The Anti-Inflammatory and Antioxidant Properties of Acebuche Oil Exert a Retinoprotective Effect in a Murine Model of High-Tension Glaucoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Dietary Supplementation
2.3. Weight, Water and Food Intake
2.4. MCE Injection
2.5. IOP Measurement
2.6. Measurement of Scotopic and Photopic Electroretinogram (ERG)
2.7. Measurement of Pattern ERG (PERG)
2.8. Retina Dissection
2.9. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.10. Protein Extraction and Western Blot
2.11. Statistical Analyses
3. Results
3.1. ACE Oil-Enriched Diet Does Not Affect Food and Water Intake or Animals’ Weight
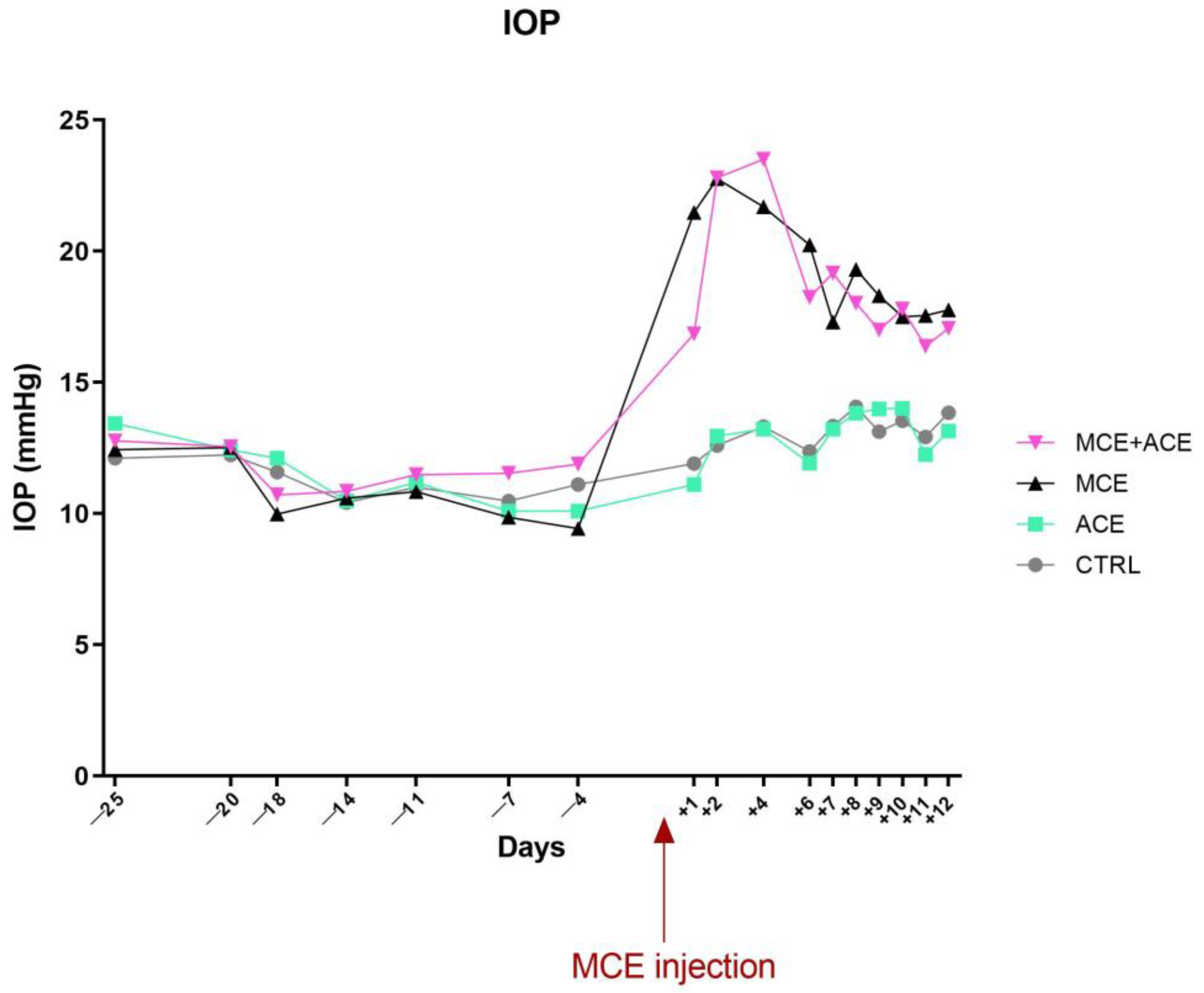
3.2. ACE Oil-Enriched Diet Does Not Prevent IOP Elevation
3.3. ACE Oil-Enriched Diet Suppresses Müller Cells and Microglia Activation in the Glaucomatous Retina
3.4. ACE Oil-Enriched Diet Efficiently Reduces Glaucoma-Related Inflammation in the Glaucomatous Retina
3.5. ACE Oil-Enriched Diet Restored a Control-like Expression of Oxidative Stress Markers in the Glaucomatous Retina
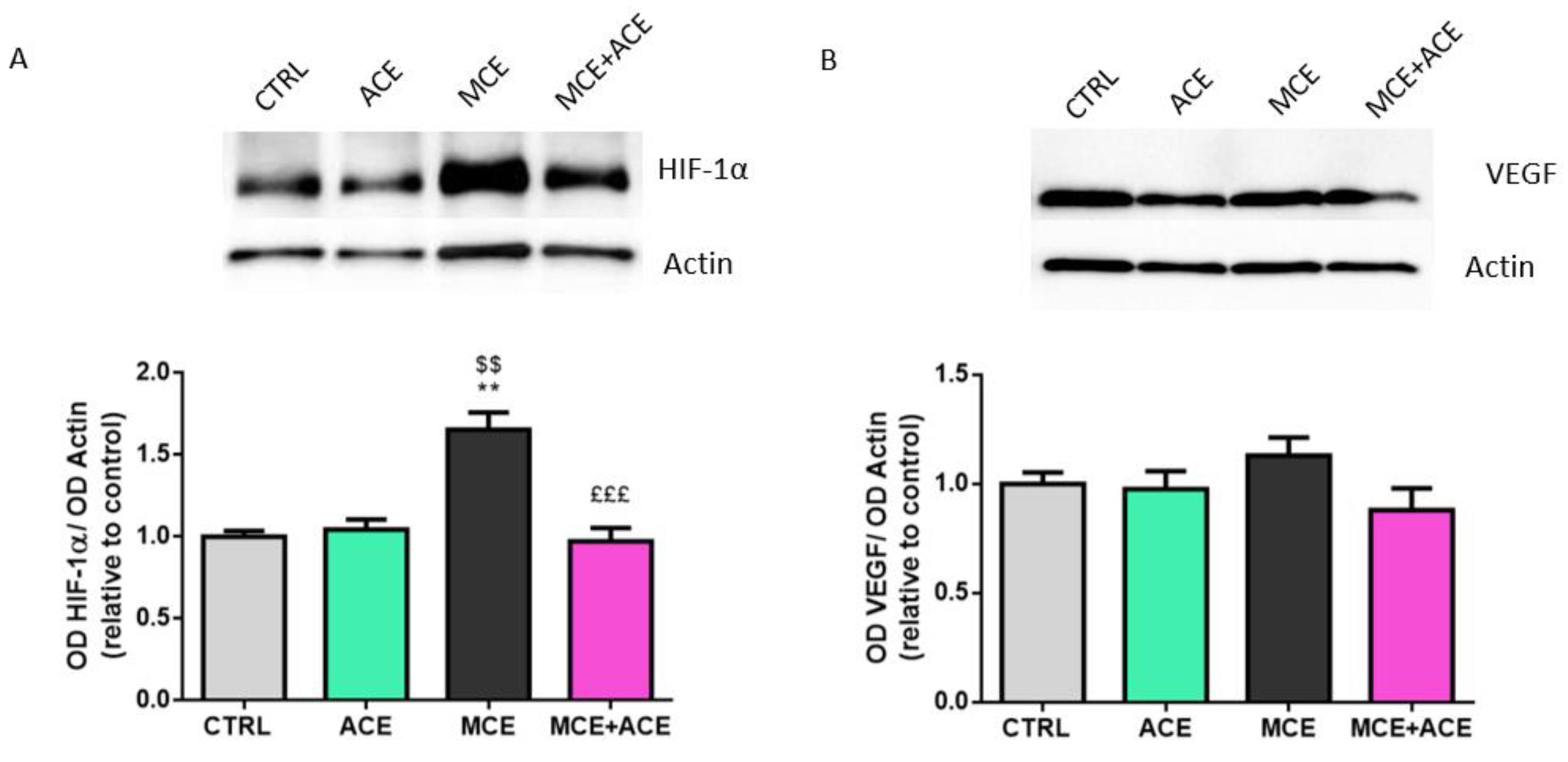
3.6. ACE Oil-Enriched Diet Alleviates Ischemia in the Glaucomatous Retina
3.7. ACE Oil-Enriched Diet Prevents Apoptosis in the Glaucomatous Retina
3.8. ACE Oil-Enriched Diet Restores Retina Electrophysiological Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Colton, T.; Ederer, F. The distribution of intraocular pressures in the general population. Surv. Ophthalmol. 1980, 25, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Mackey, D.A. Glaucoma-risk factors and current challenges in the diagnosis of a leading cause of visual impairment. Maturitas 2022, 163, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, D.K.; Sapra, A. Physiology, Aqueous Humor Circulation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Costagliola, C.; dell’Omo, R.; Agnifili, L.; Bartollino, S.; Fea, A.M.; Uva, M.G.; Zeppa, L.; Mastropasqua, L. How many aqueous humor outflow pathways are there? Surv. Ophthalmol. 2020, 65, 144–170. [Google Scholar] [CrossRef] [PubMed]
- Killer, H.E.; Pircher, A. Normal tension glaucoma: Review of current understanding and mechanisms of the pathogenesis. Eye 2018, 32, 924–930. [Google Scholar] [CrossRef]
- Salvetat, M.L.; Pellegrini, F.; Spadea, L.; Salati, C.; Zeppieri, M. Pharmaceutical Approaches to Normal Tension Glaucoma. Pharmaceuticals 2023, 16, 1172. [Google Scholar] [CrossRef]
- Guglielmi, P.; Carradori, S.; Campestre, C.; Poce, G. Novel therapies for glaucoma: A patent review (2013–2019). Expert Opin. Ther. Pat. 2019, 29, 769–780. [Google Scholar] [CrossRef]
- Sheybani, A.; Scott, R.; Samuelson, T.W.; Kahook, M.Y.; Bettis, D.I.; Ahmed, I.I.K.; Stephens, J.D.; Kent, D.; Ferguson, T.J.; Herndon, L.W. Open-Angle Glaucoma: Burden of Illness, Current Therapies, and the Management of Nocturnal IOP Variation. Ophthalmol. Ther. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Cvenkel, B.; Kolko, M. Current Medical Therapy and Future Trends in the Management of Glaucoma Treatment. J. Ophthalmol. 2020, 2020, 6138132. [Google Scholar] [CrossRef]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef]
