Enhancing Bone Health with Conjugated Linoleic Acid: Mechanisms, Challenges, and Innovative Strategies
Abstract
:1. Introduction
2. Databases and Literature Search Strategy
3. CLA and Bone Health
3.1. Pre-Clinical Studies
3.2. Clinical Trial
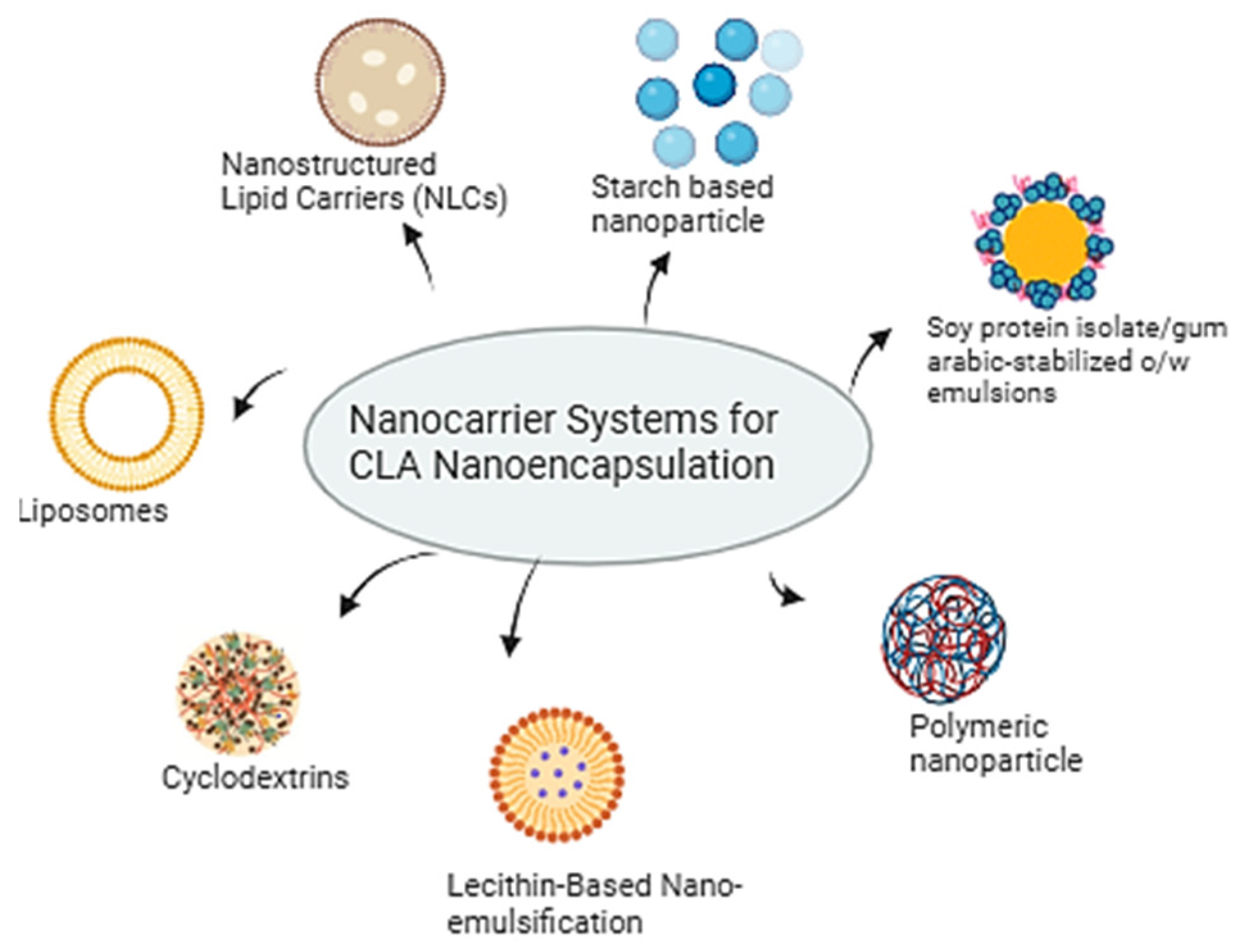
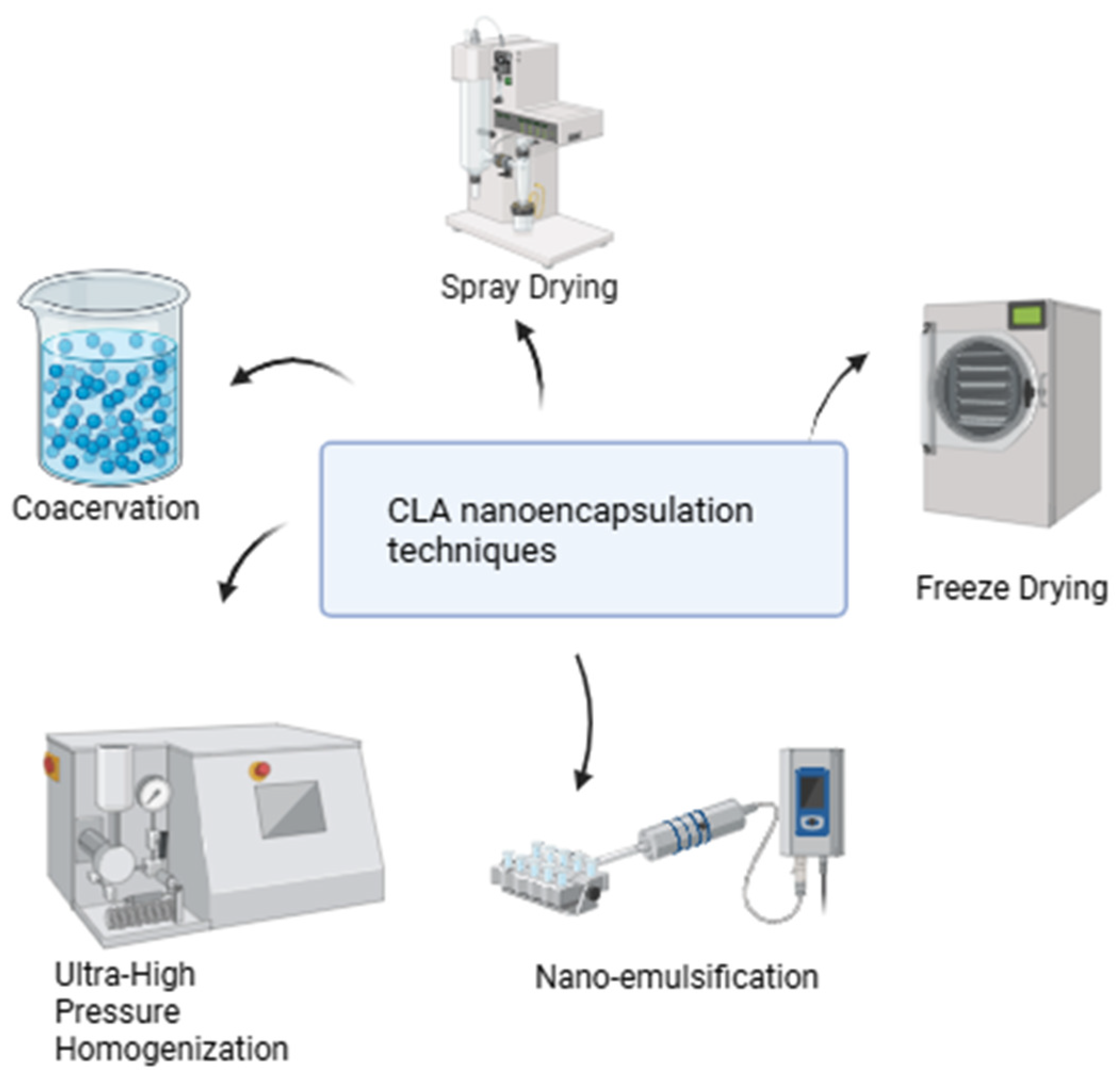
4. Enhancing CLA Efficacy Through Nanoparticle-Delivery Systems
5. Electrical Stimulation (ES) as an Effective Strategy in Maintaining Bone Health
5.1. Combination of Electrical Stimulation and CLA on Bone Health
5.2. Optimizing Osteogenesis and Diminishing Inflammation with Combined Electrical Stimulation and Conjugated Linoleic Acid Techniques
5.3. The Potential for Electrical Stimulation to Enhance Bone Tissue Absorption and CLA Effectiveness
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Badawy, S.; Liu, Y.; Guo, M.; Liu, Z.; Xie, C.; Marawan, M.A.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Maximiliano, J.-E.; et al. Conjugated linoleic acid (CLA) as a functional food: Is it beneficial or not? Food Res. Int. 2023, 172, 113158. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Wei, W.; Hu, Y.; Jin, Q.; Wang, X. Structure determination of conjugated linoleic and linolenic acids. J. Chromatogr. B 2020, 1153, 122292. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, S.; Provenza, F.D.; Kronberg, S.L. Health-Promoting Phytonutrients Are Higher in Grass-Fed Meat and Milk. Front. Sustain. Food Syst. 2021, 4, 555426. [Google Scholar] [CrossRef]
- McCrorie, T.A.; Keaveney, E.M.; Wallace, J.M.W.; Binns, N.; Livingstone, M.B.E. Human health effects of conjugated linoleic acid from milk and supplements. Nutr. Res. Rev. 2011, 24, 206–227. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Wang, H.; Dai, J.; Li, X.; Guan, M.; Gao, S.; Ding, Q.; Wang, H.; Fang, H. Conjugated linoleic acid prevents age-induced bone loss in mice by regulating both osteoblastogenesis and adipogenesis. Biochem. Biophys. Res. Commun. 2017, 490, 813–820. [Google Scholar] [CrossRef]
- Iorizzo, M.; Di Martino, C.; Letizia, F.; Crawford, T.W.; Paventi, G. Production of Conjugated Linoleic Acid (CLA) by Lactiplantibacillus plantarum: A Review with Emphasis on Fermented Foods. Foods 2024, 13, 975. [Google Scholar] [CrossRef]
- Gaullier, J.-M.; Halse, J.; Høye, K.; Kristiansen, K.; Fagertun, H.; Vik, H.; Gudmundsen, O. Supplementation with Conjugated Linoleic Acid for 24 Months Is Well Tolerated by and Reduces Body Fat Mass in Healthy, Overweight Humans. J. Nutr. 2005, 135, 778–784. [Google Scholar] [CrossRef]
- Nakamura, Y.K.; Flintoff-Dye, N.; Omaye, S.T. Conjugated linoleic acid modulation of risk factors associated with atherosclerosis. Nutr. Metab. 2008, 5, 22. [Google Scholar] [CrossRef]
- Park, Y.; Kim, J.; Scrimgeour, A.G.; Condlin, M.L.; Kim, D.; Park, Y. Conjugated linoleic acid and calcium co-supplementation improves bone health in ovariectomised mice. Food Chem. 2013, 140, 280–288. [Google Scholar] [CrossRef]
- Maciel, G.B.M.; Maciel, R.M.; Danesi, C.C. Bone cells and their role in physiological remodeling. Mol. Biol. Rep. 2023, 50, 2857–2863. [Google Scholar] [CrossRef]
- Walsh, J.S. Normal bone physiology, remodelling and its hormonal regulation. Surgery 2015, 33, 1–6. [Google Scholar] [CrossRef]
- Rahman, M.M.; Fernandes, G.; Williams, P. Conjugated Linoleic Acid Prevents Ovariectomy-Induced Bone Loss in Mice by Modulating Both Osteoclastogenesis and Osteoblastogenesis. Lipids 2014, 49, 211–224. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.; Lee, S.-H.; Park, Y. trans-10,cis-12 Conjugated linoleic acid promotes bone formation by inhibiting adipogenesis by peroxisome proliferator activated receptor-γ-dependent mechanisms and by directly enhancing osteoblastogenesis from bone marrow mesenchymal stem cells. J. Nutr. Biochem. 2013, 24, 672–679. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bhattacharya, A.; Fernandes, G. Conjugated linoleic acid inhibits osteoclast differentiation of RAW264.7 cells by modulating RANKL signaling. J. Lipid Res. 2006, 47, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Brownbill, R.A.; Petrosian, M.; Ilich, J.Z. Association between dietary conjugated linoleic acid and bone mineral density in postmenopausal women. J. Am. Coll. Nutr. 2005, 24, 177–181. [Google Scholar] [CrossRef]
- Doyle, L.; Jewell, C.; Mullen, A.; Nugent, A.P.; Roche, H.M.; Cashman, K.D. Effect of dietary supplementation with conjugated linoleic acid on markers of calcium and bone metabolism in healthy adult men. Eur. J. Clin. Nutr. 2005, 59, 432–440. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Banu, J.; Rahman, M.; Causey, J.; Fernandes, G. Biological effects of conjugated linoleic acids in health and disease. J. Nutr. Biochem. 2006, 17, 789–810. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Overman, A.; LaPoint, K.; Hopkins, R.; West, T.; Chuang, C.-C.; Martinez, K.; Bell, D.; McIntosh, M. Conjugated linoleic acid-mediated inflammation and insulin resistance in human adipocytes are attenuated by resveratrol. J. Lipid Res. 2009, 50, 225–232. [Google Scholar] [CrossRef]
