Curcumin: A Natural Warrior Against Inflammatory Liver Diseases
Abstract
:1. Introduction: Hepatic Inflammation
2. Curcumin
3. Biological Activity of Curcumin
4. IFALD
4.1. Ileum Loss
4.2. Gut Dysbiosis
4.3. Intravenous Administration of Phytosterols
4.4. Other PN-Associated Risk Factors
4.5. IFALD: Diagnosis and Treatment
5. MASLD
6. Curcumin’s Potential in IFALD and MASLD Therapy
7. Curcumin’s Nanoformulations
8. Biocompatibility of Curcumin’s Nanoformulations
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CUR | Curcumin |
| IFALD | Intestinal failure-associated liver disease |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| NAFLD | Non-alcoholic fatty liver disease |
| MAFLD | Metabolic dysfunction-associated fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| MASH | Metabolic steatohepatitis |
| NF-κB | Nuclear factor-kappa B |
| IL-1β | Interleukin-1 beta |
| DMC | Demethoxycurcumin |
| BMC | Bisdemethoxycurcumin |
| logP | Oil–water partition coefficient |
| t1/2 | Half-life time |
| DMSO | Dimethyl sulfoxide |
| ROS | Reactive oxygen species |
| HO-1 | Heme oxygenase 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| ALAT | Aminotransferase |
| AST | Aspartate aminotransferase |
| GGT | Gamma-glutamyl transferase |
| CRP | C-reactive protein |
| IL-6 | Interleukin 6 |
| ALP | Alkaline phosphatase |
| HRQoL | health-related quality of life |
| TC | Total cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| TG | Triglycerides |
| TNF-α | Tumor necrosis factor |
| TGF-β1 | Growth factor-beta1 |
| TβR II | TGF-β type II receptor |
| PN | Parenteral nutrition |
| IF | Intestinal failure |
| CYP7A1 | Cholesterol-7a-hydroxylase |
| FGF19 | Fibroblast growth factor 19 |
| FXR | Farnesoid X receptor |
| LPS | Lipopolysaccharides |
| ILE | Intravenous lipid emulsion |
| LRH-1 | Liver receptor homolog-1 |
| ABCC2/MRP2 | Multi-drug resistance protein 2 |
| SHP | Small heterodimer partner |
| BSEP | Bile salt export pump |
| ABCG5/8 | ATP-binding cassette transporters G5 and G8 |
| LXR | Liver x receptor |
| PUFAs | polyunsaturated fatty acids |
| PPARα | Peroxisomal proliferator-activated receptor alpha |
| CPT1 | Carnitine palmitoyltransferase 1 |
| UDCA | Ursodeoxycholic acid |
| GLP-2 | Glucagon-like peptide-2 |
| NASH | Nonalcoholic steatohepatitis |
| SOD | Superoxide dismutase |
| AMPK | Activated protein kinase |
| JNK | c-Jun N-terminal kinase |
| MCP-1 | Monocyte chemoattractant protein |
| EGF | Epidermal growth factor |
| IFN-γ | Interferon γ |
| VEGF | Vascular endothelial growth factor |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| HbA1c | Glycated hemoglobin |
| GSK-3β | Apoptosis—glycogen synthase kinase-3 β |
| IAPP | Islet amyloid polypeptide |
| SIRT1 | Deacetylase sirtuin-1 |
| PGC-1α | Activated receptor-gamma coactivator-1 alpha |
| TLR4 | Toll-like receptor 4 |
| MyD88 | Myeloid differentiation primary response 88 |
| SLN | Solid lipid nanoparticles |
| HFn | Human heavy chain apoferritin |
| TPP | Triphenylphosphine |
| CXCR4 | Chemokine receptor type 4 |
| BUN | Blood urea nitrogen |
| PLGA | Polylactic-co-glycolic acid |
| SREBP-1c | Sterol regulatory element-binding protein 1c |
| BMI | Body mass index |
| ACC1 | Acetylo-CoA carboxylase |
| STAT3 | Signal transducer and activator of transcription 3 |
| HIF | Hypoxia inducible factor |
| Smad7 | Mother against decapentaplegic 7 |
| Smad3 | Mother against decapentaplegic 3 |
| CCN2 | Connective tissue growth factor |
| FGF | Fibroblast growth factor |
| HGF | Hepatocyte growth factor |
| NGF | Nerve growth factor |
| PDGF | Platelet-derived growth factor |
| PhK | Phosphorylase kinase |
| PKCε | Protein kinase C epsilon |
| Pp60c-tk | pp60c-src tyrosine kinase |
| PAK | Protamine kinase |
| CAMK | Ca2+/calmodulin-dependent protein kinase |
| MaIP 1α | Macrophage inflammatory protein 1α |
| MMIF | Macrophage migration inhibitory factor |
| MCP | Monocyte chemoattractant protein |
| AR | Androgen receptor |
| AHR | Aryl hydrocarbon receptor |
| CXCR4 | Chemokine receptor 4 |
| H2R | Histamine H2 receptor |
| HER-2 | Human epidermal growth factor receptor-2 |
| IR | Integrin receptor |
| DR5 | Death receptor-5 |
| EPCR | Endothelial protein C receptor |
| FR | Fas receptor |
| PPAR-γ | Peroxisome proliferator-activated receptor γ |
| COX-2 | Cyclooxygenase-2 |
| LOX | Lipoxygenase |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| ODC | Ornithine decarboxylase |
| HAT | Histone acetyltransferase |
| FPT | Farnesyl protein transferase |
| AATF-1 | Arylamine N-acetyltransferase-1 |
| iNOS | Inducible nitric oxide synthase |
| NQO-1 | NAD(P)H:quinone oxidoreductase |
| PhpD | Phospholipase D |
| SRC-2 | Src homology 2 domain-containing tyrosine phosphatase 2 |
| GST | Glutathione-S-transferase |
References
- Tacke, F.; Zimmermann, H.W. Macrophage Heterogeneity in Liver Injury and Fibrosis. J. Hepatol. 2014, 60, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Tacke, F. Hepatic Macrophages in Homeostasis and Liver Diseases: From Pathogenesis to Novel Therapeutic Strategies. Cell. Mol. Immunol. 2016, 13, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Schwabe, R.F. Hepatic Inflammation and Fibrosis: Functional Links and Key Pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Evolving Challenges in Hepatic Fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 425–436. [Google Scholar] [CrossRef]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-Associated Immune Dysfunction: Distinctive Features and Clinical Relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From Kitchen to Clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Đoković, J.B.; Savić, S.M.; Mitrović, J.R.; Nikolic, I.; Marković, B.D.; Randjelović, D.V.; Antic-Stankovic, J.; Božić, D.; Cekić, N.D.; Stevanović, V.; et al. Curcumin Loaded PEGylated Nanoemulsions Designed for Maintained Antioxidant Effects and Improved Bioavailability: A Pilot Study on Rats. Int. J. Mol. Sci. 2021, 22, 7991. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of Curcumin in Buffer Solutions and Characterization of Its Degradation Products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Griesser, M.; Pistis, V.; Suzuki, T.; Tejera, N.; Pratt, D.A.; Schneider, C. Autoxidative and Cyclooxygenase-2 Catalyzed Transformation of the Dietary Chemopreventive Agent Curcumin. J. Biol. Chem. 2011, 286, 1114–1124. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Curcumin as a Functional Food-Derived Factor: Degradation Products, Metabolites, Bioactivity, and Future Perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Rahman, M.A.; Mittal, V.; Wahab, S.; Alsayari, A.; Bin Muhsinah, A.; Almaghaslah, D. Intravenous Nanocarrier for Improved Efficacy of Quercetin and Curcumin against Breast Cancer Cells: Development and Comparison of Single and Dual Drug–Loaded Formulations Using Hemolysis, Cytotoxicity and Cellular Uptake Studies. Membranes 2022, 12, 713. [Google Scholar] [CrossRef]
