Early Nutritional Intervention in Patients with Non-Small Cell Lung Cancer Receiving Concurrent Chemoradiotherapy: A Phase II Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Treatment
2.3. Nutritional Intervention
2.4. Matched External Controls
2.5. Data Acquisition
2.6. Microbiome Analysis: Fecal DNA Extraction and 16S rRNA Sequencing
2.7. Statistical Analysis
3. Results
3.1. Patient Baseline Demographics
3.2. Treatment Details
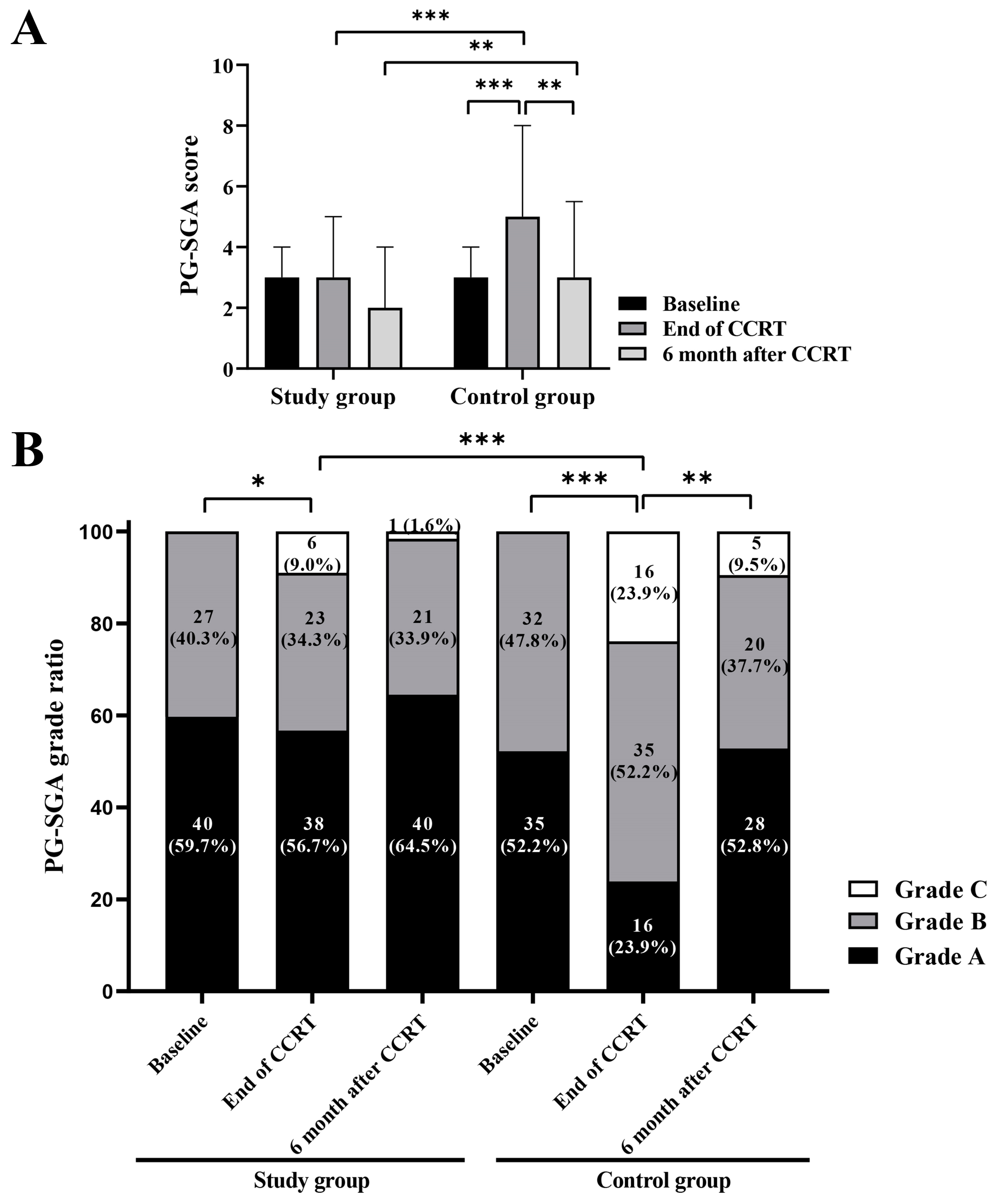
3.3. Changes of Nutritional and Inflammatory Parameters in the Two Groups
3.4. QoL Scores of the Study Group
3.5. Treatment Toxicity
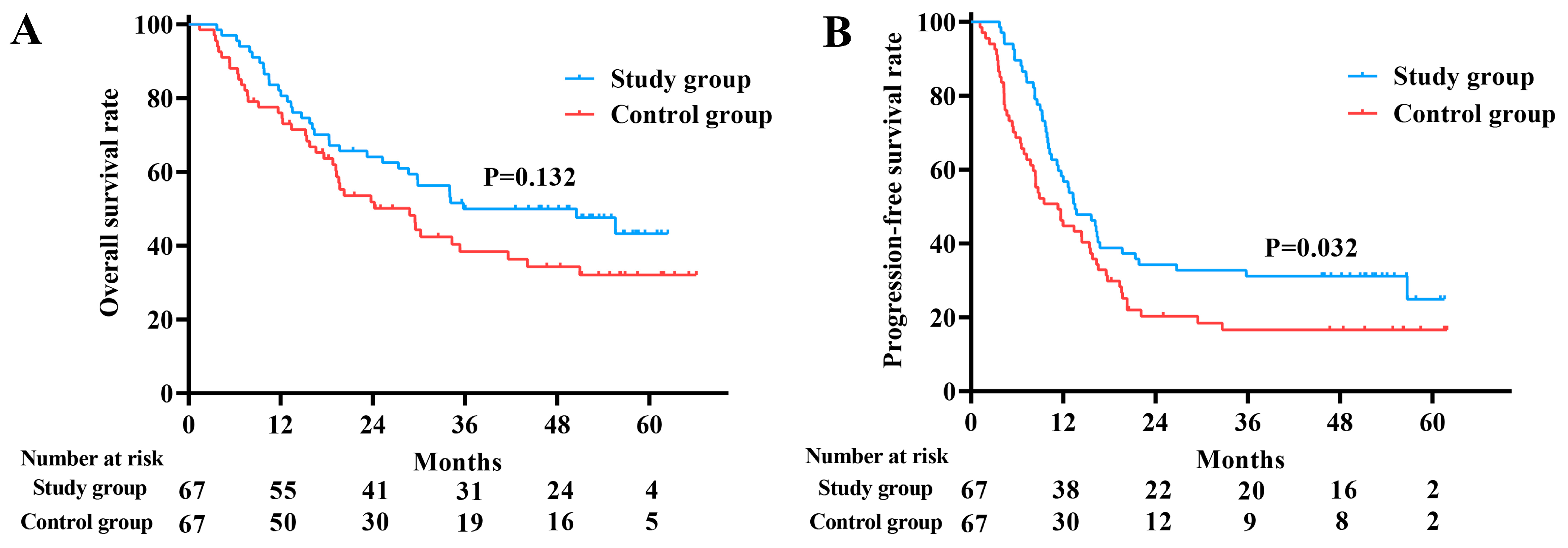
3.6. Survival Outcome
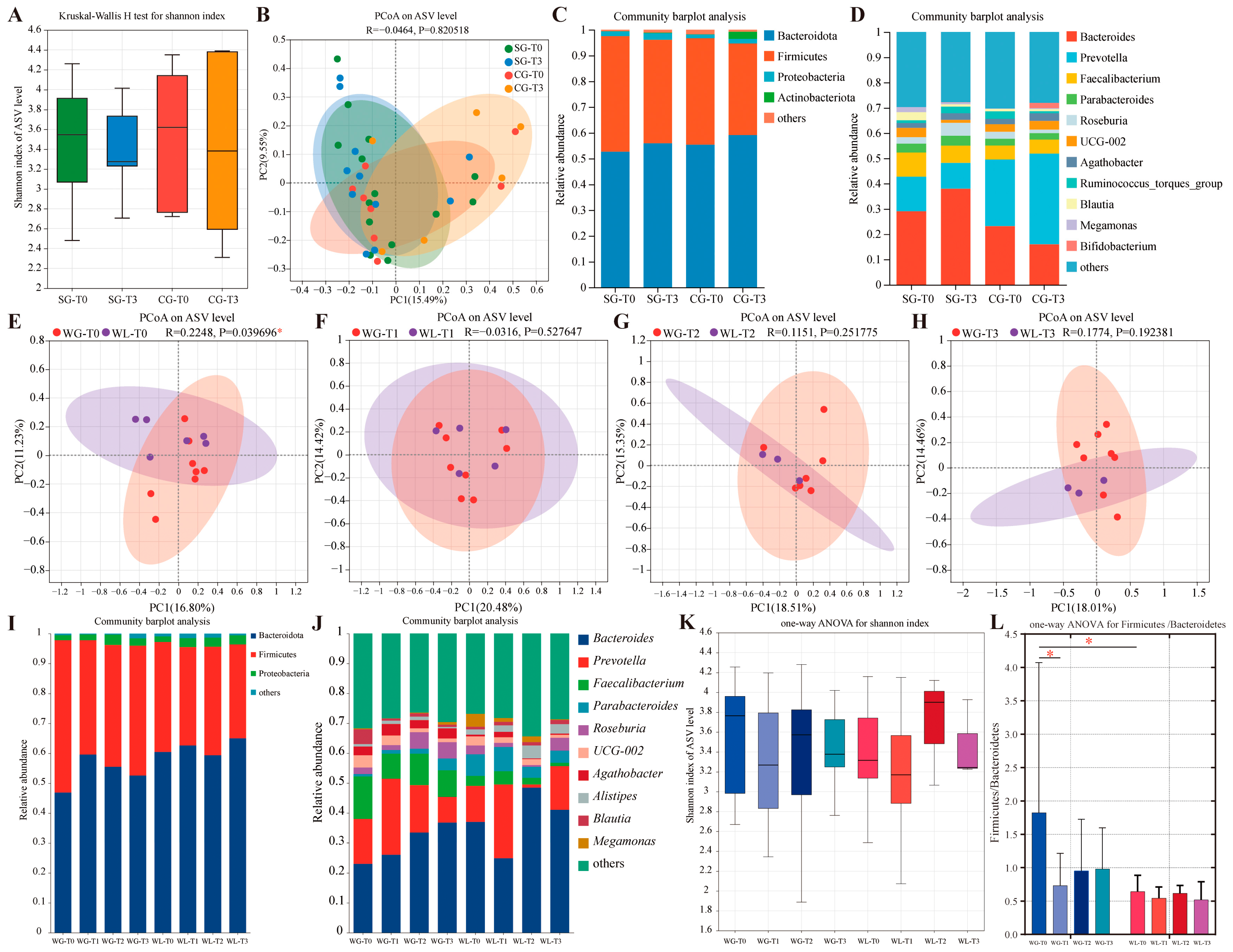
3.7. Microbiota Profiling and Exploratory Insights
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bovio, G.; Montagna, G.; Bariani, C.; Baiardi, P. Upper gastrointestinal symptoms in patients with advanced cancer: Relationship to nutritional and performance status. Support. Care Cancer 2009, 17, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.; Isenring, E.; Gough, K.; Krishnasamy, M. The prevalence of weight loss during (chemo)radiotherapy treatment for lung cancer and associated patient- and treatment-related factors. Clin. Nutr. 2014, 33, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Unsal, D.; Mentes, B.; Akmansu, M.; Uner, A.; Oguz, M.; Pak, Y. Evaluation of nutritional status in cancer patients receiving radiotherapy: A prospective study. Am. J. Clin. Oncol. 2006, 29, 183–188. [Google Scholar] [CrossRef]
- Luo, J.; Chen, Y.J.; Narsavage, G.L.; Ducatman, A. Predictors of survival in patients with non-small cell lung cancer. Oncol. Nurs. Forum 2012, 39, 609–616. [Google Scholar] [CrossRef]
- Sánchez-Lara, K.; Turcott, J.G.; Juárez, E.; Guevara, P.; Núñez-Valencia, C.; Oñate-Ocaña, L.F.; Flores, D.; Arrieta, O. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: A prospective study. Nutr. Cancer 2012, 64, 526–534. [Google Scholar] [CrossRef]
