Relationship Between Dietary Habits and Stress Responses Exerted by Different Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Questionnaire Data Acquisition and Processing
2.3. Gut Microbiota
2.4. Data Analysis
3. Results
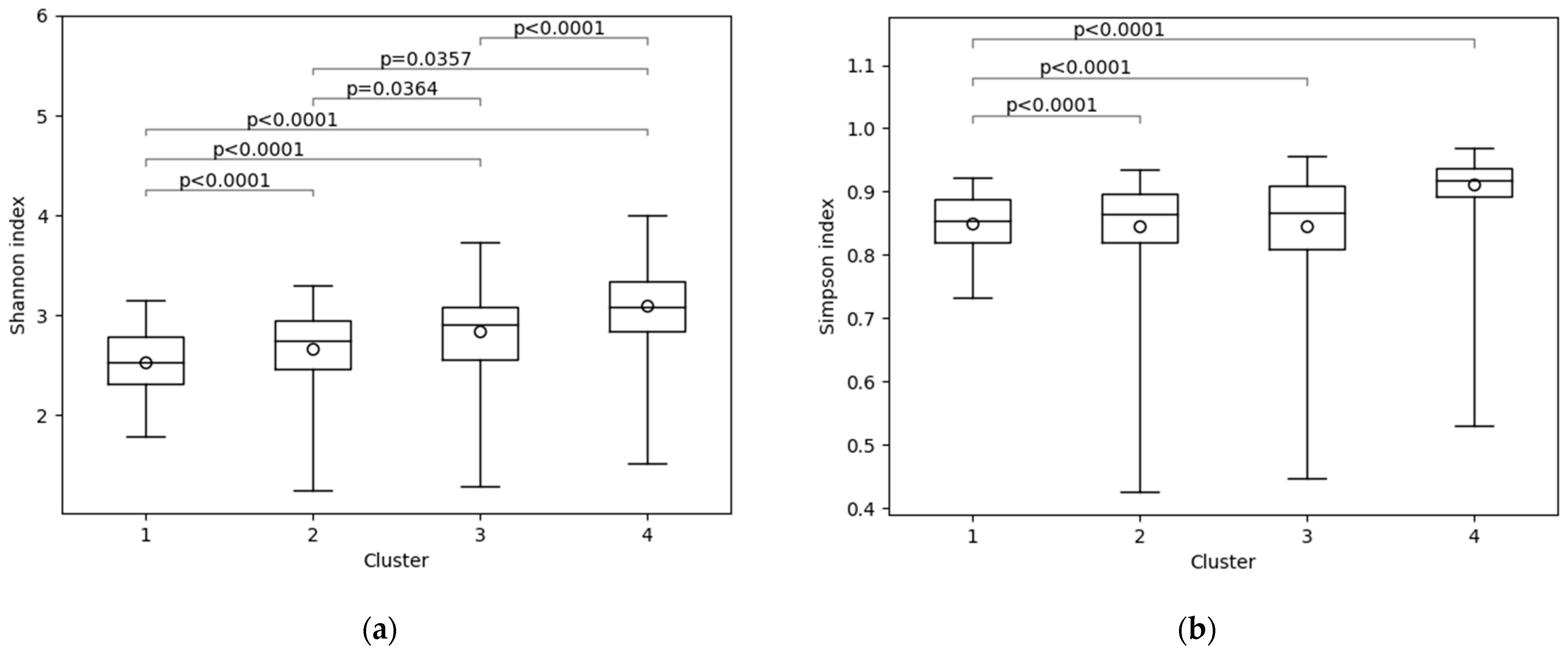
3.1. Gut Microbiota of Subjects
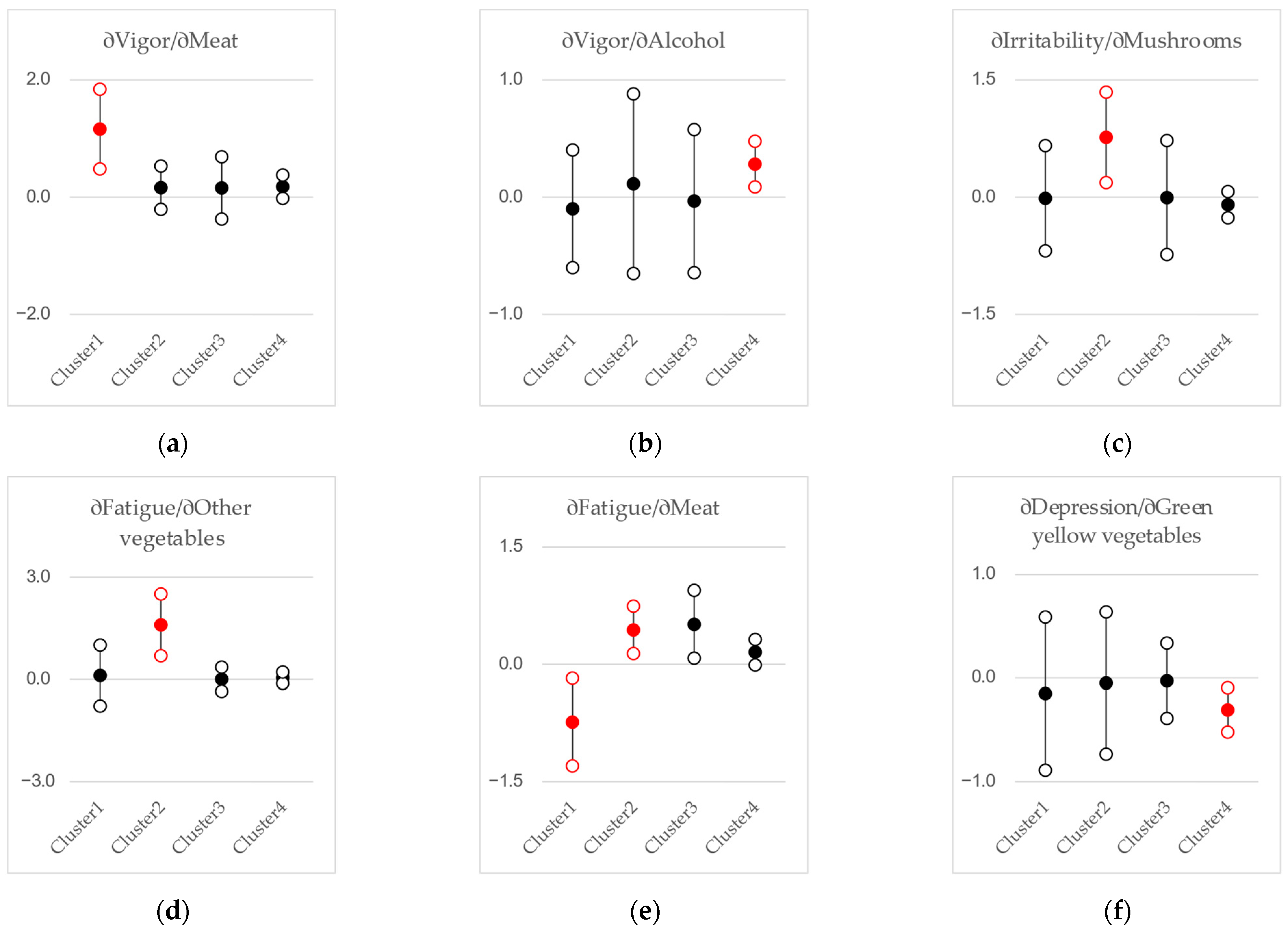
3.2. Regression Analyses
4. Discussion
4.1. Gut Microbiota Clustering
4.2. Gut Microbiota, Diet, and Stress
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, A.; Inslicht, S.S.; Bhargava, A. Gut-Brain Axis: Role of Microbiome, Metabolomics, Hormones, and Stress in Mental Health Disorders. Cells 2024, 13, 1436. [Google Scholar] [CrossRef]
- Gates, E.J.; Bernath, A.K.; Klegeris, A. Modifying the diet and gut microbiota to prevent and manage neurodegenerative diseases. Rev. Neurosci. 2022, 33, 767–787. [Google Scholar] [CrossRef] [PubMed]
- Altveş, S.; Yildiz, H.K.; Vural, H.C. Interaction of the microbiota with the human body in health and diseases. Biosci. Microbiota Food Health 2020, 39, 23–32. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Tsuchiya, S.; Dohi, O.; Yoshida, N.; Kamada, K.; et al. Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J. Gastroenterol. 