- Benjamin, S.; Spener, F. Conjugated linoleic acids as functional food: An insight into their health benefits. Nutr. Metab. 2009, 6, 36. [Google Scholar] [CrossRef]
- Sebo, Z.L.; Rendina-Ruedy, E.; Ables, G.P.; Lindskog, D.M.; Rodeheffer, M.S.; Fazeli, P.K.; Horowitz, M.C. Bone Marrow Adiposity: Basic and Clinical Implications. Endocr. Rev. 2019, 40, 1187–1206. [Google Scholar] [CrossRef]
- Kennedy, A.; Martinez, K.; Schmidt, S.; Mandrup, S.; LaPoint, K.; McIntosh, M. Antiobesity mechanisms of action of conjugated linoleic acid. J. Nutr. Biochem. 2010, 21, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Platt, I.D.; El-Sohemy, A. Regulation of osteoblast and adipocyte differentiation from human mesenchymal stem cells by conjugated linoleic acid. J. Nutr. Biochem. 2009, 20, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Abshirini, M.; Ilesanmi-Oyelere, B.L.; Kruger, M.C. Potential modulatory mechanisms of action by long-chain polyunsaturated fatty acids on bone cell and chondrocyte metabolism. Prog. Lipid Res. 2021, 83, 101113. [Google Scholar] [CrossRef] [PubMed]
- Sharav, O.; Shim, Y.Y.; Okinyo-Owiti, D.P.; Sammynaiken, R.; Reaney, M.J.T. Effect of cyclolinopeptides on the oxidative stability of flaxseed oil. J. Agric. Food Chem. 2014, 62, 88–96. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef]
- Hörmann, K.; Zimmer, A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J. Control. Release 2016, 223, 85–98. [Google Scholar] [CrossRef]
- Leppik, L.; Oliveira, K.M.C.; Bhavsar, M.B.; Barker, J.H. Electrical stimulation in bone tissue engineering treatments. Eur. J. Trauma Emerg. Surg. 2020, 46, 231–244. [Google Scholar] [CrossRef]
- Watkins, B.A.; Seifert, M.F. Conjugated Linoleic Acid and Bone Biology. J. Am. Coll. Nutr. 2000, 19, 478S–486S. [Google Scholar] [CrossRef]
- Banu, J.; Bhattacharya, A.; Rahman, M.; O’Shea, M.; Fernandes, G. Effects of conjugated linoleic acid and exercise on bone mass in young male Balb/C mice. Lipids Health Dis. 2006, 5, 7. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bhattacharya, A.; Banu, J.; Fernandes, G. Conjugated linoleic acid protects against age-associated bone loss in C57BL/6 female mice. J. Nutr. Biochem. 2007, 18, 467–474. [Google Scholar] [CrossRef]
- Kelly, O.; Cashman, K.D. The effect of conjugated linoleic acid on calcium absorption and bone metabolism and composition in adult ovariectomised rats. Prostaglandins Leukot. Essent. Fat. Acids 2004, 71, 295–301. [Google Scholar] [CrossRef]
- Halade, G.V.; Rahman, M.M.; Williams, P.J.; Fernandes, G. Combination of conjugated linoleic acid with fish oil prevents age-associated bone marrow adiposity in C57Bl/6J mice. J. Nutr. Biochem. 2011, 22, 459–469. [Google Scholar] [CrossRef]
- Rahman, M.M.; Halade, G.V.; Williams, P.J.; Fernandes, G. t10c12-CLA maintains higher bone mineral density during aging by modulating osteoclastogenesis and bone marrow adiposity. J. Cell. Physiol. 2011, 226, 2406–2414. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Palou, A.; Serra, F. Body fat loss induced by calcium in co-supplementation with conjugated linoleic acid is associated with increased expression of bone formation genes in adult mice. J. Nutr. Biochem. 2015, 26, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Platt, I.; Rao, L.G.; El-Sohemy, A. Isomer-specific effects of conjugated linoleic acid on mineralized bone nodule formation from human osteoblast-like cells. Exp. Biol. Med. 2007, 232, 246–252. [Google Scholar]
- Kim, J.; Park, Y.; Park, Y. trans-10,cis-12 CLA promotes osteoblastogenesis via SMAD mediated mechanism in bone marrow mesenchymal stem cells. J. Funct. Foods 2014, 8, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R. Pathogenesis of bone and cartilage destruction in rheumatoid arthritis. Rheumatology 2003, 42, ii11–ii16. [Google Scholar] [CrossRef]
- Darestani, R.T.; Darestani, A.T.; Kazemian, G.H.; Tahbaz, F. Effect of Dietary Supplementation with Conjugated Linoleic Acid on Bone Mineral Density, Bone Metabolism Markers and Inflammatory Markers in Healthy Post-menopausal Women: A Randomized Double Blind Placebo Controlled Trial. Nov. Biomed. 2013, 1, 66–72. [Google Scholar]
- Aryaeian, N.; Shahram, F.; Djalali, M. CLA Has a Useful Effect on Bone Markers in Patients with Rheumatoid Arthritis. Lipids 2016, 51, 1397–1405. [Google Scholar] [CrossRef]
- Aryaeian, N.; Djalali, M.; Shahram, F.; Djazayery, A.; Eshragian, M.R. Effect of conjugated linoleic Acid, vitamin e, alone or combined on immunity and inflammatory parameters in adults with active rheumatoid arthritis: A randomized controlled trial. Int. J. Prev. Med. 2014, 5, 1567–1577. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25709792 (accessed on 15 January 2025).
