Abstract
A high-fat diet leads to metabolic disturbances, which are important factors in the development of obesity. Gut microbial composition and diversity are altered by a high-fat diet. In general, a high-fat diet resulted in increased Firmicutes abundance and decreased alpha diversity. Bile acids (BAs) are involved in the digestion and absorption of fats in the small intestine and are also the metabolic substrates of microorganisms with bile salt hydrolase (BSH) activity. High-fat diets (HFDs) have been shown to alter gut microbiota composition and BA profiles in murine models. Similarly, probiotic supplementation reverses HFD-induced adverse effects. This review focuses on the energy composition characteristics of a high-fat diet and its effects on body weight, plasma lipid-related biochemical markers, changes in gut microbiome characteristics, and the important role of BAs. The regular mechanism by which a high-fat diet affects the intestinal microenvironment was attempted to be found.
1. Introduction
Obesity is a recognized global public health problem and is influenced by many factors such as age, gender, genetics, diet, geographic location, etc. [1,2,3]. A strong link between diet and obesity has been observed by changing dietary patterns [4,5]. Dietary patterns can generally be divided into three types: 1. Healthy diet, 2. Western diet, 3. Mixed diet [6]. A healthy diet is high in vegetables, fruits, and whole legumes. The Western diet is characterized by refined grains, solid fats, and snacks. A mixed diet is somewhere be-tween a healthy diet and a Western diet. It is well known that the Western diet is the main dietary pattern that contributes to obesity. Therefore, the simple summary is a high-fat diet.
The digestion and absorption of nutrients in the gut is a dynamic process in which microorganisms play an important role. The gut microbiota is an important environmental factor that affects the host’s energy acquisition and storage from the diet and can induce de novo synthesis of fat in the liver by promoting the absorption of monosaccharides in the gut [7]. There were differences in the number of microorganisms in different intestinal segments. An amount of 104–108 microorganisms per mL were present in the contents of the small intestine, and microbial colonization was limited due to the short residence time of the chyme. The microbial density of the large intestine can reach 1010 per mL, which is the intestinal segment with the highest intestinal microbial density [8]. Microbiome study is popular all over the world, and the rapid development of high-throughput sequencing technology makes it possible to observe the gut microbial environment [9]. About 90% of the microorganisms in the large intestine belong to the phyla Bacteroidetes and Firmicutes and also include Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia [10].
A translational perspective is established through systematic analysis of high-fat diet-induced physiological and microbial alterations, focusing on three critical aspects: (1) the operational definition of high-fat diets, (2) identification of diet-associated gut microbiota signatures, and (3) evaluation of therapeutic potential from microbial supplementation strategies.
2. Nutrient Characteristics of a High-Fat Diet
2.1. High-Fat Diet Induces Fat Deposition
High-fat diets are characterized by low carbohydrate levels and relatively unchanged protein levels, with a fat composition of more than 30% of total dietary energy (Table 1). Body weight and adipose tissue weight increased significantly, and plasma cholesterol concentrations increased. Dietary fats are emulsified by bile salts in the small intestine, and then pancreatic juice secreted by the pancreas begins to digest the lipids. The free fatty acids produced by lipolysis are absorbed by the small intestine, resynthesize triglycerides (TGs), and enter the blood circulation in the form of chylomicrons. The TGs released in the liver are combined with apolipoproteins and transported to extrahepatic tissues for utilization [11]. At the same time, a high-fat diet also caused significant upregulation of the lipase genes adipose triglyceride lipase (ATGL), hormone-sensitive triglyceride lipase (HSL), and fatty acid synthase gene FASN in the liver of mice, indicating that the lipid metabolism activity of mice fed a high-fat diet was more rigorous than that of the mice fed a low-fat diet [12].
A short-term (within 2 weeks) high-fat diet has no significant effect on the body weight of mice, and the deposition of fat in the body takes a period of time. When dietary fat was increased to 50% for one week, there were no significant differences in body weight, body fat, and fat mass compared with a diet containing 30% fat [13]. Diets containing 30% vegetable oil significantly increased body weight compared with 20% [14]. A 4-week high-fat diet experiment, designed to have significantly higher cholesterol levels than the control group, then measured weekly body weights in Sprague-Dawley rats, which were nearly the same weight as the control group in the first and second weeks and began to rise slowly after the second week [15]. The feeding efficiency of C57BL/6 mice fed a high-fat diet increased, but the body mass was almost no different from the control group at 0–15 days, and the increase was less than 1 g at 15–20 days [16].

Table 1.
Body weight, body composition, or serum biochemical markers affected by high-fat diet.
Table 1.
Body weight, body composition, or serum biochemical markers affected by high-fat diet.
| Reference | Species | Sex | Age or Weight | Groups | Energy Content | Main Fat Source of High-Fat Diet | Term | Effects |
|---|---|---|---|---|---|---|---|---|
| Nagai et al., 2005 [17] | human | Men | 23.6 years old | Low-fat meal | 70% Carbohydrates, 10% protein, and 20% fat | Butter, high-fat cream | 210 min after meal | Thermoregulatory sympathetic nervous system (SNS) activity and a greater level of fat oxidation ↑ (p < 0.05) |
| High-fat meal | 20% carbohydrates, 10% protein, and 70% fat | |||||||
| Meksawan et al., 2004 [13] | Men and women | Male (24.8 ± 1.0 years old) Female (22.3 ± 1.3 years old) | Regular diet | 54% carbohydrates, 16% protein, and 30% fat | Not found | 7 d | HDL ↑ (p < 0.05); no difference in body weight | |
| High-fat diet | 31% carbohydrates, 19% protein, and 50% fat | |||||||
| Rowlands and Hopkins, 2002 [18] | Men | 27 ± 5 years old | High-carbohydrate diet | 70% carbohydrate, 15% protein, and 15% fat | High-fat meats, eggs and dairy products, nuts and seeds, low-starch vegetable products, and oils | Three 2-week dietary treatment | During exercise: 10%–20% plasma-glucose concentration ↑ (p < 0.01); plasma triacylglycerol ↑ (p < 0.05); 2.5–2.9 fold increase in the peak fat-oxidation rate (p < 0.0001) | |
| High-fat diet | 15% carbohydrate, 15% protein, and 70% fat | |||||||
| Linehan et al., 2018 [15] | Sprague–Dawley rats | Male | 3 weeks old | Standard diet | 58.5% carbohydrates, 28.7% protein, and 12.7% fat | Not found | 7~28 d | No difference in body weight |
| High-fat diet | 44% Carbohydrates, 16% protein, and 40% fat | |||||||
| Woodie and Blythe, 2018 [19] | 6 weeks old | Control diet | 44.3% carbohydrate and 5.8% fat | Not found | 63 d | Final body weight, body weight change, fat pad weight, food intake, and kcal consumed ↑ (p < 0.05); no difference in fasting blood glucose | ||
| High-fat diet | 20% carbohydrate and 60% fat | |||||||
| Cheng et al., 2017 [20] | Sprague–Dawley rats | Male | 3 weeks old | Control diet | 70% carbohydrates, 20% protein, and 10% lipid | Corn oil, milk fat | 56 d | Central obesity, systolic and diastolic hypertension, impaired fasting glucose, hypertriglyceridaemia, and elevated non-HDL cholesterol level |
| High-fat diet | 20% carbohydrates, 20% protein, and 60% lipid | |||||||
| Huang et al., 2004 [21] | Sprague–Dawley rats | Male | 7 weeks old | Standard diet | 57.99% carbohydrates, 28.50% protein, and 13.49% fat | Lard | 56 d | Body weight, liver weight, adipose tissue, and relative liver weight ↑ (p < 0.05); the plasma cholesterol concentration, a-Amylase, b-Hydroxybutyrate, and Leptin ↑ |
| High-fat diet | AIN-76 diet, containing 20% fat | |||||||
| Kanthe et al., 2021 [22] | albino Wistar rats | Not found | 180–220 g | Control diet | 60% carbohydrates, 18% protein, and 20% fat | Not found | 21 d | Body weight ↑ (p < 0.05) |
| High-fat diet | 50% carbohydrates, 18% protein, and 30% fat | |||||||
| Patil et al., 2019 [14] | Control group | 60% carbohydrate, 18% protein, and 20% fat | Vegetable oil | 22 d | Final body weight ↑ (p < 0.05); lipid peroxidation and oxidative stress ↑ | |||
| High-fat diet | 50% carbohydrate, 18% protein, and 30% fat | |||||||
| Maejima et al., 2020 [23] | Wistar rats | Male | 8-week-old | Normal chow diet | 20.5% protein and 10.1% fat | Not found | 72 d | Body weight, energy intake, visceral fat, and subcutaneous fat ↑ (p < 0.05); muscle ↓ |
| High-fat diet | 20.5% protein and 56.7% fat | |||||||
| He et al., 2020 [24] | Wistar rats | Male | Not found | Standard diet | 55.5% carbohydrates, 33.3% protein, and 11.2% fat | Not found | 70 d | No difference in TG, TC, LDL-C, and HDL-C (serum) |
| High-fat diet | 28.6% carbohydrates, 26.2% protein, and 45.2% fat | |||||||
| Schanuel et al., 2019 [16] | C57BL/6 mice | Male | 12 weeks old | Standard chow | 76% carbohydrates, 14% protein, and 10% lipids | Soybean oil, lard | 20 d | Body weight and average fasting blood glucose were no significant differences between groups; inflammatory and fibroblast-like cells ↑ (10 days after, p < 0.05) |
| High-fat chow | 26% carbohydrates, 14% protein, and 60% lipids | |||||||
| Emelyanova et al., 2019 [25] | C57BL/6 mice | Male | 6 weeks old | Standard chow | 64.5% carbohydrate, 23.6% protein, and 11.9% fat | Lard | 70 d | Body weight, gain of body weight ↑ (p < 0.05) |
| High-fat diet | 20% carbohydrate, 20% protein, and 60% fat | |||||||
| Pang et al., 2016 [26] | 8 weeks old | Normal diet | 65.42% carbohydrate, 22.47% protein, and 12.11% fat | Not found | 90 d | Energy efficiency ↑ (p < 0.01); epididymal and perirenal fat weight ↑ (p < 0.01); insulin and glucose concentrations ↑ (p < 0.05) | ||
| High-fat diet | 20% carbohydrate, 20% protein, and 60% fat | |||||||
| Topal et al., 2019 [27] | Swiss albino mice | Female | 8–10 weeks old | Standard chow | 66% carbohydrate, 24% protein, and 10% fat | Not found | 63 d | Body weight, intraperitoneal adipose tissue; adrenal gland weight ↑ (p < 0.01) |
| High-fat diet | 23% carbohydrate, 17% protein, and 60% fat |
Note: ↑ and ↓ represent higher (↑) or lower (↓) values in the HFD group compared with the standard diet group or the low-fat diet group.
Feeding a high-fat diet for more than 8 weeks resulted in obesity in mice, regardless of whether the dietary fat source was lard, vegetable oil, peanut oil, corn oil, or soybean oil. High fat led to lower food intake but higher body weight and total fat pad weight [19]. Normal obesity on a high-fat diet in childhood may develop into overweight obesity in adulthood [23].
2.2. Changes in Serum Lipid Indexes
TG molecules represent the major storage and transport form of fatty acids within cells and in plasma. The liver is the central organ of fatty acid metabolism. Dietary fat is mainly hydrolyzed by pancreatic lipase and then emulsified by bile acid (BA), and the resulting lipid molecules are absorbed to synthesize TG [28]. Up to 70% of dietary fat is ingested by the body, and plasma TG levels are elevated during exercise [18]. The diet containing 60% fat was fed to mice for 56 days, and the mice developed hypertriglyceridemia [20]. However, one study showed that 45.2% dietary fat did not affect the plasma levels of total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) in male Wistar rats [24].
The type of lipids in the diet affects the levels of plasma lipid profiles. A total of 21% lard in the diet induced a significant increase in body weight gain in Wistar rats and a significant increase in TG content in the plasma lipid profile, while other parameters were not affected [29]. C57BL/6 mice were fed diets containing lard, sunflower oil, soybean oil, lard mixed with sunflower oil, and lard mixed with soybean oil for 12 weeks and found that the lard diet resulted in increased TG levels, although the vegetable oil diet did not cause obesity but will cause cholesterol metabolism disorder [30]. Oils rich in unsaturated fatty acids, such as safflower oil, sunflower oil, and rapeseed, are more effective at lowering LDL-C than foods rich in saturated fatty acids, such as butter or lard [31].