2019, 54, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Watanabe, S.; Yamasaki, H.; Sakuma, H.; Takeda, A.K.; Yamashita, T.; Hirata, K.I. Average gut flora in healthy Japanese subjects stratified by age and body mass index. Biosci. Microbiota Food Health 2022, 41, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Flandroy, L.; Poutahidis, T.; Berg, G.; Clarke, G.; Dao, M.C.; Decaestecker, E.; Furman, E.; Haahtela, T.; Massart, S.; Plovier, H.; et al. The impact of human activities and lifestyles on the interlinked microbiota and health of humans and of ecosystems. Sci. Total Environ. 2018, 15, 1018–1038. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Gorvitovskaia, A.; Holmes, S.P.; Huse, S.M. Interpreting Prevotella and Bacteroides as biomarkers of diet and lifestyle. Microbiome 2016, 4, 15. [Google Scholar] [CrossRef]
- Nakayama, J.; Watanabe, K.; Jiang, J.; Matsuda, K.; Chao, S.H.; Haryono, P.; La-Ongkham, O.; Sarwoko, M.A.; Sujaya, I.N.; Zhao, L.; et al. Diversity in gut bacterial community of school-age children in Asia. Sci. Rep. 2015, 5, 8397, Erratum in Sci. Rep. 2015, 9, 6530. https://doi.org/10.1038/s41598-019-42780-z. [Google Scholar] [CrossRef]