- Ma, L.; Ye, W.; Li, Q.; Wang, B.; Luo, G.; Chen, Z.; Guo, S.; Qiu, B.; Liu, H. Subjective Global Assessment (SGA) Score Could Be a Predictive Factor for Radiation Pneumonitis in Lung Cancer Patients With Normal Pulmonary Function Treated by Intensity-Modulated Radiation Therapy and Concurrent Chemotherapy. Clin. Lung Cancer 2018, 19, e211–e217. [Google Scholar] [CrossRef]
- Isenring, E.A.; Capra, S.; Bauer, J.D. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br. J. Cancer 2004, 91, 447–452. [Google Scholar] [CrossRef]
- Ravasco, P.; Monteiro-Grillo, I.; Marques Vidal, P.; Camilo, M.E. Impact of nutrition on outcome: A prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005, 27, 659–668. [Google Scholar] [CrossRef]
- Ravasco, P.; Monteiro-Grillo, I.; Vidal, P.M.; Camilo, M.E. Dietary counseling improves patient outcomes: A prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J. Clin. Oncol. 2005, 23, 1431–1438. [Google Scholar] [CrossRef]
- Kiss, N.K.; Krishnasamy, M.; Isenring, E.A. The effect of nutrition intervention in lung cancer patients undergoing chemotherapy and/or radiotherapy: A systematic review. Nutr. Cancer 2014, 66, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.J.; Hendriks, L.E.; Troost, E.G.; Bootsma, G.P.; Houben, R.M.; Schols, A.M.; Dingemans, A.-M.C. Early Weight Loss during Chemoradiotherapy Has a Detrimental Impact on Outcome in NSCLC. J. Thorac. Oncol. 2016, 11, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Qiu, B.; Xiong, M.; Liu, Y.; Peng, K.; Luo, Y.; Wang, D.; Liu, F.; Chen, N.; Guo, J.; et al. Hypofractionated Radiotherapy followed by Hypofractionated Boost with weekly concurrent chemotherapy for Unresectable Stage III Non-Small Cell Lung Cancer: Results of A Prospective Phase II Study (GASTO-1049). Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Xiong, M.; Luo, Y.; Li, Q.; Chen, N.; Chen, L.; Guo, S.; Wang, B.; Huang, X.; Lin, M.; et al. Hypofractionated Intensity Modulated Radiation Therapy With Concurrent Chemotherapy in Locally Advanced Non-Small Cell Lung Cancer: A Phase II Prospective Clinical Trial (GASTO1011). Pract. Radiat. Oncol. 2021, 11, 374–383. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Kiss, N.; Krishnasamy, M.; Everitt, S.; Gough, K.; Duffy, M.; Isenring, E. Dosimetric factors associated with weight loss during (chemo)radiotherapy treatment for lung cancer. Eur. J. Clin. Nutr. 2014, 68, 1309–1314. [Google Scholar] [CrossRef]
- Kiss, N.; Isenring, E.; Gough, K.; Wheeler, G.; Wirth, A.; Campbell, B.A.; Krishnasamy, M. Early and Intensive Dietary Counseling in Lung Cancer Patients Receiving (Chemo)Radiotherapy-A Pilot Randomized Controlled Trial. Nutr. Cancer 2016, 68, 958–967. [Google Scholar] [CrossRef]
- Bargetzi, L.; Brack, C.; Herrmann, J.; Bargetzi, A.; Hersberger, L.; Bargetzi, M.; Kaegi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; et al. Nutritional support during the hospital stay reduces mortality in patients with different types of cancers: Secondary analysis of a prospective randomized trial. Ann. Oncol. 2021, 32, 1025–1033. [Google Scholar] [CrossRef]
- Kamp, C.M.H.O.D.; De Ruysscher, D.K.M.; Heuvel, M.v.D.; Elferink, M.; Houben, R.M.A.; Oberije, C.J.G.; Bootsma, G.P.; Geraedts, W.H.; Pitz, C.C.M.; Langen, R.C.; et al. Early body weight loss during concurrent chemo-radiotherapy for non-small cell lung cancer. J. Cachexia Sarcopenia Muscle 2014, 5, 127–137. [Google Scholar] [CrossRef]
