Plasma Leukocyte Cell-Derived Chemotaxin-2 as a Risk Factor of Sarcopenia: Korean Frailty and Aging Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurement of the Skeletal Muscle Mass and Muscle Strength
2.3. Clinical and Biochemical Parameters
2.4. Statistical Analysis
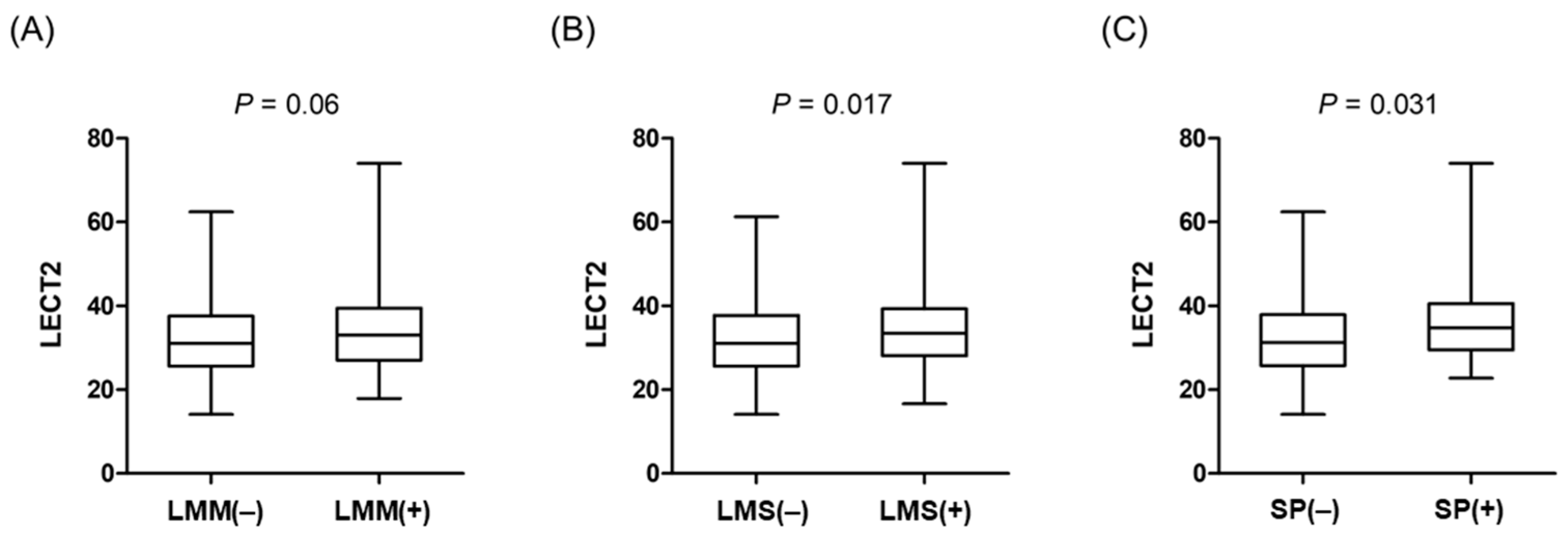
3. Results
3.1. Baseline Characteristics
3.2. Correlation
3.3. Independent Association
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Kirk, B.; Cawthon, P.M.; Arai, H.; Ávila-Funes, J.A.; Barazzoni, R.; Bhasin, S.; Binder, E.F.; Bruyere, O.; Cederholm, T.; Chen, L.-K.; et al. The Conceptual Definition of Sarcopenia: Delphi Consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing 2024, 53, afae052. [Google Scholar] [CrossRef]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef]
- Duell, P.B.; Welty, F.K.; Miller, M.; Chait, A.; Hammond, G.; Ahmad, Z.; Cohen, D.E.; Horton, J.D.; Pressman, G.S.; Toth, P.P. Nonalcoholic Fatty Liver Disease and Cardiovascular Risk: A Scientific Statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e168–e185. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol. Metab. 2008, 19, 371–379. [Google Scholar] [CrossRef]
- Roh, E.; Hwang, S.Y.; Yoo, H.J.; Baik, S.H.; Cho, B.; Park, Y.S.; Kim, H.J.; Lee, S.G.; Kim, B.J.; Jang, H.C.; et al. Association of plasma FGF21 levels with muscle mass and muscle strength in a national multicentre cohort study: Korean Frailty and Aging Cohort Study. Age Ageing 2021, 50, 1971–1978. [Google Scholar] [CrossRef]
- Kim, J.A.; Choi, K.M. Sarcopenia and fatty liver disease. Hepatol. Int. 2019, 13, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, P.; Hevener, A.L.; Karlamangla, A.S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010, 5, e10805. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Jung, K.S.; Kim, S.U.; Yoon, H.J.; Yun, Y.J.; Lee, B.W.; Kang, E.S.; Han, K.H.; Lee, H.C.; Cha, B.S. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011). J. Hepatol. 2015, 63, 486–493. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.E.; Lee, Y.B.; Jun, J.E.; Ahn, J.; Bae, J.C.; Jin, S.M.; Hur, K.Y.; Jee, J.H.; Lee, M.K.; et al. Relationship Between Relative Skeletal Muscle Mass and Nonalcoholic Fatty Liver Disease: A 7-Year Longitudinal Study. Hepatology 2018, 68, 1755–1768. [Google Scholar] [CrossRef]
- Kang, S.; Moon, M.K.; Kim, W.; Koo, B.K. Association between muscle strength and advanced fibrosis in non-alcoholic fatty liver disease: A Korean nationwide survey. J. Cachexia Sarcopenia Muscle 2020, 11, 1232–1241. [Google Scholar] [CrossRef]
- Hong, H.C.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Relationship between sarcopenia and nonalcoholic fatty liver disease: The Korean Sarcopenic Obesity Study. Hepatology 2014, 59, 1772–1778. [Google Scholar] [CrossRef]