- Yao, H.; Flanagan, B.M.; Williams, B.A.; Wu, X.; Mikkelsen, D.; Gidley, M.J. Differential effects of pectin-based dietary fibre type and gut microbiota composition on in vitro fermentation outcomes. Carbohydr. Polym. 2024, 339, 122284. [Google Scholar] [CrossRef]
- Adamberg, S.; Adamberg, K. Prevotella enterotype associates with diets supporting acidic faecal pH and production of propionic acid by microbiota. Heliyon 2024, 10, e31134. [Google Scholar] [CrossRef] [PubMed]
- Pihelgas, S.; Ehala-Aleksejev, K.; Adamberg, S.; Kazantseva, J.; Adamberg, K. The gut microbiota of healthy individuals remains resilient in response to the consumption of various dietary fibers. Sci. Rep. 2024, 14, 22208. [Google Scholar] [CrossRef] [PubMed]
- WHO. Mental Health. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-health-strengthening-our-response (accessed on 17 February 2025).
- Nagata, T.; Mori, K.; Ohtani, M.; Nagata, M.; Kajiki, S.; Fujino, Y.; Matsuda, S.; Loeppke, R. Total Health-Related Costs Due to Absenteeism, Presenteeism, and Medical and Pharmaceutical Expenses in Japanese Employers. J. Occup. Environ. Med. 2018, 60, e273–e280. [Google Scholar] [CrossRef]
- National Institute of Health and Nutrition. Health Japan 21. Available online: https://www.nibiohn.go.jp/eiken/kenkounippon21/en/kenkounippon21/index.html (accessed on 17 February 2025).