- Pinkoski, C.; Chilibeck, P.D.; Candow, D.G.; Esliger, D.; Ewaschuk, J.B.; Facci, M.; Farthing, J.P.; Zello, G.A. The effects of conjugated linoleic acid supplementation during resistance training. Med. Sci. Sports Exerc. 2006, 38, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, M.; Zimmer, A.; Paikin, J.; Safdar, A.; Aboud, A.; Pearce, E.; Roy, B.; Doherty, T. Creatine monohydrate and conjugated linoleic acid improve strength and body composition following resistance exercise in older adults. PLoS ONE 2007, 2, e991. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Kanazawa, I.; Yamamoto, M.; Kurioka, S.; Yamauchi, M.; Yano, S.; Sugimoto, T. Associations between components of the metabolic syndrome versus bone mineral density and vertebral fractures in patients with type 2 diabetes. Bone 2009, 45, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J.; Klibanski, A. Bone, fat, and body composition: Evolving concepts in the pathogenesis of osteoporosis. Am. J. Med. 2009, 122, 409–414. [Google Scholar] [CrossRef]
- Jain, R.K.; Vokes, T. Visceral Adipose Tissue is Negatively Associated with Bone Mineral Density in NHANES 2011–2018. J. Endocr. Soc. 2023, 7, bvad008. [Google Scholar] [CrossRef]
- Mądry, E.; Malesza, I.J.; Subramaniapillai, M.; Czochralska-Duszyńska, A.; Walkowiak, M.; Miśkiewicz-Chotnicka, A.; Walkowiak, J.; Lisowska, A. Body Fat Changes and Liver Safety in Obese and Overweight Women Supplemented with Conjugated Linoleic Acid: A 12-Week Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2020, 12, 1811. [Google Scholar] [CrossRef]
- Chang, H.; Gan, W.; Liao, X.; Wei, J.; Lu, M.; Chen, H.; Wang, S.; Ma, Y.; Wu, Q.; Yu, Y.; et al. Conjugated linoleic acid supplements preserve muscle in high-body-fat adults: A double-blind, randomized, placebo trial. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1777–1784. [Google Scholar] [CrossRef]
- Kang, E.K.; Park, H.W.; Baek, S.; Lim, J.Y. The Association between Trunk Body Composition and Spinal Bone Mineral Density in Korean Males versus Females: A Farmers’ Cohort for Agricultural Work-Related Musculoskeletal Disorders (FARM) Study. J. Korean Med. Sci. 2016, 31, 1595–1603. [Google Scholar] [CrossRef]
- Yamamoto, J.; Bergstrom, J.; Davis, A.; Wing, D.; Schousboe, J.T.; Nichols, J.F.; Kado, D.M. Trunk lean mass and its association with 4 different measures of thoracic kyphosis in older community dwelling persons. PLoS ONE 2017, 12, e0174710. [Google Scholar] [CrossRef]
- Jamka, M.; Czochralska-Duszyńska, A.; Mądry, E.; Lisowska, A.; Jończyk-Potoczna, K.; Cielecka-Piontek, J.; Bogdański, P.; Walkowiak, J. The Effect of Conjugated Linoleic Acid Supplementation on Densitometric Parameters in Overweight and Obese Women-A Randomised Controlled Trial. Medicina 2023, 59, 1690. [Google Scholar] [CrossRef]
- Gaullier, J.-M.; Halse, J.; Høivik, H.O.; Høye, K.; Syvertsen, C.; Nurminiemi, M.; Hassfeld, C.; Einerhand, A.; O’Shea, M.; Gudmundsen, O. Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Br. J. Nutr. 2007, 97, 550–560. [Google Scholar] [CrossRef]
- Forte, Y.S.; Renovato-Martins, M.; Barja-Fidalgo, C. Cellular and Molecular Mechanisms Associating Obesity to Bone Loss. Cells 2023, 12, 521. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, C.; Bu, J. Relationship between Selected Serum Metallic Elements and Obesity in Children and Adolescent in the U.S. Nutrients 2017, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Paik, H.-D.; Shin, M.-J.; Park, E. Eight weeks of conjugated linoleic acid supplementation has no effect on antioxidant status in healthy overweight/obese Korean individuals. Eur. J. Nutr. 2012, 51, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.A. The Role of Interleukin-6 in Bone. J. Endocr. Soc. 2020, 4, bvaa112. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; Hangoc, G.; Girasole, G.; Passeri, G.; Williams, D.C.; Abrams, J.S.; Boyce, B.; Broxmeyer, H.; Manolagas, S.C. Increased osteoclast development after estrogen loss: Mediation by interleukin-6. Science 1992, 257, 88–91. [Google Scholar] [CrossRef]
- Harmer, D.; Falank, C.; Reagan, M.R. Interleukin-6 Interweaves the Bone Marrow Microenvironment, Bone Loss, and Multiple Myeloma. Front. Endocrinol. 2018, 9, 788. [Google Scholar] [CrossRef]
- Riminucci, M.; Kuznetsov, S.A.; Cherman, N.; Corsi, A.; Bianco, P.; Gehron Robey, P. Osteoclastogenesis in fibrous dysplasia of bone: In situ and in vitro analysis of IL-6 expression. Bone 2003, 33, 434–442. [Google Scholar] [CrossRef]
- Sims, N.A.; Lorenzo, J.A. Local regulators of bone. In Principles of Bone Biology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1205–1246. [Google Scholar] [CrossRef]
- Aslani, M.R.; Matin, S.; Nemati, A.; Mesgari-Abbasi, M.; Ghorbani, S.; Ghobadi, H. Effects of conjugated linoleic acid supplementation on serum levels of interleukin-6 and sirtuin 1 in COPD patients. Avicenna J. Phytomed. 2020, 10, 305–315. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32523885 (accessed on 23 January 2025).
- Brown, A.W.; Trenkle, A.H.; Beitz, D.C. Diets high in conjugated linoleic acid from pasture-fed cattle did not alter markers of health in young women. Nutr. Res. 2011, 31, 33–41. [Google Scholar] [CrossRef]
- Deguire, J.R.; Makarem, N.; Vanstone, C.A.; Morin, S.; Duque, G.; Weiler, H.A. Conjugated linoleic acid is related to bone mineral density but does not affect parathyroid hormone in men. Nutr. Res. 2012, 32, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Racine, N.M.; Watras, A.C.; Carrel, A.L.; Allen, D.B.; McVean, J.J.; Clark, R.R.; O’Brien, A.R.; O’Shea, M.; Scott, C.E.; Schoeller, D.A. Effect of conjugated linoleic acid on body fat accretion in overweight or obese children. Am. J. Clin. Nutr. 2010, 91, 1157–1164. [Google Scholar] [CrossRef]
- Moon, H.-S.; Lee, H.-G.; Chung, C.-S.; Choi, Y.-J.; Cho, C.-S. Physico-chemical modifications of conjugated linoleic acid for ruminal protection and oxidative stability. Nutr. Metab. 2008, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Limeres, M.J.; Moretton, M.A.; Bernabeu, E.; Chiappetta, D.A.; Cuestas, M.L. Thinking small, doing big: Current success and future trends in drug delivery systems for improving cancer therapy with special focus on liver cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Maliha, A.; Tahsin, M.; Fabia, T.Z.; Rahman, S.M.; Rahman, M.M. Pro-resolving metabolites: Future of the fish oil supplements. J. Funct. Foods 2024, 121, 106439. [Google Scholar] [CrossRef]
- Martinez, K.; Kennedy, A.; West, T.; Milatovic, D.; Aschner, M.; McIntosh, M. trans-10,cis-12-Conjugated linoleic acid instigates inflammation in human adipocytes compared with preadipocytes. J. Biol. Chem. 2010, 285, 17701–17712. [Google Scholar] [CrossRef]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Al-Ghouti, M.A.; Abou-Saleh, H.; Rahman, M.M. Unraveling the Omega-3 Puzzle: Navigating Challenges and Innovations for Bone Health and Healthy Aging. Mar. Drugs 2024, 22, 446. [Google Scholar] [CrossRef]