- Yuan, R.; Li, Y.; Han, S.; Chen, X.; Chen, J.; He, J.; Gao, H.; Yang, Y.; Yang, S.; Yang, Y. Fe-Curcumin Nanozyme-Mediated Reactive Oxygen Species Scavenging and Anti-Inflammation for Acute Lung Injury. ACS Cent. Sci. 2022, 8, 10–21. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose Escalation of a Curcuminoid Formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Liao, S.-C.; Hsu, W.-H.; Huang, Z.-Y.; Chuang, K.-L.; Lin, K.-T.; Tseng, C.-L.; Tsai, T.-H.; Dao, A.-H.; Su, C.-L.; Huang, C.-Y.F. Bioactivity Evaluation of a Novel Formulated Curcumin. Nutrients 2019, 11, 2982. [Google Scholar] [CrossRef]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a Golden Spice with a Low Bioavailability. J. Herb. Med. 2015, 5, 55–70. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Paxton, J.W.; Wu, Z. Co-Delivery Using pH-Sensitive Liposomes to Pancreatic Cancer Cells: The Effects of Curcumin on Cellular Concentration and Pharmacokinetics of Gemcitabine. Pharm. Res. 2021, 38, 1209–1219. [Google Scholar] [CrossRef]
- Gong, F.; Ma, J.-C.; Jia, J.; Li, F.-Z.; Wu, J.-L.; Wang, S.; Teng, X.; Cui, Z.-K. Synergistic Effect of the Anti-PD-1 Antibody with Blood Stable and Reduction Sensitive Curcumin Micelles on Colon Cancer. Drug Deliv. 2021, 28, 930–942. [Google Scholar] [CrossRef]
- Karabasz, A.; Lachowicz, D.; Karewicz, A.; Mezyk-Kopec, R.; Stalińska, K.; Werner, E.; Cierniak, A.; Dyduch, G.; Bereta, J.; Bzowska, M. Analysis of Toxicity and Anticancer Activity of Micelles of Sodium Alginate-Curcumin. Int. J. Nanomed. 2019, 14, 7249–7262. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, R.; Arshad, M.; Khushtar, M.; Ahmad, M.A.; Muazzam, M.; Akhter, M.S.; Gupta, G.; Muzahid, M. A Comprehensive Review on Physiological Effects of Curcumin. Drug Res. 2020, 70, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Mirzaei, H.; Shakeri, A.; Rashidi, B.; Jalili, A.; Banikazemi, Z.; Sahebkar, A. Phytosomal Curcumin: A Review of Pharmacokinetic, Experimental and Clinical Studies. Biomed. Pharmacother. 2017, 85, 102–112. [Google Scholar] [CrossRef]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Curcumin in Metabolic Health and Disease. Nutrients 2021, 13, 4440. [Google Scholar] [CrossRef]
- Hernández-Aquino, E.; Quezada-Ramírez, M.A.; Silva-Olivares, A.; Ramos-Tovar, E.; Flores-Beltrán, R.E.; Segovia, J.; Shibayama, M.; Muriel, P. Curcumin Downregulates Smad Pathways and Reduces Hepatic Stellate Cells Activation in Experimental Fibrosis. Ann. Hepatol. 2020, 19, 497–506. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Funamoto, M.; Shimizu, K.; Shimizu, S.; Sari, N.; Katanasaka, Y.; Miyazaki, Y.; Kakeya, H.; Hasegawa, K.; Morimoto, T. Curcumin, an Inhibitor of P300-HAT Activity, Suppresses the Development of Hypertension-Induced Left Ventricular Hypertrophy with Preserved Ejection Fraction in Dahl Rats. Nutrients 2021, 13, 2608. [Google Scholar] [CrossRef]
- Abdelhamid, F.M.; Mahgoub, H.A.; Ateya, A.I. Ameliorative Effect of Curcumin against Lead Acetate-Induced Hemato-Biochemical Alterations, Hepatotoxicity, and Testicular Oxidative Damage in Rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 10950–10965. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Cheng, D.; Guo, Y.; Wu, R.; Yin, R.; Li, S.; Kuo, H.-C.; Hudlikar, R.; Yang, H.; et al. Pharmacokinetics and Pharmacodynamics of Three Oral Formulations of Curcumin in Rats. J. Pharmacokinet. Pharmacodyn. 2020, 47, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Costa, A.P.; Xu, X.; Lee, S.-L.; Cruz, C.N.; Bao, Q.; Burgess, D.J. Formulation and Characterization of Curcumin Loaded Polymeric Micelles Produced via Continuous Processing. Int. J. Pharm. 2020, 583, 119340. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Tamaddoni, A.; Qujeq, D.; Nasseri, E.; Zayeri, F.; Zand, H.; Gholami, M.; Mir, S.M. An Investigation of the Effects of Curcumin on Iron Overload, Hepcidin Level, and Liver Function in β-Thalassemia Major Patients: A Double-Blind Randomized Controlled Clinical Trial. Phytother. Res. 2018, 32, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Kheiripour, N.; Fathi Jouzdani, A.; Ghasemi, H.; Soleimani Asl, S.; Ghafouri-Khosrowshahi, A.; Ranjbar, A. Nanocurcumin Improves Lipid Status, Oxidative Stress, and Function of the Liver in Aluminium Phosphide-Induced Toxicity: Cellular and Molecular Mechanisms. BioMed Res. Int. 2022, 2022, 7659765. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Y.; Xia, Y.; Wu, Q.; Yan, H.; Tong, H.; Chu, M.; Wen, Z. Polybrominated Biphenyls Induce Liver Injury by Disrupting the KEAP1/Nrf2/SLC7A11 Axis Leading to Impaired GSH Synthesis and Ferroptosis in Hepatocytes. Arch. Toxicol. 2025, 99, 1545–1559. [Google Scholar] [CrossRef]
- Ayyat, M.S.; Ayyat, A.M.N.; Abd El-Latif, K.M.; Hessein, A.A.A.; Al-Sagheer, A.A. Inorganic Mercury and Dietary Safe Feed Additives Enriched Diet Impacts on Growth, Immunity, Tissue Bioaccumulation, and Disease Resistance in Nile Tilapia (Oreochromis niloticus). Aquat. Toxicol. 2020, 224, 105494. [Google Scholar] [CrossRef]
- Gbolahan, O.B.; O’Neil, B.H.; McRee, A.J.; Sanoff, H.K.; Fallon, J.K.; Smith, P.C.; Ivanova, A.; Moore, D.T.; Dumond, J.; Asher, G.N. A Phase I Evaluation of the Effect of Curcumin on Dose-limiting Toxicity and Pharmacokinetics of Irinotecan in Participants with Solid Tumors. Clin. Transl. Sci. 2022, 15, 1304–1315. [Google Scholar] [CrossRef]
- Tauil, R.B.; Golono, P.T.; de Lima, E.P.; de Alvares Goulart, R.; Guiguer, E.L.; Bechara, M.D.; Nicolau, C.C.T.; Yanaguizawa Junior, J.L.; Fiorini, A.M.R.; Méndez-Sánchez, N.; et al. Metabolic-Associated Fatty Liver Disease: The Influence of Oxidative Stress, Inflammation, Mitochondrial Dysfunctions, and the Role of Polyphenols. Pharmaceuticals 2024, 17, 1354. [Google Scholar] [CrossRef]
- Zhai, F.; Wang, J.; Wan, X.; Liu, Y.; Mao, X. Dual Anti-Inflammatory Effects of Curcumin and Berberine on Acetaminophen-Induced Liver Injury in Mice by Inhibiting NF-κB Activation via PI3K/AKT and PPARγ Signaling Pathways. Biochem. Biophys. Res. Commun. 2024, 734, 150772. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Farrell, G.C.; Sempoux, C.; dela Peña, A.; Horsmans, Y. Curcumin Inhibits NF-κB Activation and Reduces the Severity of Experimental Steatohepatitis in Mice. J. Hepatol. 2004, 41, 926–934. [Google Scholar] [CrossRef]