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar] [PubMed] [PubMed Central]
- Apostolakopoulou, X.A.; Petinaki, E.; Kapsoritakis, A.N.; Bonotis, K. A Narrative Review of the Association Between Healthy Dietary Patterns and Depression. Cureus 2024, 16, e60920. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Mo, L.; Luo, J.; Shen, Q.; Quan, W. Association between Western Dietary Patterns, Typical Food Groups, and Behavioral Health Disorders: An Updated Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2023, 16, 125. [Google Scholar] [CrossRef]
- Wang, M.; Yan, X.; Li, Y.; Li, Q.; Xu, Y.; Huang, J.; Gan, J.; Yang, W. Association between plasma polyunsaturated fatty acids and depressive among US adults. Front. Nutr. 2024, 11, 1342304. [Google Scholar] [CrossRef] [PubMed]
- Carnegie, R.; Borges, M.C.; Jones, H.J.; Zheng, J.; Haycock, P.; Evans, J.; Martin, R.M. Omega-3 fatty acids and major depression: A Mendelian randomization study. Transl. Psychiatry 2024, 14, 222. [Google Scholar] [CrossRef]
- Hamazaki, K.; Matsuoka, Y.J.; Yamaji, T.; Sawada, N.; Mimura, M.; Nozaki, S.; Shikimoto, R.; Tsugane, S. Plasma phospholipid n-3 polyunsaturated fatty acids and major depressive disorder in Japanese elderly: The Japan Public Health Center-based Prospective Study. Sci. Rep. 2021, 11, 4003. [Google Scholar] [CrossRef]
- Kagami-Katsuyama, H.; Sato-Ueshima, M.; Satoh, K.; Tousen, Y.; Takimoto, H.; Maeda-Yamamoto, M.; Nishihira, J. The Relationship between Mental and Physical Minor Health Complaints and the Intake of Dietary Nutrients. Nutrients 2023, 15, 865. [Google Scholar] [CrossRef]
- Ministry of Health, Labor and Welfare. The Brief Job Stress Questionnaire. Available online: https://stresscheck.mhlw.go.jp/material.html (accessed on 17 February 2025).
- Tourlousse, D.M.; Narita, K.; Miura, T.; Sakamoto, M.; Ohashi, A.; Shiina, K.; Matsuda, M.; Miura, D.; Shimamura, M.; Ohyama, Y.; et al. Validation and standardization of DNA extraction and library construction methods for metagenomics-based human fecal microbiome measurements. Microbiome 2021, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Leviatan, S.; Shoer, S.; Rothschild, D.; Gorodetski, M.; Segal, E. An expanded reference map of the human gut microbiome reveals hundreds of previously unknown species. Nat. Commun. 2022, 13, 3863. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Lu, J.; Breitwieser, F.P.; Thielen, P.; Salzberg, S.L. Bracken: Estimating species abundance in metagenomics data. PeerJ Comput. Sci. 2017, 3, e104. [Google Scholar] [CrossRef]