- Leung, D.Y.L.; Tham, C.C. Normal-tension glaucoma: Current concepts and approaches—A review. Clin. Exp. Ophthalmol. 2022, 50, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.S.; Sharma, S.; Londhe, V.Y. Bio-tactics for neuroprotection of retinal ganglion cells in the treatment of glaucoma. Life Sci. 2020, 243, 117303. [Google Scholar] [CrossRef]
- Almasieh, M.; Levin, L.A. Neuroprotection in Glaucoma: Animal Models and Clinical Trials. Annu. Rev. Vis. Sci. 2017, 3, 91–120. [Google Scholar] [CrossRef] [PubMed]
- Rusciano, D.; Pezzino, S.; Mutolo, M.G.; Giannotti, R.; Librando, A.; Pescosolido, N. Neuroprotection in Glaucoma: Old and New Promising Treatments. Adv. Pharmacol. Sci. 2017, 2017, 4320408. [Google Scholar] [CrossRef]
- Morrone, L.A.; Rombola, L.; Adornetto, A.; Corasaniti, M.T.; Russo, R. Rational Basis for Nutraceuticals in the Treatment of Glaucoma. Curr. Neuropharmacol. 2018, 16, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Coleman, A.L.; Stone, K.L.; Kodjebacheva, G.; Yu, F.; Pedula, K.L.; Ensrud, K.E.; Cauley, J.A.; Hochberg, M.C.; Topouzis, F.; Badala, F.; et al. Glaucoma risk and the consumption of fruits and vegetables among older women in the study of osteoporotic fractures. Am. J. Ophthalmol. 2008, 145, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Giaconi, J.A.; Yu, F.; Stone, K.L.; Pedula, K.L.; Ensrud, K.E.; Cauley, J.A.; Hochberg, M.C.; Coleman, A.L.; Study of Osteoporotic Fractures Research Group. The association of consumption of fruits/vegetables with decreased risk of glaucoma among older African-American women in the study of osteoporotic fractures. Am. J. Ophthalmol. 2012, 154, 635–644. [Google Scholar] [CrossRef]
- Loskutova, E.; O’Brien, C.; Loskutov, I.; Loughman, J. Nutritional supplementation in the treatment of glaucoma: A systematic review. Surv. Ophthalmol. 2019, 64, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Kassa, A.; Konrad, H.; Geburek, T. Molecular diversity and gene flow within and among different subspecies of the wild olive (Olea europaea L.): A review. Flora 2019, 250, 18–26. [Google Scholar] [CrossRef]
- Reyes-Goya, C.; Santana-Garrido, Á.; Espinosa-Martín, P.; Vázquez, C.M.; Mate, A. Wild and cultivated olive trees: Nutraceutical insights of extra virgin olive oils in cardiovascular and ocular diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166904. [Google Scholar] [CrossRef] [PubMed]
- Santana-Garrido, Á.; Reyes-Goya, C.; Pérez-Camino, M.C.; André, H.; Mate, A.; Vázquez, C.M. Retinoprotective Effect of Wild Olive (Acebuche) Oil-Enriched Diet against Ocular Oxidative Stress Induced by Arterial Hypertension. Antioxidants 2020, 9, 885. [Google Scholar] [CrossRef]