- Sriprablom, J.; Luangpituksa, P.; Wongkongkatep, J.; Pongtharangkul, T.; Suphantharika, M. Influence of pH and ionic strength on the physical and rheological properties and stability of whey protein stabilized o/w emulsions containing xanthan gum. J. Food Eng. 2019, 242, 141–152. [Google Scholar] [CrossRef]
- Alkhair, S.; Zouari, N.; Ibrahim Ahmad Ibrahim, M.; Al-Ghouti, M.A. Efficacy of adsorption processes employing green nanoparticles for bisphenol A decontamination in water: A review. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100963. [Google Scholar] [CrossRef]
- Al-Karablieh, H.A.; Zouari, N.; Shomar, B.; Al-Ghouti, M.A. Functionalized cellulose nanocrystals extracted from date pits as agricultural waste for the efficient remediation of molybdenum from groundwater: Performance and mechanistic studies. Groundw. Sustain. Dev. 2024, 25, 101134. [Google Scholar] [CrossRef]
- Sangor, F.I.M.S.; Al-Ghouti, M.A. Waste-to-value: Synthesis of nano-aluminum oxide (nano-γ-Al2O3) from waste aluminum foils for efficient adsorption of methylene blue dye. Case Stud. Chem. Environ. Eng. 2023, 8, 100394. [Google Scholar] [CrossRef]
- Eldos, H.I.; Khan, M.; Zouari, N.; Saeed, S.; Al-Ghouti, M.A. Adsorptive removal of volatile petroleum hydrocarbons from aqueous solution by zeolite imidazole framework (ZIF-8) and iron oxide (Fe3O4) nanoparticles. Environ. Technol. Innov. 2023, 32, 103382. [Google Scholar] [CrossRef]
- Gill, R.; Al-Badr, M.; Alghouti, M.; Mohamed, N.A.; Abou-Saleh, H.; Rahman, M.M. Revolutionizing Cardiovascular Health with Nano Encapsulated Omega-3 Fatty Acids: A Nano-Solution Approach. Mar. Drugs 2024, 22, 256. [Google Scholar] [CrossRef] [PubMed]
- Pentak, D.; Sułkowski, W.W.; Sułkowska, A. Influence of some physical properties of 5-fluorouracil on encapsulation efficiency in liposomes. J. Therm. Anal. Calorim. 2012, 108, 67–71. [Google Scholar] [CrossRef]
- Pentak, D. Evaluation of the physicochemical properties of liposomes as potential carriers of anticancer drugs: Spectroscopic study. J. Nanoparticle Res. 2016, 18, 126. [Google Scholar] [CrossRef]
- Pentak, D.; Ploch-Jankowska, A.; Zięba, A.; Kozik, V. The Advances and Challenges of Liposome-Assisted Drug Release in the Presence of Serum Albumin Molecules: The Influence of Surrounding pH. Materials 2022, 15, 1568. [Google Scholar] [CrossRef]
- Pande, S. Liposomes for drug delivery: Review of vesicular composition, factors affecting drug release and drug loading in liposomes. Artif. Cells Nanomed. Biotechnol. 2023, 51, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.; Ming, L.; Lee, K.; Yuen, K. Influence of the Encapsulation Efficiency and Size of Liposome on the Oral Bioavailability of Griseofulvin-Loaded Liposomes. Pharmaceutics 2016, 8, 25. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Labhasetwar, V. Nanotech approaches to drug delivery and imaging. Drug Discov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Grazia Calvagno, M.; Celia, C.; Paolino, D.; Cosco, D.; Iannone, M.; Castelli, F.; Doldo, P.; Fresta, M. Effects of Lipid Composition and Preparation Conditions on Physical-Chemical Properties, Technological Parameters and In Vitro Biological Activity of Gemcitabine-Loaded Liposomes. Curr. Drug Deliv. 2007, 4, 89–101. [Google Scholar] [CrossRef]
- Yang, B.; Geng, S.-Y.; Liu, X.-M.; Wang, J.-T.; Chen, Y.-K.; Wang, Y.-L.; Wang, J.-Y. Positively charged cholesterol derivative combined with liposomes as an efficient drug delivery system, in vitro and in vivo study. Soft Matter 2012, 8, 518–525. [Google Scholar] [CrossRef]
- Vélez, M.A.; Perotti, M.C.; Zanel, P.; Hynes, E.R.; Gennaro, A.M. Soy PC liposomes as CLA carriers for food applications: Preparation and physicochemical characterization. J. Food Eng. 2017, 212, 174–180. [Google Scholar] [CrossRef]
- Vélez, M.A.; Perotti, M.C.; Hynes, E.R.; Gennaro, A.M. Effect of lyophilization on food grade liposomes loaded with conjugated linoleic acid. J. Food Eng. 2019, 240, 199–206. [Google Scholar] [CrossRef]
- Hashemi, F.S.; Farzadnia, F.; Aghajani, A.; Ahmadzadeh NobariAzar, F.; Pezeshki, A. Conjugated linoleic acid loaded nanostructured lipid carrier as a potential antioxidant nanocarrier for food applications. Food Sci. Nutr. 2020, 8, 4185–4195. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, R.; Rasul, A.; Asghar, S.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. Int. J. Mol. Sci. 2023, 24, 15764. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.N.; Baghel, D.S.; Kumar, B.; Pandey, N.K.; Singh, S.; Sudhakar, K.; Charyulu, R.N. Cyclodextrin as Solubilizer and Targeting Agent for Drugs. Int. J. Appl. Pharm. 2024, 16, 15–22. [Google Scholar] [CrossRef]
- Szente, L.; Singhal, A.; Domokos, A.; Song, B. Cyclodextrins: Assessing the Impact of Cavity Size, Occupancy, and Substitutions on Cytotoxicity and Cholesterol Homeostasis. Molecules 2018, 23, 1228. [Google Scholar] [CrossRef]
- Szente, L.; Fenyvesi, É. Cyclodextrin-Lipid Complexes: Cavity Size Matters. Struct. Chem. 2017, 28, 479–492. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, G.B.; Kang, C.B.; Park, S.D.; Jung, M.Y.; Kim, J.O.; Ha, Y.L. Improvement of Oxidative Stability of Conjugated Linoleic Acid (CLA) by Microencapsulation in Cyclodextrins. J. Agric. Food Chem. 2000, 48, 3922–3929. [Google Scholar] [CrossRef]
- Kim, D.; Park, J.-H.; Kweon, D.-J.; Han, G.D. Bioavailability of nanoemulsified conjugated linoleic acid for an antiobesity effect. Int. J. Nanomed. 2013, 8, 451–459. [Google Scholar] [CrossRef]
- Xiang, S.; Yao, X.; Zhang, W.; Zhang, K.; Fang, Y.; Nishinari, K.; Phillips, G.O.; Jiang, F. Gum Arabic-stabilized conjugated linoleic acid emulsions: Emulsion properties in relation to interfacial adsorption behaviors. Food Hydrocoll. 2015, 48, 110–116. [Google Scholar] [CrossRef]
- Cheng, W.; McClements, D.J. Biopolymer-stabilized conjugated linoleic acid (CLA) oil-in-water emulsions: Impact of electrostatic interactions on formation and stability of pectin-caseinate-coated lipid droplets. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 511, 172–179. [Google Scholar] [CrossRef]
- Heo, W.; Kim, J.H.; Pan, J.H.; Kim, Y.J. Lecithin-Based Nano-emulsification Improves the Bioavailability of Conjugated Linoleic Acid. J. Agric. Food Chem. 2016, 64, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, H.; Gu, Z.; Cheng, L.; Li, C.; Li, Z.; Hong, Y. Conjugated linoleic acid loaded starch-based emulsion nanoparticles: In vivo gastrointestinal controlled release. Food Hydrocoll. 2020, 101, 105477. [Google Scholar] [CrossRef]