Nonalcoholic fatty liver disease (NAFLD) is caused by excessive fat deposition in liver cells caused by a high-fat diet. A mixed diet of lard and soybean oil attenuated low-fat–high-carbohydrate diet-induced NAFLD by modulating genes and BA profiles in C57BL/6 mice. A lard–soybean oil mixture alleviates NAFLD by down-regulating fatty acid binding protein 2 (FABP2), fatty acid synthase (FAS), tumor necrosis factor receptor-associated factor 2 (TRAF2), activator protein-1 (AP-1), mitochondrially encoded cytochrome b (MT-Cytb), interleukin 6 (IL-6), and interleukin 1 (IL-1) genes; upregulating protein kinase AMP-activated catalytic subunit alpha 2 (AMPKα2) and HSL genes and promoting the binding of BAs and BAs signaling receptor takeda G protein-coupled receptor 5 (TGR5) protein [31].
3. High-Fat Diet Alters Host Gut Microbiota Abundance and Diversity
3.1. High-Fat Diet Affects Gut Microbiome Composition
A high-fat diet will affect the composition and abundance of intestinal microbiota. The digested chyme stays in the large intestine for a long time. Therefore, more articles are studying the microbes of the large intestine and feces. Microbial composition in mouse and rat large intestines and feces was analyzed using 16S rRNA sequencing technology. When the taxonomic level is phylum, Firmicutes, Bacteroidetes, and Proteobacteria account for more than 90%. At the genus level, Parabacteroides, Lachnoclostridium, Oscillibacter, Lactobacillus, Akkermansia, Bacteroides, and Alistipes appeared more frequently (Table 2).

Table 2.
Gut microbial composition under high-fat diet treatment.
The ratio of F/B (Firmicutes/Bacteroidetes) is generally considered to be associated with obesity [51]. Some studies suggest that F/B is elevated in obese individuals, while others show no significant change in F/B [52]. This suggests that elevated F/B in obese individuals is not inevitable. If we focus solely on the changes in both phyla, the level of dietary fat content will have an impact on composition. Notably, gut microbiota composition in high-fat diet-induced obese mice exhibited distinct shifts in phylum-level dominance, with Firmicutes and Bacteroidetes showing differential responsiveness to dietary fat content. While Firmicutes prevalence was generally amplified in mice consuming elevated dietary fat levels, Bacteroidetes abundance displayed an inverse correlation pattern under equivalent conditions. However, the results in some studies demonstrated outcomes contrary to the predominant trend described above.
A high-fat diet caused increased body weight, body composition, serum TGs, and cholesterol levels in mice. The gut microenvironment is influenced by diet, and microbial composition and diversity are altered to varying degrees. Therefore, there may be a link between changes in the microbiome and body weight and serum markers. A high-fat diet can affect the composition and abundance of the gut microbiota, triggering disturbances in the gut microbiota, which may cause a range of health problems. Christensenellaceae, Porphyromonadaceae, Rikenellaceae, Ruminococcaceae UCG 014, and Ruminococcaceae UCG 005 were negatively associated with obesity [50].
3.2. Alterations in Alpha Diversity May Be Related to Lipid Types
A considerable number of studies have shown that a high-fat diet can lead to a decrease in the Simpson, Shannon, and Chao index of gut microbes [35,37,39,41,43,47,53]. The decrease in gut microbial alpha diversity may not only be due to the effect of a high-fat diet but may also be related to interactions between lipid metabolism, inter-microbial, and microbial metabolites. Contrary to the popular belief that a high-fat diet reduces gut microbial diversity, it was elevated in a high-fat diet with lard as the main fat source compared with a low-fat diet, and linear discriminant analysis effect size (LEfSe) results indicated that the taxa features that best characterize the differences between the high-fat diet and low-fat diet groups were mainly those of the Rikenellaceae, Deferribacteraceae, Streptococcaceae, Christensenellaceae, and Peptococcaceae families [12].
High-fat diets typically use corn oil, peanut oil, soybean oil, and lard as the main fat sources. However, the fat source of the diet inducing obesity in mice was mainly lard. Different fat treatments affected the community composition of gut microbiota; for example, the abundance of Proteobacteria showed a decreasing trend in the lard, walnut, and peanut oil intervention groups, while flaxseed oil, olive oil, and canola oil showed a downward trend or increasing trend [54]. Lard has a synergistic effect with Coriobacteriaceae_UCG-002 in the cecum of Kunming mice (half male and female), and vegetable oil has a synergistic effect with Akkermansia, Roseburia, and Enteractinococcus. Among them, Coriobacteriaceae_UCG-002 showed a significant negative correlation with Glycolysis/Gluconeogenesis. Roseburia was most strongly associated with starch and sucrose metabolism [55].
4. Bile Acid—Fat Metabolism and Microbial Action
By binding to glycine (human) or taurine (rodent), BAs help limit passive reabsorption, promote micelle formation, and facilitate digestion and absorption of fats in the small intestine [56]. Bile acidolysis conjugation is carried out by bacteria with bile salt hydrolase (BSH) activity, such as Lactobacillus, Bifidobacterium, Clostridium, and Bacteroidetes with this functional BSH, resulting in a small amount of BA not being reabsorbed by the intestine back to the liver [57]. Another microbial metabolic pathway for BAs is catalyzed by bacteria with hydroxysteroid dehydrogenases found in Actinobacteria, Proteobacteria, Firmicutes, and Bacteroidetes [58].
In general, impaired gut microbial diversity affected by a high-fat diet was characterized by the highest abundance of Firmicutes, but elevated microbial diversity was characterized by the highest abundance of Bacteroidetes (Table 2). Lactobacillus is a member of the Firmicutes phylum. Lactobacillus abundance was positively correlated with the concentration of free BAs, which inhibit gut bacteria and modulate gut microflora [59]. Parabacteroides distasonis alleviates obesity-related metabolic dysregulation by producing succinate, which directly activates intestinal gluconeogenesis via fructose-1,6-bisphosphatase binding, paired with BA-mediated farnesoid X receptor (FXR) signaling to synergistically improve glucose and lipid homeostasis [60].
There is an interaction between microorganisms and BAs, and BAs inhibit the growth of BA-sensitive bacteria by promoting the growth of BA-metabolizing bacteria. BAs exert direct antibacterial effects through bacterial membrane damage, a mechanism demonstrable both in vitro and in vivo within the gut, and indirectly by activating ileal epithelial FXR signaling, which induces antimicrobial peptide expression in vivo [61,62]. This is one of the reasons for the altered diversity of the microbiota. In addition, a study suggests that BA metabolites produced by colonic microbes build a proinflammatory gut microenvironment that may further develop into various types of intestinal inflammation [63]. The enrichment of intestinal Clostridia causes an increase in the free BA content in the intestine, which stimulates the gastrointestinal tract and causes diarrhea [64].
5. Beneficial Effects of Probiotics on Mice Fed with High-Fat Diet
HFD has been shown to alter gut microbiota composition and BA profiles in murine models. Similarly, probiotic supplementation reverses HFD-induced adverse effects. The advent of high-throughput sequencing technologies has facilitated a surge in gut microbiome studies. Recognized as the “second genome” of animals, gut microbiota regulates a spectrum of physiological and biochemical processes. However, given the intricate regulatory networks among microbial communities, research focusing on strain-specific interventions to counteract HFD-associated dysbiosis has gained significant attention.
5.1. Lactobacillus
Certain strains within the Lactobacillus (specific strains to be subsequently described) have been identified as probiotics and have attracted considerable attention due to their ability to alleviate obesity and adipose tissue accumulation. Different strains have different effects on high-fat diet mice (Supplementary Table S1). Lactiplantibacillus plantarum FZU3013 reduced body weight and serum TG, TC, and LDL-c and increased the mRNA levels of liver cholesterol 7α-hydroxylase (CYP7A1) and bile salt export pump (BSEP), indicating that BA synthesis was enhanced and the excretion of BA through feces was promoted [65]. Lactiplantibacillus plantarum NKK20 reduced TC and TG concentrations, increased the abundance of colonic Akkermansia and the concentration of short-chain fatty acids (SCFAs), and regulated BA anabolism [66]. Lactiplantibacillus plantarum strain CNCM I-4459 reduced LDL-c concentrations and downregulated liver FAS, perilipin (PLIN), and carnitine palmitoyltransferase-I-alpha (CPTIα) genes [67]. Lactiplantibacillus plantarum FRT10 reduced body weight, fat weight, and liver TG concentration; upregulated the mRNA expression levels of liver peroxisome proliferator-activated receptor alpha (PPARα) and carnitine palmitoyltransferase-1 alpha (CPT1α); and downregulated the mRNA expression levels of liver sterol regulatory element-binding protein 1 (SREBP-1) and diacylglycerol acyltransferase 1 (DGAT1) [68]. Lactiplantibacillus plantarum NCHBL-004 induced glucagon-like peptide 1 (GLP-1) production and increased fecal SCFA levels [69]. Lactiplantibacillus plantarum CQPC01 inhibited the increase in adipocyte volume, increased IL-4 and IL-10 content, downregulated the expression of CCAAT/enhancer binding protein alpha (C/EBP-α) and peroxisome proliferator-activated receptor gamma (PPARγ) mRNA, and upregulated the expression of CYP7A1, CPT1, lipoprotein lipase (LPL), catalase (CAT), superoxide dismutase 1 (SOD1), and SOD2 [70]. Lactiplantibacillus plantarum SKO-001 promoted the increase in serum adiponectin, decreased the levels of leptin, insulin, TC, LDL-c, free fatty acid (FFA), and TG, and decreased the mRNA levels of SREBP-1c and PPARγ [71]. The extract of Lactiplantibacillus plantarum LMT1-48 reduced liver weight and TG levels and downregulated the lipogenic genes PPARγ, HSL, stearoyl-CoA desaturase-1 (SCD-1), and fatty acid translocase (FAT or CD36) in the liver, leading to a reduction in body weight and fat volume [72]. Lactiplantibacillus plantarum CQPC03 alleviated inflammation by increasing the levels of IL-4 and IL-10 and reducing the levels of proinflammatory factors, including IL-6, IL-1β, tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) [73]. Lactiplantibacillus plantarum Shinshu N-07 reduces epididymal adipose tissue weight and adipocyte area and inhibits hepatic steatosis [74]. Lactiplantibacillus plantarum Y44 inhibited the expression of FAS and acetyl CoA carboxylases (ACC) in the liver of obese mice, upregulated the expression of colonic tight junction proteins such as claudin-1 and occludin, reduced serum IL-8 and TNF-α levels, and increased the content of SCFA in feces [75]. Lactiplantibacillus plantarum HF02 inhibited pancreatic lipase activity in small intestinal contents, increased fecal TG levels, and reduced serum lipopolysaccharide (LPS), IL-1β, and TNF-α levels [76]. Lactiplantibacillus plantarum E2_MCCKT leads to upregulation of PPAR-α mRNA, downregulation of adipogenesis and fatty acid synthesis genes (SREBP-1c, ACC, and FAS), and downregulation of proinflammatory cytokine (IL-1Ra and TNF-α) expression [77]. Lactiplantibacillus plantarum KAD 8 restores metabolic health by normalizing glycemia, lipidomics, liver parameters, oxidative stress, and inflammatory parameters [78]. Lactiplantibacillus plantarum BXM2 reversed intestinal dysbiosis by increasing the ratio of villus height to crypt depth and the number of intestinal goblet cells and normalizing the mRNA expression of TNF-α and IL-6 [79]. Lactiplantibacillus plantarum DSR330 reduced the expression of SREBP-1c, ACC1, FAS, 1-aminocyclopropane-1-carboxylicacid oxidase (ACO), PPARα, and CPT-1 in hepatocytes [80]. Lactiplantibacillus plantarum A29 downregulated the expression of lipogenic genes (PPAR-γ, C/EBP-α, and C/EBP-β) in adipocytes and alleviated the development of obesity by increasing phosphorylation and activation of p38 mitogen-activated protein kinase (MAPK), p44/42, and AMPK-α [81]. Lactiplantibacillus plantarum dfa1 reduced inflammatory cytokines in blood and colon tissue and decreased the relative abundance of Proteobacteria [82]. Lactiplantibacillus plantarum strain Ln4 reduced body weight and epididymal fat mass and decreased the protein levels of C-reactive protein (CRP), insulin-like growth factor binding protein-3 (IGFBP-3), and monocyte chemoattractant protein-1 (MCP-1) in white adipose tissue [83]. Lactiplantibacillus plantarum DSM20174 improved glucose and lipid homeostasis and reduced white adipose inflammation [84]. Lactiplantibacillus plantarum KC28 significantly upregulated PPAR-gamma co-activator-1 alpha (PGC1-α) and CPT1-α in the liver and downregulated ACOX-1, PPAR-γ, and FAS expression in mesenteric adipose tissue [85].