- Santana-Garrido, Á.; Reyes-Goya, C.; Milla-Navarro, S.; de la Villa, P.; André, H.; Vázquez, C.M.; Mate, A. Anti-Inflammatory Action of Dietary Wild Olive (Acebuche) Oil in the Retina of Hypertensive Mice. Foods 2021, 10, 1993. [Google Scholar] [CrossRef] [PubMed]
- Santana-Garrido, Á.; Reyes-Goya, C.; André, H.; Vázquez, C.M.; Mate, A. Exploring the Potential of Wild Olive (Acebuche) Oil as a Pharm-Food to Prevent Ocular Hypertension and Fibrotic Events in the Retina of Hypertensive Mice. Mol. Nutr. Food Res. 2023, e2200623. [Google Scholar] [CrossRef]
- Pang, I.H.; Clark, A.F. Inducible rodent models of glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.D.; Cai, F.Y. Development of experimental chronic intraocular hypertension in the rabbit. Aust. N. Z. J. Ophthalmol. 1992, 20, 225–234. [Google Scholar] [CrossRef]
- Dal Monte, M.; Cammalleri, M.; Amato, R.; Pezzino, S.; Corsaro, R.; Bagnoli, P.; Rusciano, D. A Topical Formulation of Melatoninergic Compounds Exerts Strong Hypotensive and Neuroprotective Effects in a Rat Model of Hypertensive Glaucoma. Int. J. Mol. Sci. 2020, 21, 9267. [Google Scholar] [CrossRef]
- Cammalleri, M.; Dal Monte, M.; Amato, R.; Bagnoli, P.; Rusciano, D. A Dietary Combination of Forskolin with Homotaurine, Spearmint and B Vitamins Protects Injured Retinal Ganglion Cells in a Rodent Model of Hypertensive Glaucoma. Nutrients 2020, 12, 1189. [Google Scholar] [CrossRef]
- Santana-Garrido, Á.; Reyes-Goya, C.; Arroyo-Barrios, A.; André, H.; Vázquez, C.M.; Mate, A. Hypertension secondary to nitric oxide depletion produces oxidative imbalance and inflammatory/fibrotic outcomes in the cornea of C57BL/6 mice. J. Physiol. Biochem. 2022, 78, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Millar, J.C.; Pang, I.H.; Wax, M.B.; Clark, A.F. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4617–4621. [Google Scholar] [CrossRef] [PubMed]
- Porciatti, V. Electrophysiological assessment of retinal ganglion cell function. Exp. Eye Res. 2015, 141, 164–170. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Melecchi, A.; Amato, R.; Dal Monte, M.; Rusciano, D.; Bagnoli, P.; Cammalleri, M. Restored retinal physiology after administration of niacin with citicoline in a mouse model of hypertensive glaucoma. Front. Med. (Lausanne) 2023, 10, 1230941. [Google Scholar] [CrossRef]
- Dal Monte, M.; Cammalleri, M.; Pezzino, S.; Corsaro, R.; Pescosolido, N.; Bagnoli, P.; Rusciano, D. Hypotensive Effect of Nanomicellar Formulation of Melatonin and Agomelatine in a Rat Model: Significance for Glaucoma Therapy. Diagnostics 2020, 10, 138. [Google Scholar] [CrossRef]
- Amato, R.; Canovai, A.; Melecchi, A.; Maci, S.; Quintela, F.; Fonseca, B.A.; Cammalleri, M.; Dal Monte, M. Efficacy of a Spearmint (Mentha spicata L.) Extract as Nutritional Support in a Rat Model of Hypertensive Glaucoma. Transl. Vis. Sci. Technol. 2023, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Cho, K.S.; Yi, I.; To, C.H.; Chen, D.F.; Do, C.W. Baicalein, Baicalin, and Wogonin: Protective Effects against Ischemia-Induced Neurodegeneration in the Brain and Retina. Oxidative Med. Cell. Longev. 2021, 2021, 8377362. [Google Scholar] [CrossRef]