- Musso, G.; Pinach, S.; Mariano, F.; Saba, F.; De Michieli, F.; Framarin, L.; Berrutti, M.; Paschetta, E.; Parente, R.; Lizet Castillo, Y.; et al. Effect of Phospholipid Curcumin Meriva on Liver Histology and Kidney Disease in Nonalcoholic Steatohepatitis: A Randomized, Double-Blind, Placebo-Controlled Trial. Hepatology 2025, 81, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Ranneh, Y.; Bedir, A.S.; Abu-Elsaoud, A.M.; Al Raish, S. Polyphenol Intervention Ameliorates Non-Alcoholic Fatty Liver Disease: An Updated Comprehensive Systematic Review. Nutrients 2024, 16, 4150. [Google Scholar] [CrossRef] [PubMed]
- Adhvaryu, M.R.; Reddy, N.; Vakharia, B.C. Prevention of Hepatotoxicity Due to Anti Tuberculosis Treatment: A Novel Integrative Approach. World J. Gastroenterol. 2008, 14, 4753–4762. [Google Scholar] [CrossRef] [PubMed]
- Petagine, L.; Zariwala, M.G.; Somavarapu, S.; Chan, S.H.Y.; Kaya, E.A.; Patel, V.B. Oxidative Stress in a Cellular Model of Alcohol-Related Liver Disease: Protection Using Curcumin Nanoformulations. Sci. Rep. 2025, 15, 7752. [Google Scholar] [CrossRef]
- Teng, C.-F.; Yu, C.-H.; Chang, H.-Y.; Hsieh, W.-C.; Wu, T.-H.; Lin, J.-H.; Wu, H.-C.; Jeng, L.-B.; Su, I.-J. Chemopreventive Effect of Phytosomal Curcumin on Hepatitis B Virus-Related Hepatocellular Carcinoma in A Transgenic Mouse Model. Sci. Rep. 2019, 9, 10338. [Google Scholar] [CrossRef]
- Nouri-Vaskeh, M.; Malek Mahdavi, A.; Afshan, H.; Alizadeh, L.; Zarei, M. Effect of Curcumin Supplementation on Disease Severity in Patients with Liver Cirrhosis: A Randomized Controlled Trial. Phytother. Res. 2020, 34, 1446–1454. [Google Scholar] [CrossRef]
- Nouri-Vaskeh, M.; Afshan, H.; Malek Mahdavi, A.; Alizadeh, L.; Fan, X.; Zarei, M. Curcumin Ameliorates Health-Related Quality of Life in Patients with Liver Cirrhosis: A Randomized, Double-Blind Placebo-Controlled Trial. Complement. Ther. Med. 2020, 49, 102351. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Bimonte, S.; Barbieri, A.; Leongito, M.; Piccirillo, M.; Giudice, A.; Pivonello, C.; de Angelis, C.; Granata, V.; Palaia, R.; Izzo, F. Curcumin AntiCancer Studies in Pancreatic Cancer. Nutrients 2016, 8, 433. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, C.; Xi, H.; Gao, Y.; Xu, D. Curcumin Induces Apoptosis in Pancreatic Cancer Cells through the Induction of Forkhead Box O1 and Inhibition of the PI3K/Akt Pathway. Mol. Med. Rep. 2015, 12, 5415–5422. [Google Scholar] [CrossRef]
- Kim, S.G.; Veena, M.S.; Basak, S.K.; Han, E.; Tajima, T.; Gjertson, D.W.; Starr, J.; Eidelman, O.; Pollard, H.B.; Srivastava, M.; et al. Curcumin Treatment Suppresses IKKβ Kinase Activity of Salivary Cells of Patients with Head and Neck Cancer: A Pilot Study. Clin. Cancer Res. 2011, 17, 5953–5961. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ding, H.; Liang, M.; Chen, X.; Yan, Y.; Wan, N.; Chen, Q.; Zhang, J.; Cao, J. Curcumin Induces Ferroptosis in Non-Small-Cell Lung Cancer via Activating Autophagy. Thorac. Cancer 2021, 12, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Wan Mohd Tajuddin, W.N.B.; Lajis, N.H.; Abas, F.; Othman, I.; Naidu, R. Mechanistic Understanding of Curcumin’s Therapeutic Effects in Lung Cancer. Nutrients 2019, 11, 2989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, X.; Xu, S.; Bao, J.; Yu, H. Curcumin Induces Endoplasmic Reticulum Stress-Associated Apoptosis in Human Papillary Thyroid Carcinoma BCPAP Cells via Disruption of Intracellular Calcium Homeostasis. Medicine 2018, 97, e11095. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; He, K.; Cao, T.; Song, D.; Yang, H.; Li, L.; Lin, J. The Apoptosis of Liver Cancer Cells Promoted by Curcumin/TPP-CZL Nanomicelles With Mitochondrial Targeting Function. Front. Bioeng. Biotechnol. 2022, 10, 804513. [Google Scholar] [CrossRef]
- Liang, W.-F.; Gong, Y.-X.; Li, H.-F.; Sun, F.-L.; Li, W.-L.; Chen, D.-Q.; Xie, D.-P.; Ren, C.-X.; Guo, X.-Y.; Wang, Z.-Y.; et al. Curcumin Activates ROS Signaling to Promote Pyroptosis in Hepatocellular Carcinoma HepG2 Cells. Vivo 2021, 35, 249–257. [Google Scholar] [CrossRef]
- Zhao, Z.; Malhotra, A.; Seng, W.Y. Curcumin Modulates Hepatocellular Carcinoma by Reducing UNC119 Expression. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 195–203. [Google Scholar] [CrossRef]
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A Phase 1 Dose-Escalation Study on the Safety, Tolerability and Activity of Liposomal Curcumin (LipocurcTM) in Patients with Locally Advanced or Metastatic Cancer. Cancer Chemother. Pharmacol. 2018, 82, 695–706. [Google Scholar] [CrossRef]
- Wan, X.; Wang, D. Curcumin: Epigenetic Modulation and Tumor Immunity in Antitumor Therapy. Planta Med. 2025, 4, 146–221. [Google Scholar] [CrossRef]
- Jiang, Y.; Hui, D.; Pan, Z.; Yu, Y.; Liu, L.; Yu, X.; Wu, C.; Sun, M. Curcumin Promotes Ferroptosis in Hepatocellular Carcinoma via Upregulation of ACSL4. J. Cancer Res. Clin. Oncol. 2024, 150, 429. [Google Scholar] [CrossRef]
- Sadegh Malvajerd, S.; Azadi, A.; Izadi, Z.; Kurd, M.; Dara, T.; Dibaei, M.; Sharif Zadeh, M.; Akbari Javar, H.; Hamidi, M. Brain Delivery of Curcumin Using Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Preparation, Optimization, and Pharmacokinetic Evaluation. ACS Chem. Neurosci. 2019, 10, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, P.H.; Bagger, J.I.; Carlander, K.R.; Forman, J.; Chabanova, E.; Svenningsen, J.S.; Holst, J.J.; Gillum, M.P.; Vilsbøll, T.; Knop, F.K. The Effect of Curcumin on Hepatic Fat Content in Individuals with Obesity. Diabetes Obes. Metab. 2022, 24, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Bagherniya, M.; Khoram, Z.; Ebrahimi Varzaneh, A.; Atkin, S.L.; Jamialahmadi, T.; Sahebkar, A.; Askari, G. Efficacy of Curcumin plus Piperine Co-Supplementation in Moderate-to-High Hepatic Steatosis: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. Phytother. Res. 2023, 37, 2217–2229. [Google Scholar] [CrossRef]
- Zanzer, Y.C.; Batista, Â.G.; Dougkas, A.; Tovar, J.; Granfeldt, Y.; Östman, E. Difficulties in Translating Appetite Sensations Effect of Turmeric-Based Beverage When Given Prior to Isoenergetic Medium- or High-Fat Meals in Healthy Subjects. Nutrients 2019, 11, 736. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Farimani, A.R.; Dehhabe, M.; Bidkhori, M.; Hariri, M.; Ghouchani, B.F.; Abdollahi, F. Effect of Phytosomal Curcumin on Circulating Levels of Adiponectin and Leptin in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Gastrointestin. Liver Dis. 2019, 28, 183–189. [Google Scholar] [CrossRef]