- Chayes, F. On correlation between variables of constant sum. J. Geophys. Res. 1960, 65, 4185–4193. [Google Scholar] [CrossRef]
- Park, J.; Kato, K.; Murakami, H.; Hosomi, K.; Tanisawa, K.; Nakagata, T.; Ohno, H.; Konishi, K.; Kawashima, H.; Chen, Y.A.; et al. Comprehensive analysis of gut microbiota of a healthy population and covariates affecting microbial variation in two large Japanese cohorts. BMC Microbiol. 2021, 21, 151. [Google Scholar] [CrossRef]
- Takagi, T.; Inoue, R.; Oshima, A.; Sakazume, H.; Ogawa, K.; Tominaga, T.; Mihara, Y.; Sugaya, T.; Mizushima, K.; Uchiyama, K.; et al. Typing of the Gut Microbiota Community in Japanese Subjects. Microorganisms 2022, 10, 664. [Google Scholar] [CrossRef]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’Sullivan, O.; Fouhy, F.; Clarke, S.F.; O’Toole, P.W.; Quigley, E.M.; Stanton, C.; et al. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Bauer, L.L.; Gourineni, V.; Pelkman, C.L.; Fahey, G.C., Jr.; Swanson, K.S. Agave Inulin Supplementation Affects the Fecal Microbiota of Healthy Adults Participating in a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Martin, B.R.; Schoterman, M.H.; Nakatsu, C.H.; McCabe, L.D.; McCabe, G.P.; Wastney, M.E.; van den Heuvel, E.G.; Weaver, C.M. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: A double-blind cross-over trial. Br. J. Nutr. 2013, 110, 1292–1303. [Google Scholar] [CrossRef]
- Takahashi, S.; Anzawa, D.; Takami, K.; Ishizuka, A.; Mawatari, T.; Kamikado, K.; Sugimura, H.; Nishijima, T. Effect of Bifidobacterium animalis ssp. lactis GCL2505 on visceral fat accumulation in healthy Japanese adults: A randomized controlled trial. Biosci. Microbiota Food Health 2016, 35, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, R.; Li, X.F.; Wang, R.L. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br. J. Nutr. 2012, 107, 1429–1434. [Google Scholar] [CrossRef]
- Popoola, A.A.; Frediani, J.K.; Hartman, T.J.; Paynabar, K. Mitigating underreported error in food frequency questionnaire data using a supervised machine learning method and error adjustment algorithm. BMC Med. Inform. Decis. Mak. 2023, 23, 178. [Google Scholar] [CrossRef]
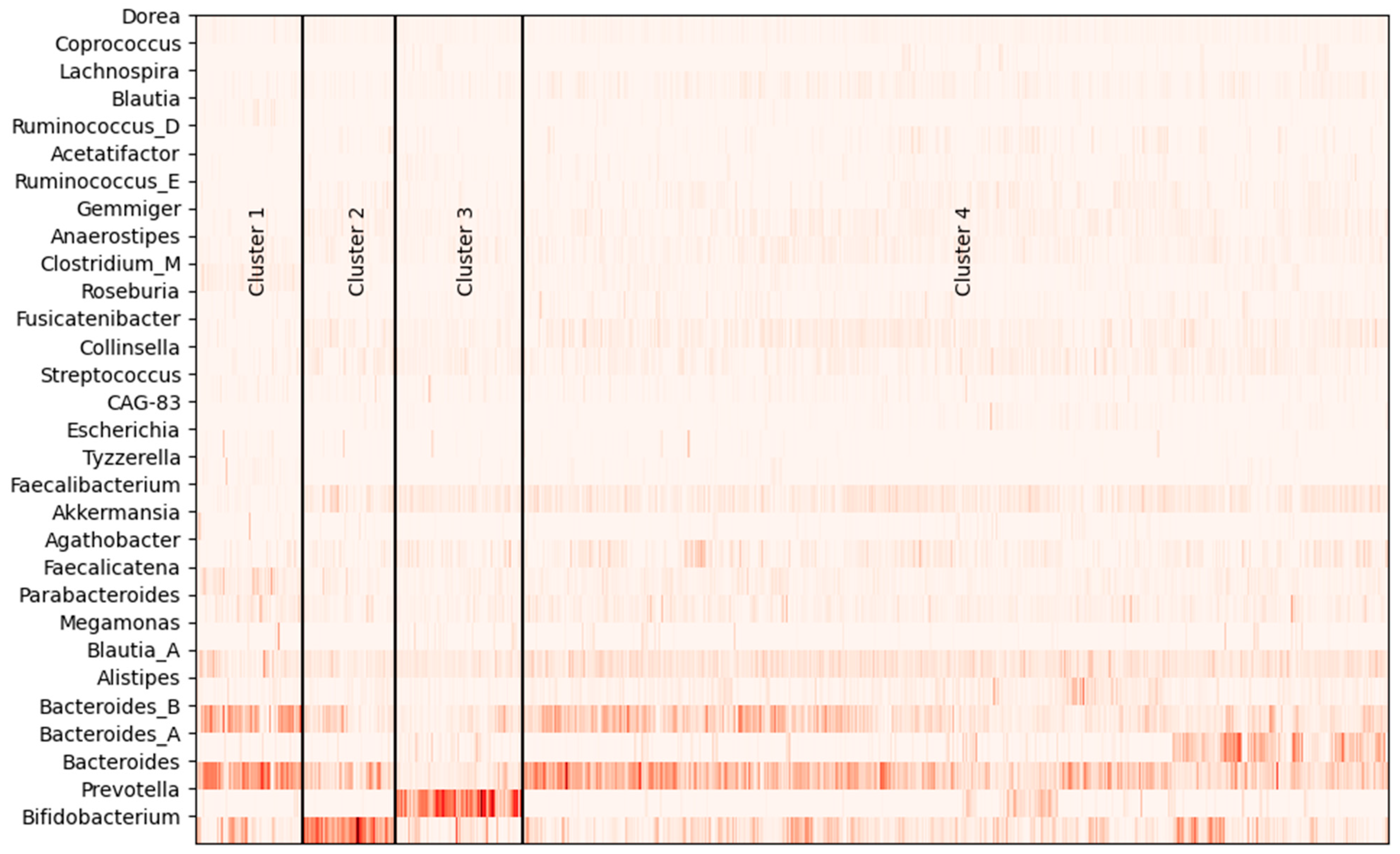

| Cluster | N | Agathobacter | Alistipes | Anaerostipes | Bacteroides | Bacteroides_A | Bacteroides_B |