- Crowston, J.G.; Kong, Y.X.; Trounce, I.A.; Dang, T.M.; Fahy, E.T.; Bui, B.V.; Morrison, J.C.; Chrysostomou, V. An acute intraocular pressure challenge to assess retinal ganglion cell injury and recovery in the mouse. Exp. Eye Res. 2015, 141, 3–8. [Google Scholar] [CrossRef]
- Bui, B.V.; He, Z.; Vingrys, A.J.; Nguyen, C.T.; Wong, V.H.; Fortune, B. Using the electroretinogram to understand how intraocular pressure elevation affects the rat retina. J. Ophthalmol. 2013, 2013, 262467. [Google Scholar] [CrossRef]
- Prencipe, M.; Perossini, T.; Brancoli, G.; Perossini, M. The photopic negative response (PhNR): Measurement approaches and utility in glaucoma. Int. Ophthalmol. 2020, 40, 3565–3576. [Google Scholar] [CrossRef]
- Saleh, M.; Nagaraju, M.; Porciatti, V. Longitudinal evaluation of retinal ganglion cell function and IOP in the DBA/2J mouse model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4564–4572. [Google Scholar] [CrossRef] [PubMed]
- Cvenkel, B.; Sustar, M.; Perovšek, D. Ganglion cell loss in early glaucoma, as assessed by photopic negative response, pattern electroretinogram, and spectral-domain optical coherence tomography. Doc. Ophthalmol. 2017, 135, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Gao, F.; Hu, F.; Huang, J.; Wang, M.; Xu, P.; Zhang, R.; Chen, J.; Sun, X.; Zhang, S.; et al. Asiatic Acid Prevents Retinal Ganglion Cell Apoptosis in a Rat Model of Glaucoma. Front. Neurosci. 2018, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Locri, F.; Cammalleri, M.; Dal Monte, M.; Rusciano, D.; Bagnoli, P. Protective Efficacy of a Dietary Supplement Based on Forskolin, Homotaurine, Spearmint Extract, and Group B Vitamins in a Mouse Model of Optic Nerve Injury. Nutrients 2019, 11, 2931. [Google Scholar] [CrossRef]
- Adornetto, A.; Russo, R.; Parisi, V. Neuroinflammation as a target for glaucoma therapy. Neural Regen. Res. 2019, 14, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Inman, D.M.; Horner, P.J. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia 2007, 55, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, I.C.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflamm. 2015, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; López-Cuenca, I.; Rojas, P.; Triviño, A.; Ramírez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; Ramírez, A.I.; de Hoz, R.; López-Villarín, N.; Salobrar-García, E.; López-Cuenca, I.; Licastro, E.; Inarejos-García, A.M.; Almodóvar, P.; Pinazo-Durán, M.D.; et al. Neuroprotective and Anti-Inflammatory Effects of a Hydrophilic Saffron Extract in a Model of Glaucoma. Int. J. Mol. Sci. 2019, 20, 4110. [Google Scholar] [CrossRef]
- Amato, R.; Rossino, M.G.; Cammalleri, M.; Timperio, A.M.; Fanelli, G.; Dal Monte, M.; Pucci, L.; Casini, G. The Potential of Lisosan G as a Possible Treatment for Glaucoma. Front. Pharmacol. 2021, 12, 719951. [Google Scholar] [CrossRef]
