A Gut Microbial Metabolite HYA Ameliorates Adipocyte Hypertrophy by Activating AMP-Activated Protein Kinase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Study
2.2. Histological Analysis
2.3. Cell Culture
2.4. Oil Red O Staining
2.5. RT-qPCR Analysis
2.6. Immunoblot Analysis
2.7. Calcium Imaging
2.8. Statistical Analysis
3. Result
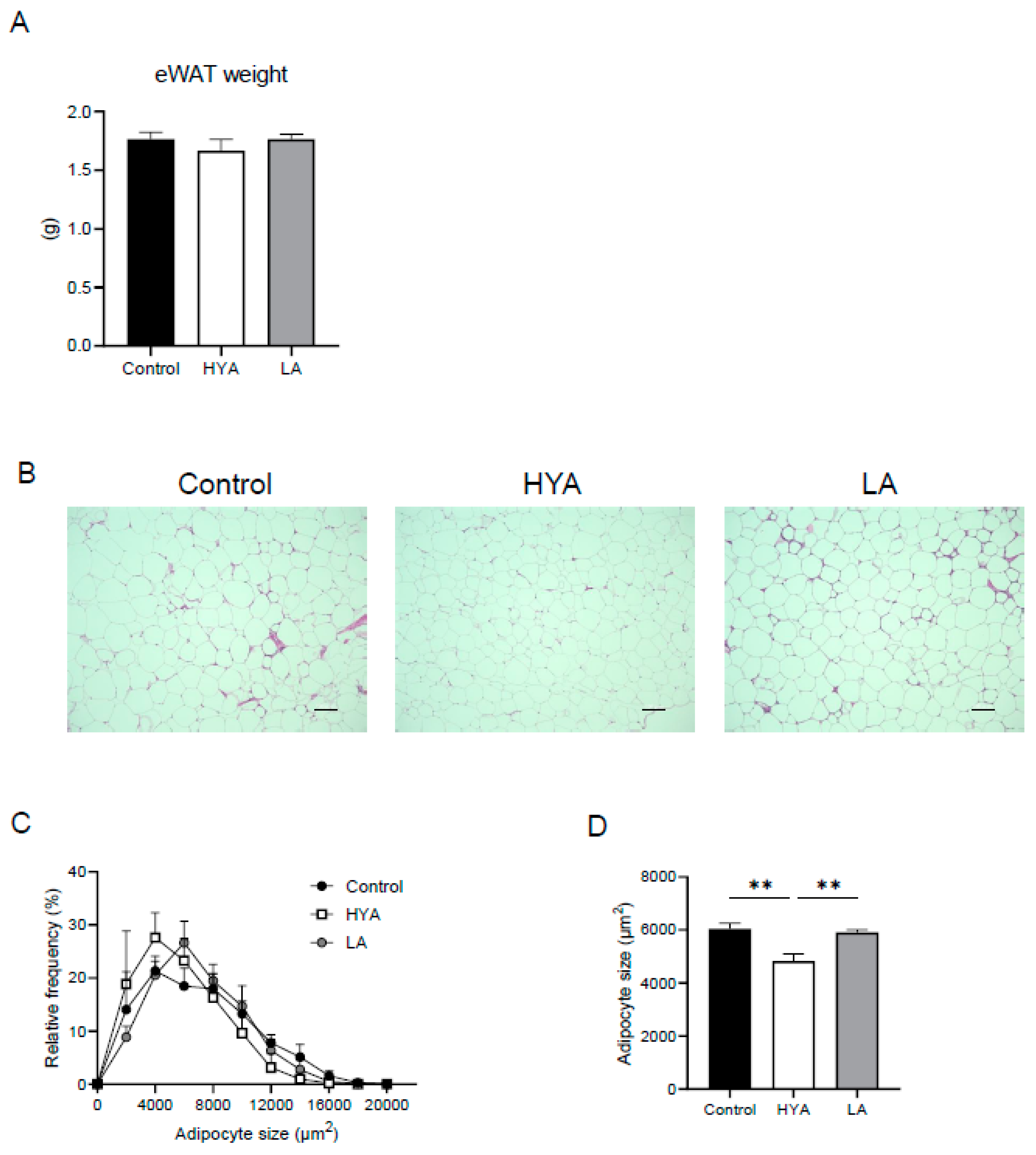
3.1. HYA Inhibits Adipocyte Hypertrophy in Mice Fed a High-Fat Diet
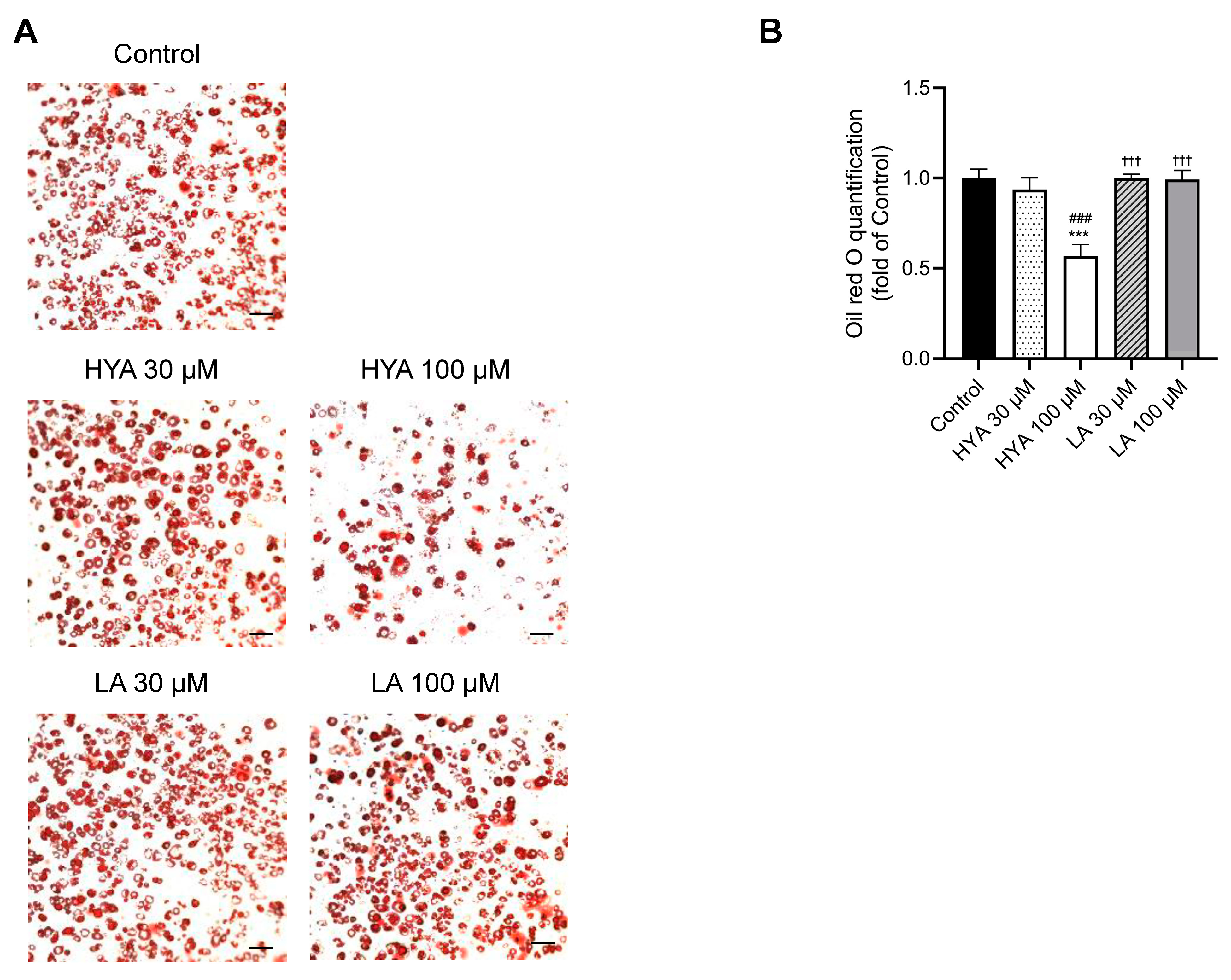
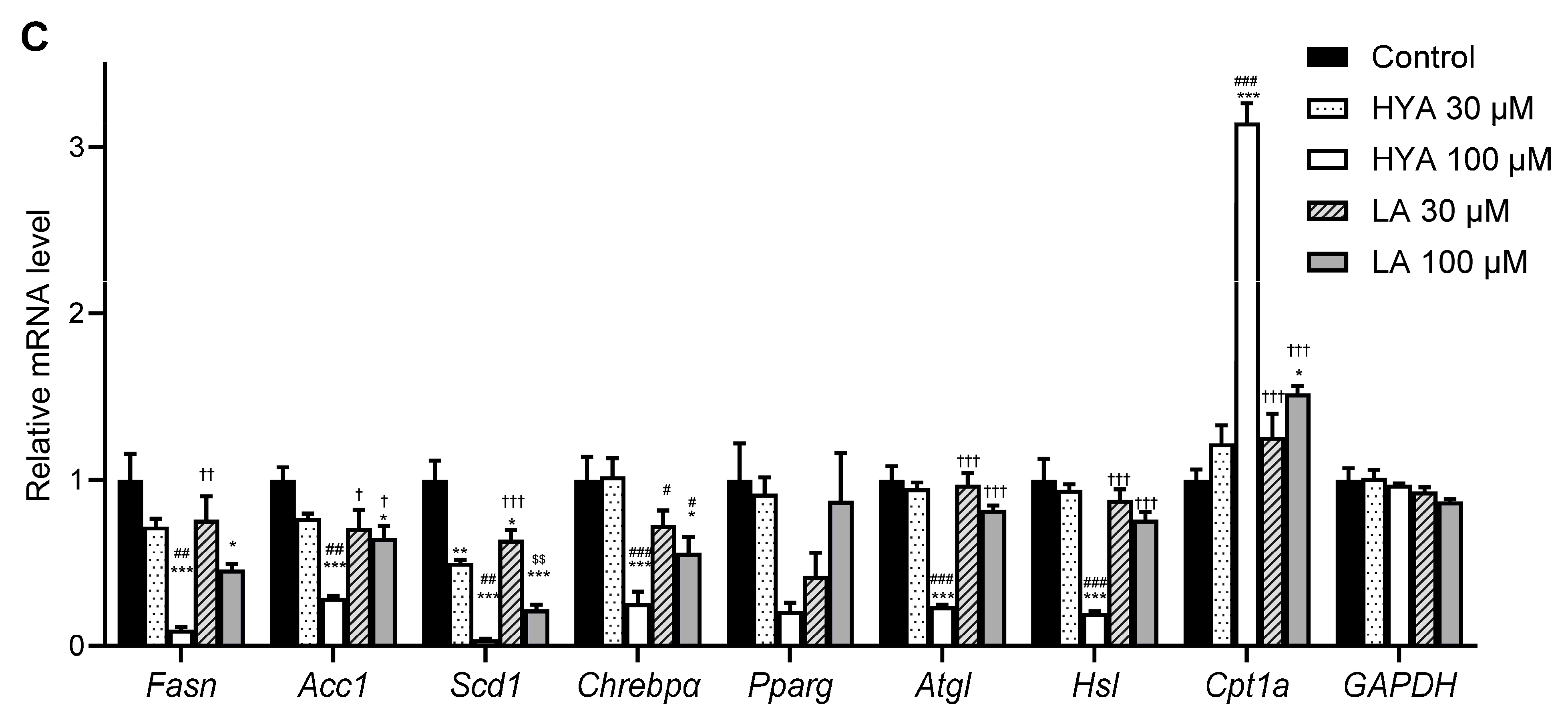
3.2. HYA Decreases the Amount of Lipid Content in Cultured Adipocytes
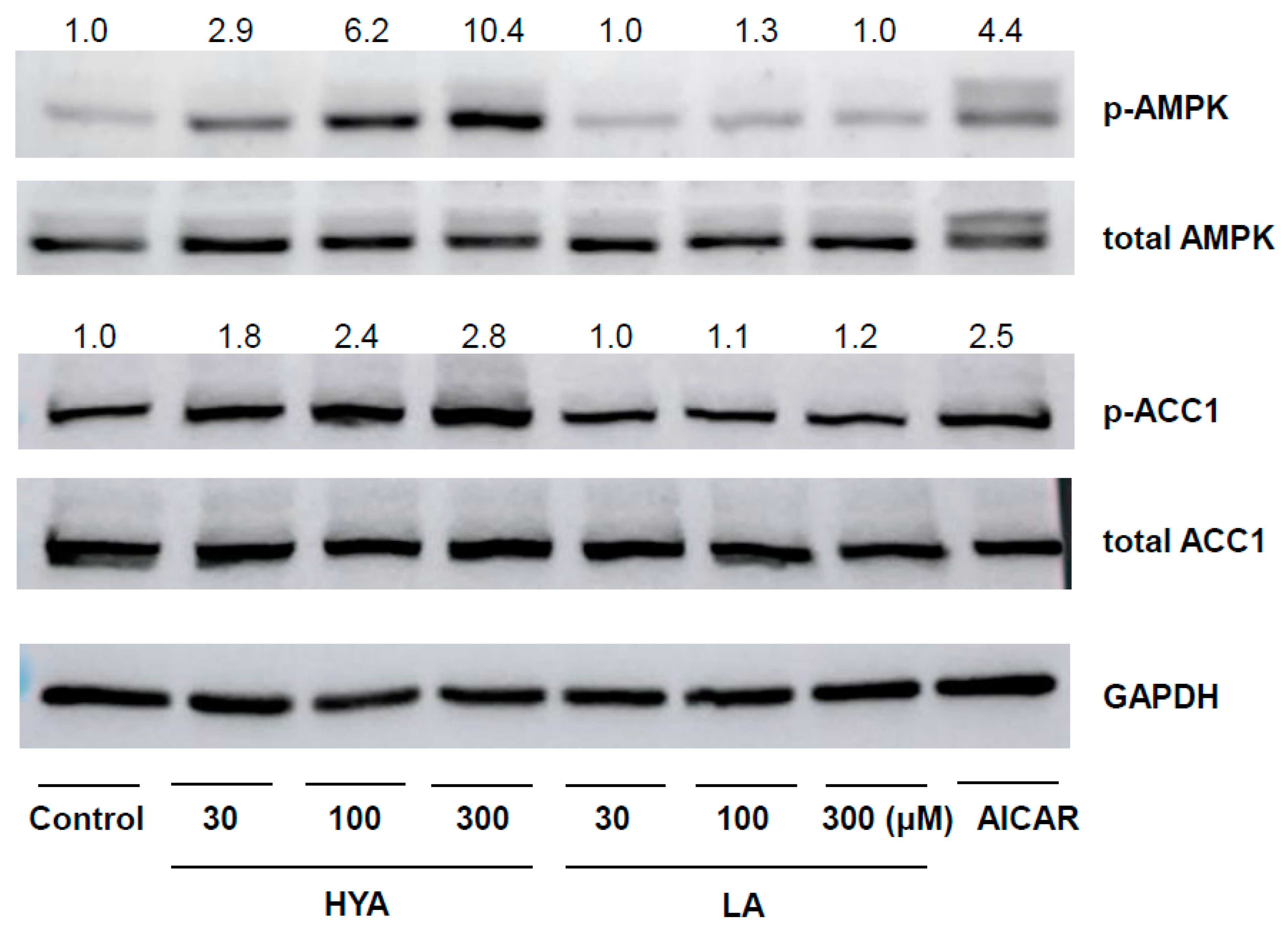
3.3. HYA Activates AMP Kinase in Adipocytes
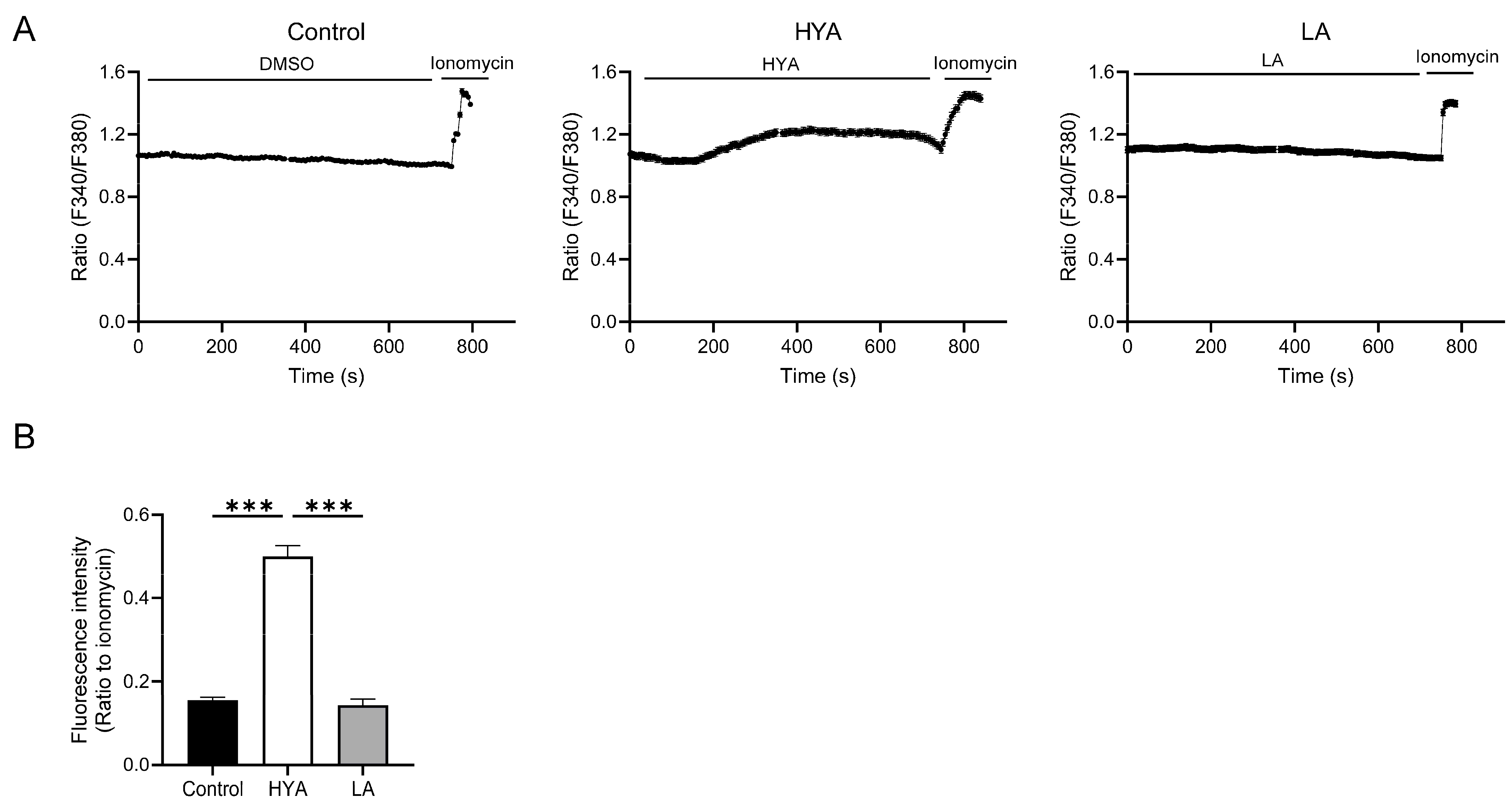
3.4. HYA Activates AMPK by Increasing Intracellular Ca2+ Concentration
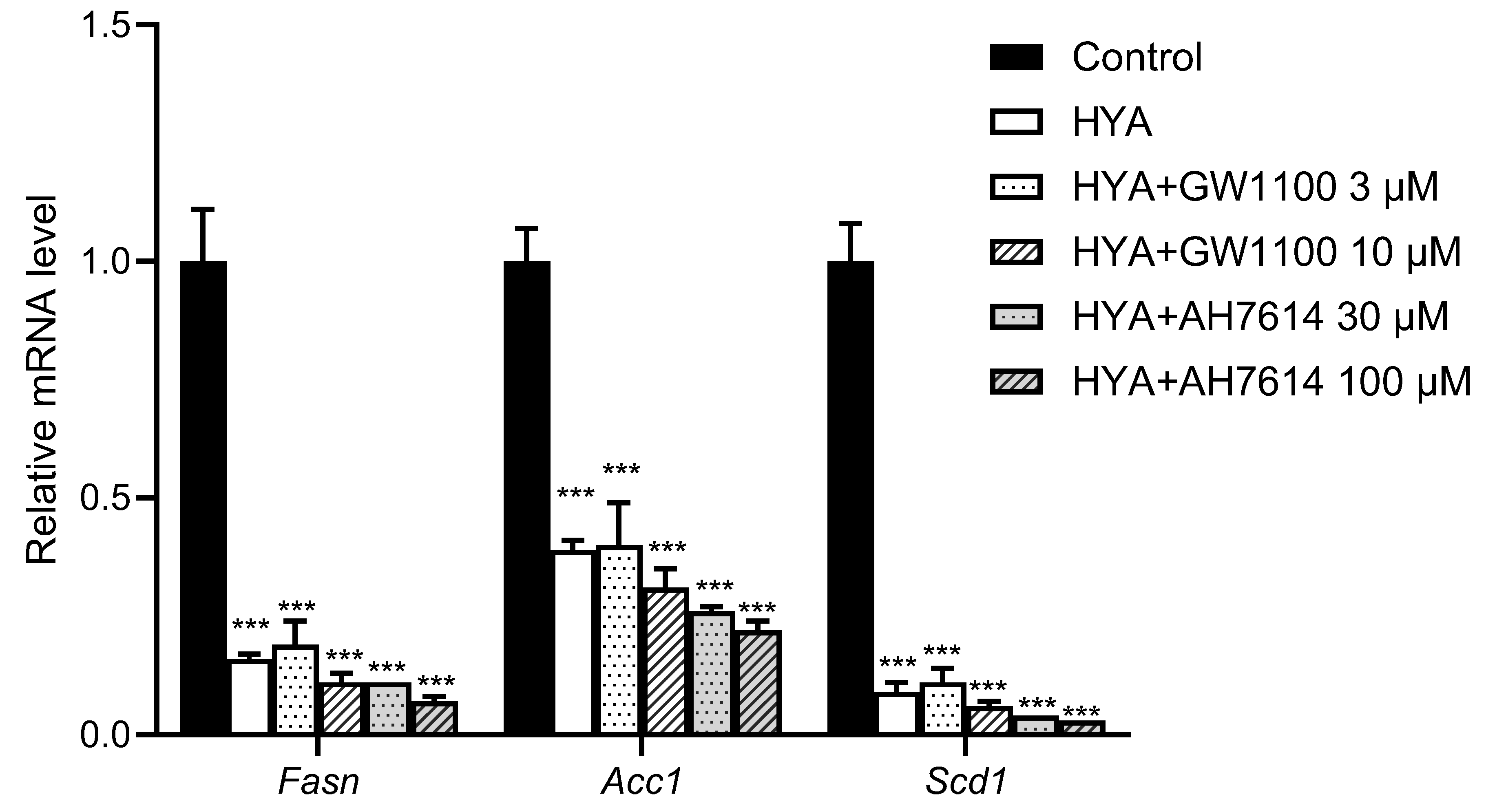
3.5. HYA Reduces Lipid Content in Adipocytes by a Mechanism Independent of GPR40 and GPR120
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC1 | acetyl-CoA carboxylase 1 |
| AhR | aryl hydrocarbon receptor |
| ATGL | adipose triglyceride lipase |
| ATP | adenosine triphosphate |
| AMP | adenosine monophosphate |
| CLA-HY | conjugated linoleic acid hydrase |
| ChREBPα | carbohydrate-responsive element-binding protein α |
| CPT1A | carnitine acyltransferase 1A |