- Ferro, Y.; Pujia, R.; Mazza, E.; Lascala, L.; Lodari, O.; Maurotti, S.; Pujia, A.; Montalcini, T. A New Nutraceutical (Livogen Plus®) Improves Liver Steatosis in Adults with Non-Alcoholic Fatty Liver Disease. J. Transl. Med. 2022, 20, 377. [Google Scholar] [CrossRef]
- Afshar Ghahremani, S.; Raisi, A.; Minaei Beirami, S.; Kahroba, H.; Mardani, M.; Dezfoulian, O.; Tarhriz, V. Curcumin Alleviates Inflammatory Effects of Ketamine Anesthesia in Postnatal Rats. Vet. Res. Forum 2024, 15, 473–480. [Google Scholar] [CrossRef]
- Abo-Salem, O.M.; Harisa, G.I.; Ali, T.M.; El-Sayed, E.-S.M.; Abou-Elnour, F.M. Curcumin Ameliorates Streptozotocin-Induced Heart Injury in Rats. J. Biochem. Mol. Toxicol. 2014, 28, 263–270. [Google Scholar] [CrossRef]
- Guo, S.; Meng, X.-W.; Yang, X.-S.; Liu, X.-F.; Ou-Yang, C.-H.; Liu, C. Curcumin Administration Suppresses Collagen Synthesis in the Hearts of Rats with Experimental Diabetes. Acta Pharmacol. Sin. 2018, 39, 195–204. [Google Scholar] [CrossRef]
- Ma, J.; Chen, W.; Vaishnani, D.K.; Wang, C.; Xue, S.; Yang, Q.; Tong, Y.; Lei, N.; Zhao, Z.; Ying, F. Curcumin Analog J7 Attenuates Liver Fibrosis and Metabolic Dysregulation in a Rat Model of Type 2 Diabetes via Modulation of TGF-β/Smad and NF-κB/BCL-2/BAX Pathways. Drug Des. Dev. Ther. 2025, 19, 2411–2432. [Google Scholar] [CrossRef]
- Fleming, C.R.; Remington, M.; Hill, G. Nutrition and the Surgical Patient; Churchill Livingstone: New York, NY, USA, 1981; pp. 219–235. [Google Scholar]
- Grainger, J.T.; Maeda, Y.; Donnelly, S.C.; Vaizey, C.J. Assessment and Management of Patients with Intestinal Failure: A Multidisciplinary Approach. Clin. Exp. Gastroenterol. 2018, 11, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zafirovska, M.; Zafirovski, A.; Rotovnik Kozjek, N. Current Insights Regarding Intestinal Failure-Associated Liver Disease (IFALD): A Narrative Review. Nutrients 2023, 15, 3169. [Google Scholar] [CrossRef] [PubMed]
- Inayet, N.; Neild, P. Parenteral Nutrition. J. R. Coll. Physicians Edinb. 2015, 45, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Chew, K.S.; Ng, R.T.; Kasmi, K.E.; Sokol, R.J. Intestinal Failure-Associated Liver Disease (IFALD): Insights into Pathogenesis and Advances in Management. Hepatol. Int. 2020, 14, 305–316. [Google Scholar] [CrossRef]
- Pereira-Fantini, P.M.; Lapthorne, S.; Joyce, S.A.; Dellios, N.L.; Wilson, G.; Fouhy, F.; Thomas, S.L.; Scurr, M.; Hill, C.; Gahan, C.G.M.; et al. Altered FXR Signalling Is Associated with Bile Acid Dysmetabolism in Short Bowel Syndrome-Associated Liver Disease. J. Hepatol. 2014, 61, 1115–1125. [Google Scholar] [CrossRef]
- Mutanen, A.; Nissinen, M.J.; Lohi, J.; Heikkilä, P.; Gylling, H.; Pakarinen, M.P. Serum Plant Sterols, Cholestanol, and Cholesterol Precursors Associate with Histological Liver Injury in Pediatric Onset Intestinal Failure1234. Am. J. Clin. Nutr. 2014, 100, 1085–1094. [Google Scholar] [CrossRef]
- Manithody, C.S.; Van Nispen, J.; Murali, V.; Jain, S.; Samaddar, A.; Armstrong, A.; Jain, A. Role of Bile Acids and Gut Microbiota in Parenteral Nutrition Associated Injury. J. Hum. Nutr. 2020, 4, 286. [Google Scholar] [CrossRef]
- Mutanen, A.; Lohi, J.; Heikkilä, P.; Jalanko, H.; Pakarinen, M.P. Loss of Ileum Decreases Serum Fibroblast Growth Factor 19 in Relation to Liver Inflammation and Fibrosis in Pediatric Onset Intestinal Failure. J. Hepatol. 2015, 62, 1391–1397. [Google Scholar] [CrossRef]
- Xiao, Y.-T.; Cao, Y.; Zhou, K.-J.; Lu, L.-N.; Cai, W. Altered Systemic Bile Acid Homeostasis Contributes to Liver Disease in Pediatric Patients with Intestinal Failure. Sci. Rep. 2016, 6, 39264. [Google Scholar] [CrossRef]
- Khalaf, R.T.; Sokol, R.J. New Insights into Intestinal Failure–Associated Liver Disease in Children. Hepatology 2020, 71, 1486–1498. [Google Scholar] [CrossRef]
- Ciobârcă, D.; Cătoi, A.F.; Gavrilaș, L.; Banc, R.; Miere, D.; Filip, L. Natural Bioactive Compounds in the Management of Type 2 Diabetes and Metabolic (Dysfunction)-Associated Steatotic Liver Disease. Pharmaceuticals 2025, 18, 279. [Google Scholar] [CrossRef] [PubMed]
- McGrath, K.H.; Pitt, J.; Bines, J.E. Small Intestinal Bacterial Overgrowth in Children with Intestinal Failure on Home Parenteral Nutrition. JGH Open 2019, 3, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Mutanen, A.; Salonen, A.; Savilahti, E.; de Vos, W.M.; Pakarinen, M.P. Intestinal Microbiota Signatures Associated with Histological Liver Steatosis in Pediatric-Onset Intestinal Failure. J. Parenter. Enteral. Nutr. 2017, 41, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Rochling, F.A. Intravenous Lipid Emulsions in the Prevention and Treatment of Liver Disease in Intestinal Failure. Nutrients 2021, 13, 895. [Google Scholar] [CrossRef]
- Carter, B.A.; Taylor, O.A.; Prendergast, D.R.; Zimmerman, T.L.; Von Furstenberg, R.; Moore, D.D.; Karpen, S.J. Stigmasterol, a Soy Lipid-Derived Phytosterol, Is an Antagonist of the Bile Acid Nuclear Receptor FXR. Pediatr. Res. 2007, 62, 301–306. [Google Scholar] [CrossRef]
- Chen, X.; Memory Kunda, L.S.; Li, X.; Wang, N.; Huang, Y.; Hao, Y.; He, Q.; Liao, W.; Chen, J. A Comprehensive Review of Beneficial Effects of Phytosterols on Glycolipid Metabolism and Related Mechanisms. J. Agric. Food Chem. 2025, 73, 3826–3841. [Google Scholar] [CrossRef]
- El Kasmi, K.C.; Anderson, A.L.; Devereaux, M.W.; Vue, P.M.; Zhang, W.; Setchell, K.D.R.; Karpen, S.J.; Sokol, R.J. Phytosterols Promote Liver Injury and Kupffer Cell Activation in Parenteral Nutrition-Associated Liver Disease. Sci. Transl. Med. 2013, 5, 206ra137. [Google Scholar] [CrossRef]
- Ghosh, S.; Devereaux, M.W.; Anderson, A.L.; Gehrke, S.; Reisz, J.A.; D’Alessandro, A.; Orlicky, D.J.; Lovell, M.; El Kasmi, K.C.; Shearn, C.T.; et al. NF-κB Regulation of LRH-1 and ABCG5/8 Potentiates Phytosterol Role in the Pathogenesis of Parenteral Nutrition-Associated Cholestasis. Hepatology 2021, 74, 3284–3300. [Google Scholar] [CrossRef]
- Sanchez, S.E.; Braun, L.P.; Mercer, L.D.; Sherrill, M.; Stevens, J.; Javid, P.J. The Effect of Lipid Restriction on the Prevention of Parenteral Nutrition-Associated Cholestasis in Surgical Infants. J. Pediatr. Surg. 2013, 48, 573–578. [Google Scholar] [CrossRef]
- Nandivada, P.; Cowan, E.; Carlson, S.J.; Chang, M.; Gura, K.M.; Puder, M. Mechanisms for the Effects of Fish Oil Lipid Emulsions in the Management of Parenteral Nutrition-Associated Liver Disease. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 153–158. [Google Scholar] [CrossRef]
- El Kasmi, K.C.; Vue, P.M.; Anderson, A.L.; Devereaux, M.W.; Ghosh, S.; Balasubramaniyan, N.; Fillon, S.A.; Dahrenmoeller, C.; Allawzi, A.; Woods, C.; et al. Macrophage-Derived IL-1β/NF-κB Signaling Mediates Parenteral Nutrition-Associated Cholestasis. Nat. Commun. 2018, 9, 1393. [Google Scholar] [CrossRef] [PubMed]