- Bosco, A.; Breen, K.T.; Anderson, S.R.; Steele, M.R.; Calkins, D.J.; Vetter, M.L. Glial coverage in the optic nerve expands in proportion to optic axon loss in chronic mouse glaucoma. Exp. Eye Res. 2016, 150, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Guttenplan, K.A.; Stafford, B.K.; El-Danaf, R.N.; Adler, D.I.; Münch, A.E.; Weigel, M.K.; Huberman, A.D.; Liddelow, S.A. Neurotoxic Reactive Astrocytes Drive Neuronal Death after Retinal Injury. Cell Rep. 2020, 31, 107776. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G. Immune regulation toward immunomodulation for neuroprotection in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 23–31. [Google Scholar] [CrossRef]
- Silverman, S.M.; Wong, W.T. Microglia in the Retina: Roles in Development, Maturity, and Disease. Annu. Rev. Vis. Sci. 2018, 4, 45–77. [Google Scholar] [CrossRef]
- Wang, M.; Liu, H.; Xia, N.; Li, H.; van Beers, T.; Gericke, A.; Prokosch, V. Intraocular Pressure-Induced Endothelial Dysfunction of Retinal Blood Vessels Is Persistent, but Does Not Trigger Retinal Ganglion Cell Loss. Antioxidants 2022, 11, 1864. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E.M. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog. Retin. Eye Res. 2021, 83, 100916. [Google Scholar] [CrossRef]
- Sappington, R.M.; Chan, M.; Calkins, D.J. Interleukin-6 protects retinal ganglion cells from pressure-induced death. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2932–2942. [Google Scholar] [CrossRef]
- Johnson, E.C.; Doser, T.A.; Cepurna, W.O.; Dyck, J.A.; Jia, L.; Guo, Y.; Lambert, W.S.; Morrison, J.C. Cell proliferation and interleukin-6-type cytokine signaling are implicated by gene expression responses in early optic nerve head injury in rat glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 504–518. [Google Scholar] [CrossRef]
- Chidlow, G.; Wood, J.P.; Ebneter, A.; Casson, R.J. Interleukin-6 is an efficacious marker of axonal transport disruption during experimental glaucoma and stimulates neuritogenesis in cultured retinal ganglion cells. Neurobiol. Dis. 2012, 48, 568–581. [Google Scholar] [CrossRef]
- Sims, S.M.; Holmgren, L.; Cathcart, H.M.; Sappington, R.M. Spatial regulation of interleukin-6 signaling in response to neurodegenerative stressors in the retina. Am. J. Neurodegener. Dis. 2012, 1, 168–179. [Google Scholar]
- Echevarria, F.D.; Formichella, C.R.; Sappington, R.M. Interleukin-6 Deficiency Attenuates Retinal Ganglion Cell Axonopathy and Glaucoma-Related Vision Loss. Front. Neurosci. 2017, 11, 318. [Google Scholar] [CrossRef]
- Ng, T.K.; Chu, K.O.; Wang, C.C.; Pang, C.P. Green Tea Catechins as Therapeutic Antioxidants for Glaucoma Treatment. Antioxidants 2023, 12, 1320. [Google Scholar] [CrossRef] [PubMed]
- Merelli, A.; Repetto, M.; Lazarowski, A.; Auzmendi, J. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J. Alzheimers Dis. 2021, 82, S109–S126. [Google Scholar] [CrossRef] [PubMed]