Lacticaseibacillus paracasei S0940 and Streptococcus thermophilus ldbm1 reduced serum and liver TC and TG levels in high-fat-fed mice [86]. Bifidobacterium longum BORI reduced the body weight of mice, Lactobacillus acidophilus AD031 and Bifidobacterium bifidum BGN4 reduced the TG level in the liver of mice, while Bifidobacterium longum BORI reduced the TC level in the liver [87]. Lacticaseibacillus paracasei 24 reduced body weight and fat deposition, decreased the ratio of Firmicutes/Bacteroidetes, and increased the abundance of Akkermansia [88]. Lacticaseibacillus paracasei K56 reduced the expression of FAS and PPAR-γ in the liver [89]. Lacticaseibacillus paracasei N1115 reduced visceral fat, liver weight, serum insulin, and leptin levels; altered intestinal microbiota; and increased SCFA content [90]. Lacticaseibacillus paracasei X-1, Lacticaseibacillus paracasei X-17, and Limosilactobacillus fermentum BM-325 inhibited the growth of adipocyte volume and stabilized fasting blood glucose [91]. Lacticaseibacillus paracasei BEPC22 and Lactiplantibacillus plantarum BELP53 reduced white adipose tissue volume and adipocyte size, reduced the expression of PPARγ in the liver, and increased the expression of PPARα in white adipose tissue [92]. Lacticaseibacillus paracasei FZU103 reduced epididymal adipocyte hypertrophy, promoted fecal excretion of BAs, and increased the relative abundance of Ruminococcus, Alistipes, Pseudoflavonifractor and Helicobacter [93]. Lacticaseibacillus paracasei AO356 alters the relative abundance of microorganisms involved in lipid metabolism pathways and obesity-related markers, such as Lactobacillus, Bacteroides, and Oscillospira [94]. Lactobacillus casei CRL 431 exerts beneficial effects by reducing the proinflammatory cytokines IL-6, IL-17, and TNF-α [95].
Lactobacillus sakei MJM60958 reduced the expression of FAS, ACC, and SREBP-1 in the liver; upregulated the expression of PPARα and CPT1A; and affected the regulation of intestinal flora by increasing the production of acetate [96]. Latilactobacillus sakei QC9 increased the abundance of butyrate-producing bacteria and the content of SCFA to mediate the microbiota-gut-liver axis, affecting the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway in the liver and alleviating the development of T2DM [97]. Latilactobacillus sakei WIKIM31 reduced body weight gain, epididymal fat mass, TG, and TC levels; significantly decreased the expression of lipogenesis-related genes in epididymal adipose tissue and liver; and promoted the production of intestinal short-chain fatty acids (such as butyrate and propionate) [98]. Lactobacillus sakei OK67 reduced LPS levels in blood and colon contents, colonic TNF-α and IL-1β expression, and nuclear factor-kappaB (NF-κB) activation; increased IL-10 and tight junction protein expression; and downregulated PPARγ, FAS, and TNF-α expression in adipose tissue [99,100]. Lactobacillus sakei ADM14 reduced body weight gain and blood glucose levels, decreased the expression of lipid-related genes in the epididymal fat pad, decreased the ratio of Firmicutes to Bacteroidetes, and increased the relative abundance of Bacteroides faecichinchillae and Alistipes [40]. Lactobacillus sakei CJLS03 reduced the average size of adipocytes, decreased the gene expression of SREBP-1c, FAS, and SCD1 in epididymal adipose tissue, and increased the levels of SCFA in serum and feces [101].
Lactobacillus acidophilus reduces body weight, fat mass, inflammation, and insulin resistance and inhibits the toll-like receptor 4 (TLR4)/NF-κB signaling pathway [102]. Lactobacillus acidophilus NX2-6 activated the insulin signaling pathway; promoted glucose uptake, glycolysis, and intestinal gluconeogenesis; inhibited hepatic gluconeogenesis; effectively lowered blood glucose levels and improved glucose tolerance; and improved liver energy metabolism through the fibroblast growth factor 21 (FGF21)/AMPKα/PGC-1α/ nuclear respiratory factor 1 (NRF1) pathway [103]. Lactobacillus acidophilus NS1 increased the expression of SREBP2 and low-density lipoprotein receptor (LDLR) in the liver [104]. Lactobacillus acidophilus LA5 reduced obesity, intestinal permeability defect, endotoxemia, and serum cytokines in mice and increased the relative abundance of Akkermansia muciniphila [105]. Lactobacillus acidophilus GOLDGUT-LA100 has high BSH activity, good gastric acid and bile salt tolerance, and alleviates the pathophysiological symptoms of high-fat diet-induced obese mice [106].
Lactobacillus fermentum CECT5716 increased the relative abundance of Akkermansia sp. and the proportion of Bacteroidetes [107]. Limosilactobacillus fermentum HNU312 reduced body weight, serum TG, TC, and LDL-c levels, significantly reduced fat accumulation in the liver and adipose tissue, and increased SCFA production [108]. Limosilactobacillus fermentum MG4231 reduced the expression of PPARγ, C/EBPα, FAS, PLIN2, and LPL in epididymal tissue and reduced SREBP1-c and FAS in liver tissue [109]. Limosilactobacillus fermentum MG4294 and Lactiplantibacillus plantarum MG5289 reduced the levels of proinflammatory cytokines TNF-α, IL-1β, and IL-6 in intestinal tissues [110]. Lactobacillus fermentum LM1016 improved glucose clearance and fatty liver and reduced inflammation in gonadal white adipose tissue in mice fed a high-fat diet [111]. Lactobacillus fermentum CKCC1858, Lactobacillus fermentum CKCC1369, Lactiplantibacillus plantarum CKCC1312, and Lactobacillus gasseri CKCC1913 alleviated liver and pancreatic damage, reduced blood lipids and the secretion of proinflammatory cytokines, increased liver antioxidant enzymes, and improved hyperlipidemia, inflammation, and oxidative stress [112].
Lactobacillus rhamnosus reduced serum IL-6 levels [113]. Lactobacillus rhamnosus TR08 promoted the increase in the relative abundance of Bifidobacteria and Bacteroidetes, thereby reshaping the intestinal flora, reducing the abundance of pathogenic bacteria Enterococci, and increasing the content of SCFAs [114]. Lactobacillus rhamnosus strain LRH05 modulated white adipose tissue monoacylglycerol O-acyltransferase 1 (MOGAT1), insulin-like growth factor-1 (IGF-1), MCP-1, and F4/80 mRNA expression and increased butyrate- and propionate-producing bacteria (Lachnoclostridium, Romboutsia, and Fusobacterium) [115]. Lactobacillus rhamnosus LA68 significantly reduced TC and HDL levels, while Lactiplantibacillus plantarum WCFS1 was more effective in reducing TG and LDL levels [116]. Lactobacillus rhamnosus GG restored exogenous leptin responsiveness, increased the ratio of villus height to crypt depth, and reduced the proportion of Proteobacteria in the fecal microbiota [117]. Lactobacillus rhamnosus LRa05 reduced body weight, blood lipid levels, and lipid accumulation in hepatocytes and epididymal adipose tissue; reduced the abundance of the pathogen-promoting bacterium Streptococcus; and inhibited blood and liver glucose content [118]. Lactobacillus rhamnosus GR-1 reduced the development of oxidative stress and chronic inflammation in a dose-dependent manner [119].
Lactobacillus gasseri SBT2055 reduced the number of macrophages in adipose tissue, the ratio of M1 macrophages to total macrophages was significantly reduced, and the expression of C-C motif ligand 2 (CCL2), C-C chemokine receptor 2 (CCR2), and leptin (LEP) was downregulated [120]. Lactobacillus paragasseri SBT2055 increased small intestinal lipid excretion into feces by reducing the mRNA levels of FABP1, FABP2, fatty-acid transport protein 4 (FATP4), CD36, and apolipoprotein B48 (APOB48) [121].
Lactobacillus johnsonni 3121 and Lactobacillus rhamnosus 86 downregulated the expression of genes related to adipogenesis and normalized the obesity-associated intestinal microbiota [122]. Lactobacillus johnsonii JNU3402 reduced the expression of hepatic SREBP-1c, FAS, and ACC; inhibited SREBP-1c transcriptional activity by enhancing protein kinase A (PKA)-mediated phosphorylation; and reduced the expression of its lipogenic target genes in alpha mouse liver 12 (AML12) and HepG2 cells, thereby attenuating hepatic lipid accumulation [123].
Lactobacillus curvatus HY7601 and Lactiplantibacillus plantarum KY1032 upregulate the expression of cholesterol transport genes in the liver and jejunum, including liver X receptors alpha (LXRα), ATP-binding cassette transporters G (ABCG) 5 and ABCG8, and CYP7A1 [124]. Lactobacillus curvatus HY7601 and Lactiplantibacillus plantarum KY1032 upregulate the expression of fatty acid oxidation-related genes (PGC1α, CPT1, CPT2, and ACOX1) in the liver and reduce the expression of proinflammatory genes (TNFα, IL6, IL1β, and MCP1) in adipose tissue [125].
Lactobacillus reuteri FN04 reduces hepatic FAS overexpression, increases SREBP1c expression, improves intestinal epithelial barrier function, and induces the intestinal microbiota to produce SCFAs [126]. Limosilactobacillus reuteri BIO7251 reduced subcutaneous adipose tissue mass, glucose absorption, and food intake [127]. Lactobacillus rhamnosus FJSYC4-1 and Lactobacillus reuteri FGSZY33L6 alleviated weight gain, blood sugar, and lipid disorders; regulated intestinal flora; and produced SCFA [128].
Lactobacillus amylovorus KU4 increased the expression of uncoupling protein 1 (UCP1), PPARγ, and PGC-1α in subcutaneous inguinal white adipose tissue, reduced receptor-interacting protein 140 (RIP140) expression, and released RIP140 to stimulate UCP1 expression, thereby increasing the interaction between PPARγ and PGC-1α, thereby promoting the browning of white adipocytes [129]. Ligilactobacillus Salivarius LCK11 inhibited food intake by significantly increasing the transcription and translation levels of peptide tyrosine tyrosine (PYY) and, ultimately, the serum PYY level, which is attributed to the activation of the TLR2/NF-κB signaling pathway in enteroendocrine L cells by the peptidoglycan of LCK11 [130]. Lactobacillus kefiri DH5 upregulated the expression of PPAR-α, FABP4 and CPT1 in epididymal adipose tissue, stimulated fatty acid oxidation, and reduced obesity [131]. Lactobacillus pentosus S-PT84 improved intestinal integrity by maintaining tight junction protein expression to inhibit LPS from entering the blood and reduced the secretion of TNF-α and MCP-1 to inhibit systemic inflammatory response [132]. Lactobacillus delbrueckii subsp. lactis CKDB001 reduced liver TG and TC levels without significantly affecting the expression of genes related to lipid metabolism [133]. Lactobacillus coryniformis supsp. torquens T3 inhibits liver inflammation and oxidative stress damage by regulating the LPS inflammatory pathway in the liver, enhances the mechanical function of the intestinal barrier, and increases the content of short-chain fatty acids [134].
5.2. Bifidobacterium (Supplementary Table S2)
Bifidobacterium longum subsp. infantis YB0411 reduced body weight and fat weight [135]. Bifidobacterium longum subsp. infantis FB3-14 reduced the Firmicutes/Bacteroidetes ratio and increased the abundance of Akkermansia muciniphila, unclassified_Muribaculaceae, Lachnospiraceae_NK4A136_group, and Bifidobacterim [136]. Lactiplantibacillus plantarum LC27 and Bifidobacterium longum LC67 increased the expression of claudin-1 and occludin in the colon and reduced the level of Firmicutes and Proteobacteria and fecal LPS [137]. Bifidobacterium longum subsp. longum BL21 reduced serum TC, TG, and LDL-c levels, improved fat vacuolization in hepatocytes and epididymal fat accumulation, and reduced the Firmicutes/Bacteroidetes ratio [138].