| FAS | fatty acid synthase |
| GLP-1 | glucagon-like peptide-1 |
| GPCR | G protein-coupled receptor |
| GPR40 | G protein-coupled receptor 40 |
| GPR120 | G protein-coupled receptor 120 |
| HSL | hormone-sensitive lipase |
| HYA | 10-hydroxy-cis-12-octadecenoic acid |
| LA | linoleic acid |
| MASH | metabolic dysfunction-associated steatohepatitis |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| SCD1 | stearoyl-CoA desaturase 1 |
| TGF-β | transforming growth factor-β |
| TRPV1 | transient receptor potential vanilloid 1 |
References
- Reaven, G.M. Insulin resistance and human disease: A short history. J. Basic. Clin. Physiol. Pharmacol. 1998, 9, 387–406. [Google Scholar] [CrossRef]
- Kaplan, N.M. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch. Intern. Med. 1989, 149, 1514–1520. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Jiao, L.; Berrington de Gonzalez, A.; Hartge, P.; Pfeiffer, R.M.; Park, Y.; Freedman, D.M.; Gail, M.H.; Alavanja, M.C.; Albanes, D.; Beane Freeman, L.E.; et al. Body mass index, effect modifiers, and risk of pancreatic cancer: A pooled study of seven prospective cohorts. Cancer Causes Control 2010, 21, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R. Triglycerides and toggling the tummy. Nat. Genet. 2000, 25, 6–7. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Flier, J.S. Obesity and the regulation of energy balance. Cell 2001, 104, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Santoro, A.; McGraw, T.E.; Kahn, B.B. Insulin action in adipocytes, adipose remodeling, and systemic effects. Cell Metab. 2021, 33, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Descamps, H.C.; Herrmann, B.; Wiredu, D.; Thaiss, C.A. The path toward using microbial metabolites as therapies. EBioMedicine 2019, 44, 747–754. [Google Scholar] [CrossRef]
- Rastogi, S.; Singh, A. Gut microbiome and human health: Exploring how the probiotic genus Lactobacillus modulate immune responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Zhang, D.; Qi, S.; Liu, Y. Metabolite interactions between host and microbiota during health and disease: Which feeds the other? Biomed. Pharmacother. 2023, 160, 114295. [Google Scholar] [CrossRef]
- Kishino, S.; Takeuchi, M.; Park, S.B.; Hirata, A.; Kitamura, N.; Kunisawa, J.; Kiyono, H.; Iwamoto, R.; Isobe, Y.; Arita, M.; et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc. Natl. Acad. Sci. USA 2013, 110, 17808–17813. [Google Scholar] [CrossRef]
- Kaikiri, H.; Miyamoto, J.; Kawakami, T.; Park, S.B.; Kitamura, N.; Kishino, S.; Yonejima, Y.; Hisa, K.; Watanabe, J.; Ogita, T.; et al. Supplemental feeding of a gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, alleviates spontaneous atopic dermatitis and modulates intestinal microbiota in NC/nga mice. Int. J. Food Sci. Nutr. 2017, 68, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Mizukure, T.; Park, S.B.; Kishino, S.; Kimura, I.; Hirano, K.; Bergamo, P.; Rossi, M.; Suzuki, T.; Arita, M.; et al. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J. Biol. Chem. 2015, 290, 2902–2918. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.I.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019, 10, 4007. [Google Scholar] [CrossRef]
- Nanthirudjanar, T.; Furumoto, H.; Zheng, J.; Kim, Y.I.; Goto, T.; Takahashi, N.; Kawada, T.; Park, S.B.; Hirata, A.; Kitamura, N.; et al. Gut Microbial Fatty Acid Metabolites Reduce Triacylglycerol Levels in Hepatocytes. Lipids 2015, 50, 1093–1102. [Google Scholar] [CrossRef]
- Kasahara, N.; Imi, Y.; Amano, R.; Shinohara, M.; Okada, K.; Hosokawa, Y.; Imamori, M.; Tomimoto, C.; Kunisawa, J.; Kishino, S.; et al. A gut microbial metabolite of linoleic acid ameliorates liver fibrosis by inhibiting TGF-beta signaling in hepatic stellate cells. Sci. Rep. 2023, 13, 18983. [Google Scholar] [CrossRef]