- Shu, D.Y.; Chaudhary, S.; Cho, K.S.; Lennikov, A.; Miller, W.P.; Thorn, D.C.; Yang, M.; McKay, T.B. Role of Oxidative Stress in Ocular Diseases: A Balancing Act. Metabolites 2023, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.C.; Izzotti, A.; Rossi, P.; Traverso, C. Glaucomatous outflow pathway and oxidative stress. Exp. Eye Res. 2007, 84, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidative Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, Z.L. Activation of Nrf2/HO-1 pathway protects retinal ganglion cells from a rat chronic ocular hypertension model of glaucoma. Int. Ophthalmol. 2019, 39, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.N.; Amin, R.H.; Puklin, J.E. Antioxidant enzymes in the macular retinal pigment epithelium of eyes with neovascular age-related macular degeneration. Am. J. Ophthalmol. 1999, 127, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Flammer, J.; Orgül, S.; Costa, V.P.; Orzalesi, N.; Krieglstein, G.K.; Serra, L.M.; Renard, J.P.; Stefánsson, E. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye Res. 2002, 21, 359–393. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Wax, M.B. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch. Ophthalmol. 2004, 122, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.M.; Chuang, M.J.; Liu, J.H.; Liu, X.Q.; Ho, L.K.; Pan, W.H.; Zhang, X.M.; Liu, C.M.; Tsai, S.K.; Kong, C.W.; et al. Baicalein protects against retinal ischemia by antioxidation, antiapoptosis, downregulation of HIF-1α, VEGF, and MMP-9 and upregulation of HO-1. J. Ocul. Pharmacol. Ther. 2013, 29, 539–549. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Ergorul, C.; Ray, A.; Huang, W.; Darland, D.; Luo, Z.K.; Grosskreutz, C.L. Levels of vascular endothelial growth factor-A165b (VEGF-A165b) are elevated in experimental glaucoma. Mol. Vis. 2008, 14, 1517–1524. [Google Scholar]
- Zhou, J.; Chen, F.; Yan, A.; Xia, X. Role of mammalian target of rapamycin in regulating HIF-1α and vascular endothelial growth factor signals in glaucoma. Arch. Physiol. Biochem. 2021, 127, 44–50. [Google Scholar] [CrossRef]
- Lip, P.L.; Felmeden, D.C.; Blann, A.D.; Matheou, N.; Thakur, S.; Cunliffe, I.A.; Lip, G.Y. Plasma vascular endothelial growth factor, soluble VEGF receptor FLT-1, and von Willebrand factor in glaucoma. Br. J. Ophthalmol. 2002, 86, 1299–1302. [Google Scholar] [CrossRef]
- Hu, D.N.; Ritch, R.; Liebmann, J.; Liu, Y.; Cheng, B.; Hu, M.S. Vascular endothelial growth factor is increased in aqueous humor of glaucomatous eyes. J. Glaucoma 2002, 11, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Kim, Y.K.; Kim, Y.W.; Jeoung, J.W.; Kim, S.H.; Heo, J.W.; Yu, H.G.; Park, K.H. Rate of Macular Ganglion Cell-inner Plexiform Layer Thinning in Glaucomatous Eyes With Vascular Endothelial Growth Factor Inhibition. J. Glaucoma 2017, 26, 980–986. [Google Scholar] [CrossRef]