Bifidobacterium adolescentis IM38 increased colonic IL-10 and tight junction protein expression, downregulated NF-κB activation and TNF expression, and reduced blood and colonic content LPS levels, as well as the ratio of Proteobacteria to Bacteroidetes [139]. Bifidobacterium adolescentis (BA3, BA5, Z25) and Lactobacillus rhamnosus (LGG, L7-1, L10-1) increased the concentration of SCFA in the intestine of mice, among which Lactobacillus rhamnosus LGG regulated energy metabolism and lipid metabolism, and Lactobacillus rhamnosus L10-1 reduced liver inflammation [140].
Bifidobacterium animalis subsp. lactis lkm512 improved hepatic lipid accumulation and intestinal barrier function [141]. Bifidobacterium animalis subsp. lactis MN-Gup significantly reduced fasting blood glucose levels, increased SCFA levels, increased the relative abundance of Bifidobacterium, and reduced the relative abundance of Escherichia-Shigella and Staphylococcus [142].
Bifidobacterium lactis IDCC 4301 reduced body weight and adipose tissue weight, increased blood lipid levels, and downregulated mRNA expression of adipogenesis-related genes [143]. Bifidobacterium breve strain B-3 dose-dependently inhibited body weight and epididymal fat accumulation, increased serum TC, fasting blood glucose, and insulin levels, and significantly increased intestinal bifidobacterium counts [144]. Bifidobacterium CECT 7765 reduced serum levels of leptin, IL-6, and monocyte chemotactic protein-1 while increasing IL-4 levels [145]. Bifidobacterium bifidum DS0908 reduced body weight and epididymal fat accumulation and serum TG, LDL-c, and TC levels [146].
5.3. Other Probiotics (Supplementary Table S3)
Bacillus coagulans BC69 reduced body weight and increased acetate and butyrate concentrations in feces [147]. A probiotic mixture consisting of five different Bacillus species (sonorensis JJY12-3, paralicheniformis JJY12-8, sonorensis JJY13-1, sonorensis JJY 13-3, and sonorensis JJY 13-8) increased the hepatic expression of lipid oxidation genes, downregulated the expression of genes for lipid uptake and lipogenesis, and reduced lipid accumulation in subcutaneous and mesenteric adipose tissue [148]. Bacillus amyloliquefaciens SC06 improved the antioxidant capacity of mice through the Nrf2/Keap1 signaling pathway and reduced the ratio of Firmicutes/Bacteroidetes [149]. Bacillus coagulans T4 inhibits the accumulation of macrophages in white adipose tissue, converts M1 macrophages into M2 macrophages, reduces TLR4 gene mRNA expression, increases the number of Lactobacillus and Faecalibacterium, and increases propionate and acetate levels [150]. Bacillus licheniformis reduces body weight, serum and liver TG, and epididymal fat weight; reduces liver fat deposition; and significantly changes the colonic bacterial community of obese mice [151].
Bacteroides vulgatus leads to reduced 5-hydroxytryptamine (5-HT) synthesis in jejunal enterochromaffin cells and reduced chylomicron uptake in the jejunal mesentery after HFD in Tph1ΔIEC, thereby alleviating HFD-induced obesity and metabolic dysfunction [152]. Bacteroides thetaiotaomicron increases the proportion of polyunsaturated fatty acids in the liver and prevents hepatic steatohepatitis and liver damage [153]. Bacteroides ovatus reduces serum LPS, CD163, IL-1β, and TNF-α levels and downregulates genes for de novo lipogenesis in the liver (SREBF1, ACC, SCD1, and FAS), accompanied by upregulation of genes related to fatty acid oxidation (PPARα) [154].
Pediococcus pentosaceus PP04 reduces serum TC, TG, LDL-C, FFA, leptin, LPS, and TNF-α levels; downregulates liver SREBP-1c, FAS, and SCD1 to inhibit lipogenesis; and significantly increases the expression of tight junction proteins such as occludin, claudin-1 and zonula occludens-1 (ZO-1) to improve the abnormal increase in intestinal permeability, thereby reducing liver LPS concentration and alleviating intestinal inflammation caused by a high-fat diet through the NF-κB/Nrf2 signaling pathway [155,156]. Clostridium cochlearium reduces body weight, fat mass, fasting blood glucose, and SCFA levels [157]. Clostridium tyrobutyricum reduces liver PPARγ expression, upregulates AMPK, PPARα, ATGL, and HSL expression, reduces the expression of TNF-α, IL-6, and IL-1β in the colon, and upregulates the expression of tight junction proteins [158].
Enterococcus faecium SF68 improves intestinal barrier integrity and function in obese mice by increasing the expression of tight junction proteins and intestinal butyrate transporter [159]. Blautia producta can inhibit cellular lipid accumulation and improve hyperlipidemia [160]. Leuconostoc mesenteroides subsp. mesenteroides SD23 reduces the height of intestinal villi, reduces the expression of TNF-α in the liver, and increases the expression of IL-10 [161]. Roseburia hominis inhibits the expansion of white adipose tissue in mice fed a high-fat diet, which is partly attributed to the production of nicotinamide riboside and the upregulation of the Sirtuin1/mTOR signaling pathway [162]. Coprococcus can effectively reverse HFD-induced hepatic lipid accumulation, inflammation, and fibrosis in mice [163]. Akkermansia muciniphila alleviated high-fat diet-induced weight gain, hepatic steatosis, and liver damage; decreased Alistipes, Lactobacilli, Tyzzerella, Butyricimonas, and Blautia; and increased Clostridium, Osclibacter, Allobaculum, Anaeroplasma, and Rikenella. Akkermansia muciniphila regulated the intestinal FXR-FGF15 axis and remodeled BA construction, reducing secondary BAs in the cecum and liver, including deoxycholic acid (DCA) and lithocholic acid (LCA) [164].
6. Summary
A high-fat diet is the main cause of obesity. Containing more than 30% dietary fat can be called a high-fat diet. Short-term high-fat diets of less than 2 weeks resulted in almost no weight gain, while more than 8 weeks resulted in a significant increase in fat deposition. Dietary fat is digested and absorbed into the blood in the small intestine, and when it reaches a certain amount, it will cause an increase in TC, TG, LDL-C, and HDL-C in plasma, which is related to many metabolic diseases.
High-fat diets lead to altered gut microbiome profiles. Firmicutes and Bacteroidetes usually account for more than 90%. The F/B ratio in obese mice is generally thought to be elevated, but there are exceptions. Gut microbiota composition in high-fat diet-induced obese mice exhibited distinct shifts in phylum-level dominance, with Firmicutes and Bacteroidetes showing differential responsiveness to dietary fat content. A high-fat diet leads to a decrease in the alpha diversity of gut microbes, and different lipid types may influence changes in microbial abundance. Family-level microorganisms such as Rikenellaceae, Deferribacteraceae, Streptococcaceae, Christensenellaceae, and Peptococcaceae may be important biomarkers that differentiate high-fat diets from normal diets.
BAs are key to the emulsification of fats in the small intestine. Small amounts of BAs are conjugated by BSH-active microorganisms in the gut, including Lactobacillus, Bifidobacterium, Clostridium, and Bacteroides, thereby preventing their entry into the enterohepatic circulation. Decreased gut microbial alpha diversity related to the levels of BAs. Adequate BA levels are beneficial for maintaining gut health and weight management. Supplementing probiotics can reverse the negative effects of obesity caused by a high-fat diet in mice, including weight gain, increased serum TG, TC, and LDL contents, increased adipocyte area, increased gene expression of lipogenic genes in the liver and adipose tissue, and a damaged intestinal barrier.
The murine model of HFD is widely used in obesity-related research. HFD-induced metabolic disorders and inflammatory processes exhibit bidirectional interplay with the gut microbiota (Figure 1). The advancement of high-throughput technologies has led to a growing number of studies investigating microbial strains that mitigate the adverse effects of HFD, although most of these strains belong to the Lactobacillus. However, research on strains from genera identified by 16S rRNA sequencing and metagenomic analyses as taxa potentially linked to probiotic properties, such as Bifidobacterium, Akkermansia, Prevotella, and Osclibacter, remains limited, as these techniques primarily reveal taxonomic associations rather than direct evidence of probiotic functions. Future studies should prioritize mechanistic investigations of strains within these genera to evaluate their probiotic potential.
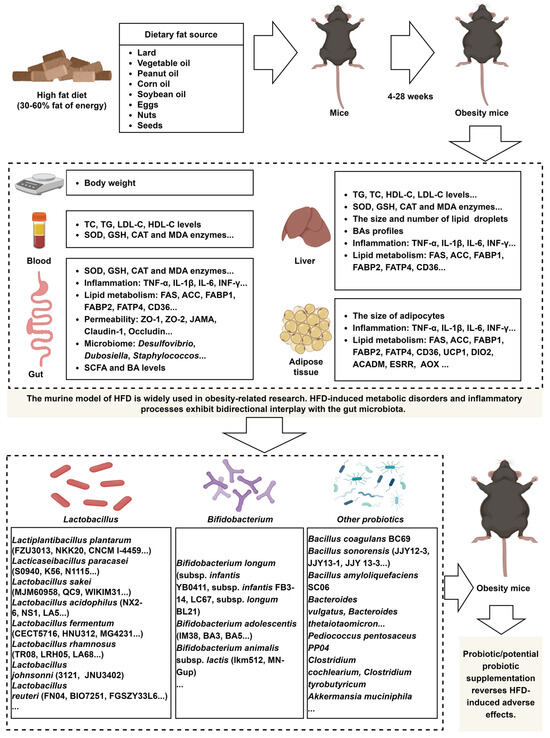
Figure 1.
A HFD induces dysregulation of lipid metabolism and immune responses in mice, and probiotic supplementation effectively reverses these detrimental effects. The image material in Figure 1 comes from www.biorender.com.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17091402/s1, Table S1: Effects of oral administration of a strain (belonging to the Lactobacillus) on mice fed a high-fat diet; Table S2: Effects of oral administration of a strain (belonging to the Bifidobacterium) on mice fed a high-fat diet; Table S3: Effects of oral administration of a strain (other probiotics/potential probiotic) on mice fed a high-fat diet.
Author Contributions
Writing—original draft, L.Y. and Z.L.; Visualization, H.X. and D.S.; Writing—review and editing, Y.H., H.P. and Y.Z.; Supervision, H.Z.; Conceptualization, S.Z., H.W. and M.Y.; Funding acquisition, S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2024YFD1800404), the National Natural Science Foundation of China (32360808, 31760645, 31260592, 31060331), the Major Science and Technology Project of Yunnan Province (202102AA310054 and 202202AE090032), and the Sci-Tech Service Station of Farmer Academician in Fuyuan County.