- Hosooka, T.; Noguchi, T.; Kotani, K.; Nakamura, T.; Sakaue, H.; Inoue, H.; Ogawa, W.; Tobimatsu, K.; Takazawa, K.; Sakai, M.; et al. Dok1 mediates high-fat diet-induced adipocyte hypertrophy and obesity through modulation of PPAR-gamma phosphorylation. Nat. Med. 2008, 14, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Kita, T.; Uchida, K.; Kato, K.; Suzuki, Y.; Tominaga, M.; Yamazaki, J. FK506 (tacrolimus) causes pain sensation through the activation of transient receptor potential ankyrin 1 (TRPA1) channels. J. Physiol. Sci. 2019, 69, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Hardie, D.G. New insights into activation and function of the AMPK. Nat. Rev. Mol. Cell Biol. 2023, 24, 255–272. [Google Scholar] [CrossRef]
- Hardie, D.G. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008, 582, 81–89. [Google Scholar] [CrossRef] [PubMed]
- McAloon, L.M.; Muller, A.G.; Nay, K.; Lu, E.L.; Smeuninx, B.; Means, A.R.; Febbraio, M.A.; Scott, J.W. CaMKK2: Bridging the gap between Ca2+ signaling and energy-sensing. Essays Biochem. 2024, 68, 309–320. [Google Scholar]
- Oteng, A.B.; Liu, L. GPCR-mediated effects of fatty acids and bile acids on glucose homeostasis. Front. Endocrinol. 2023, 14, 1206063. [Google Scholar] [CrossRef]
- Kim, M.; Furuzono, T.; Yamakuni, K.; Li, Y.; Kim, Y.I.; Takahashi, H.; Ohue-Kitano, R.; Jheng, H.F.; Takahashi, N.; Kano, Y.; et al. 10-oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, enhances energy metabolism by activation of TRPV1. FASEB J. 2017, 31, 5036–5048. [Google Scholar] [CrossRef]