- Roh, E.; Hwang, S.Y.; Yoo, H.J.; Baik, S.H.; Lee, J.H.; Son, S.J.; Kim, H.J.; Park, Y.S.; Lee, S.G.; Cho, B.L.; et al. Impact of non-alcoholic fatty liver disease on the risk of sarcopenia: A nationwide multicenter prospective study. Hepatol. Int. 2022, 16, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Meex, R.C.R.; Watt, M.J. Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. [Google Scholar] [CrossRef]
- Wu, H.T.; Ou, H.Y.; Hung, H.C.; Su, Y.C.; Lu, F.H.; Wu, J.S.; Yang, Y.C.; Wu, C.L.; Chang, C.J. A novel hepatokine, HFREP1, plays a crucial role in the development of insulin resistance and type 2 diabetes. Diabetologia 2016, 59, 1732–1742. [Google Scholar] [CrossRef]
- Lan, F.; Misu, H.; Chikamoto, K.; Takayama, H.; Kikuchi, A.; Mohri, K.; Takata, N.; Hayashi, H.; Matsuzawa-Nagata, N.; Takeshita, Y.; et al. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes 2014, 63, 1649–1664. [Google Scholar] [CrossRef]
- Yoo, H.J.; Hwang, S.Y.; Choi, J.H.; Lee, H.J.; Chung, H.S.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; et al. Association of leukocyte cell-derived chemotaxin 2 (LECT2) with NAFLD, metabolic syndrome, and atherosclerosis. PLoS ONE 2017, 12, e0174717. [Google Scholar] [CrossRef]
- Won, C.W.; Lee, S.; Kim, J.; Chon, D.; Kim, S.; Kim, C.O.; Kim, M.K.; Cho, B.; Choi, K.M.; Roh, E.; et al. Korean frailty and aging cohort study (KFACS): Cohort profile. BMJ Open 2020, 10, e035573. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.R.; Shardell, M.D.; Alley, D.E.; Cawthon, P.M.; Fragala, M.S.; Harris, T.B.; Kenny, A.M.; Peters, K.W.; Ferrucci, L.; Guralnik, J.M.; et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: The foundation for the National Institutes of Health (FNIH) sarcopenia project. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, G.; Xia, L.; Yang, X.; Zhang, B.; Liu, F.; Ma, J.; Hu, Z.; Li, Y.; Li, W.; et al. Relative Handgrip Strength Is Inversely Associated with Metabolic Profile and Metabolic Disease in the General Population in China. Front. Physiol. 2018, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Kim, K.M.; Kim, J.H.; Moon, J.H.; Choi, S.H.; Lim, S.; Lim, J.Y.; Kim, K.W.; Park, K.S.; Jang, H.C. Predictive Values of the New Sarcopenia Index by the Foundation for the National Institutes of Health Sarcopenia Project for Mortality among Older Korean Adults. PLoS ONE 2016, 11, e0166344. [Google Scholar] [CrossRef]