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
The following abbreviations are used in this manuscript.
| 5-HT | 5-hydroxytryptamine |
| ABCG | ATP-binding cassette transporters G |
| ACC | acetyl CoA carboxylases |
| ACO | 1-aminocyclopropane-1-carboxylicacid oxidase |
| AML12 | alpha mouse liver 12 |
| AMPKα2 | kinase AMP-activated catalytic subunit alpha 2 |
| AP-1 | activator protein-1 |
| APOB48 | apolipoprotein B48 |
| ATGL | adipose triglyceride lipase |
| BA | bile acid |
| BSEP | bile salt export pump |
| BSH | bile salt hydrolase |
| C/EBP-α | CCAAT/enhancer binding protein alpha |
| CAT | catalase |
| CCL2 | C-C motif ligand 2 |
| CCR2 | C-C chemokine receptor 2 |
| CPT1α | carnitine palmitoyltransferase-1 alpha |
| CPTIα | carnitine palmitoyltransferase-I-alpha |
| CRP | C-reactive protein |
| CYP7A1 | cholesterol 7α-hydroxylase |
| DCA | deoxycholic acid |
| DGAT1 | diacylglycerol acyltransferase 1 |
| F/B | Firmicutes/Bacteroidetes |
| FABP2 | fatty acid binding protein 2 |
| FAS | fatty acid synthase |
| FAT/CD36 | fatty acid translocase |
| FATP4 | fatty-acid transport protein 4 |
| FFA | free fatty acid |
| FGF21 | fibroblast growth factor 21 |
| FXR | farnesoid X receptor |
| GLP-1 | glucagon-like peptide 1 |
| HDL-C | high-density lipoprotein cholesterol |
| HFD | high fat diet |
| HSL | hormone-sensitive triglyceride lipase |
| IFN-γ | interferon-gamma |
| IGF-1 | insulin-like growth factor-1 |
| IGFBP-3 | insulin-like growth factor binding protein-3 |
| IL-1 | interleukin 1 |
| IL-6 | interleukin 6 |
| LCA | lithocholic acid |
| LDL-C | low-density lipoprotein cholesterol |
| LDLR | low-density lipoprotein receptor |
| LEfSe | linear discriminant analysis effect size |
| LEP | leptin |
| LPL | lipoprotein lipase |
| LPS | lipopolysaccharide |
| LXRα | liver X receptors alpha |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemoattractant protein-1 |
| MOGAT1 | monoacylglycerol O-acyltransferase 1 |
| MT-Cytb | mitochondrially encoded cytochrome b |
| NAFLD | nonalcoholic fatty liver disease |
| NF-κB | nuclear factor-kappaB |
| NRF1 | nuclear respiratory factor 1 |
| PGC1-α | PPAR-gamma co-activator-1 alpha |
| PI3K | phosphatidylinositol 3-kinase |
| PKA | protein kinase A |
| PLIN | perilipin |
| PPARα | peroxisome proliferator-activated receptor alpha |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| PYY | peptide tyrosine tyrosine |
| RIP140 | receptor-interacting protein 140 |
| SCD-1 | stearoyl-CoA desaturase-1 |
| SCFAs | short-chain fatty acids |
| SNS | sympathetic nervous system |
| SOD1 | superoxide dismutase 1 |
| SREBP-1 | sterol regulatory element-binding protein 1 |
| TC | total cholesterol |
| TG | triglyceride |
| TGR5 | takeda G protein-coupled receptor 5 |
| TLR4 | toll-like receptor 4 |
| TNF-α | tumor necrosis factor-alpha |
| TRAF2 | tumor necrosis factor receptor-associated factor 2 |
| UCP1 | uncoupling protein 1 |
| ZO-1 | zonula occludens-1 |
References
- Lotrean, L.M.; Stan, O.; Lencu, C.; Laza, V. Dietary patterns, physical activity, body mass index, weight-related behaviours and their interrelationship among Romanian university students-trends from 2003 to 2016. Nutr. Hosp. 2018, 35, 375–383. [Google Scholar] [PubMed]
- Rocha, N.P.; Milagres, L.C.; Filgueiras, M.S.; Suhett, L.G.; Silva, M.A.; Albuquerque, F.M.; Ribeiro, A.Q.; Vieira, S.A.; Novaes, J.F. Association of dietary patterns with excess weight and body adiposity in Brazilian children: The Pase-Brasil study. Arq. Bras. Cardiol. 2019, 113, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ang, Q.Y.; Alba, D.L.; Upadhyay, V.; Bisanz, J.E.; Cai, J.; Lee, H.L.; Barajas, E.; Wei, G.; Noecker, C.; Patterson, A.D.; et al. The East Asian gut microbiome is distinct from colocalized White subjects and connected to metabolic health. Elife 2021, 10, e70349. [Google Scholar] [CrossRef]
- Lampuré, A.; Castetbon, K.; Hanafi, M.; Deglaire, A.; Schlich, P.; Péneau, S.; Hercberg, S.; Méjean, C. Relative influence of socioeconomic, psychological and sensory characteristics, physical activity and diet on 5-year weight gain in French adults. Nutrients 2017, 9, 1179. [Google Scholar] [CrossRef]
- Viola, P.C.A.F.; Carvalho, C.A.; Bragança, M.L.B.M.; França, A.K.T.D.C.; Alves, M.T.S.S.B.E.; da Silva, A.A.M. High consumption of ultra-processed foods is associated with lower muscle mass in Brazilian adolescents in the RPS birth cohort. Nutrition 2020, 79–80, 110983. [Google Scholar] [CrossRef] [PubMed]
- Aghayan, M.; Asghari, G.; Yuzbashian, E.; Mahdavi, M.; Mirmiran, P.; Azizi, F. Secular trend in dietary patterns of Iranian adults from 2006 to 2017: Tehran lipid and glucose study. Nutr. J. 2020, 19, 110. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA. 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Requena, T.; Velasco, M. The human microbiome in sickness and in health. Rev. Clínica Española 2021, 221, 233–240. [Google Scholar] [CrossRef]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the human gut microbiome: An international review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef]
- Mancabelli, L.; Milani, C.; Lugli, G.A.; Turroni, F.; Ferrario, C.; van Sinderen, D.; Ventura, M. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ. Microbiol. 2017, 19, 1379–1390. [Google Scholar] [CrossRef]
- Jing, Y.S.; Ma, Y.F.; Pan, F.B.; Li, M.S.; Zheng, Y.G.; Wu, L.F.; Zhang, D.S. An insight into antihyperlipidemic effects of polysaccharides from natural resources. Molecules 2022, 27, 1903. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-fat diet increases gut microbiota biodiversity and energy expenditure due to nutrient difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef] [PubMed]
- Meksawan, K.; Pendergast, D.R.; Leddy, J.J.; Mason, M.; Horvath, P.J.; Awad, A.B. Effect of low and high fat diets on nutrient intakes and selected cardiovascular risk factors in sedentary men and women. J. Am. Coll. Nutr. 2004, 23, 131–140. [Google Scholar] [CrossRef]
- Patil, B.S.; Kanthe, P.S.; Reddy, C.R.; Das, K.K. Emblica officinalis (Amla) ameliorates high-fat diet induced alteration of cardiovascular pathophysiology. Cardiovasc. Hematol. Agents Med. Chem. 2019, 17, 52–63. [Google Scholar] [CrossRef]
- Linehan, V.; Fang, L.Z.; Hirasawa, M. Short-term high-fat diet primes excitatory synapses for long-term depression in orexin neurons. J. Physiol. 2018, 596, 305–316. [Google Scholar] [CrossRef]
- Schanuel, F.S.; Romana-Souza, B.; Monte-Alto-Costa, A. Short-term administration of a high-fat diet impairs wound repair in mice. Lipids 2020, 55, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Sakane, N.; Moritani, T. Metabolic responses to high-fat or low-fat meals and association with sympathetic nervous system activity in healthy young men. J. Nutr. Sci. Vitaminol. 2005, 51, 355–360. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Hopkins, W.G. Effects of high-fat and high-carbohydrate diets on metabolism and performance in cycling. Metabolism 2002, 51, 678–690. [Google Scholar] [CrossRef]
- Woodie, L.; Blythe, S. The differential effects of high-fat and high-fructose diets on physiology and behavior in male rats. Nutr. Neurosci. 2018, 21, 328–336. [Google Scholar] [CrossRef]
- Cheng, H.S.; Ton, S.H.; Phang, S.C.W.; Tan, J.B.L.; Abdul Kadir, K. Increased susceptibility of post-weaning rats on high-fat diet to metabolic syndrome. J. Adv. Res. 2017, 8, 743–752. [Google Scholar] [CrossRef]
- Huang, B.W.; Chiang, M.T.; Yao, H.T.; Chiang, W. The effect of high-fat and high-fructose diets on glucose tolerance and plasma lipid and leptin levels in rats. Diabetes Obes. Metab. 2004, 6, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Kanthe, P.S.; Patil, B.S.; Das, K.K. Terminalia arjuna supplementation ameliorates high fat diet-induced oxidative stress in nephrotoxic rats. J. Basic Clin. Physiol. Pharmacol. 2021, 33, 409–417. [Google Scholar] [CrossRef]
- Maejima, Y.; Yokota, S.; Horita, S.; Shimomura, K. Early life high-fat diet exposure evokes normal weight obesity. Nutr. Metab. 2020, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shi, M.; Wu, J.; Sun, Z.; Guo, J.; Liu, Y.; Han, D. Effects of a high-fat diet on intracellular calcium (Ca2+) handling and cardiac remodeling in Wistar rats without hyperlipidemia. Ultrastruct. Pathol. 2020, 44, 42–51. [Google Scholar] [CrossRef]
- Emelyanova, L.; Boukatina, A.; Myers, C.; Oyarzo, J.; Lustgarten, J.; Shi, Y.; Jahangir, A. High calories but not fat content of lard-based diet contribute to impaired mitochondrial oxidative phosphorylation in C57BL/6J mice heart. PLoS ONE 2019, 14, e0217045. [Google Scholar] [CrossRef]
- Pang, J.; Xi, C.; Huang, X.; Cui, J.; Gong, H.; Zhang, T. Effects of excess energy intake on glucose and lipid metabolism in C57BL/6 mice. PLoS ONE 2016, 11, e0146675. [Google Scholar] [CrossRef]
- Topal, F.; Goren, H.; Yucel, F.; Sahinturk, V.; Aydar, Y. Effect of consuming high-fat diet on the morphological parameters of adrenal gland. Bratisl. Med. J. 2019, 120, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride metabolism in the liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Muniz, L.B.; Alves-Santos, A.M.; Camargo, F.; Martins, D.B.; Celes, M.R.N.; Naves, M.M.V. High-lard and high-cholesterol diet, but not high-lard diet, leads to metabolic disorders in a modified dyslipidemia model. Arq. Bras. De Cardiol. 2019, 113, 896–902. [Google Scholar] [CrossRef]
- Yan, S.; Liu, S.; Qu, J.; Li, X.; Hu, J.; Zhang, L.; Liu, X.; Li, X.; Wang, X.; Wen, L.; et al. A lard and soybean oil mixture alleviates low-fat-high-carbohydrate diet-induced nonalcoholic fatty liver disease in mice. Nutrients 2022, 14, 560. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Bogensberger, B.; Benčič, A.; Knüppel, S.; Boeing, H.; Hoffmann, G. Effects of oils and solid fats on blood lipids: A systematic review and network meta-analysis. J. Lipid Res. 2018, 59, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Shen, X.; Wang, F.; Li, Y.; Zheng, X. Black current anthocyanins improve lipid metabolism and modulate gut microbiota in high-fat diet-induced obese mice. Mol. Nutr. Food Res. 2021, 65, e2001090. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Lee, H.H.; Gil, H.S.; Chung, K.S.; Kim, J.K.; Kim, D.H.; Yoon, J.; Chung, E.K.; Lee, J.K.; Yang, W.M.; et al. Standardized hot water extract from the leaves of Hydrangea serrata (Thunb.) Ser. alleviates obesity via the AMPK pathway and modulation of the gut microbiota composition in high fat diet-induced obese mice. Food Funct. 2021, 12, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Tang, M.; Li, J.; Xie, Y.; Li, Y.; Xie, J.; Zhou, L.; Liu, Y.; Yu, X. Gut microbiota and serum metabolic signatures of high-fat-induced bone loss in mice. Front. Cell. Infect. Microbiol. 2021, 11, 788576. [Google Scholar] [CrossRef]