- Oswalt, C.; Liu, Y.; Pang, H.; Le-Rademacher, J.; Wang, X.; Crawford, J. Associations between body mass index, weight loss and overall survival in patients with advanced lung cancer. J. Cachexia Sarcopenia Muscle 2022, 13, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Pereira, J.; Chen, J.; Liu, J.; Guba, S.; John, W.; Orlando, M.; Scagliotti, G.; Bonomi, P. Relationship between efficacy outcomes and weight gain during treatment of advanced, non-squamous, non-small-cell lung cancer patients. Ann. Oncol. 2016, 27, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Le-Rademacher, J.; Lopez, C.; Wolfe, E.; Foster, N.R.; Mandrekar, S.J.; Wang, X.; Kumar, R.; Adjei, A.; Jatoi, A. Weight loss over time and survival: A landmark analysis of 1000+ prospectively treated and monitored lung cancer patients. J. Cachexia Sarcopenia Muscle 2020, 11, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.; Pardo, J.; Jara, C.; Zugazabeitia, L.; Carulla, J.; Peñas, R.d.L.; García-Cabrera, E.; Azuara, M.L.; Casadó, J.; Gómez-Candela, C. An epidemiological evaluation of the prevalence of malnutrition in Spanish patients with locally advanced or metastatic cancer. Clin. Nutr. 2005, 24, 801–814. [Google Scholar] [CrossRef]
- Kawai, H.; Ota, H. Low perioperative serum prealbumin predicts early recurrence after curative pulmonary resection for non-small-cell lung cancer. World J. Surg. 2012, 36, 2853–2857. [Google Scholar] [CrossRef]
- Szczyrek, M.; Bitkowska, P.; Chunowski, P.; Czuchryta, P.; Krawczyk, P.; Milanowski, J. Diet, Microbiome, and Cancer Immunotherapy-A Comprehensive Review. Nutrients 2021, 13, 2217. [Google Scholar] [CrossRef]
- Ubachs, J.; Ziemons, J.; Soons, Z.; Aarnoutse, R.; van Dijk, D.P.; Penders, J.; van Helvoort, A.; Smidt, M.L.; Kruitwagen, R.F.; Baade-Corpelijn, L.; et al. Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J. Cachexia Sarcopenia Muscle 2021, 12, 2007–2021. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]

| Characteristic | Study Group | Control Group | p Value |
|---|---|---|---|
| (n = 67) | (n = 67) | ||
| Age, yrs | |||
| Median (IQR) | 58 (51–65) | 58 (52–67) | 0.561 |