- Sun, W.; Yu, Z.; Yang, S.; Jiang, C.; Kou, Y.; Xiao, L.; Tang, S.; Zhu, T. A Transcriptomic Analysis Reveals Novel Patterns of Gene Expression During 3T3-L1 Adipocyte Differentiation. Front. Mol. Biosci. 2020, 7, 564339. [Google Scholar] [CrossRef]
- Sladekova, L.; Mani, S.; Dvorak, Z. Ligands and agonists of the aryl hydrocarbon receptor AhR: Facts and myths. Biochem. Pharmacol. 2023, 213, 115626. [Google Scholar] [CrossRef]
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef]
- Denison, M.S.; Soshilov, A.A.; He, G.; DeGroot, D.E.; Zhao, B. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 2011, 124, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Perdew, G.H. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes 2020, 12, 1859812. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.L.; Ganem, L.G.; Fernandez-Salguero, P.; Gonzalez, F.; Jefcoate, C.R. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J. Cell Sci. 1998, 111 Pt 22, 3311–3322. [Google Scholar] [CrossRef]
- Dou, H.; Duan, Y.; Zhang, X.; Yu, Q.; Di, Q.; Song, Y.; Li, P.; Gong, Y. Aryl hydrocarbon receptor (AhR) regulates adipocyte differentiation by assembling CRL4B ubiquitin ligase to target PPARgamma for proteasomal degradation. J. Biol. Chem. 2019, 294, 18504–18515. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: The diverse biology of PPARgamma. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Goransson, O.; Kopietz, F.; Rider, M.H. Metabolic control by AMPK in white adipose tissue. Trends Endocrinol. Metab. 2023, 34, 704–717. [Google Scholar] [CrossRef]
- Kim, S.J.; Tang, T.; Abbott, M.; Viscarra, J.A.; Wang, Y.; Sul, H.S. AMPK Phosphorylates Desnutrin/ATGL and Hormone-Sensitive Lipase To Regulate Lipolysis and Fatty Acid Oxidation within Adipose Tissue. Mol. Cell Biol. 2016, 36, 1961–1976. [Google Scholar] [CrossRef]
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| Fasn | GCTGGCATTCGTGATGGAGTCGT | AGGCCACCAGTGATGATGTAACTCT |
| Acc1 | GATGAACCATCTCCGTTGGC | GACCCAATTATGAATCGGGAGTG |
| Scd1 | TTCTTGCGATACACTCTGGTGC | CGGGATTGAATGTTCTTGTCGT |
| Chrebpα | CACCTCTTCGAGTGCTTGAG | CATAGCAACTTGAGGCCTTTG |
| Pparg | GGGGATGTCTCACAATGCCA | CAGACTCTGGGTTCAGCTGG |
| Atgl | GCCAACGCCACTCACATCTA | AATGTTGGCACCTGCTTCAC |
| Hsl | TATGGAGTGAGGGTGCCAGA | ATGGTCCTCTGCCTCTGTCC |
| Cpt1a | GCTGCTTCCCCTCACAAGTTCC | GCTTTGGCTGCCTGTGTCAGTATGC |
| Gapdh | AACTTTGGCATTGTGGAAGG | GGATGCAGGGATGATGTTCT |