- Jiao, Q.; Liu, Z.; Li, B.; Tian, B.; Zhang, N.; Liu, C.; Feng, Z.; Jiang, B. Development of Antioxidant and Stable Conjugated Linoleic Acid Pickering Emulsion with Protein Nanofibers by Microwave-Assisted Self-Assembly. Foods 2021, 10, 1892. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Liao, C.-C.; Lin, Z.-C.; Alalaiwe, A.; Hwang, E.; Lin, T.-W.; Fang, J.-Y. Facile adipocyte uptake and liver/adipose tissue delivery of conjugated linoleic acid-loaded tocol nanocarriers for a synergistic anti-adipogenesis effect. J. Nanobiotechnol. 2024, 22, 50. [Google Scholar] [CrossRef]
- Rafi Azari, S.; Hojjatoleslamy, M.; Mousavi, Z.E.; Kiani, H.; Jalali, S.M.A. The effect of microencapsulation of microbial oil containing CLA by the complex coacervation on the physicochemical and sensory characteristics of buttermilk. Food Chem. Adv. 2024, 5, 100757. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. Fabrication methods of biopolymeric microgels and microgel-based hydrogels. Food Hydrocoll. 2017, 62, 262–272. [Google Scholar] [CrossRef]
- Venugopalan, V.K.; Gopakumar, L.R.; Kumaran, A.K.; Chatterjee, N.S.; Soman, V.; Peeralil, S.; Mathew, S.; McClements, D.J.; Nagarajarao, R.C. Encapsulation and Protection of Omega-3-Rich Fish Oils Using Food-Grade Delivery Systems. Foods 2021, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Perez-Palacios, T.; Ruiz-Carrascal, J.; Solomando, J.C.; De-la-Haba, F.; Pajuelo, A.; Antequera, T. Recent Developments in the Microencapsulation of Fish Oil and Natural Extracts: Procedure, Quality Evaluation and Food Enrichment. Foods 2022, 11, 3291. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; González, C.; Maestro, A.; Solè, I.; Pey, C.M.; Nolla, J. Nano-emulsions: New applications and optimization of their preparation. Curr. Opin. Colloid Interface Sci. 2008, 13, 245–251. [Google Scholar] [CrossRef]
- Salager, J.-L. Basic theory, measurement, applications. Encycl. Emuls. Technol. 1988, 3, 79–134. [Google Scholar]
- Bernardi, D.S.; Pereira, T.A.; Maciel, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; Rocha-Filho, P.A. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: In vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 44. [Google Scholar] [CrossRef]
- Gonçalves, A.; Nikmaram, N.; Roohinejad, S.; Estevinho, B.N.; Rocha, F.; Greiner, R.; McClements, D.J. Production, properties, and applications of solid self-emulsifying delivery systems (S-SEDS) in the food and pharmaceutical industries. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 538, 108–126. [Google Scholar] [CrossRef]
- Leong, T.S.H.; Wooster, T.J.; Kentish, S.E.; Ashokkumar, M. Minimising oil droplet size using ultrasonic emulsification. Ultrason. Sonochem. 2009, 16, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Mahdi Jafari, S.; He, Y.; Bhandari, B. Nano-Emulsion Production by Sonication and Microfluidization—A Comparison. Int. J. Food Prop. 2006, 9, 475–485. [Google Scholar] [CrossRef]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for Formulation of Nanoemulsion Drug Delivery System: A Review. Prev. Nutr. Food Sci. 2019, 24, 225–234. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- Solans, C.; Morales, D.; Homs, M. Spontaneous emulsification. Curr. Opin. Colloid Interface Sci. 2016, 22, 88–93. [Google Scholar] [CrossRef]
- Solè, I.; Pey, C.M.; Maestro, A.; González, C.; Porras, M.; Solans, C.; Gutiérrez, J.M. Nano-emulsions prepared by the phase inversion composition method: Preparation variables and scale up. J. Colloid Interface Sci. 2010, 344, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Duttaroy, A.K. Conjugated Linoleic Acid and Its Beneficial Effects in Obesity, Cardiovascular Disease, and Cancer. Nutrients 2020, 12, 1913. [Google Scholar] [CrossRef]
- den Hartigh, L.J. Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Zhao, Z.; Liu, M.; Yin, X.; Zhou, Y.; Wu, Y.; Peng, Q. Highly effective lead (II) removal by sustainable alkaline activated β-lactoglobulin nanofibrils from whey protein. J. Clean. Prod. 2020, 255, 120297. [Google Scholar] [CrossRef]
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering emulsions: Versatility of colloidal particles and recent applications. Curr. Opin. Colloid Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Cheng, J.; Huang, Q. Food-grade Pickering emulsions stabilized by ovotransferrin fibrils. Food Hydrocoll. 2019, 94, 592–602. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.; Wang, M.; Wu, S.; Wang, X.; Li, D.; Liu, C.; Feng, Z.; Chi, Y. Direct separation and purification of α-lactalbumin from cow milk whey by aqueous two-phase flotation of thermo-sensitive polymer/phosphate. J. Sci. Food Agric. 2021, 101, 4173–4182. [Google Scholar] [CrossRef]
- Bolder, S.G.; Vasbinder, A.J.; Sagis, L.M.C.; van der Linden, E. Heat-induced whey protein isolate fibrils: Conversion, hydrolysis, and disulphide bond formation. Int. Dairy J. 2007, 17, 846–853. [Google Scholar] [CrossRef]
- Bao, Y.; Pignitter, M. Mechanisms of lipid oxidation in water-in-oil emulsions and oxidomics-guided discovery of targeted protective approaches. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2678–2705. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef] [PubMed]
- Rescignano, N.; Fortunati, E.; Armentano, I.; Hernandez, R.; Mijangos, C.; Pasquino, R.; Kenny, J.M. Use of alginate, chitosan and cellulose nanocrystals as emulsion stabilizers in the synthesis of biodegradable polymeric nanoparticles. J. Colloid Interface Sci. 2015, 445, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, C.; Xiong, W.; Song, Y.; Wang, Q.; Zhang, H.; Guo, S.; Yang, S.; Liu, H. Advances in electroactive biomaterials: Through the lens of electrical stimulation promoting bone regeneration strategy. J. Orthop. Transl. 2024, 47, 191–206. [Google Scholar] [CrossRef]
- Pettersen, E.; Anderson, J.; Ortiz-Catalan, M. Electrical stimulation to promote osseointegration of bone anchoring implants: A topical review. J. Neuroeng. Rehabil. 2022, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xu, B.; Li, Q.; Peng, C.; Yang, K. The role of mechanically sensitive ion channel Piezo1 in bone remodeling. Front. Bioeng. Biotechnol. 2024, 12, 1342149. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, B.; Fan, Y.; Liu, Y.; Feng, S.; Cong, Q.; Zhang, X.; Zhou, Y.; Yadav, P.S.; Lin, J.; et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. eLife 2020, 9, e52779. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Basu, B.; Maiti, P.; Dubey, A.K. Interplay of piezoelectricity and electrical stimulation in tissue engineering and regenerative medicine. Appl. Mater. Today 2024, 39, 102332. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Huang, X.; Zhu, X.; Zhi, W.; Wang, J.; Sun, D.; Chen, X.; Zhu, X.; Zhang, X. Accelerated osteogenesis of bone graft by optimizing the bone microenvironment formed by electrical signals dependent on driving micro vibration stimulation. Mater. Today Bio 2023, 23, 100891. [Google Scholar] [CrossRef]