- Blanco-Reina, E.; Ocaña-Riola, R.; Ariza-Zafra, G.; García-Merino, M.R.; Aguilar-Cano, L.; Valdellós, J.; Torres-Blanco, C.; Bellido-Estévez, I. Prevalence and Clinical Conditions Related to Sarcopaenia among Older Persons Living in the Community. J. Clin. Med. 2022, 11, 3814. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J. Intern. Med. 2016, 31, 643–650. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Lee, E.J.; Chung, H.S.; Yoo, H.J.; Kang, H.J.; Song, W.; Baik, S.H.; Choi, K.M. Comparisons of three different methods for defining sarcopenia: An aspect of cardiometabolic risk. Sci. Rep. 2017, 7, 6491. [Google Scholar] [CrossRef]
- Yamagoe, S.; Yamakawa, Y.; Matsuo, Y.; Minowada, J.; Mizuno, S.; Suzuki, K. Purification and primary amino acid sequence of a novel neutrophil chemotactic factor LECT2. Immunol. Lett. 1996, 52, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.H.; Liu, Y.J.; Li, C.Y.; Tao, F.; Yang, G.J.; Chen, J. The emerging roles of leukocyte cell-derived chemotaxin-2 in immune diseases: From mechanisms to therapeutic potential. Front. Immunol. 2023, 14, 1158083. [Google Scholar] [CrossRef]
- Nagai, H.; Hamada, T.; Uchida, T.; Yamagoe, S.; Suzuki, K. Systemic expression of a newly recognized protein, LECT2, in the human body. Pathol. Int. 1998, 48, 882–886. [Google Scholar] [CrossRef]
- Zhu, S.; Bennett, S.; Li, Y.; Liu, M.; Xu, J. The molecular structure and role of LECT2 or CHM-II in arthritis, cancer, and other diseases. J. Cell. Physiol. 2022, 237, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Chikamoto, K.; Misu, H.; Takayama, H.; Kikuchi, A.; Ishii, K.A.; Lan, F.; Takata, N.; Tajima-Shirasaki, N.; Takeshita, Y.; Tsugane, H.; et al. Rapid response of the steatosis-sensing hepatokine LECT2 during diet-induced weight cycling in mice. Biochem. Biophys. Res. Commun. 2016, 478, 1310–1316. [Google Scholar] [CrossRef]
- Park, C.Y.; Lee, S.K.; Kim, J.; Kim, D.; Choe, H.; Jeong, J.H.; Choi, K.C.; Park, H.S.; Han, S.N.; Jang, Y.J. Endoplasmic reticulum stress increases LECT2 expression via ATF4. Biochem. Biophys. Res. Commun. 2021, 585, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Afroze, D.; Kumar, A. ER stress in skeletal muscle remodeling and myopathies. FEBS J. 2019, 286, 379–398. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, H.; Lin, J.; Hu, Y.; Yang, R.; Sun, J.; Chen, R.; Chen, H. Circulating LECT2 levels in newly diagnosed type 2 diabetes mellitus and their association with metabolic parameters: An observational study. Medicine 2018, 97, e0354. [Google Scholar] [CrossRef]
- Wei, M.; Liu, J.; Pan, H.; Zhou, Z.; Guo, K. Plasma Leukocyte Cell-Derived Chemotaxin 2 (LECT2) as a Risk Factor of Coronary Artery Disease: A Cross-Sectional Study. Angiology 2022, 73, 265–274. [Google Scholar] [CrossRef]