| Rplp0 | GAGGAATCAGATGAGGATATGGGA | AAGCAGGCTGACTTGGTTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsushita, R.; Sato, K.; Uchida, K.; Imi, Y.; Amano, R.; Kasahara, N.; Kitao, Y.; Oishi, Y.; Kawaai, H.; Tomimoto, C.; et al. A Gut Microbial Metabolite HYA Ameliorates Adipocyte Hypertrophy by Activating AMP-Activated Protein Kinase. Nutrients 2025, 17, 1393. https://doi.org/10.3390/nu17081393
Matsushita R, Sato K, Uchida K, Imi Y, Amano R, Kasahara N, Kitao Y, Oishi Y, Kawaai H, Tomimoto C, et al. A Gut Microbial Metabolite HYA Ameliorates Adipocyte Hypertrophy by Activating AMP-Activated Protein Kinase. Nutrients. 2025; 17(8):1393. https://doi.org/10.3390/nu17081393
Chicago/Turabian StyleMatsushita, Rino, Kaori Sato, Kunitoshi Uchida, Yukiko Imi, Reina Amano, Nanaho Kasahara, Yuki Kitao, Yuki Oishi, Honoka Kawaai, Chiaki Tomimoto, and et al. 2025. "A Gut Microbial Metabolite HYA Ameliorates Adipocyte Hypertrophy by Activating AMP-Activated Protein Kinase" Nutrients 17, no. 8: 1393. https://doi.org/10.3390/nu17081393
APA StyleMatsushita, R., Sato, K., Uchida, K., Imi, Y., Amano, R., Kasahara, N., Kitao, Y., Oishi, Y., Kawaai, H., Tomimoto, C., Hosokawa, Y., Kishino, S., Ogawa, J., & Hosooka, T. (2025). A Gut Microbial Metabolite HYA Ameliorates Adipocyte Hypertrophy by Activating AMP-Activated Protein Kinase. Nutrients, 17(8), 1393. https://doi.org/10.3390/nu17081393