- Wu, L.; Yan, Q.; Chen, F.; Cao, C.; Wang, S. Bupleuri radix extract ameliorates impaired lipid metabolism in high-fat diet-induced obese mice via gut microbia-mediated regulation of FGF21 signaling pathway. Biomed. Pharmacother. 2021, 135, 111187. [Google Scholar] [CrossRef]
- Islam, T.; Koboziev, I.; Albracht-Schulte, K.; Mistretta, B.; Scoggin, S.; Yosofvand, M.; Moussa, H.; Zabet-Moghaddam, M.; Ramalingam, L.; Gunaratne, P.H.; et al. Curcumin reduces adipose tissue inflammation and alters gut microbiota in diet-induced obese male mice. Mol. Nutr. Food Res. 2021, 65, e2100274. [Google Scholar] [CrossRef]
- Peng, C.; Xu, X.; Li, Y.; Li, X.; Yang, X.; Chen, H.; Zhu, Y.; Lu, N.; He, C. Sex-specific association between the gut microbiome and high-fat diet-induced metabolic disorders in mice. Biol. Sex Differ. 2020, 11, 5. [Google Scholar] [CrossRef]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes. 2020, 44, 213–225. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Zhang, Z.; Feng, Y. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKα/STAT3 signaling in diet-induced obesity. Theranostics 2020, 10, 11302–11323. [Google Scholar] [CrossRef] [PubMed]
- Won, S.M.; Chen, S.; Lee, S.Y.; Lee, K.E.; Park, K.W.; Yoon, J.H. Lactobacillus sakei ADM14 induces anti-obesity effects and changes in gut microbiome in high-fat diet-induced obese mice. Nutrients 2020, 12, 3703. [Google Scholar] [CrossRef]
- Van Hul, M.; Karnik, K.; Canene-Adams, K.; De Souza, M.; Van den Abbeele, P.; Marzorati, M.; Delzenne, N.M.; Everard, A.; Cani, P.D. Comparison of the effects of soluble corn fiber and fructooligosaccharides on metabolism, inflammation, and gut microbiome of high-fat diet-fed mice. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E779–E791. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Liu, C.; Zheng, N.; Jia, W.; Zhang, W.; Li, H. Metabolic and gut microbial characterization of obesity-prone mice under a high-fat diet. J. Proteome Res. 2019, 18, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhao, Y.; Zhu, H.; Liang, N.; Ma, K.Y.; Lei, L.; He, W.S.; et al. Beneficial effects of tea water extracts on the body weight and gut microbiota in C57BL/6J mice fed with a high-fat diet. Food Funct. 2019, 10, 2847–2860. [Google Scholar] [CrossRef]
- Cao, W.; Chin, Y.; Chen, X.; Mi, Y.; Xue, C.; Wang, Y.; Tang, Q. The role of gut microbiota in the resistance to obesity in mice fed a high fat diet. Int. J. Food Sci. Nutr. 2020, 71, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Igarashi, M.; Li, X.; Nakatani, A.; Miyamoto, J.; Inaba, Y.; Sutou, A.; Saito, T.; Sato, T.; Tachibana, N.; et al. Dietary soybean protein ameliorates high-fat diet-induced obesity by modifying the gut microbiota-dependent biotransformation of bile acids. PLoS ONE 2018, 13, e0202083. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, G.; Li, Q.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitin oligosaccharide modulates gut microbiota and attenuates high-fat-diet-induced metabolic syndrome in mice. Mar. Drugs 2018, 16, 66. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, N.; Sun, Y.; Wu, S.; Tian, W.; Lai, Y.; Li, P.; Du, B. Supplementation of Bacillus sp. DU-106 reduces hypercholesterolemia and ameliorates gut dysbiosis in high-fat diet rats. Appl. Microbiol. Biotechnol. 2021, 105, 287–299. [Google Scholar]
- Li, D.; Wu, H.; Dou, H.; Guo, L.; Huang, W. Microcapsule of sweet orange essential oil changes gut microbiota in diet-induced obese rats. Biochem. Biophys. Res. Commun. 2018, 505, 991–995. [Google Scholar] [CrossRef]
- Choi, S.; Hwang, Y.J.; Shin, M.J.; Yi, H. Difference in the gut microbiome between ovariectomy-induced obesity and diet-induced obesity. J. Microbiol. Biotechnol. 2017, 27, 2228–2236. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Knight, R.; Leibel, R.L. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol. Metab. 2015, 26, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Jing, N.; Liu, X.; Jin, M.; Yang, X.; Hu, X.; Li, C.; Zhao, K. Fubrick tea attenuates high-fat diet induced fat deposition and metabolic disorder by regulating gut microbiota and caffeine metabolism. Food Funct. 2020, 11, 6971–6986. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Wang, Q.; Yi, S.; Liu, X.; Jin, H.; Xu, J.; Wen, G.; Zhu, J.; Tuo, B. The source of the fat significantly affects the results of high-fat diet intervention. Sci. Rep. 2022, 12, 4315. [Google Scholar] [CrossRef]
- Qiao, B.; Li, X.; Wu, Y.; Guo, T.; Tan, Z. Comparative analysis of the gut microbiota in mice under lard or vegetable blend oil diet. J. Oleo Sci. 2022, 71, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Monte, M.J.; Marin, J.J.; Antelo, A.; Vazquez-Tato, J. Bile acids: Chemistry, physiology, and pathophysiology. World J. Gastroenterol. 2009, 15, 804–816. [Google Scholar] [CrossRef]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.; Marchesi, J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 13580–13585. [Google Scholar] [CrossRef]
- Kisiela, M.; Skarka, A.; Ebert, B.; Maser, E. Hydroxysteroid dehydrogenases (HSDs) in bacteria: A bioinformatic perspective. J. Steroid Biochem. Mol. Biol. 2012, 129, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hao, C.; Yao, W.; Zhu, D.; Lu, H.; Li, L.; Ma, B.; Sun, B.; Xue, D.; Zhang, W. Intestinal flora imbalance affects bile acid metabolism and is associated with gallstone formation. BMC Gastroenterol. 2020, 20, 59. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef]
- Inagaki, T.; Moschetta, A.; Lee, Y.K.; Peng, L.; Zhao, G.; Downes, M.; Yu, R.T.; Shelton, J.M.; Richardson, J.A.; Repa, J.J.; et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 3920–3925. [Google Scholar] [CrossRef]
- Kurdi, P.; Kawanishi, K.; Mizutani, K.; Yokota, A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J. Bacteriol. 2006, 188, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, Z.; Zhang, X.; Zhu, F.; Liu, Y.; Jin, C.; Du, X.; Xu, C.; Chen, Y.; Cai, W.; et al. Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation. Gut Microbes 2020, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, W.; Chen, Y.; Huang, F.; Lu, L.; Lin, C.; Huang, T.; Ning, Z.; Zhai, L.; Zhong, L.L.; et al. Clostridia-rich microbiota enhances bile acid excretion in diarrhea-predominant irritable bowel syndrome. J. Clin. Investig. 2020, 130, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, W.L.; Li, Q.Y.; Xu, J.X.; Cao, Y.J.; Liu, B.; Yu, X.D.; Rao, P.F.; Ni, L.; Lv, X.C. The protective mechanism of Lactobacillus plantarum FZU3013 against non-alcoholic fatty liver associated with hyperlipidemia in mice fed a high-fat diet. Food Funct. 2020, 11, 3316–3331. [Google Scholar] [CrossRef]
- Sun, C.; Qiu, C.; Zhang, Y.; Yan, M.; Tan, J.; He, J.; Yang, D.; Wang, D.; Wu, L. Lactiplantibacillus plantarum NKK20 Alleviates High-Fat-Diet-Induced Nonalcoholic Fatty Liver Disease in Mice through Regulating Bile Acid Anabolism. Molecules 2023, 28, 4042. [Google Scholar] [CrossRef]
- Jacouton, E.; Mondot, S.; Langella, P.; Bermúdez-Humarán, L.G. Impact of Oral Administration of Lactiplantibacillus plantarum Strain CNCM I-4459 on Obesity Induced by High-Fat Diet in Mice. Bioengineering 2023, 10, 1151. [Google Scholar] [CrossRef]
- Cai, H.; Wen, Z.; Li, X.; Meng, K.; Yang, P. Lactobacillus plantarum FRT10 alleviated high-fat diet-induced obesity in mice through regulating the PPARα signal pathway and gut microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 5959–5972. [Google Scholar] [CrossRef]
- Jang, A.R.; Jung, D.H.; Lee, T.S.; Kim, J.K.; Lee, Y.B.; Lee, J.Y.; Kim, S.Y.; Yoo, Y.C.; Ahn, J.H.; Hong, E.H.; et al. Lactobacillus plantarum NCHBL-004 modulates high-fat diet-induced weight gain and enhances GLP-1 production for blood glucose regulation. Nutrition 2024, 128, 112565. [Google Scholar] [CrossRef]
- Gan, Y.; Tang, M.W.; Tan, F.; Zhou, X.R.; Fan, L.; Xie, Y.X.; Zhao, X. Anti-obesity effect of Lactobacillus plantarum CQPC01 by modulating lipid metabolism in high-fat diet-induced C57BL/6 mice. J. Food Biochem. 2020, 44, e13491. [Google Scholar] [CrossRef]
- Choi, M.J.; Yu, H.; Kim, J.I.; Seo, H.; Kim, J.G.; Kim, S.K.; Lee, H.S.; Cheon, H.G. Anti-obesity effects of Lactiplantibacillus plantarum SKO-001 in high-fat diet-induced obese mice. Eur. J. Nutr. 2023, 62, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Dong, H.J.; Jeong, H.U.; Ryu, D.W.; Song, S.M.; Kim, Y.R.; Jung, H.H.; Kim, T.H.; Kim, Y.H. Lactobacillus plantarum LMT1-48 exerts anti-obesity effect in high-fat diet-induced obese mice by regulating expression of lipogenic genes. Sci. Rep. 2020, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Chen, H.; Zhou, X.R.; Chu, L.L.; Ran, W.T.; Tan, F.; Zhao, X. Regulating effect of Lactobacillus plantarum CQPC03 on lipid metabolism in high-fat diet-induced obesity in mice. J. Food Biochem. 2020, 44, e13495. [Google Scholar]
- Yin, T.; Bayanjargal, S.; Fang, B.; Inaba, C.; Mutoh, M.; Kawahara, T.; Tanaka, S.; Watanabe, J. Lactobacillus plantarum Shinshu N-07 isolated from fermented Brassica rapa L. attenuates visceral fat accumulation induced by high-fat diet in mice. Benef. Microbes 2020, 11, 655–667. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Ma, F.; Sun, M.; Mu, G.; Tuo, Y. The ameliorative effect of Lactobacillus plantarum Y44 oral administration on inflammation and lipid metabolism in obese mice fed with a high fat diet. Food Funct. 2020, 11, 5024–5039. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, H.; Qi, X.; Sun, Y.; Ma, Y.; Li, Q. Lactobacillus plantarum HF02 alleviates lipid accumulation and intestinal microbiota dysbiosis in high-fat diet-induced obese mice. J. Sci. Food Agric. 2023, 103, 4625–4637. [Google Scholar] [CrossRef]
- Das, T.K.; Kar, P.; Panchali, T.; Khatun, A.; Dutta, A.; Ghosh, S.; Chakrabarti, S.; Pradhan, S.; Mondal, K.C.; Ghosh, K. Anti-obesity potentiality of Lactiplantibacillus plantarum E2_MCCKT isolated from a fermented beverage, haria: A high fat diet-induced obese mice model study. World J. Microbiol. Biotechnol. 2024, 40, 168. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, A.J.; Islam, R.; Sarkar, S.; Saha, T. Lactobacillus plantarum KAD protects against high-fat diet-induced hepatic complications in Swiss albino mice: Role of inflammation and gut integrity. PLoS ONE 2024, 19, e0313548. [Google Scholar] [CrossRef]
- Cai, X.; Huang, J.; Yu, T.; Guan, X.; Sun, M.; Zhao, D.; Zheng, Y.; Wang, Q. Lactiplantibacillus plantarum BXM2 Treatment Alleviates Disorders Induced by a High-Fat Diet in Mice by Improving Intestinal Health and Modulating the Gut Microbiota. Nutrients 2025, 17, 407. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, Y.; Shin, D.S.; Ra, J.; Choi, Y.M.; Ryu, B.H.; Lee, J.; Park, E.; Paik, H.D. Hepatoprotective Effect of Lactiplantibacillus plantarum DSR330 in Mice with High Fat Diet-Induced Nonalcoholic Fatty Liver Disease. J. Microbiol. Biotechnol. 2024, 34, 399–406. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Kuppusamy, P.; Srisesharam, S.; Lee, J.C.; Sivanesan, R.; Kim, D.; Choi, K.C. Positive metabolic effects of selected probiotic bacteria on diet-induced obesity in mice are associated with improvement of dysbiotic gut microbiota. FASEB J. 2020, 34, 12289–12307. [Google Scholar] [CrossRef]