- Gabe, S.M.; Culkin, A. Abnormal Liver Function Tests in the Parenteral Nutrition Fed Patient. Frontline Gastroenterol. 2010, 1, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Fousekis, F.S.; Mitselos, I.V.; Christodoulou, D.K. New Insights into Intestinal Failure-Associated Liver Disease in Adults: A Comprehensive Review of the Literature. Saudi J. Gastroenterol. 2021, 27, 3–12. [Google Scholar] [CrossRef]
- Trotta, E.; Bortolotti, S.; Fugazzotto, G.; Gellera, C.; Montagnese, S.; Amodio, P. Familial Vitamin E Deficiency: Multiorgan Complications Support the Adverse Role of Oxidative Stress. Nutrition 2019, 63–64, 57–60. [Google Scholar] [CrossRef]
- Ng, K.; Stoll, B.; Chacko, S.; Saenz de Pipaon, M.; Lauridsen, C.; Gray, M.; Squires, E.J.; Marini, J.; Zamora, I.J.; Olutoye, O.O.; et al. Vitamin E in New-Generation Lipid Emulsions Protects Against Parenteral Nutrition-Associated Liver Disease in Parenteral Nutrition-Fed Preterm Pigs. J. Parenter. Enteral. Nutr. 2016, 40, 656–671. [Google Scholar] [CrossRef]
- Buchman, A.L.; Ament, M.E.; Sohel, M.; Dubin, M.; Jenden, D.J.; Roch, M.; Pownall, H.; Farley, W.; Awal, M.; Ahn, C. Choline Deficiency Causes Reversible Hepatic Abnormalities in Patients Receiving Parenteral Nutrition: Proof of a Human Choline Requirement: A Placebo-Controlled Trial. J. Parenter. Enter. Nutr. 2001, 25, 260–268. [Google Scholar] [CrossRef]
- Sentongo, T.A.; Kumar, P.; Karza, K.; Keys, L.; Iyer, K.; Buchman, A.L. Whole-Blood-Free Choline and Choline Metabolites in Infants Who Require Chronic Parenteral Nutrition Therapy. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 194–199. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, T.; Chen, F.; Yan, J.; Chen, F.; Zhang, Q.; Wang, J.; Yan, W.; Yu, T.; Tang, Q.; et al. Choline Protects Against Intestinal Failure-Associated Liver Disease in Parenteral Nutrition-Fed Immature Rats. J. Parenter. Enteral. Nutr. 2018, 42, 436–445. [Google Scholar] [CrossRef]
- Gura, K.M.; Mulberg, A.E.; Mitchell, P.D.; Yap, J.; Kim, C.Y.; Chen, M.; Potemkin, A.; Puder, M. Pediatric Intestinal Failure–Associated Liver Disease: Challenges in Identifying Clinically Relevant Biomarkers. J. Parenter. Enter. Nutr. 2018, 42, 455–462. [Google Scholar] [CrossRef]
- Lacaille, F.; Gupte, G.; Colomb, V.; D’Antiga, L.; Hartman, C.; Hojsak, I.; Kolacek, S.; Puntis, J.; Shamir, R.; ESPGHAN Working Group of Intestinal Failure and Intestinal Transplantation. Intestinal Failure-Associated Liver Disease: A Position Paper of the ESPGHAN Working Group of Intestinal Failure and Intestinal Transplantation. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Mutanen, A.; Lohi, J.; Merras-Salmio, L.; Koivusalo, A.; Pakarinen, M.P. Prediction, Identification and Progression of Histopathological Liver Disease Activity in Children with Intestinal Failure. J. Hepatol. 2021, 74, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Beath, S.; Pironi, L.; Gabe, S.; Horslen, S.; Sudan, D.; Mazeriegos, G.; Steiger, E.; Goulet, O.; Fryer, J. Collaborative Strategies to Reduce Mortality and Morbidity in Patients with Chronic Intestinal Failure Including Those Who Are Referred for Small Bowel Transplantation. Transplantation 2008, 85, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Woodward, J.M.; Priest, A.N.; Hollingsworth, K.G.; Lomas, D.J. Clinical Application of Magnetic Resonance Spectroscopy of the Liver in Patients Receiving Long-Term Parenteral Nutrition. J. Parenter. Enter. Nutr. 2009, 33, 669–676. [Google Scholar] [CrossRef]
- Huijbers, A.; Wanten, G.; Dekker, H.M.; van der Graaf, M. Noninvasive Quantitative Assessment of Hepatic Steatosis by Proton Magnetic Resonance Spectroscopy Among Adult Patients Receiving Home Parenteral Nutrition. J. Parenter. Enter. Nutr. 2018, 42, 778–785. [Google Scholar] [CrossRef]
- Fligor, S.C.; Tsikis, S.T.; Hirsch, T.I.; Jain, A.; Sun, L.; Rockowitz, S.; Gura, K.M.; Puder, M. Inflammation Drives Pathogenesis of Early Intestinal Failure-Associated Liver Disease. Sci. Rep. 2024, 14, 4240. [Google Scholar] [CrossRef]
- Secor, J.D.; Yu, L.; Tsikis, S.; Fligor, S.; Puder, M.; Gura, K.M. Current Strategies for Managing Intestinal Failure-Associated Liver Disease. Expert Opin. Drug Saf. 2021, 20, 307–320. [Google Scholar] [CrossRef]
- Beau, P.; Labat-Labourdette, J.; Ingrand, P.; Beauchant, M. Is Ursodeoxycholic Acid an Effective Therapy for Total Parenteral Nutrition-Related Liver Disease? J. Hepatol. 1994, 20, 240–244. [Google Scholar] [CrossRef]
- De Marco, G.; Sordino, D.; Bruzzese, E.; Di Caro, S.; Mambretti, D.; Tramontano, A.; Colombo, C.; Simoni, P.; Guarino, A. Early Treatment with Ursodeoxycholic Acid for Cholestasis in Children on Parenteral Nutrition Because of Primary Intestinal Failure. Aliment. Pharmacol. Ther. 2006, 24, 387–394. [Google Scholar] [CrossRef]
- Yano, K.; Kaji, T.; Onishi, S.; Machigashira, S.; Nagai, T.; Harumatsu, T.; Yamada, K.; Yamada, W.; Muto, M.; Nakame, K.; et al. Novel Effect of Glucagon-like Peptide-2 for Hepatocellular Injury in a Parenterally Fed Rat Model of Short Bowel Syndrome. Pediatr. Surg. Int. 2019, 35, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Naimi, R.M.; Hvistendahl, M.; Nerup, N.; Ambrus, R.; Achiam, M.P.; Svendsen, L.B.; Grønbæk, H.; Møller, H.J.; Vilstrup, H.; Steensberg, A.; et al. Effects of Glepaglutide, a Novel Long-Acting Glucagon-like Peptide-2 Analogue, on Markers of Liver Status in Patients with Short Bowel Syndrome: Findings from a Randomised Phase 2 Trial. EBioMedicine 2019, 46, 444–451. [Google Scholar] [CrossRef]
- Fligor, S.C.; Tsikis, S.T.; Hirsch, T.I.; Pan, A.; Moskowitzova, K.; Rincon-Cruz, L.; Whitlock, A.E.; Mitchell, P.D.; Nedder, A.P.; Gura, K.M.; et al. A Medium-Chain Fatty Acid Analogue Prevents Intestinal Failure–Associated Liver Disease in Preterm Yorkshire Piglets. Gastroenterology 2023, 165, 733–745.e9. [Google Scholar] [CrossRef]
- Hawksworth, J.S.; Desai, C.S.; Khan, K.M.; Kaufman, S.S.; Yazigi, N.; Girlanda, R.; Kroemer, A.; Fishbein, T.M.; Matsumoto, C.S. Visceral Transplantation in Patients with Intestine-Failure Associated Liver Disease: Evolving Indications, Graft Selection, And Outcomes. Am. J. Transplant. 2018, 18, 1312–1320. [Google Scholar] [CrossRef]
- Kaufman, S.S.; Avitzur, Y.; Beath, S.V.; Ceulemans, L.J.; Gondolesi, G.E.; Mazariegos, G.V.; Pironi, L. New Insights Into the Indications for Intestinal Transplantation: Consensus in the Year 2019. Transplantation 2020, 104, 937–946. [Google Scholar] [CrossRef]
- Horslen, S.P.; Ahn, Y.S.; Wood, N.L.; Schnellinger, E.M.; Gauntt, K.; McDermott, M. OPTN/SRTR 2022 Annual Data Report: Intestine. Am. J. Transplant. 2024, 24, S266–S304. [Google Scholar] [CrossRef]