| Gender, n (%) | |||
| Male | 53 (79.1) | 50 (74.6) | 0.539 |
| Female | 14 (20.9) | 17 (25.4) | |
| ECOG, n (%) | |||
| 0 | 30 (44.8) | 34 (50.7) | 0.489 |
| 1 | 37 (55.2) | 33 (49.3) | |
| Smoking history, n (%) | |||
| Smoker | 45 (67.2) | 41 (61.2) | 0.471 |
| Non-smoker | 22 (32.8) | 26 (38.8) | |
| Diabetes mellitus, n (%) | |||
| Yes | 9 (13.4) | 8 (11.9) | 0.795 |
| No | 58 (86.6) | 59 (88.1) | |
| Histology, n (%) | |||
| Adenocarcinoma | 23 (34.3) | 23 (34.3) | 0.971 |
| Squamous cell carcinoma | 32 (47.8) | 33 (49.3) | |
| Others | 12 (17.9) | 11 (16.4) | |
| Disease stage, n (%) | |||
| IIIA | 24 (35.8) | 25 (37.3) | 0.938 |
| IIIB | 26 (38.8) | 24 (35.8) | |
| IIIC | 17 (25.4) | 18 (26.9) | |
| PG-SGA, n (%) | |||
| A | 40 (59.7) | 35 (52.2) | 0.384 |
| B | 27 (40.3) | 32 (47.8) | |
| BMI, kg/m2 | |||
| Mean (SD) | 23.79 (2.79) | 23.65 (2.97) | 0.772 |
| Variables | Baseline | End of CCRT | 6 Months After CCRT | |
|---|---|---|---|---|
| Median (IQR)/Mean (SD) | ||||
| Weight (kg) | Study group | 62.61 (8.58) | 63.67 (8.18) | 63.73 (7.94) |
| Control group | 62.00 (9.53) | 60.00 (8.52) | 60.35 (6.85) | |
| p value | 0.708 | 0.024 | 0.082 | |
| BMI (kg/m2) | Study group | 23.79 (2.79) | 24.13 (2.75) | 24.38 (2.80) |
| Control group | 23.65 (2.97) | 23.03 (2.51) | 23.27 (2.44) | |
| p value | 0.772 | 0.034 | 0.106 | |
| ALB (g/L) | Study group | 43.68 (2.90) | 41.24 (3.49) | 43.23 (3.57) |
| Control group | 43.19 (3.50) | 41.21 (2.24) | 42.02 (3.94) | |
| p value | 0.381 | 0.965 | 0.145 | |
| PA (mg/dL) | Study group | 26.53 (4.68) | 28.59 (5.04) | 26.92 (4.92) |
| Control group | 25.30 (5.38) | 25.86 (5.92) | 25.49 (6.51) | |
| p value | 0.189 | 0.014 | 0.352 | |
| LY × 109/L | Study group | 1.77 (0.58) | 0.58 (0.21) | 1.26 (0.53) |
| Control group | 1.71 (0.64) | 0.52 (0.22) | 1.16 (0.59) | |
| p value | 0.600 | 0.152 | 0.435 | |