- Tanisawa, K.; Taniguchi, H.; Sun, X.; Ito, T.; Kawakami, R.; Sakamoto, S.; Higuchi, M. Visceral fat area is a strong predictor of leukocyte cell-derived chemotaxin 2, a potential biomarker of dyslipidemia. PLoS ONE 2017, 12, e0173310. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.K.; Kim, D.; Lee, E.; Park, C.Y.; Choe, H.; Kang, M.J.; Kim, H.J.; Kim, J.H.; Hong, J.P.; et al. Adipose tissue LECT2 expression is associated with obesity and insulin resistance in Korean women. Obesity 2022, 30, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]

| Total | Men | Women | p-Value | |
|---|---|---|---|---|
| N | 400 | 189 | 211 | |
| Age (years) | 75 (73, 79) | 75 (73, 79) | 75 (72, 79) | 0.998 |
| BMI (kg/m2) | 24.2 (22.3, 25.7) | 23.7 (22.1, 25.6) | 24.4 (22.8, 25.8) | 0.031 |
| WC (cm) | 87 ± 8.1 | 87.9 ± 7.9 | 86.2 ± 8.2 | 0.030 |
| SBP (mmHg) | 129 (119, 138.8) | 129 (118.3, 138) | 129.3 (119.3, 139.7) | 0.483 |
| DBP (mmHg) | 76 (70.7, 81.3) | 77 (71, 81.3) | 75.3 (70.3, 82) | 0.283 |
| AST (IU/L) | 21 (18, 25) | 21 (19, 24) | 21 (18, 25) | 0.392 |
| ALT (IU/L) | 17 (13, 21) | 16 (13, 21) | 17 (13, 21) | 0.946 |
| GGT (IU/L) | 18 (14, 25) | 20 (17, 28) | 16 (13, 22) | <0.001 |
| FPG (mg/dL) | 96 (88, 110) | 96 (88, 110) | 96 (88, 109) | 0.908 |
| HOMA-IR | 1.4 (0.9, 2.3) | 1.3 (0.8, 1.8) | 1.6 (1.1, 2.8) | <0.001 |
| HbA1c | 5.8 (5.5, 6.3) | 5.7 (5.4, 6.1) | 5.8 (5.5, 6.4) | 0.028 |
| TC (mg/dL) | 171.7 ± 35 | 164.4 ± 35.5 | 178.2 ± 33.2 | <0.001 |
| LDL-C (mg/dL) | 103.5 (79.5, 125) | 100 (77, 121) | 108 (81, 130) | 0.009 |
| HDL-C (mg/dL) | 51 (42, 60) | 49 (40, 59) | 53 (44, 62) | 0.007 |
| TG (mg/dL) | 105 (78.5, 139.5) | 96 (74, 132) | 113 (83, 159) | 0.001 |
| hs-CRP (mg/dL) | 0.7 (0.5, 1.2) | 0.7 (0.4, 1.3) | 0.7 (0.5, 1.2) | 0.953 |
| BUN (mg/dL) | 16 (13, 19) | 16 (14, 19) | 15 (13, 18) | 0.068 |
| Creatinine (mg/dL) | 0.8 (0.7, 0.9) | 0.9 (0.8, 1.1) | 0.7 (0.6, 0.8) | <0.001 |
| Vitamin D (ng/mL) | 22.8 (17.2, 30.8) | 23.3 (18.4, 30.1) | 21.9 (15.7, 31.8) | 0.244 |
| Current smoker (n, %) | 26 (6.5) | 24 (12.7) | 2 (1) | <0.001 |
| Regular drinking(n, %) | 52 (13) | 45 (23.8) | 7 (3.3) | <0.001 |
| Hypertension (n, %) | 253 (63.3) | 110 (58.2) | 143 (67.8) | 0.047 |
| Dyslipidemia (n, %) | 155 (38.8) | 56 (29.6) | 99 (46.9) | <0.001 |
| Diabetes mellitus (n, %) | 92 (23) | 44 (23.3) | 48 (22.8) | 0.900 |
| NAFLD (n, %) | 142 (35.5) | 68 (36) | 74 (35.1) | 0.850 |
| LECT2 (ng/mL) | 31.7 (25.9, 38.2) | 27 (23.7, 32) | 36.5 (30.7, 41.5) | <0.001 |
| ASM (kg) | 15 (12.3, 17.9) | 18 (16.7, 19.7) | 12.6 (11.3, 13.9) | <0.001 |
| ASM/BMI (m2) | 0.61 (0.52, 0.75) | 0.75 (0.7, 0.82) | 0.52 (0.48, 0.58) | <0.001 |
| HGS (kg) | 25.8 (21.5, 32.6) | 33.1 (28.8, 36.9) | 21.8 (19, 24) | <0.001 |
| HGS/BMI (m2) | 1.08 (0.89, 1.35) | 1.36 (1.19, 1.54) | 0.9 (0.78, 1.01) | <0.001 |
| r | p | |
|---|---|---|
| Age | −0.080 | 0.109 |
| Sex (men) | −0.515 | <0.001 |
| BMI | 0.261 | <0.001 |