- Hariri, M.; Gholami, A.; Mirhafez, S.R.; Bidkhori, M.; Sahebkar, A. A Pilot Study of the Effect of Curcumin on Epigenetic Changes and DNA Damage among Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Complement. Ther. Med. 2020, 51, 102447. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. Ann. Hepatol. 2024, 29, 101133. [Google Scholar] [CrossRef]
- Sabini, J.H.; Timotius, K.H. Hepatoprotective and Fat-Accumulation-Reductive Effects of Curcumin on Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Curr. Issues Mol. Biol. 2025, 47, 159. [Google Scholar] [CrossRef]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and Inflammation in Non-Alcoholic Fatty Liver Disease: A Randomized, Placebo Controlled Clinical Trial. BMC Gastroenterol. 2019, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- Naseri, K.; Saadati, S.; Yari, Z.; Askari, B.; Mafi, D.; Hoseinian, P.; Asbaghi, O.; Hekmatdoost, A.; de Courten, B. Curcumin Offers No Additional Benefit to Lifestyle Intervention on Cardiometabolic Status in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 3224. [Google Scholar] [CrossRef] [PubMed]
- Pipitone, R.M.; Zito, R.; Lupo, G.; Javed, A.; La Mantia, C.; Di Maria, G.; Pratelli, G.; Di Salvo, F.; Fontana, S.; Pucci, M.; et al. Curcumin and Andrographolide Co-Administration Safely Prevent Steatosis Induction and ROS Production in HepG2 Cell Line. Molecules 2023, 28, 1261. [Google Scholar] [CrossRef]
- Seidita, A.; Cusimano, A.; Giuliano, A.; Meli, M.; Carroccio, A.; Soresi, M.; Giannitrapani, L. Oxidative Stress as a Target for Non-Pharmacological Intervention in MAFLD: Could There Be a Role for EVOO? Antioxidants 2024, 13, 731. [Google Scholar] [CrossRef]
- Du, S.; Zhu, X.; Zhou, N.; Zheng, W.; Zhou, W.; Li, X. Curcumin Alleviates Hepatic Steatosis by Improving Mitochondrial Function in Postnatal Overfed Rats and Fatty L02 Cells through the SIRT3 Pathway. Food Funct. 2022, 13, 2155–2171. [Google Scholar] [CrossRef]
- Feng, D.; Zou, J.; Su, D.; Mai, H.; Zhang, S.; Li, P.; Zheng, X. Curcumin Prevents High-Fat Diet-Induced Hepatic Steatosis in ApoE−/− Mice by Improving Intestinal Barrier Function and Reducing Endotoxin and Liver TLR4/NF-κB Inflammation. Nutr. Metab. 2019, 16, 79. [Google Scholar] [CrossRef]
- Tong, C.; Wu, H.; Gu, D.; Li, Y.; Fan, Y.; Zeng, J.; Ding, W. Effect of Curcumin on the Non-Alcoholic Steatohepatitis via Inhibiting the M1 Polarization of Macrophages. Hum. Exp. Toxicol. 2021, 40, S310–S317. [Google Scholar] [CrossRef]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- Fu, Y.; Li, X.; Zeng, Y.; Zhang, A.; Qiu, S. Arctiin Attenuated NASH by Inhibiting Glycolysis and Inflammation via FGFR2/CSF1R Signaling. Eur. J. Pharmacol. 2025, 996, 177424. [Google Scholar] [CrossRef]
- Powell, E.E. A New Treatment and Updated Clinical Practice Guidelines for MASLD. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 88–89. [Google Scholar] [CrossRef]
- Martins, A.S.d.P.; Araújo, O.R.P.d.; Gomes, A.d.S.; Araujo, F.L.C.; Oliveira Junior, J.; Vasconcelos, J.K.G.d.; Rodrigues Junior, J.I.; Cerqueira, I.T.; Lins Neto, M.Á.d.F.; Bueno, N.B.; et al. Effect of Curcumin Plus Piperine on Redox Imbalance, Fecal Calprotectin and Cytokine Levels in Inflammatory Bowel Disease Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pharmaceuticals 2024, 17, 849. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of Phytosomal Curcumin on Anthropometric Parameters, Insulin Resistance, Cortisolemia and Non-Alcoholic Fatty Liver Disease Indices: A Double-Blind, Placebo-Controlled Clinical Trial. Eur. J. Nutr. 2020, 59, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Saadati, S.; Hatami, B.; Yari, Z.; Shahrbaf, M.A.; Eghtesad, S.; Mansour, A.; Poustchi, H.; Hedayati, M.; Aghajanpoor-Pasha, M.; Sadeghi, A.; et al. The Effects of Curcumin Supplementation on Liver Enzymes, Lipid Profile, Glucose Homeostasis, and Hepatic Steatosis and Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Eur. J. Clin. Nutr. 2019, 73, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Tang, D.; Du, Y.L.; Cao, C.Y.; Nie, Y.Q.; Cao, J.; Zhou, Y.J. Fatty Liver Mediated by Peroxisome Proliferator-Activated Receptor-α DNA Methylation Can Be Reversed by a Methylation Inhibitor and Curcumin. J. Dig. Dis. 2018, 19, 421–430. [Google Scholar] [CrossRef]
- Lee, D.E.; Lee, S.J.; Kim, S.J.; Lee, H.-S.; Kwon, O.-S. Curcumin Ameliorates Nonalcoholic Fatty Liver Disease through Inhibition of O-GlcNAcylation. Nutrients 2019, 11, 2702. [Google Scholar] [CrossRef]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.-J. Nano-Curcumin Improves Glucose Indices, Lipids, Inflammation, and Nesfatin in Overweight and Obese Patients with Non-Alcoholic Fatty Liver Disease (NAFLD): A Double-Blind Randomized Placebo-Controlled Clinical Trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef]
- Aliyari, M.; Ghoflchi, S.; Hashemy, S.I.; Hashemi, S.F.; Reihani, A.; Hosseini, H. The PI3K/Akt Pathway: A Target for Curcumin’s Therapeutic Effects. J. Diabetes Metab. Disord. 2025, 24, 52. [Google Scholar] [CrossRef]
- Saberi-Karimian, M.; Keshvari, M.; Ghayour-Mobarhan, M.; Salehizadeh, L.; Rahmani, S.; Behnam, B.; Jamialahmadi, T.; Asgary, S.; Sahebkar, A. Effects of Curcuminoids on Inflammatory Status in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Complement. Ther. Med. 2020, 49, 102322. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Tan, X.; Li, Q.; Yu, Z.; Wu, W.; Ma, X.; Zeng, J.; Wang, X. Protective Role of Curcumin in Disease Progression from Non-Alcoholic Fatty Liver Disease to Hepatocellular Carcinoma: A Meta-Analysis. Front. Pharmacol. 2024, 15, 1343193. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Rezai, A.; Dehabeh, M.; Nobakht M. Gh, B.F.; Bidkhori, M.; Sahebkar, A.; Hariri, M. Efficacy of Phytosomal Curcumin among Patients with Non-Alcoholic Fatty Liver Disease. Int. J. Vitam. Nutr. Res. 2021, 91, 278–286. [Google Scholar] [CrossRef]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-Alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-Controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Curcumin Lowers Serum Lipids and Uric Acid in Subjects with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. J. Cardiovasc. Pharmacol. 2016, 68, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Azimi-Nezhad, M.; Dehabeh, M.; Hariri, M.; Naderan, R.D.; Movahedi, A.; Abdalla, M.; Sathyapalan, T.; Sahebkar, A. The Effect of Curcumin Phytosome on the Treatment of Patients with Non-Alcoholic Fatty Liver Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. Adv. Exp. Med. Biol. 2021, 1308, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Chashmniam, S.; Mirhafez, S.R.; Dehabeh, M.; Hariri, M.; Azimi Nezhad, M.; Nobakht M Gh, B.F. A Pilot Study of the Effect of Phospholipid Curcumin on Serum Metabolomic Profile in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Eur. J. Clin. Nutr. 2019, 73, 1224–1235. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, Y.; Zhang, X.; Aa, J.; Wang, G.; Xie, Y. Curcumin Regulates Endogenous and Exogenous Metabolism via Nrf2-FXR-LXR Pathway in NAFLD Mice. Biomed. Pharmacother. 2018, 105, 274–281. [Google Scholar] [CrossRef]