- Albanese, A.; Daly, L.A.; Mennerich, D.; Kietzmann, T.; Sée, V. The Role of Hypoxia-Inducible Factor Post-Translational Modifications in Regulating Its Localisation, Stability, and Activity. Int. J. Mol. Sci. 2020, 22, 268. [Google Scholar] [CrossRef]
- Spitz, A.Z.; Gavathiotis, E. Physiological and pharmacological modulation of BAX. Trends Pharmacol. Sci. 2022, 43, 206–220. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef]
- Buckingham, B.P.; Inman, D.M.; Lambert, W.; Oglesby, E.; Calkins, D.J.; Steele, M.R.; Vetter, M.L.; Marsh-Armstrong, N.; Horner, P.J. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J. Neurosci. 2008, 28, 2735–2744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, G.; Yang, B.; Wu, J. Quercetin Enhances Inhibitory Synaptic Inputs and Reduces Excitatory Synaptic Inputs to OFF- and ON-Type Retinal Ganglion Cells in a Chronic Glaucoma Rat Model. Front. Neurosci. 2019, 13, 672. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, A.; Martine, L.; Grégoire, S.; Nury, T.; Meddeb, W.; Camus, E.; Badreddine, A.; Durand, P.; Namsi, A.; Yammine, A.; et al. Profile of Fatty Acids, Tocopherols, Phytosterols and Polyphenols in Mediterranean Oils (Argan Oils, Olive Oils, Milk Thistle Seed Oils and Nigella Seed Oil) and Evaluation of their Antioxidant and Cytoprotective Activities. Curr. Pharm. Des. 2019, 25, 1791–1805. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.L.; Peng, P.H.; Hsu, S.Y.; Chen, C.F. Dietary deficiency of vitamin E aggravates retinal ganglion cell death in experimental glaucoma of rats. Curr. Eye Res. 2010, 35, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Engin, K.N.; Engin, G.; Kucuksahin, H.; Oncu, M.; Engin, G.; Guvener, B. Clinical evaluation of the neuroprotective effect of alpha-tocopherol against glaucomatous damage. Eur. J. Ophthalmol. 2007, 17, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Tang, X.; Guan, T.; Li, Y.; Sun, H. Neuroprotection by Combined Administration with Maslinic Acid, a Natural Product from Olea europaea, and MK-801 in the Cerebral Ischemia Model. Molecules 2016, 21, 1093. [Google Scholar] [CrossRef]
- Wang, Z.H.; Mong, M.C.; Yang, Y.C.; Yin, M.C. Asiatic acid and maslinic acid attenuated kainic acid-induced seizure through decreasing hippocampal inflammatory and oxidative stress. Epilepsy Res. 2018, 139, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Kyung, H.; Kwong, J.M.; Bekerman, V.; Gu, L.; Yadegari, D.; Caprioli, J.; Piri, N. Celastrol supports survival of retinal ganglion cells injured by optic nerve crush. Brain Res. 2015, 1609, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kwong, J.M.K.; Yadegari, D.; Yu, F.; Caprioli, J.; Piri, N. The effect of celastrol on the ocular hypertension-induced degeneration of retinal ganglion cells. Neurosci. Lett. 2018, 670, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.O.; Antunes, E.; de Nucci, G.; Lovisolo, S.M.; Zatz, R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension 1992, 20, 298–303. [Google Scholar] [CrossRef]