| WC (cm) | 0.156 | 0.002 |
| SBP (mmHg) | 0.129 | 0.010 |
| GGT (IU/L) | 0.068 | 0.172 |
| TC (mg/dL) | 0.203 | <0.001 |
| TG (mg/dL) | 0.314 | <0.001 |
| FPG (mg/dL) | 0.155 | 0.002 |
| HOMA-IR | 0.288 | <0.001 |
| Creatinine (mg/dL) | −0.245 | <0.001 |
| hs-CRP (mg/dL) | 0.135 | 0.007 |
| Vitamin D (ng/mL) | −0.089 | 0.075 |
| ASM (kg) | −0.384 | <0.001 |
| ASM/BMI (m2) | −0.506 | <0.001 |
| HGS (kg) | −0.379 | <0.001 |
| HGS/BMI (m2) | −0.474 | <0.001 |
| LMM | ORs (95% CIs) | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p for Trend | |
| Model 1 | 1 (ref) | 2.53 (1.12–5.71) | 3.0 (1.34–6.74) | 2.96 (1.31–6.68) | 0.008 |
| Model 2 | 1 (ref) | 2.56 (1.13–5.8) | 2.97 (1.32–6.69) | 3.07 (1.35–6.97) | 0.007 |
| Model 3 | 1 (ref) | 2.54 (1.11–5.79) | 3.04 (1.34–6.89) | 3.22 (1.39–7.50) | 0.006 |
| Model 4 | 1 (ref) | 2.59 (1.13–5.95) | 3.12 (1.37–7.11) | 3.31 (1.41–7.75) | 0.005 |
| LMS | ORs (95% CIs) | ||||
| Q1 | Q2 | Q3 | Q4 | p for trend | |
| Model 1 | 1 (ref) | 0.83 (0.36–1.92) | 2.28 (1.08–4.82) | 2.86 (1.36–6.01) | 0.001 |
| Model 2 | 1 (ref) | 0.83 (0.36–1.94) | 2.28 (1.08–4.82) | 2.94 (1.4–6.19) | 0.001 |
| Model 3 | 1 (ref) | 0.83 (0.35–1.94) | 2.2 (1.03–4.7) | 2.73 (1.27–5.88) | 0.001 |
| Model 4 | 1 (ref) | 0.87 (0.36–2.07) | 2.32 (1.07–5.05) | 2.85 (1.29–6.26) | 0.001 |
| Sarcopenia | ORs (95% CIs) | ||||
| Q1 | Q2 | Q3 | Q4 | p for trend | |
| Model 1 | 1 (ref) | 1.55 (0.42–5.73) | 1.97 (0.55–7.05) | 4.78 (1.47–15.56) | 0.010 |
| Model 2 | 1 (ref) | 1.59 (0.43–5.93) | 2.1 (0.58–7.57) | 5.38 (1.63–17.75) | 0.006 |
| Model 3 | 1 (ref) | 1.59 (0.42–6.0) | 2.05 (0.56–7.49) | 5.0 (1.47–17.02) | 0.010 |
| Model 4 | 1 (ref) | 1.85 (0.48–7.2) | 2.32 (0.62–8.69) | 5.48 (1.57–19.05) | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roh, E.; Hwang, S.Y.; Kim, M.; Won, C.W.; Choi, K.M. Plasma Leukocyte Cell-Derived Chemotaxin-2 as a Risk Factor of Sarcopenia: Korean Frailty and Aging Cohort Study. Nutrients 2025, 17, 1342. https://doi.org/10.3390/nu17081342
Roh E, Hwang SY, Kim M, Won CW, Choi KM. Plasma Leukocyte Cell-Derived Chemotaxin-2 as a Risk Factor of Sarcopenia: Korean Frailty and Aging Cohort Study. Nutrients. 2025; 17(8):1342. https://doi.org/10.3390/nu17081342
Chicago/Turabian StyleRoh, Eun, Soon Young Hwang, Miji Kim, Chang Won Won, and Kyung Mook Choi. 2025. "Plasma Leukocyte Cell-Derived Chemotaxin-2 as a Risk Factor of Sarcopenia: Korean Frailty and Aging Cohort Study" Nutrients 17, no. 8: 1342. https://doi.org/10.3390/nu17081342
APA StyleRoh, E., Hwang, S. Y., Kim, M., Won, C. W., & Choi, K. M. (2025). Plasma Leukocyte Cell-Derived Chemotaxin-2 as a Risk Factor of Sarcopenia: Korean Frailty and Aging Cohort Study. Nutrients, 17(8), 1342. https://doi.org/10.3390/nu17081342