- Belka, M.; Gostyńska-Stawna, A.; Stawny, M.; Krajka-Kuźniak, V. Activation of Nrf2 and FXR via Natural Compounds in Liver Inflammatory Disease. Int. J. Mol. Sci. 2024, 25, 11213. [Google Scholar] [CrossRef]
- Jiménez-Flores, L.M.; López-Briones, S.; Macías-Cervantes, M.H.; Ramírez-Emiliano, J.; Pérez-Vázquez, V. A PPARγ, NF-κB and AMPK-Dependent Mechanism May Be Involved in the Beneficial Effects of Curcumin in the Diabetic Db/Db Mice Liver. Molecules 2014, 19, 8289–8302. [Google Scholar] [CrossRef]
- Yang, Q.; Wan, Q.; Wang, Z. Curcumin Mitigates Polycystic Ovary Syndrome in Mice by Suppressing TLR4/MyD88/NF-κB Signaling Pathway Activation and Reducing Intestinal Mucosal Permeability. Sci. Rep. 2024, 14, 29848. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef]
- Moradi Kelardeh, B.; Rahmati-Ahmadabad, S.; Farzanegi, P.; Helalizadeh, M.; Azarbayjani, M.-A. Effects of Non-Linear Resistance Training and Curcumin Supplementation on the Liver Biochemical Markers Levels and Structure in Older Women with Non-Alcoholic Fatty Liver Disease. J. Bodyw. Mov. Ther. 2020, 24, 154–160. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Sun, C.-Y.; Adu-Frimpong, M.; Yu, J.-N.; Xu, X.-M. Glutathione-Sensitive PEGylated Curcumin Prodrug Nanomicelles: Preparation, Characterization, Cellular Uptake and Bioavailability Evaluation. Int. J. Pharm. 2019, 555, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; van Nostrum, C.F.; Kok, R.J.; Storm, G.; Hennink, W.E.; Heger, M. Utility of Intravenous Curcumin Nanodelivery Systems for Improving In Vivo Pharmacokinetics and Anticancer Pharmacodynamics. Mol. Pharm. 2022, 19, 3057–3074. [Google Scholar] [CrossRef] [PubMed]
- Dibaei, M.; Rouini, M.-R.; Sheikholeslami, B.; Gholami, M.; Dinarvand, R. The Effect of Surface Treatment on the Brain Delivery of Curcumin Nanosuspension: In Vitro and in Vivo Studies. Int. J. Nanomed. 2019, 14, 5477–5490. [Google Scholar] [CrossRef] [PubMed]
- Hocking, A.; Tommasi, S.; Sordillo, P.; Klebe, S. The Safety and Exploration of the Pharmacokinetics of Intrapleural Liposomal Curcumin. Int. J. Nanomed. 2020, 15, 943–952. [Google Scholar] [CrossRef]
- Lollo, G.; Ullio-Gamboa, G.; Fuentes, E.; Matha, K.; Lautram, N.; Benoit, J.-P. In Vitro Anti-Cancer Activity and Pharmacokinetic Evaluation of Curcumin-Loaded Lipid Nanocapsules. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 859–867. [Google Scholar] [CrossRef]
- Chirio, D.; Peira, E.; Dianzani, C.; Muntoni, E.; Gigliotti, C.L.; Ferrara, B.; Sapino, S.; Chindamo, G.; Gallarate, M. Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution. Nanomaterials 2019, 9, 230. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, X.; Li, Y.; Zhai, J.; Ma, Y. Excogitation and Assessment of Curcumin-Vitamin E Self-Assembly PEGylated Nanoparticles by the Route of Oral Administration. J. Pharm. Sci. 2021, 110, 146–154. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, N.; Cheng, R.; Zhao, C.; Liu, Z.; Li, X.; Liu, J.; Tian, Z. pH Multistage Responsive Micellar System with Charge-Switch and PEG Layer Detachment for Co-Delivery of Paclitaxel and Curcumin to Synergistically Eliminate Breast Cancer Stem Cells. Biomaterials 2017, 147, 53–67. [Google Scholar] [CrossRef]
- Bagheri, M.; Fens, M.H.; Kleijn, T.G.; Capomaccio, R.B.; Mehn, D.; Krawczyk, P.M.; Scutigliani, E.M.; Gurinov, A.; Baldus, M.; van Kronenburg, N.C.H.; et al. In Vitro and In Vivo Studies on HPMA-Based Polymeric Micelles Loaded with Curcumin. Mol. Pharm. 2021, 18, 1247–1263. [Google Scholar] [CrossRef]
- Kundu, M.; Sadhukhan, P.; Ghosh, N.; Chatterjee, S.; Manna, P.; Das, J.; Sil, P.C. pH-Responsive and Targeted Delivery of Curcumin via Phenylboronic Acid-Functionalized ZnO Nanoparticles for Breast Cancer Therapy. J. Adv. Res. 2019, 18, 161–172. [Google Scholar] [CrossRef]
- Ji, P.; Wang, X.; Yin, J.; Mou, Y.; Huang, H.; Ren, Z. Selective Delivery of Curcumin to Breast Cancer Cells by Self-Targeting Apoferritin Nanocages with pH-Responsive and Low Toxicity. Drug Deliv. 2022, 29, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Asghar, S.; Hu, Z.; Qiu, Y.; Zhang, J.; Shao, F.; Xiao, Y. Understanding the Cellular Uptake and Biodistribution of a Dual-Targeting Carrier Based on Redox-Sensitive Hyaluronic Acid-Ss-Curcumin Micelles for Treating Brain Glioma. Int. J. Biol. Macromol. 2019, 136, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, Y.; Sun, J.; Han, Y.; Gong, W.; Li, Y.; Feng, Y.; Wang, H.; Yang, M.; Li, Z.; et al. Neuronal Mitochondria-Targeted Delivery of Curcumin by Biomimetic Engineered Nanosystems in Alzheimer’s Disease Mice. Acta Biomater. 2020, 108, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, S.; Zhang, F.; Ma, S.; Heng, B.C.; Wang, Y.; Zhu, J.; Xu, M.; He, Y.; Wei, Y.; et al. Cell Membrane Vesicles with Enriched CXCR4 Display Enhances Their Targeted Delivery as Drug Carriers to Inflammatory Sites. Adv. Sci. 2021, 8, 2101562. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.; Li, Y.; Xia, Q.; Li, Z.; Hou, X.; Feng, N. Tumor Cell Membrane-Derived Nano-Trojan Horses Encapsulating Phototherapy and Chemotherapy Are Accepted by Homologous Tumor Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111670. [Google Scholar] [CrossRef]
- Do, X.-H.; Nguyen, T.D.; Le, T.T.H.; To, T.T.; Bui, T.V.K.; Pham, N.H.; Lam, K.; Hoang, T.M.N.; Ha, P.T. High Biocompatibility, MRI Enhancement, and Dual Chemo- and Thermal-Therapy of Curcumin-Encapsulated Alginate/Fe3O4 Nanoparticles. Pharmaceutics 2023, 15, 1523. [Google Scholar] [CrossRef]
- Liu, X.; Mu, X.; Wang, Y.; Liu, Z.; Li, Y.; Lan, J.; Feng, S.; Wang, S.; Zhao, Q. Metal-Based Mesoporous Polydopamine with Dual Enzyme-like Activity as Biomimetic Nanodrug for Alleviating Liver Fibrosis. J. Colloid. Interface Sci. 2025, 684, 586–599. [Google Scholar] [CrossRef]
- Czerniel, J.; Gostyńska-Stawna, A.; Sommerfeld-Klatta, K.; Przybylski, T.; Krajka-Kuźniak, V.; Stawny, M. Development and Validation of In Vitro Assessment Protocol of Novel Intravenous Nanoemulsions for Parenteral Nutrition. Pharmaceutics 2025, 17, 493. [Google Scholar] [CrossRef]