| HGB (g/L) | Study group | 130 (19) | 127 (15) | 135 (15) |
| Control group | 129 (16) | 125 (11) | 131 (20) | |
| p value | 0.671 | 0.391 | 0.364 | |
| NLR | Study group | 2.07 (1.58–3.28) | 6.34 (4.70–8.53) | 3.42 (2.29–5.01) |
| Control group | 2.52 (1.85–3.83) | 8.00 (5.96–10.66) | 4.17 (2.44–6.51) | |
| p value | 0.062 | 0.011 | 0.285 | |
| CRP (mg/L) | Study group | 2.93 (1.60–9.00) | 4.27 (2.12–10.74) | 7.12 (2.84–23.29) |
| Control group | 4.01 (1.47–17.49) | 5.38 (2.88–13.82) | 6.46 (2.52–24.28) | |
| p value | 0.368 | 0.185 | 0.910 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Luo, Q.; Xi, Y.; Zhang, P.; Wu, Y.; Guo, S.; Dong, Y.; Wang, D.; Wu, Q.; Liu, H.; et al. Early Nutritional Intervention in Patients with Non-Small Cell Lung Cancer Receiving Concurrent Chemoradiotherapy: A Phase II Prospective Study. Nutrients 2025, 17, 1389. https://doi.org/10.3390/nu17081389
Liu F, Luo Q, Xi Y, Zhang P, Wu Y, Guo S, Dong Y, Wang D, Wu Q, Liu H, et al. Early Nutritional Intervention in Patients with Non-Small Cell Lung Cancer Receiving Concurrent Chemoradiotherapy: A Phase II Prospective Study. Nutrients. 2025; 17(8):1389. https://doi.org/10.3390/nu17081389
Chicago/Turabian StyleLiu, Fangjie, Qiaoting Luo, Yu Xi, Pengxin Zhang, Yingjia Wu, Suping Guo, Yaoling Dong, Daquan Wang, Qingping Wu, Hui Liu, and et al. 2025. "Early Nutritional Intervention in Patients with Non-Small Cell Lung Cancer Receiving Concurrent Chemoradiotherapy: A Phase II Prospective Study" Nutrients 17, no. 8: 1389. https://doi.org/10.3390/nu17081389
APA StyleLiu, F., Luo, Q., Xi, Y., Zhang, P., Wu, Y., Guo, S., Dong, Y., Wang, D., Wu, Q., Liu, H., Rong, Y., & Qiu, B. (2025). Early Nutritional Intervention in Patients with Non-Small Cell Lung Cancer Receiving Concurrent Chemoradiotherapy: A Phase II Prospective Study. Nutrients, 17(8), 1389. https://doi.org/10.3390/nu17081389