- Guillot-Ferriols, M.; Lanceros-Méndez, S.; Gómez Ribelles, J.L.; Gallego Ferrer, G. Electrical stimulation: Effective cue to direct osteogenic differentiation of mesenchymal stem cells? Biomater. Adv. 2022, 138, 212918. [Google Scholar] [CrossRef]
- Rahaman, S.H.; Bodhak, S.; Balla, V.K.; Bhattacharya, D. Role of in-situ electrical stimulation on early-stage mineralization and in-vitro osteogenesis of electroactive bioactive glass composites. Biomater. Adv. 2025, 166, 214062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Wu, Z.; Wu, N.; Wang, Z.; Chen, X.; Wei, Y.; Zhang, P. Modulation of Osteogenesis in MC3T3-E1 Cells by Different Frequency Electrical Stimulation. PLoS ONE 2016, 11, e0154924. [Google Scholar] [CrossRef] [PubMed]
- Kreller, T.; Zimmermann, J.; van Rienen, U.; Boccaccini, A.R.; Jonitz-Heincke, A.; Detsch, R. Alternating electric field stimulation: Phenotype analysis and osteoclast activity of differentiated RAW 264.7 macrophages on hydroxyapatite-coated Ti6Al4V surfaces and their crosstalk with MC3T3-E1 pre-osteoblasts. Biomater. Adv. 2023, 146, 213285. [Google Scholar] [CrossRef]
- Helaehil, J.V.; Huang, B.; Bartolo, P.; Santamaria-JR, M.; Caetano, G.F. Bone regeneration: The influence of composite HA/TCP scaffolds and electrical stimulation on TGF/BMP and RANK/RANKL/OPG pathways. Injury 2025, 56, 112158. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Heinemann, C.; Zheng, K.; Appali, R.; Alt, F.; Krieghoff, J.; Bernhardt, A.; Boccaccini, A.R.; van Rienen, U.; Hintze, V. The interplay of collagen/bioactive glass nanoparticle coatings and electrical stimulation regimes distinctly enhanced osteogenic differentiation of human mesenchymal stem cells. Acta Biomater. 2022, 149, 373–386. [Google Scholar] [CrossRef]
- Khaw, J.S.; Xue, R.; Cassidy, N.J.; Cartmell, S.H. Electrical stimulation of titanium to promote stem cell orientation, elongation and osteogenesis. Acta Biomater. 2022, 139, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, D.; Guo, B.; Wang, Z.; Yu, H.; Wang, Y.; Jin, S.; Yu, M.; Zhu, L.; Chen, L.; et al. Self-promoted electroactive biomimetic mineralized scaffolds for bacteria-infected bone regeneration. Nat. Commun. 2023, 14, 6963. [Google Scholar] [CrossRef]
- Sun, J.; Xie, W.; Wu, Y.; Li, Z.; Li, Y. Accelerated Bone Healing via Electrical Stimulation. Adv. Sci. 2024, e2404190. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, Y.; Xu, J.; Guo, C.; Shi, J.; Li, L.; Sun, X.; Kong, Q. The Possible Role of Electrical Stimulation in Osteoporosis: A Narrative Review. Medicina 2023, 59, 121. [Google Scholar] [CrossRef]
- Wang, T.; Ouyang, H.; Luo, Y.; Xue, J.; Wang, E.; Zhang, L.; Zhou, Z.; Liu, Z.; Li, X.; Tan, S.; et al. Rehabilitation exercise–driven symbiotic electrical stimulation system accelerating bone regeneration. Sci. Adv. 2024, 10, eadi6799. [Google Scholar] [CrossRef]
- Leppik, L.; Zhihua, H.; Mobini, S.; Thottakkattumana Parameswaran, V.; Eischen-Loges, M.; Slavici, A.; Helbing, J.; Pindur, L.; Oliveira, K.M.C.; Bhavsar, M.B.; et al. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep. 2018, 8, 6307. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.-H.; Yang, B.-Y.; Li, Y.-C.E. Remodeling Effects of the Combination of GGT Scaffolds, Percutaneous Electrical Stimulation, and Acupuncture on Large Bone Defects in Rats. Front. Bioeng. Biotechnol. 2022, 10, 832808. [Google Scholar] [CrossRef]
- Wu, H.; Dong, H.; Tang, Z.; Chen, Y.; Liu, Y.; Wang, M.; Wei, X.; Wang, N.; Bao, S.; Yu, D.; et al. Electrical stimulation of piezoelectric BaTiO3 coated Ti6Al4V scaffolds promotes anti-inflammatory polarization of macrophages and bone repair via MAPK/JNK inhibition and OXPHOS activation. Biomaterials 2023, 293, 121990. [Google Scholar] [CrossRef] [PubMed]
- Aleem, I.S.; Aleem, I.; Evaniew, N.; Busse, J.W.; Yaszemski, M.; Agarwal, A.; Einhorn, T.; Bhandari, M. Efficacy of Electrical Stimulators for Bone Healing: A Meta-Analysis of Randomized Sham-Controlled Trials. Sci. Rep. 2016, 6, 31724. [Google Scholar] [CrossRef] [PubMed]
- Klinder, A.; Möws, F.; Ziebart, J.; Su, Y.; Gabler, C.; Jonitz-Heincke, A.; van Rienen, U.; Ellenrieder, M.; Bader, R. Effects of electrical stimulation with alternating fields on the osseointegration of titanium implants in the rabbit tibia—A pilot study. Front. Bioeng. Biotechnol. 2024, 12, 1395715. [Google Scholar] [CrossRef]
- Sahm, F.; Freiin Grote, V.; Zimmermann, J.; Haack, F.; Uhrmacher, A.M.; van Rienen, U.; Bader, R.; Detsch, R.; Jonitz-Heincke, A. Long-term stimulation with alternating electric fields modulates the differentiation and mineralization of human pre-osteoblasts. Front. Physiol. 2022, 13, 965181. [Google Scholar] [CrossRef]
- Rahman, M.; Cugno, C.; Ibrahim, K.; Kizhakayil, D.; Sathappan, A.; Elanbari, M. P03-066-23 Conjugated Linoleic Acid (CLA) Attenuates the Negative Effects of Thiazolidinedione Medication on Bone Remodeling. Curr. Dev. Nutr. 2023, 7, 100577. [Google Scholar] [CrossRef]
- Ibrahim, K.; Cugno, C.; Kizhakayil, D.; Rahman, M.; Sathappan, A.; Lachica, C.A.; Herrera, S.M.; Ali, Z. IDF23-0089 Bone Remodeling Induced by Thiazolidinediones can be Reverted by CLA in vitro. Diabetes Res. Clin. Pract. 2024, 209, 111246. [Google Scholar] [CrossRef]
- Qiao, Z.; Lian, M.; Liu, X.; Zhang, X.; Han, Y.; Ni, B.; Xu, R.; Yu, B.; Xu, Q.; Dai, K. Electreted Sandwich Membranes with Persistent Electrical Stimulation for Enhanced Bone Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 31655–31666. [Google Scholar] [CrossRef]
- Fathi, E.; Farahzadi, R. Enhancement of osteogenic differentiation of rat adipose tissue-derived mesenchymal stem cells by zinc sulphate under electromagnetic field via the PKA, ERK1/2 and Wnt/β-catenin signaling pathways. PLoS ONE 2017, 12, e0173877. [Google Scholar] [CrossRef]
- Creecy, C.M.; O’Neill, C.F.; Arulanandam, B.P.; Sylvia, V.L.; Navara, C.S.; Bizios, R. Mesenchymal Stem Cell Osteodifferentiation in Response to Alternating Electric Current. Tissue Eng. Part A 2013, 19, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Ziebart, J.; Jonitz-Heincke, A.; Hansmann, D.; Dauben, T.; Bader, R. Alternating Electric Fields Modify the Function of Human Osteoblasts Growing on and in the Surroundings of Titanium Electrodes. Int. J. Mol. Sci. 2020, 21, 6944. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.C.; Wang, W.; Brighton, C.T. Up-regulation of expression of selected genes in human bone cells with specific capacitively coupled electric fields. J. Orthop. Res. 2014, 32, 894–903. [Google Scholar] [CrossRef]