- Gou, S.; Huang, Y.; Wan, Y.; Ma, Y.; Zhou, X.; Tong, X.; Huang, J.; Kang, Y.; Pan, G.; Dai, F.; et al. Multi-Bioresponsive Silk Fibroin-Based Nanoparticles with on-Demand Cytoplasmic Drug Release Capacity for CD44-Targeted Alleviation of Ulcerative Colitis. Biomaterials 2019, 212, 39–54. [Google Scholar] [CrossRef]
- Park, J.Y.; Chu, G.E.; Park, S.; Park, C.; Aryal, S.; Kang, W.J.; Cho, W.G.; Key, J. Therapeutic Efficacy of Curcumin Enhanced by Microscale Discoidal Polymeric Particles in a Murine Asthma Model. Pharmaceutics 2020, 12, 739. [Google Scholar] [CrossRef]
- Xia, X.; Wang, L.; Yang, X.; Hu, Y.; Liu, Q. Acute Damage to the Sperm Quality and Spermatogenesis in Male Mice Exposed to Curcumin-Loaded Nanoparticles. Int. J. Nanomed. 2020, 15, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Das, U.; Bhuniya, A.; Roy, A.K.; Gmeiner, W.H.; Ghosh, S. Hairpin Oligonucleotide Can Functionalize Gold Nanorods for in Vivo Application Delivering Cytotoxic Nucleotides and Curcumin: A Comprehensive Study in Combination with Near-Infrared Laser. ACS Omega 2020, 5, 28463–28474. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xu, Y.; Yao, Y.; Yue, J.; Wu, Z.; Li, H.; Shen, G.; Liao, Y.; Wang, H.; Zhou, W. Self-Oxygenation Mesoporous MnO2 Nanoparticles with Ultra-High Drug Loading Capacity for Targeted Arteriosclerosis Therapy. J. Nanobiotechnol. 2022, 20, 88. [Google Scholar] [CrossRef] [PubMed]
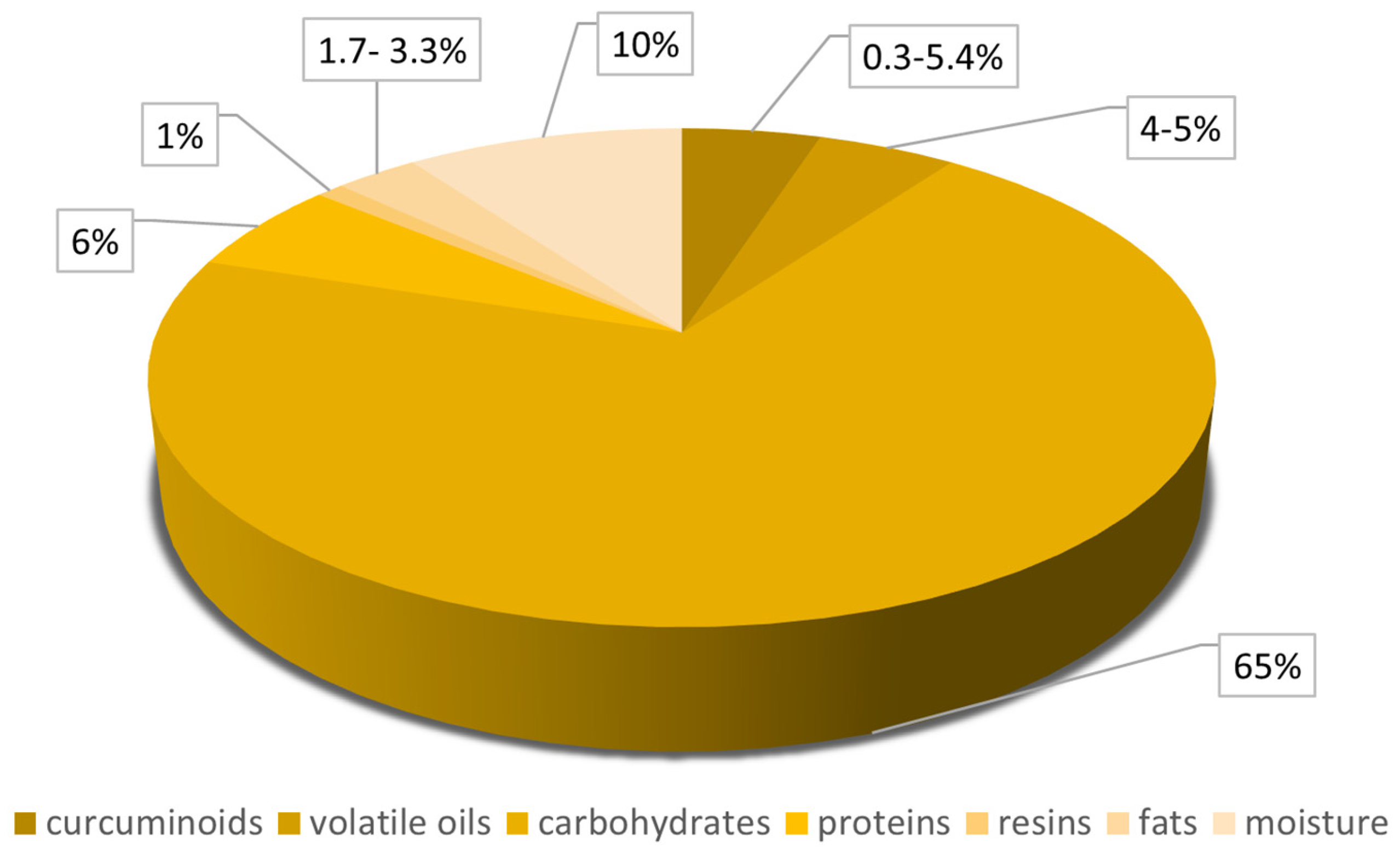
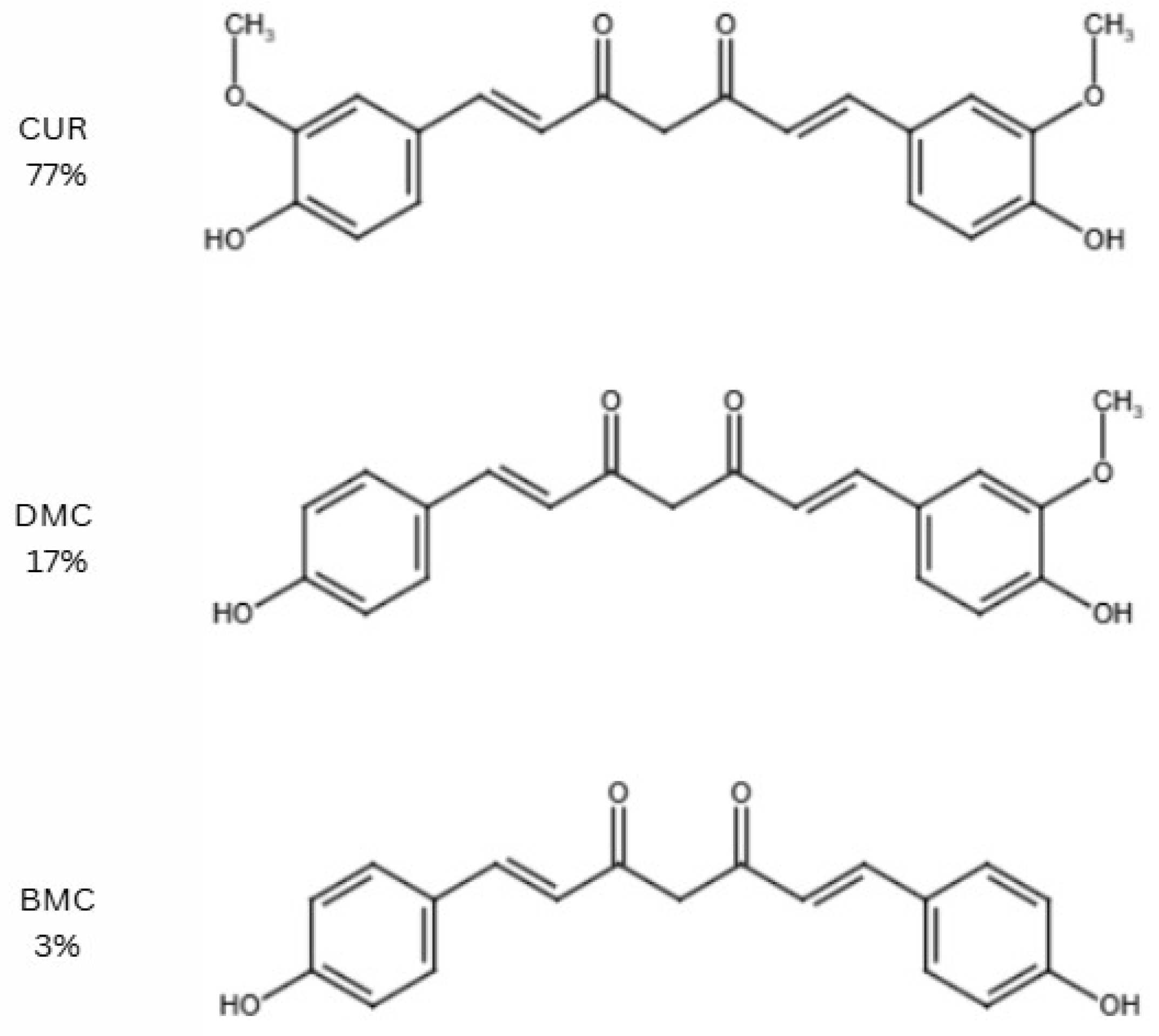
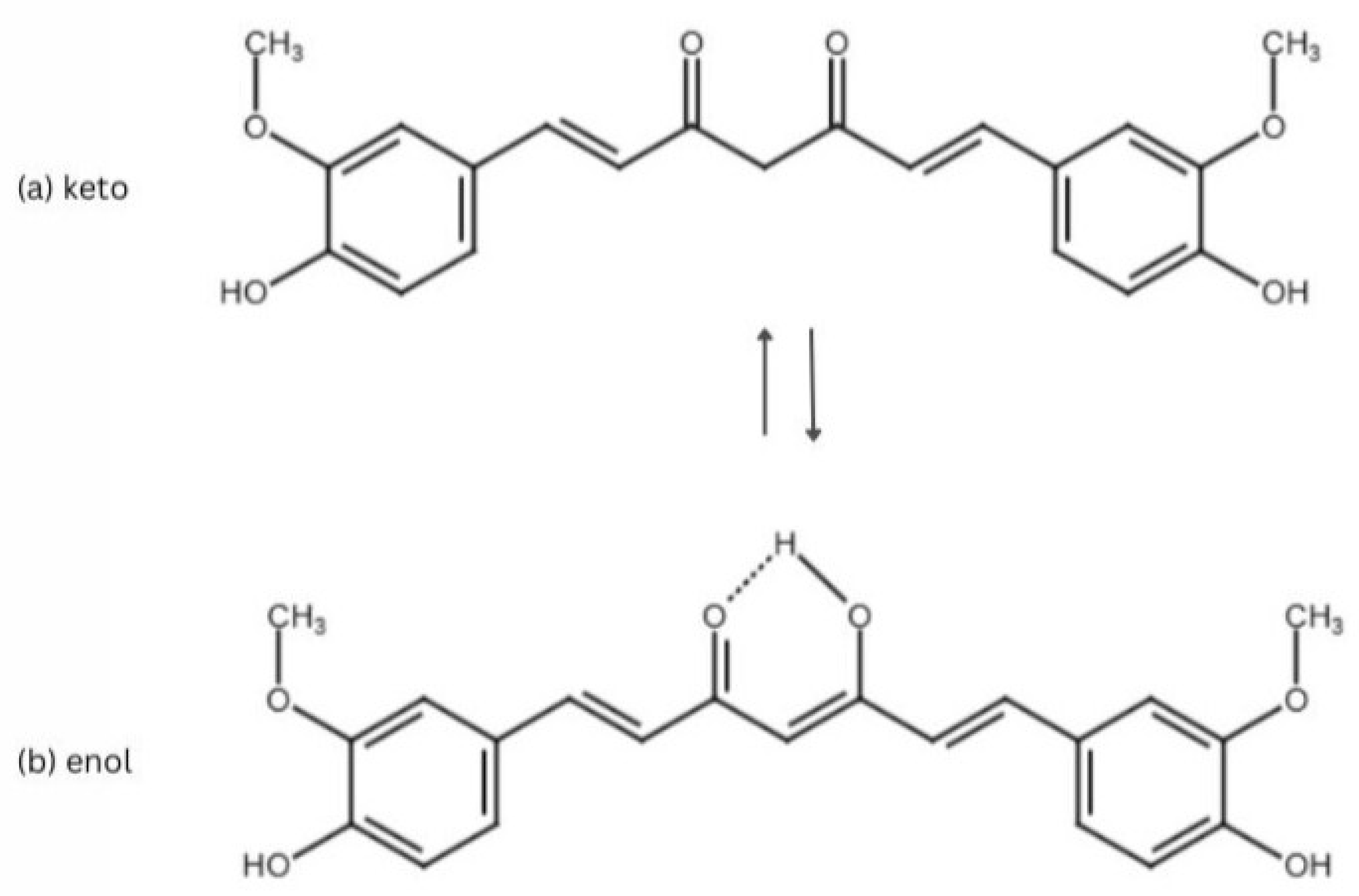

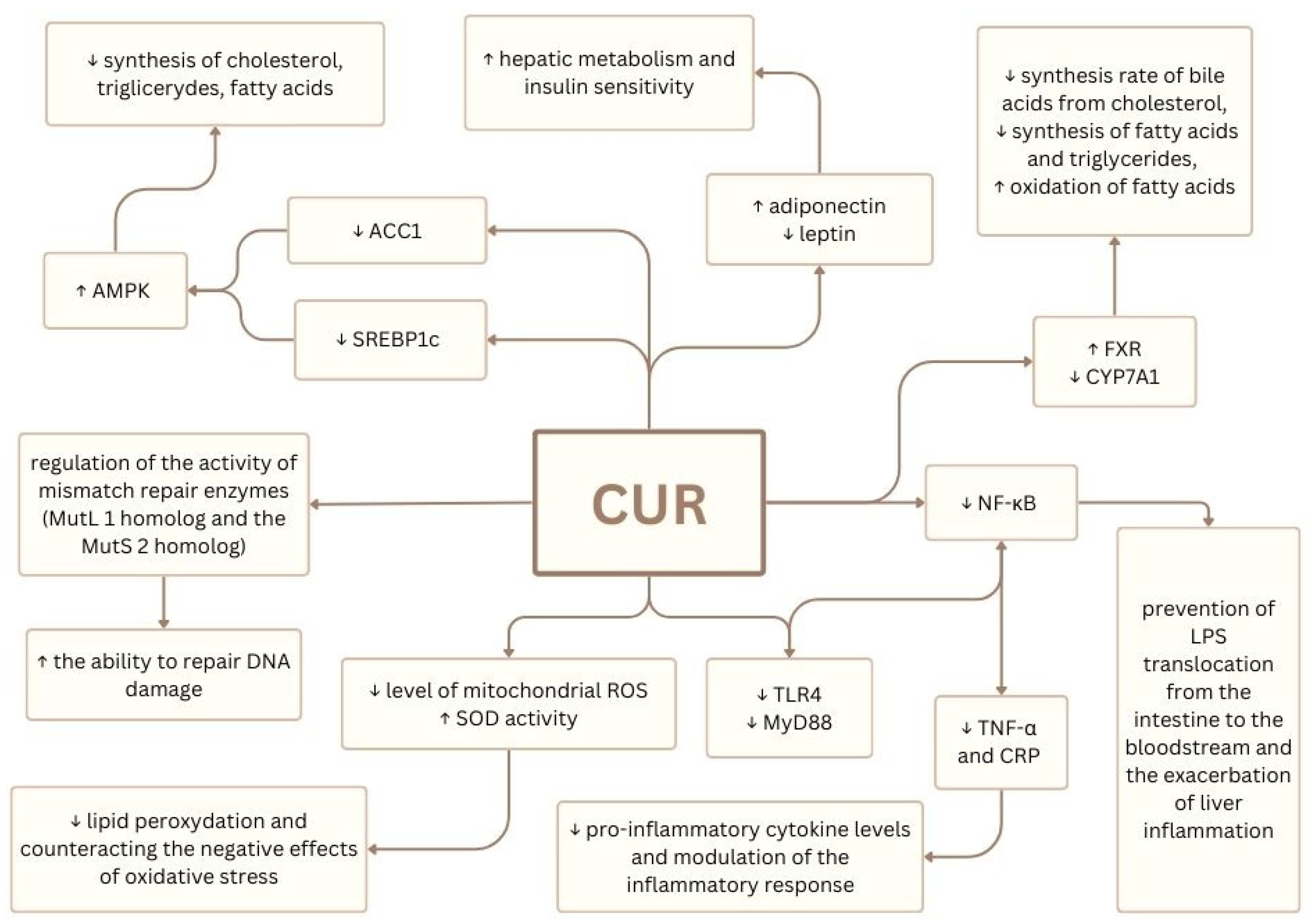
| Category | Targets | Reference |
|---|---|---|
| Transcription factors | NF-κB, Nrf2 *, AP-1, β-catenin, STAT3, HIF, Smad7, and Smad3 | [22,23,24,25,28] |
| Growth factors | CCN2, EGF, FGF, HGF, NGF, PDGF, VEGF, and TGF-β | [23,24,25,28] |
| Kinases | PhK, PKCε, PAK, Pp60c-tk, EGFR kinase, CAMK, GSK-3β, AMPK *, and JNK | [23,24,25,28] |
| Inflammatory cytokines | IL-1, IL-2, IL-5, IL-6, IL-8, IL-10, IL-12, IL-18, MCP, MaIP 1α, TNF-α, and MMIF | [22,23,24,25,28] |
| Receptors | LXR, FXR *, keratinocyte transferring receptor, AR, AHR, CXCR4, EGFR, H2R, HER-2, IR, DR5 *, EPCR *, FR *, and PPAR-γ | [23,24,25] |
| Enzymes | ATPase, COX-2, LOX, SOD *, CAT *, GPx *, ODC, HAT, DNA polymerase, FPT, AATF-1, iNOS, NQO-1, PhpD, SRC-2 *, GST *, and HO-1 * | [22,23,24,25,29] |
| Type | I—Acute Condition | II- Prolonged Acute Condition | III—Chronic Condition |
|---|---|---|---|
| Reversibility of the primary disease | Reversible. Postoperative ileus and sudden intestinal obstruction. | Reversible. It usually occurs with unstable patients who may have suffered complications as a result of major bowel or any other surgery. | May be irreversible. Stable patients, who suffer from short bowel syndrome, surgical complications, or inflammatory bowel disease. |
| Duration of the PN therapy | Short-term PN (Days) | Short-term PN (Weeks or months) | Long-term PN (Months or years, in some cases lifelong) |
| Type of Diagnostic Tool | Diagnostic Tool | Diagnostic Features | References |
|---|---|---|---|
| Physical feature | Physical examination of the patient data | Jaundice, hepatomegaly, and splenomegaly | [75] |
| Biomarkers | ALAT | Increased (>2–3 times the upper limit) | [95,101,102,103] |
| AST | Increased (>2–3 times the upper limit) 44–302 U/L | [95,101,102,103] | |
| Bilirubin | Increased (2–3 times the pre-PN levels) 5.0–45 µmol/L | [95,103] | |
| Conjugated bilirubin | Increased (2–3 times the pre-PN levels) 2.3–28 µmol/L | [103] | |
| GGT | Increased 39–179 U/L | [103,104] | |
| Citrulline | Decreased 5.0–16 µmol/L | [103] | |
| Imaging techniques | Transient elastography | Evaluation of liver stiffness | [103,105] |
| Magnetic resonance spectroscopy | Evaluation of the degree of steatosis | [106] | |
| Proton MRS | Evaluation of the degree of steatosis (quantitative liver fat content) | [107] |
| Formulation | Dose of CUR [mg/ Day] | Time [Weeks] | ALAT | AST | ALP | TNF-α | CRP | TGs | LDL | HDL | TC | FBG | IL-6 | BMI | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CUR capsules | 1000 | 12 | N.S | N.S | N.S | x | x | x | x | x | x | x | x | x | [46] |
| CUR + piperine capsules | 500 | 12 | ↓ | ↓ | x | x | ↓* | ↓ | ↓ | N.S | ↓ | ↓ | x | ↓ | [63] |
| Phytosomal/ Meriva® | 50 | 8 | N.S | N.S | x | x | x | N.S | N.S | N.S | N.S | N.S | x | ↓* | [65] |
| Livogen Plus® | x | 12 | N.S | N.S | x | N.S | N.S | N.S | x | ↑* | N.S | N.S | ↓* | ↓* | [66] |
| Amorphous dispersion of 70 mg of curcuminoids | x | 8 | ↓ | ↓ | x | x | x | ↓ | ↓ | ↓ | ↓ | N.S | x | ↓ | [142] |
| BIOCUR® | 1500 | 12 | ↓* | ↓* | x | ↓* | N.S | x | x | x | x | x | x | ↓* | [122] |
| Phytosomal/ Meriva® | 200 | 8 | ↓ | ↓ | N.S | x | x | x | x | x | x | x | x | ↓ | [150] |
| Nanomicelle | 80 | 12 | N.S | N.S | N.S | x | x | x | x | x | x | x | x | N.S | [151] |
| Phytosomal/ Meriva® | 50 | 8 | N.S | N.S | x | x | x | x | x | x | x | x | x | ↓ | [118] |
| Phytosomal/ Curserin® | 200 | 8 | x | x | x | x | x | ↓ | N.S | ↓* | N.S | ↓* | x | ↓* | [133] |
| Phytosomal/ Meriva® | 200 | 8 | x | x | x | x | x | ↓ | ↓ | ↓* | ↓ | ↓* | x | x | [143] |
| BIOCUR® | 1500 | 12 | N.S | N.S | x | x | x | N.S | N.S | N.S | ↓* | ↓* | x | ↓* | [134] |
| Phytosomal/ Meriva® | 50 | 8 | ↓ | ↓ | x | x | x | x | x | x | x | x | x | ↓ | [141] |
| Phytosomal/ Meriva® | 50 | 8 | N.S | ↓ | N.S | x | x | N.S | N.S | ↓* | N.S | N.S | x | ↓* | [144] |
| NanoCUR/ sinaCUR® | x | 12 | ↓ | ↓ | x | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | ↓ | ↓* | [137] |
| C3 Complex® + Bioperine® | x | 8 | N.S | N.S | x | ↓ | x | N.S | N.S | N.S | N.S | N.S | N.S | ↓ | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obrzut, O.; Gostyńska-Stawna, A.; Kustrzyńska, K.; Stawny, M.; Krajka-Kuźniak, V. Curcumin: A Natural Warrior Against Inflammatory Liver Diseases. Nutrients 2025, 17, 1373. https://doi.org/10.3390/nu17081373
Obrzut O, Gostyńska-Stawna A, Kustrzyńska K, Stawny M, Krajka-Kuźniak V. Curcumin: A Natural Warrior Against Inflammatory Liver Diseases. Nutrients. 2025; 17(8):1373. https://doi.org/10.3390/nu17081373
Chicago/Turabian StyleObrzut, Olga, Aleksandra Gostyńska-Stawna, Karolina Kustrzyńska, Maciej Stawny, and Violetta Krajka-Kuźniak. 2025. "Curcumin: A Natural Warrior Against Inflammatory Liver Diseases" Nutrients 17, no. 8: 1373. https://doi.org/10.3390/nu17081373
APA StyleObrzut, O., Gostyńska-Stawna, A., Kustrzyńska, K., Stawny, M., & Krajka-Kuźniak, V. (2025). Curcumin: A Natural Warrior Against Inflammatory Liver Diseases. Nutrients, 17(8), 1373. https://doi.org/10.3390/nu17081373