| 1 | 74 | 0.013 | 0.014 | 0.014 | 0.239 | 0.009 | 0.175 |
| 2 | 64 | 0.018 | 0.016 | 0.015 | 0.125 | 0.006 | 0.072 |
| 3 | 87 | 0.030 | 0.010 | 0.014 | 0.052 | 0.027 | 0.046 |
| 4 | 596 | 0.031 | 0.029 | 0.019 | 0.153 | 0.044 | 0.096 |
| p-value * | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Cluster | Bifidobacterium | Blautia | Blautia_A | Clostridium_M | Collinsella | Dorea | Faecalibacterium |
| 1 | 0.076 | 0.012 | 0.064 | 0.031 | 0.007 | 0.009 | 0.009 |
| 2 | 0.299 | 0.002 | 0.052 | 0.005 | 0.026 | 0.010 | 0.046 |
| 3 | 0.055 | 0.002 | 0.050 | 0.006 | 0.022 | 0.010 | 0.045 |
| 4 | 0.074 | 0.003 | 0.073 | 0.009 | 0.022 | 0.010 | 0.048 |
| p-value * | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 |
| Cluster | Faecalicatena | Fusicatenibacter | Gemmiger | Lachnospira | Lactobacillus | Megamonas | Parabacteroides |
| 1 | 0.055 | 0.006 | 0.005 | 0.007 | 0.000 | 0.006 | 0.040 |
| 2 | 0.020 | 0.027 | 0.023 | 0.008 | 0.012 | 0.000 | 0.023 |
| 3 | 0.017 | 0.017 | 0.012 | 0.010 | 0.000 | 0.011 | 0.021 |
| 4 | 0.020 | 0.030 | 0.019 | 0.016 | 0.000 | 0.004 | 0.029 |
| p-value * | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | 0.001 |
| Cluster | Prevotella | Roseburia | Ruminococcus_E | Tyzzerella | |||
| 1 | 0.006 | 0.005 | 0.002 | 0.014 | |||
| 2 | 0.001 | 0.005 | 0.012 | 0.002 | |||
| 3 | 0.316 | 0.008 | 0.006 | 0.002 | |||
| 4 | 0.014 | 0.011 | 0.013 | 0.003 | |||
| p-value * | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satoh, K.; Hazama, M.; Maeda-Yamamoto, M.; Nishihira, J. Relationship Between Dietary Habits and Stress Responses Exerted by Different Gut Microbiota. Nutrients 2025, 17, 1388. https://doi.org/10.3390/nu17081388
Satoh K, Hazama M, Maeda-Yamamoto M, Nishihira J. Relationship Between Dietary Habits and Stress Responses Exerted by Different Gut Microbiota. Nutrients. 2025; 17(8):1388. https://doi.org/10.3390/nu17081388
Chicago/Turabian StyleSatoh, Kouji, Makoto Hazama, Mari Maeda-Yamamoto, and Jun Nishihira. 2025. "Relationship Between Dietary Habits and Stress Responses Exerted by Different Gut Microbiota" Nutrients 17, no. 8: 1388. https://doi.org/10.3390/nu17081388
APA StyleSatoh, K., Hazama, M., Maeda-Yamamoto, M., & Nishihira, J. (2025). Relationship Between Dietary Habits and Stress Responses Exerted by Different Gut Microbiota. Nutrients, 17(8), 1388. https://doi.org/10.3390/nu17081388





