults and Teens with Celiac Disease Maintaining a Gluten-Free Diet Compared to a Nationally Representative Sample: A Cross-Sectional Study. Nutrients 2024, 16, 3067. https://doi.org/10.3390/nu16183067
Cadenhead JW, Lee AR, Nguyen TTT, Lebwohl B, Green PHR, Wolf RL. Dietary Adherence to Recommendations among a Cohort of Adults and Teens with Celiac Disease Maintaining a Gluten-Free Diet Compared to a Nationally Representative Sample: A Cross-Sectional Study. Nutrients. 2024; 16(18):3067. https://doi.org/10.3390/nu16183067
Chicago/Turabian StyleCadenhead, Jennifer W., Anne R. Lee, Thanh Thanh T. Nguyen, Benjamin Lebwohl, Peter H. R. Green, and Randi L. Wolf. 2024. "Dietary Adherence to Recommendations among a Cohort of Adults and Teens with Celiac Disease Maintaining a Gluten-Free Diet Compared to a Nationally Representative Sample: A Cross-Sectional Study" Nutrients 16, no. 18: 3067. https://doi.org/10.3390/nu16183067
APA StyleCadenhead, J. W., Lee, A. R., Nguyen, T. T. T., Lebwohl, B., Green, P. H. R., & Wolf, R. L. (2024). Dietary Adherence to Recommendations among a Cohort of Adults and Teens with Celiac Disease Maintaining a Gluten-Free Diet Compared to a Nationally Representative Sample: A Cross-Sectional Study. Nutrients, 16(18), 3067. https://doi.org/10.3390/nu16183067