- Wei, H.; Cui, J.; Lin, K.; Xie, J.; Wang, X. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res. 2022, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Tu, J.; Wang, W.; Li, Z.; Li, Y.; Yu, X.; Zhang, Z. Effects of Mechanical Stress Stimulation on Function and Expression Mechanism of Osteoblasts. Front. Bioeng. Biotechnol. 2022, 10, 830722. [Google Scholar] [CrossRef]
- Wang, A.; Ma, X.; Bian, J.; Jiao, Z.; Zhu, Q.; Wang, P.; Zhao, Y. Signalling pathways underlying pulsed electromagnetic fields in bone repair. Front. Bioeng. Biotechnol. 2024, 12, 1333566. [Google Scholar] [CrossRef]
- Staehlke, S.; Bielfeldt, M.; Zimmermann, J.; Gruening, M.; Barke, I.; Freitag, T.; Speller, S.; Van Rienen, U.; Nebe, B. Pulsed Electrical Stimulation Affects Osteoblast Adhesion and Calcium Ion Signaling. Cells 2022, 11, 2650. [Google Scholar] [CrossRef] [PubMed]
- Aifantis, I.D.; Ampadiotaki, M.-M.; Pallis, D.; Tsivelekas, K.K.; Papadakis, S.A.; Chronopoulos, E. Biophysical Enhancement in Fracture Healing: A Review of the Literature. Cureus 2023, 15, e37704. [Google Scholar] [CrossRef]
- Massari, L.; Benazzo, F.; Falez, F.; Perugia, D.; Pietrogrande, L.; Setti, S.; Osti, R.; Vaienti, E.; Ruosi, C.; Cadossi, R. Biophysical stimulation of bone and cartilage: State of the art and future perspectives. Int. Orthop. 2019, 43, 539–551. [Google Scholar] [CrossRef]
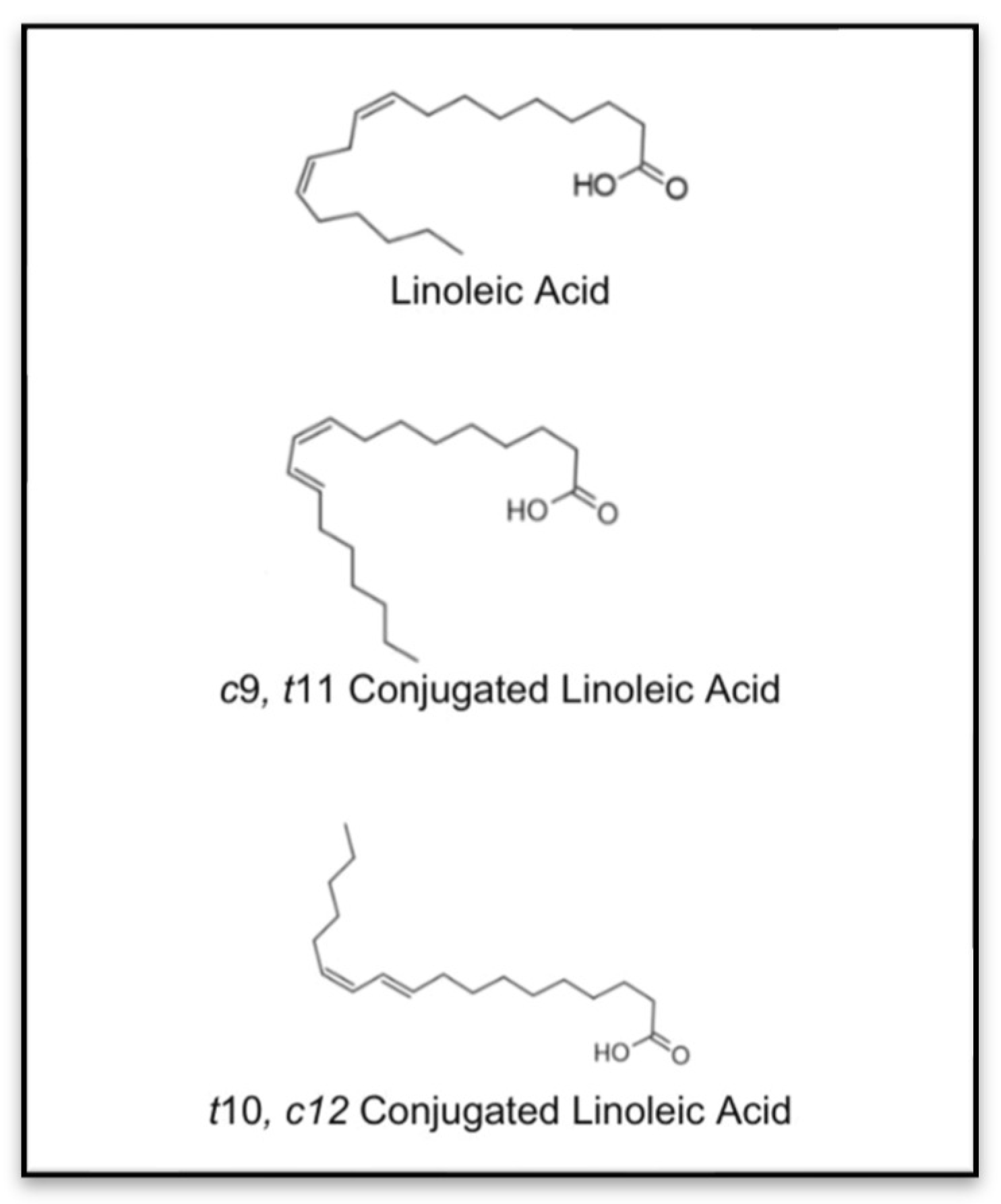
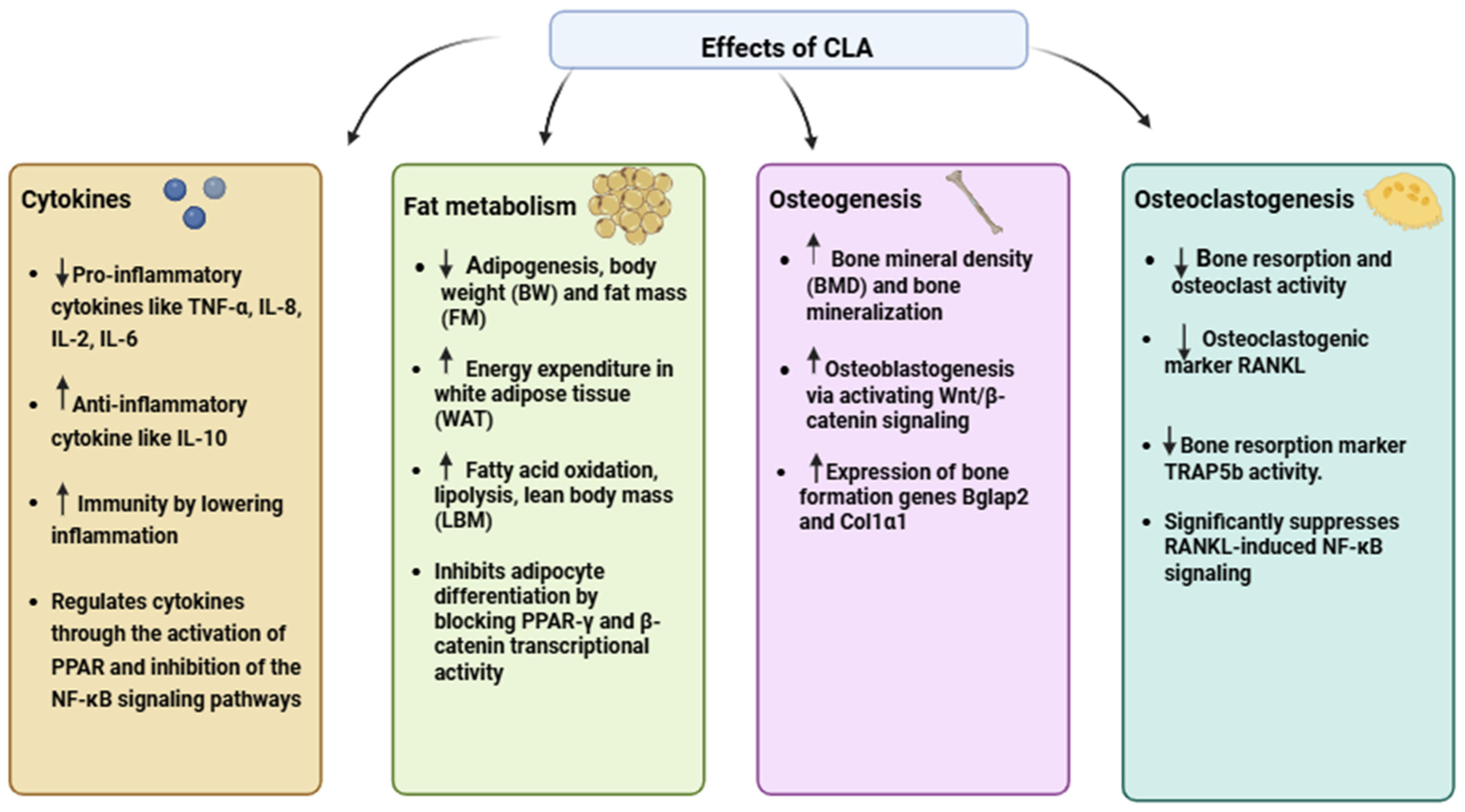

| Reference | Model Type | In Vitro/In Vivo | Treatment | Duration | Results |
|---|---|---|---|---|---|
| Kelly and Cashman, 2004 [31] | OVX rats | In Vivo |
| 9 weeks | CLA decreased bone-resorption markers but had no impact on BMD. Higher doses were more effective. |
| I. Platt et al., 2007 [35] | Human SaOS-2 cells | In Vitro |
| Not specified | 9cis,11trans CLA increased the number and size of mineralized bone nodules, whereas 10trans,12cis CLA had no such effect. |
| Rahman et al., 2007 [30] | 14-month-old C57BL/6 mice | In Vivo |
| 10 weeks | CLA increased BMD, reduced pro-inflammatory cytokines, decreased fat mass, and increased muscle mass. |
| Halade et al., 2011 [32] | 12-month-old C57BL/6J mice | In Vivo |
| 20 weeks | CLA improved BMD and muscle mass but led to insulin resistance. CLA + FO combination reduced inflammation, improved BMD, and energy metabolism. |
| Rahman et al., 2011 [33] | 12-month-old C57BL/6 mice | In Vivo |
| 26 weeks | The t10,c12 CLA isomer significantly improved BMD and reduced osteoclastogenic factors and bone marrow adiposity. |
| Park et al., 2013 [9] | 6-month-old ICR mice | In Vivo |
| 8 weeks | CLA increased BMD and bone strength in ovariectomized (OVX) mice. CLA + Calcium improved BMD and bone formation markers in comparison to controls |
| J. Kim et al., 2013 [13] | Murine mesenchymal stem cells | In Vitro |
| 4 weeks | The trans-10,cis-12 CLA isomer inhibited adipogenesis and promoted osteoblastogenesis, positively influencing bone-resorption processes. |
| Kim st al. 2014 [36] | n murinemesenchymal stem cells | In vitro |
| 28 days | The trans-10,cis-12 CLA enhances osteoblastogenesis through a SMAD8-mediated mechanism; inhibiting adipogenesis independently of SMAD8; and reducing factors involved in osteoclastogenesis. |
| Rahman et al., 2014 [12] | 8-week-old C57BL/6 mice | In Vivo |
| 24 weeks | CLA prevented bone loss in OVX mice and stimulated new bone formation. |
| Chaplin et al., 2015 [34] | C57BL/6J mice | In Vivo |
| 8 weeks | CLA alone had minimal impact, but combined with calcium, it improved bone weight and expression of bone formation genes like Bglap2 and Col1a1. |
| Lin et al., 2017 [5] | C57BL/6J mice | In Vivo |
| 1 week | CLA increased BMD and reduced bone marrow adiposity. |
| Reference | Study Population | Age/Mean ± SD Age | Treatment | Duration | Results |
|---|---|---|---|---|---|
| Tarnopolsky et al., 2007 [42] | 39 community-dwelling, older adults | 65–85 years | 5 g creatine monohydrate (CrM) + 6 g of CLA (45% c9, t11; 45% t10, c12) | 26 weeks | -The combination of CrM and CLA enhanced the benefits of resistance exercise in older adults, significantly improving strength (p < 0.001), functional capacity (p < 0.05), and muscular endurance compared to exercise alone. |
| Racine et al., 2010 [63] | 53 overweight or obese children (BMI > 85th percentile) | 6–10 years | Clarinol™ 3.0 g (80% CLA, 50% c9t11, 50% t10c12) (n = 28) Placebo: sunflower oil (n = 25) | 30 weeks | -The CLA group experienced a reduction in total body bone mineral content (BMC) accrual, with bone mineral accretion lower in the CLA group compared to the placebo group. This indicates that CLA had no notable effect on improving bone formation. |
| Brown et al., 2011 [61] | 18 healthy women BMI between 19–30 | 20–40 years | CLA diet: 1.17 g/d; control diet: 0.35 g/d | 8 weeks | -No significant changes in bone mineral density (BMD) and BMC. |
| Deguire et al., 2012 [62] | 54 community-dwelling adult men | 19–53 years | 1.5 g, 3.0 g cis-9, trans-11 CLA mixture Placebo: olive oil | 17 weeks | -Men with higher RBC c9,t11 CLA levels showed increased BMD and lean mass |
| J. Kim et al., 2012 [54] | 29 healthy overweight/obese Korean individuals | 19–65 years | 2.4 g/day CLA [36.9% of cis-9, trans-11 and 37.9% of trans-10, cis-12] | 8 weeks | -No significant impact on lipid peroxidation or antioxidant metabolism was observed, indicating no effect on bone formation. No difference in plasma TRAP levels suggests no effect on bone resorption. |
| Darestani R et al., 2013 [38] | 76 healthy postmenopausal women | 45–65 years | CLA G80 containing 3.2 g isomer blend (50:50% cis-9, trans-11: trans-10, cis-12 isomers) Placebo: oleic sunflower oil | 12 weeks | -CLA supplementation had no significant effects on markers of bone formation (serum osteocalcin, bone-specific alkaline phosphatase) or bone resorption (urine C-telopeptide). No changes were observed in PTH, urinary calcium, creatinine, or IL-6 levels, indicating no impact on overall bone metabolism or calcium. |
| Aryaeian et al., 2014 [40] | 78 patients with active rheumatoid arthritis | 18 and 69 years | 1.25 g/day 80% CLA [2 g 50:50 mix of cis-9, trans-11 and trans-10, cis-12 glycerinated CLA] | 13 weeks | -CLA’s anti-inflammatory effects help reduce inflammation-associated bone loss in rheumatoid arthritis (RA) patients, confirmed by reduced white blood cell (WBC) count, MMP-3, and TNF-α levels in active RA patients, indicating a beneficial impact on bone health. |
| Aryaeian et al., 2016 [39] | 52 patients with active rheumatoid arthritis | 19–69 years | 2 g of 9-cis 11-trans isomer and 10-cis 12-trans isomer in ratio of 50 −50 CLA in glycerinated form | 12 weeks | -CLA benefits bone health in rheumatoid arthritis patients by positively affecting telopeptide C and osteocalcin levels |