- Ondee, T.; Pongpirul, K.; Janchot, K.; Kanacharoen, S.; Lertmongkolaksorn, T.; Wongsaroj, L.; Somboonna, N.; Ngamwongsatit, N.; Leelahavanichkul, A. Lactiplantibacillus plantarum dfa1 Outperforms Enterococcus faecium dfa1 on Anti-Obesity in High Fat-Induced Obesity Mice Possibly through the Differences in Gut Dysbiosis Attenuation, despite the Similar Anti-Inflammatory Properties. Nutrients 2021, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Jung, S.R.; Lee, S.Y.; Lee, N.K.; Paik, H.D.; Lim, S.I. Lactobacillus plantarum Strain Ln4 Attenuates Diet-Induced Obesity, Insulin Resistance, and Changes in Hepatic mRNA Levels Associated with Glucose and Lipid Metabolism. Nutrients 2018, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Riezu-Boj, J.I.; Barajas, M.; Pérez-Sánchez, T.; Pajares, M.J.; Araña, M.; Milagro, F.I.; Urtasun, R. Lactiplantibacillus plantarum DSM20174 Attenuates the Progression of Non-Alcoholic Fatty Liver Disease by Modulating Gut Microbiota, Improving Metabolic Risk Factors, and Attenuating Adipose Inflammation. Nutrients 2022, 14, 5212. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Kim, S.; Park, H.; Park, S.; Ji, Y.; Todorov, S.D.; Lim, S.D.; Holzapfel, W.H. Modulation of the Gut Microbiome and Obesity Biomarkers by Lactobacillus Plantarum KC28 in a Diet-Induced Obesity Murine Model. Probiotics Antimicrob. Proteins 2021, 13, 677–697. [Google Scholar] [CrossRef]
- Kuerman, M.; Bao, Y.; Guo, M.; Jiang, S. Safety assessment of two strains and anti-obese effects on mice fed a high-cholesterol diet. Biochem. Biophys. Res. Commun. 2021, 572, 131–137. [Google Scholar] [CrossRef]
- Li, Z.; Jin, H.; Oh, S.Y.; Ji, G.E. Anti-obese effects of two Lactobacilli and two Bifidobacteria on ICR mice fed on a high fat diet. Biochem. Biophys. Res. Commun. 2016, 480, 222–227. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, X.; Wang, W.; Gu, L.; Hu, C.; Sun, H.; Xu, C.; Hou, J.; Jiang, Z. Lactobacillus paracasei 24 Attenuates Lipid Accumulation in High-Fat Diet-Induced Obese Mice by Regulating the Gut Microbiota. J. Agric. Food Chem. 2022, 70, 4631–4643. [Google Scholar] [CrossRef]
- Miao, Z.; Zheng, H.; Liu, W.H.; Cheng, R.; Lan, H.; Sun, T.; Zhao, W.; Li, J.; Shen, X.; Li, H.; et al. Lacticaseibacillus paracasei K56 Attenuates High-Fat Diet-Induced Obesity by Modulating the Gut Microbiota in Mice. Probiotics Antimicrob. Proteins 2023, 15, 844–855. [Google Scholar] [CrossRef]
- Miao, Z.H.; Wang, J.N.; Shen, X.; Zhou, Q.Q.; Luo, Y.T.; Liang, H.J.; Wang, S.J.; Qi, S.H.; Cheng, R.Y.; He, F. Long-term use of Lacticaseibacillus paracasei N1115 from early life alleviates high-fat-diet-induced obesity and dysmetabolism in mice. Benef Microbes 2022, 13, 407–416. [Google Scholar] [CrossRef]
- Galindev, U.; Erdenebold, U.; Batnasan, G.; Ganzorig, O.; Batdorj, B. Anti-obesity effects of potential probiotic Lactobacillus strains isolated from Mongolian fermented dairy products in high-fat diet-induced obese rodent model. Braz. J. Microbiol. 2024, 55, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.R.; Kwon, T.J.; Chung, E.C.; Bae, J.; Soung, S.H.; Tak, H.J.; Choi, J.Y.; Lee, Y.E.; Won Hwang, N.; Lee, J.S.; et al. Combination of Lacticaseibacillus paracasei BEPC22 and Lactiplantibacillus plantarum BELP53 attenuates fat accumulation and alters the metabolome and gut microbiota in mice with high-fat diet-induced obesity. Food Funct. 2024, 15, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.C.; Chen, M.; Huang, Z.R.; Guo, W.L.; Ai, L.Z.; Bai, W.D.; Yu, X.D.; Liu, Y.L.; Rao, P.F.; Ni, L. Potential mechanisms underlying the ameliorative effect of Lactobacillus paracasei FZU103 on the lipid metabolism in hyperlipidemic mice fed a high-fat diet. Food Res. Int. 2021, 139, 109956. [Google Scholar] [CrossRef] [PubMed]
- Song, E.J.; Lee, E.S.; Kim, Y.I.; Shin, D.U.; Eom, J.E.; Shin, H.S.; Lee, S.Y.; Nam, Y.D. Gut microbial change after administration of Lacticaseibacillus paracasei AO356 is associated with anti-obesity in a mouse model. Front. Endocrinol. 2023, 14, 1224636. [Google Scholar] [CrossRef]
- Novotny Núñez, I.; Maldonado Galdeano, C.; de Moreno de LeBlanc, A.; Perdigón, G. Lactobacillus casei CRL 431 administration decreases inflammatory cytokines in a diet-induced obese mouse model. Nutrition 2015, 31, 1000–1007. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Gu, M.; Werlinger, P.; Cho, J.H.; Cheng, J.; Suh, J.W. Lactobacillus sakei MJM60958 as a Potential Probiotic Alleviated Non-Alcoholic Fatty Liver Disease in Mice Fed a High-Fat Diet by Modulating Lipid Metabolism, Inflammation, and Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 13436. [Google Scholar] [CrossRef]
- Chen, M.; Pan, P.; Zhang, H.; Li, R.; Ren, D.; Jiang, B. Latilactobacillus sakei QC9 alleviates hyperglycaemia in high-fat diet and streptozotocin-induced type 2 diabetes mellitus mice via the microbiota-gut-liver axis. Food Funct. 2024, 15, 8008–8029. [Google Scholar] [CrossRef]
- Park, S.S.; Lim, S.K.; Lee, J.; Park, H.K.; Kwon, M.S.; Yun, M.; Kim, N.; Oh, Y.J.; Choi, H.J. Latilactobacillus sakei WIKIM31 Decelerates Weight Gain in High-Fat Diet-Induced Obese Mice by Modulating Lipid Metabolism and Suppressing Inflammation. J. Microbiol. Biotechnol. 2021, 31, 1568–1575. [Google Scholar] [CrossRef]
- Lim, S.M.; Jeong, J.J.; Woo, K.H.; Han, M.J.; Kim, D.H. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr. Res. 2016, 36, 337–348. [Google Scholar] [CrossRef]
- Jang, H.M.; Han, S.K.; Kim, J.K.; Oh, S.J.; Jang, H.B.; Kim, D.H. Lactobacillus sakei Alleviates High-Fat-Diet-Induced Obesity and Anxiety in Mice by Inducing AMPK Activation and SIRT1 Expression and Inhibiting Gut Microbiota-Mediated NF-κB Activation. Mol. Nutr. Food Res. 2019, 63, e1800978. [Google Scholar] [CrossRef]
- Ji, Y.; Park, S.; Chung, Y.; Kim, B.; Park, H.; Huang, E.; Jeong, D.; Jung, H.Y.; Kim, B.; Hyun, C.K.; et al. Amelioration of obesity-related biomarkers by Lactobacillus sakei CJLS03 in a high-fat diet-induced obese murine model. Sci. Rep. 2019, 9, 6821. [Google Scholar] [CrossRef]
- Kang, Y.; Kang, X.; Yang, H.; Liu, H.; Yang, X.; Liu, Q.; Tian, H.; Xue, Y.; Ren, P.; Kuang, X.; et al. Lactobacillus acidophilus ameliorates obesity in mice through modulation of gut microbiota dysbiosis and intestinal permeability. Pharmacol. Res. 2022, 175, 106020. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Cao, G.; Zhao, W.; Bie, X.; Lu, F.; Lu, Z.; Lu, Y. Lactobacillus acidophilus NX2-6 Improved High-Fat Diet-Induced Glucose Metabolism Disorder Independent of Promotion of Insulin Secretion in Mice. J. Agric. Food Chem. 2021, 69, 15598–15610. [Google Scholar] [CrossRef]
- Song, M.; Park, S.; Lee, H.; Min, B.; Jung, S.; Park, S.; Kim, E.; Oh, S. Effect of Lactobacillus acidophilus NS1 on plasma cholesterol levels in diet-induced obese mice. J. Dairy Sci. 2015, 98, 1492–1501. [Google Scholar] [CrossRef]
- Ondee, T.; Pongpirul, K.; Visitchanakun, P.; Saisorn, W.; Kanacharoen, S.; Wongsaroj, L.; Kullapanich, C.; Ngamwongsatit, N.; Settachaimongkon, S.; Somboonna, N.; et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci. Rep. 2021, 11, 6367. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, S.; Zhang, Z.; Zhang, T.; Teng, X.; Xiao, G.; Huang, S. Isolation of Lactobacillus acidophilus strain and its anti-obesity effect in a diet induced obese murine model. Lett. Appl. Microbiol. 2024, 77, ovae021. [Google Scholar] [CrossRef] [PubMed]
- Molina-Tijeras, J.A.; Diez-Echave, P.; Vezza, T.; Hidalgo-García, L.; Ruiz-Malagón, A.J.; Rodríguez-Sojo, M.J.; Romero, M.; Robles-Vera, I.; García, F.; Plaza-Diaz, J.; et al. Lactobacillus fermentum CECT5716 ameliorates high fat diet-induced obesity in mice through modulation of gut microbiota dysbiosis. Pharmacol. Res. 2021, 167, 105471. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Xu, Y.; Li, W.; Jiang, S.; Zhang, J.; Xue, H. Limosilactobacillus fermentum HNU312 alleviates lipid accumulation and inflammation induced by a high-fat diet: Improves lipid metabolism pathways and increases short-chain fatty acids in the gut microbiome. Food Funct. 2024, 15, 8878–8892. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, S.I.; Jang, M.; Jeong, Y.A.; Kang, C.H.; Kim, G.H. Combination of Limosilactobacillus fermentum MG4231 and MG4244 attenuates lipid accumulation in high-fat diet-fed obese mice. Benef. Microbes 2021, 12, 479–491. [Google Scholar] [CrossRef]
- Lee, J.Y.; An, M.; Heo, H.; Park, J.Y.; Lee, J.; Kang, C.H. Limosilactobacillus fermentum MG4294 and Lactiplantibacillus plantarum MG5289 Ameliorates Nonalcoholic Fatty Liver Disease in High-Fat Diet-Induced Mice. Nutrients 2023, 15, 2005. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, G.; Noh, M.G.; Park, J.H.; Jang, M.; Fang, S.; Park, H. Lactobacillus fermentum promotes adipose tissue oxidative phosphorylation to protect against diet-induced obesity. Exp. Mol. Med. 2020, 52, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Xue, H.; Zhang, Z.; Wang, J.; Li, A.; Zhang, J.; Luo, P.; Zhan, M.; Zhou, X.; Chen, L.; et al. Amelioration of Type 2 Diabetes Using Four Strains of Lactobacillus Probiotics: Effects on Gut Microbiota Reconstitution-Mediated Regulation of Glucose Homeostasis, Inflammation, and Oxidative Stress in Mice. J. Agric. Food Chem. 2023, 71, 20801–20814. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.P.; Molz, P.; Fraga, B.S.; Bondarczuk, N.H.; Silveira, P.D.; Ferri, M.H.; Crestani, T.B.; Breyer, G.M.; Guimarães, G.R.; Motta, A.S.D.; et al. Effect of Probiotic Lacticaseibacillus rhamnosus LB1.5 on Anxiety-like Behavior, Neuroprotection and Neuroinflammation Markers of Male Mice Fed a High-Fat Diet. Nutrients 2024, 16, 879. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ding, L.; Ma, G.; Zhang, Y.; Sun, Y.; Li, Z.; Tao, X.; Ali, A.; Wang, D.; Wu, L. Lactobacillus rhamnosus TR08 Improves Dyslipidemia in Mice Fed with a High Fat Diet by Regulating the Intestinal Microbiota, Reducing Systemic Inflammatory Response, and Promoting Sphingomholipid Metabolism. Molecules 2022, 27, 7357. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chiou, S.Y.; Hsu, A.H.; Lin, Y.C.; Lin, J.S. Lactobacillus rhamnosus Strain LRH05 Intervention Ameliorated Body Weight Gain and Adipose Inflammation via Modulating the Gut Microbiota in High-Fat Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2022, 66, e2100348. [Google Scholar] [CrossRef]