- Vishwaraj, C.R.; Kavitha, S.; Venkatesh, R.; Shukla, A.G.; Chandran, P.; Tripathi, S. Neuroprotection in glaucoma. Indian J. Ophthalmol. 2022, 70, 380–385. [Google Scholar] [CrossRef]






| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| GFAP | AGGGAGTGGAGGAGTCATTCG | CGGAGACGCATCACCTCT |
| IBA-1 | CGAATGCTGGAGAAACTTGG | AGCCCCACCGTGTGACAT |
| TNF α | GCCTCTTCTCATTCCTGCTTG | CACTTGGTGGTTTGCTACGAC |
| IL-6 | CCAAGAACGATAGTCAATTCCAGA | CATCAGTCCCAAGAAGGCAAC |
| IL-10 | CCAAGCCTTATCGGAAATGA | TTTTCACAGGGGAGAAATCG |
| HO-1 | AAGCCGAGAATGCTGAGTTCA | GCCGTGTAGATATGGTACAAGGA |
| NQO1 | AGGATGGGAGGTACTCGAATC | AGGCGTCCTTCCTTATATGCTA |
| RPL13a | CACTCTGGAGGAGAAACGGAAGG | GCAGGCATGAGGCAAACAGTC |
| Primary Antibody | Origin | Dilution | Secondary Antibody | Dilution | Reference |
|---|---|---|---|---|---|
| Anti-GFAP | Mouse monoclonal | 1:4000 | Goat Anti-Mouse | 1:5000 | Santa Cruz Biotechnol-ogy (SCB), Dallas, TX, USA |
| Anti-IBA-1 | Rabbit polyclonal | 1:1000 | Goat Anti-Rabbit | 1:5000 | Abcam, Cambridge, UK |
| Anti-TNF α | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | SCB |
| Anti-IL-6 | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | SCB |
| Anti-IL-10 | Mouse monoclonal | 1:100 | Goat Anti-Mouse | 1:1000 | SCB |
| Anti-HO-1 | Rabbit poly-clonal | 1:1000 | Goat Anti-Rabbit | 1:5000 | Abcam |
| Anti-NQO1 | Rabbit polyclonal | 1:500 | Goat Anti-Rabbit | 1:5000 | Abcam |
| Anti-HIF-1α | Rabbit polyclonal | 1:1000 | Goat Anti-Rabbit | 1:5000 | Abcam |
| Anti-VEGF | Rabbit polyclonal | 1:1000 | Goat Anti-Rabbit | 1:5000 | Abcam |
| Anti-Bax | Rabbit polyclonal | 1:500 | Goat Anti-Rabbit | 1:5000 | Abcam |
| Antii-Bcl2 | Rabbit polyclonal | 1:500 | Goat Anti-Rabbit | 1:5000 | Abcam |
| Anti-Cleaved Caspase3 | Rabbit polyclonal | 1:1000 | Goat Anti-Rabbit | 1:2000 | Cell Signaling, Danvers, MA, USA |
| Anti-β-actin | Mouse monoclonal | 1:20,000 | Goat Anti-Mouse | 1:30,000 | SCB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucchesi, M.; Marracci, S.; Amato, R.; Lapi, D.; Santana-Garrido, Á.; Espinosa-Martín, P.; Vázquez, C.M.; Mate, A.; Dal Monte, M. The Anti-Inflammatory and Antioxidant Properties of Acebuche Oil Exert a Retinoprotective Effect in a Murine Model of High-Tension Glaucoma. Nutrients 2024, 16, 409. https://doi.org/10.3390/nu16030409
Lucchesi M, Marracci S, Amato R, Lapi D, Santana-Garrido Á, Espinosa-Martín P, Vázquez CM, Mate A, Dal Monte M. The Anti-Inflammatory and Antioxidant Properties of Acebuche Oil Exert a Retinoprotective Effect in a Murine Model of High-Tension Glaucoma. Nutrients. 2024; 16(3):409. https://doi.org/10.3390/nu16030409
Chicago/Turabian StyleLucchesi, Martina, Silvia Marracci, Rosario Amato, Dominga Lapi, Álvaro Santana-Garrido, Pablo Espinosa-Martín, Carmen María Vázquez, Alfonso Mate, and Massimo Dal Monte. 2024. "The Anti-Inflammatory and Antioxidant Properties of Acebuche Oil Exert a Retinoprotective Effect in a Murine Model of High-Tension Glaucoma" Nutrients 16, no. 3: 409. https://doi.org/10.3390/nu16030409