| Aslani et al., 2020 [60] | 82 COPD patients | 60–65 years | 3.2 g of CLA | 6 weeks | -CLA supplementation significantly reduced serum IL-6 levels, modified inflammatory markers, and improved overall health status, confirming a positive influence on bone health |
| Chang et al., 2020 [47] | 66 Chinese adults with elevated body fat percentage | 18–45 years | 3.2 g/day CLA Placebo: Sunflower oil | 12 weeks | -CLA supplementation helps preserve muscle mass, particularly in the trunk region, and contributes to better BMD. |
| Jamka et al., 2023 [50] | 74 Caucasian Obese women | 50–55 years | 3 g (80% CLA, 50% of cis-9, trans-11, 50% of trans-10, cis-12 isomers) (n = 37) Placebo: sunflower oil (n = 37) | 13 weeks | -The CLA group showed significant increases in BMC and BMD at the lumbar spine. There were no differences in BMC and BMD at the total body and femoral neck between the CLA and placebo groups. |
| Reference | Nanoparticle Type | Loaded Compound | Findings |
|---|---|---|---|
| Kim, S. J et al., 2000 [93] | Alpha, beta, and gamma Cyclodextrins(CD) | CLA (48% cis-9, trans-11 and 48% trans-10, cis-12 isomers) | CLA/CD microencapsulation (1:4 mole ratio) fully protected CLA from oxidation, with α-, β-, and γ-CDs reducing peroxide values, confirming their protective role. |
| Kim, D et al., 2013 [94] | Nanoemulsified water-soluble conjugated linoleic acid (N-CLA) | CLA (>77% purity). | N-CLA outperformed CLA in reducing fat accumulation due to better absorption. It effectively lowered body weight, improved blood and liver lipid profiles, and enhanced CLA bioavailability, making it a potential anti-obesity agent. |
| Xiang, S et al., 2015 [95] | Gum Arabic (biopolymer emulsifier) | 80% pure CLA | Among the tested gum Arabic types (conventional GA, matured EM2/EM10), EM10 showed superior emulsifying activity and stability despite a lower surface load. |
| Cheng, W et al., 2016 [96] | Biopolymer-coated oil droplets | 75–80% CLA (50:50 ratio of c9,t11 and t10,c12 isomers) | Mixed biopolymer-coated emulsions demonstrated thermal stability (90 °C/20 min). Stability was pectin-to-caseinate ratio-dependent, with higher ratios improving aggregation and creaming resistance via enhanced steric/electrostatic repulsion. |
| Heo, W et al., 2016 [97] | Soybean lecithin | CLA (38.6% cis-9, trans-11; 43.3% trans-10, cis-12; 3.5% other isomers) | Nano-emulsification with soybean lecithin improved CLA’s thermal stability, bioavailability, and enhanced CLA absorption in the small intestine. |
| Vélez, M. A et al., 2017 [85] | Soy phosphatidylcholine (PC) liposomes | CLA isomers 9c, 11t and 10t, 12c | CLA-loaded nanoparticles remained stable for 30 days at 4 °C, preventing CLA degradation. They enhanced membrane fluidity, improved digestibility and bioavailability, and achieved over 80% encapsulation efficiency. |
| Vélez, M. A et al., 2019 [86] | Soy phosphatidylcholine (PC) liposomes | CLA isomers 9c, 11t and 10t, 12c | CLA-loaded liposomes showed improved stability and smaller size compared to control liposomes during storage while efficiently preserving CLA isomers with high encapsulation efficiency. |
| Hashemi, F.S et al., 2020 [87] | Nanostructured lipid carrier (NLC) | CLA with 80% purity, a mixture of 9-cis, 11-trans and 10-trans, 12-cis isomers. | Encapsulating CLA in NLC systems improved protection against oxidation, reduced secondary oxidation products like malondialdehydes, and enhanced stability against thermal processes, environmental conditions, and oxidation. |
| Yang, J et al., 2020 [98] | Octenyl succinic anhydride (OSA)-modified starch and xanthan gum (XG) | CLA (80% purity, cis-9, trans-11/cis-10, trans-12 octadecadienoic acids, and linoleic acid < 1%) | Encapsulation efficiencies exceeded 97%, ensuring effective CLA entrapment. Release studies showed minimal CLA release in the stomach, with most released in the small intestine. |
| Jiao, Q et al., 2021 [99] | Pickering Emulsion with Whey Protein Nanofibers | CLA | Whey protein nanofibrils (WPNFs) effectively encapsulated CLA, improving its water solubility and addressing key delivery challenges of lipophilic bioactives. |
| Hsu, C. Y et al., 2024 [100] | Tocol nanostructured lipid carriers (NLCs) | CLA (41.2% c9-t11 and t9-c11 CLA, 44.1% t10-c12 CLA, and 9.4% t10-c12) | CLA-loaded NLCs more effectively reduced fat storage, TG levels, and adipokine expression in adipocytes than free CLA. |
| Mechanism | Conjugated Linoleic Acid (CLA) | Electrical Stimulation (ES) |
|---|---|---|
| Anti-inflammatory Action | Suppresses TNF-α, IL-6, and COX-2 expression; decreases osteoclast formation | Decreases pro-inflammatory cytokines (e.g., IL-1β, TNF-α); promotes a healing environment |
| Osteoblast Differentiation | Upregulates Runx2, ALP, osteocalcin; enhances osteogenesis | Activates MAPK/ERK and calcium signaling; promotes ALP activity and mineralization |
| Osteoclast Inhibition | Inhibits RANKL and upregulates OPG; reduces bone resorption | Alters cellular bioelectric signals to suppress osteoclast activity |
| Wnt/β-Catenin Pathway | Indirect activation; supports osteoblast lineage commitment | Direct activation; promotes β-catenin nuclear translocation and gene expression |
| MSC Differentiation | Stimulates MSCs to become osteoblasts over adipocytes | Guides MSC fate via physical/electrical cues toward osteogenesis |
| Oxidative Stress Regulation | Acts as an antioxidant; protects osteoblasts from ROS-induced apoptosis | Moderate ES reduces oxidative stress, enhancing osteoblast survival and function |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoque, K.; Ali, Z.; Maliha, A.; Al-Ghouti, M.A.; Cugno, C.; Rahman, S.M.; Rahman, M.M. Enhancing Bone Health with Conjugated Linoleic Acid: Mechanisms, Challenges, and Innovative Strategies. Nutrients 2025, 17, 1395. https://doi.org/10.3390/nu17081395
Hoque K, Ali Z, Maliha A, Al-Ghouti MA, Cugno C, Rahman SM, Rahman MM. Enhancing Bone Health with Conjugated Linoleic Acid: Mechanisms, Challenges, and Innovative Strategies. Nutrients. 2025; 17(8):1395. https://doi.org/10.3390/nu17081395
Chicago/Turabian StyleHoque, Khandoker, Zayana Ali, Asma Maliha, Mohammad A. Al-Ghouti, Chiara Cugno, Shaikh Mizanoor Rahman, and Md Mizanur Rahman. 2025. "Enhancing Bone Health with Conjugated Linoleic Acid: Mechanisms, Challenges, and Innovative Strategies" Nutrients 17, no. 8: 1395. https://doi.org/10.3390/nu17081395
APA StyleHoque, K., Ali, Z., Maliha, A., Al-Ghouti, M. A., Cugno, C., Rahman, S. M., & Rahman, M. M. (2025). Enhancing Bone Health with Conjugated Linoleic Acid: Mechanisms, Challenges, and Innovative Strategies. Nutrients, 17(8), 1395. https://doi.org/10.3390/nu17081395