- Ivanovic, N.; Minic, R.; Dimitrijevic, L.; Radojevic Skodric, S.; Zivkovic, I.; Djordjevic, B. Lactobacillus rhamnosus LA68 and Lactobacillus plantarum WCFS1 differently influence metabolic and immunological parameters in high fat diet-induced hypercholesterolemia and hepatic steatosis. Food Funct. 2015, 6, 558–565. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Liu, J.R. Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity. Nutrients 2020, 12, 2557. [Google Scholar] [CrossRef]
- Sun, M.; Wu, T.; Zhang, G.; Liu, R.; Sui, W.; Zhang, M.; Geng, J.; Yin, J.; Zhang, M. Lactobacillus rhamnosus LRa05 improves lipid accumulation in mice fed with a high fat diet via regulating the intestinal microbiota, reducing glucose content and promoting liver carbohydrate metabolism. Food Funct. 2020, 11, 9514–9525. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, H.Q.; Zhang, X.; Zhang, H.; Xia, J.; Ding, K.; Fang, Z.Y. Probiotic administration of Lactobacillus rhamnosus GR-1 attenuates atherosclerotic plaque formation in ApoE−/− mice fed with a high-fat diet. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3533–3541. [Google Scholar]
- Ukibe, K.; Miyoshi, M.; Kadooka, Y. Administration of Lactobacillus gasseri SBT2055 suppresses macrophage infiltration into adipose tissue in diet-induced obese mice. Br. J. Nutr. 2015, 114, 1180–1187. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, S.K.; Han, J.W.; Kim, J.E.; Lee, M.J.; Bae, H.R.; Kwon, E.Y. Lactobacillus paragasseri SBT2055 attenuates obesity via the adipose tissue-muscle-gut axis in obese mice. Microbiol. Res. 2025, 290, 127972. [Google Scholar] [CrossRef]
- Lee, C.S.; Park, M.H.; Kim, B.K.; Kim, S.H. Antiobesity Effect of Novel Probiotic Strains in a Mouse Model of High-Fat Diet-Induced Obesity. Probiotics Antimicrob. Proteins 2021, 13, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.; Kang, H.; Yang, G.; Oh, S.; Kim, E. The PKA-SREBP1c Pathway Plays a Key Role in the Protective Effects of Lactobacillus johnsonii JNU3402 Against Diet-Induced Fatty Liver in Mice. Mol. Nutr. Food Res. 2023, 67, e2200496. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, H.J.; Kim, J.Y.; Shim, J.J.; Lee, J.H. A Mixture of Lactobacillus HY7601 and KY1032 Regulates Energy Metabolism in Adipose Tissue and Improves Cholesterol Disposal in High-Fat-Diet-Fed Mice. Nutrients 2024, 16, 2570. [Google Scholar] [CrossRef]
- Park, D.Y.; Ahn, Y.T.; Park, S.H.; Huh, C.S.; Yoo, S.R.; Yu, R.; Sung, M.K.; McGregor, R.A.; Choi, M.S. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS ONE 2013, 8, e59470. [Google Scholar] [CrossRef]
- Li, S.; Qi, C.; Zhu, H.; Yu, R.; Xie, C.; Peng, Y.; Yin, S.W.; Fan, J.; Zhao, S.; Sun, J. Lactobacillus reuteri improves gut barrier function and affects diurnal variation of the gut microbiota in mice fed a high-fat diet. Food Funct. 2019, 10, 4705–4715. [Google Scholar] [CrossRef] [PubMed]
- Abot, A.; Pomié, N.; Astre, G.; Jaomanjaka, F.; Marchand, P.; Cani, P.D.; Roudier, N.; Knauf, C. Limosilactobacillus reuteri BIO7251 administration improves metabolic phenotype in obese mice fed a high fat diet: An inter-organ crosstalk between gut, adipose tissue and nervous system. Int. J. Food. Sci. Nutr. 2024, 75, 58–69. [Google Scholar] [CrossRef]
- Zheng, F.; Wang, Z.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Lactobacillus rhamnosus FJSYC4-1 and Lactobacillus reuteri FGSZY33L6 alleviate metabolic syndrome via gut microbiota regulation. Food Funct. 2021, 12, 3919–3930. [Google Scholar] [CrossRef]
- Park, S.S.; Lee, Y.J.; Kang, H.; Yang, G.; Hong, E.J.; Lim, J.Y.; Oh, S.; Kim, E. Lactobacillus amylovorus KU4 ameliorates diet-induced obesity in mice by promoting adipose browning through PPARγ signaling. Sci. Rep. 2019, 9, 20152. [Google Scholar] [CrossRef]
- Liang, C.; Zhou, X.H.; Jiao, Y.H.; Guo, M.J.; Meng, L.; Gong, P.M.; Lyu, L.Z.; Niu, H.Y.; Wu, Y.F.; Chen, S.W.; et al. Ligilactobacillus Salivarius LCK11 Prevents Obesity by Promoting PYY Secretion to Inhibit Appetite and Regulating Gut Microbiota in C57BL/6J Mice. Mol. Nutr. Food Res. 2021, 65, e2100136. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, D.; Kang, I.B.; Kim, H.; Song, K.Y.; Seo, K.H. Dual function of Lactobacillus kefiri DH5 in preventing high-fat-diet-induced obesity: Direct reduction of cholesterol and upregulation of PPAR-α in adipose tissue. Mol. Nutr. Food Res. 2017, 61, 1700252. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, H.; Tsao, R.; Mine, Y. Lactobacillus pentosus S-PT84 Prevents Low-Grade Chronic Inflammation-Associated Metabolic Disorders in a Lipopolysaccharide and High-Fat Diet C57/BL6J Mouse Model. J. Agric. Food Chem. 2020, 68, 4374–4386. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Joung, H.; Chu, J.; Cho, M.; Kim, Y.W.; Kim, K.H.; Shin, C.H.; Lee, J.; Ha, J.H. Lactobacillus delbrueckii subsp. Lactis CKDB001 Ameliorates Metabolic Complications in High-Fat Diet-Induced Obese Mice. Nutrients 2024, 16, 4260. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, T.; Cui, X.; Li, L.; Chen, B.; Li, Y.; Yue, T. Lactobacillus coryniformis subsp. torquens T3 alleviates non-alcoholic fatty liver disease via reconstruction of the gut microbiota and redox system. J. Sci. Food Agric. 2023, 103, 6814–6825. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kang, I.; Lee, Y.; Habib, M.A.; Choi, B.J.; Kang, J.S.; Park, D.S.; Kim, Y.S. Bifidobacterium longum subsp. Infantis YB0411 Inhibits Adipogenesis in 3T3-L1 Pre-adipocytes and Reduces High-Fat-Diet-Induced Obesity in Mice. J. Agric. Food Chem. 2021, 69, 6032–6042. [Google Scholar] [CrossRef] [PubMed]
- Kou, R.; Wang, J.; Li, A.; Wang, Y.; Zhang, B.; Liu, J.; Sun, Y.; Wang, S. Ameliorating Effects of Bifidobacterium longum subsp. Infantis FB3-14 against High-Fat-Diet-Induced Obesity and Gut Microbiota Disorder. Nutrients 2023, 15, 4104. [Google Scholar] [CrossRef]
- In Kim, H.; Kim, J.K.; Kim, J.Y.; Jang, S.E.; Han, M.J.; Kim, D.H. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 simultaneously alleviate high-fat diet-induced colitis, endotoxemia, liver steatosis, and obesity in mice. Nutr. Res. 2019, 67, 78–89. [Google Scholar] [CrossRef]
- Wu, T.; Sun, M.; Liu, R.; Sui, W.; Zhang, J.; Yin, J.; Fang, S.; Zhu, J.; Zhang, M. Bifidobacterium longum subsp. Longum Remodeled Roseburia and Phosphatidylserine Levels and Ameliorated Intestinal Disorders and liver Metabolic Abnormalities Induced by High-Fat Diet. J. Agric. Food Chem. 2020, 68, 4632–4640. [Google Scholar]
- Lim, S.M.; Kim, D.H. Bifidobacterium adolescentis IM38 ameliorates high-fat diet-induced colitis in mice by inhibiting NF-κB activation and lipopolysaccharide production by gut microbiota. Nutr. Res. 2017, 41, 86–96. [Google Scholar] [CrossRef]
- Wang, G.; Jiao, T.; Xu, Y.; Li, D.; Si, Q.; Hao, J.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium adolescentis and Lactobacillus rhamnosus alleviate non-alcoholic fatty liver disease induced by a high-fat, high-cholesterol diet through modulation of different gut microbiota-dependent pathways. Food Funct. 2020, 11, 6115–6127. [Google Scholar] [CrossRef]
- Ma, L.; Zheng, A.; Ni, L.; Wu, L.; Hu, L.; Zhao, Y.; Fu, Z.; Ni, Y. Bifidobacterium animalis subsp. lactis lkm512 Attenuates Obesity-Associated Inflammation and Insulin Resistance Through the Modification of Gut Microbiota in High-Fat Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2022, 66, e2100639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fang, B.; Zhang, N.; Zhang, Q.; Niu, T.; Zhao, L.; Sun, E.; Wang, J.; Xiao, R.; He, J.; et al. The Effect of Bifidobacterium animalis subsp. Lactis MN-Gup on Glucose Metabolism, Gut Microbiota, and Their Metabolites in Type 2 Diabetic Mice. Nutrients 2024, 16, 1691. [Google Scholar] [CrossRef] [PubMed]
- Ban, O.H.; Lee, M.; Bang, W.Y.; Nam, E.H.; Jeon, H.J.; Shin, M.; Yang, J.; Jung, Y.H. Bifidobacterium lactis IDCC 4301 Exerts Anti-Obesity Effects in High-Fat Diet-Fed Mice Model by Regulating Lipid Metabolism. Mol. Nutr. Food Res. 2023, 67, e2200385. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Xiao, J.Z.; Satoh, T.; Odamaki, T.; Takahashi, S.; Sugahara, H.; Yaeshima, T.; Iwatsuki, K.; Kamei, A.; Abe, K. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci Biotechnol. Biochem. 2010, 74, 1656–1661. [Google Scholar] [CrossRef]
- Cano, P.G.; Santacruz, A.; Trejo, F.M.; Sanz, Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity 2013, 21, 2310–2321. [Google Scholar] [CrossRef]
- Rahman, M.S.; Lee, Y.; Park, D.S.; Kim, Y.S. Bifidobacterium bifidum DS0908 and Bifidobacterium longum DS0950 Culture-Supernatants Ameliorate Obesity-Related Characteristics in Mice with High-Fat Diet-Induced Obesity. J. Microbiol. Biotechnol. 2023, 33, 96–105. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, R.; Wang, Y. Effects of the probiotic Bacillus coagulans BC69 on the metabolic and histological alterations induced by a high-sugar and high-fat diet in C57BL/6J mice. Food Funct. 2023, 14, 6596–6609. [Google Scholar] [CrossRef]
- Kim, B.; Kwon, J.; Kim, M.S.; Park, H.; Ji, Y.; Holzapfel, W.; Hyun, C.K. Protective effects of Bacillus probiotics against high-fat diet-induced metabolic disorders in mice. PLoS ONE 2018, 13, e0210120. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, B.; Xu, H.; Mei, X.; Xu, X.; Zhang, X.; Ni, J.; Li, W. Bacillus amyloliquefaciens SC06 Protects Mice Against High-Fat Diet-Induced Obesity and Liver Injury via Regulating Host Metabolism and Gut Microbiota. Front. Microbiol. 2019, 10, 161. [Google Scholar] [CrossRef]
- Hashemnia, S.M.R.; Meshkani, R.; Zamani-Garmsiri, F.; Shabani, M.; Tajabadi-Ebrahimi, M.; Ragerdi Kashani, I.; Siadat, S.D.; Mohassel Azadi, S.; Emamgholipour, S. Amelioration of obesity-induced white adipose tissue inflammation by Bacillus coagulans T4 in a high-fat diet-induced obese murine model. Life Sci. 2023, 314, 121286. [Google Scholar] [CrossRef]
- Cao, G.T.; Dai, B.; Wang, K.L.; Yan, Y.; Xu, Y.L.; Wang, Y.X.; Yang, C.M. Bacillus licheniformis, a potential probiotic, inhibits obesity by modulating colonic microflora in C57BL/6J mice model. J. Appl. Microbiol. 2019, 127, 880–888. [Google Scholar] [CrossRef]
- Wen, X.; Feng, X.; Xin, F.; An, R.; Huang, H.; Mao, L.; Liu, P.; Zhang, J.; Huang, H.; Liu, X.; et al. B. vulgatus ameliorates high-fat diet-induced obesity through modulating intestinal serotonin synthesis and lipid absorption in mice. Gut Microbes 2024, 16, 2423040. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.K.; Tang, M.; Lei, L.; Li, J.R.; Sun, H.; Jiang, J.; Dong, B.; Li, H.Y.; Jiang, J.D.; et al. Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism. Gut Microbes 2024, 16, 2304159. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xiong, X.; Liu, M.; Liang, Q.; Zhao, Q.; Wei, G.; Shi, J.; Li, X. Bacteroides ovatus alleviates high-fat and high-cholesterol-induced nonalcoholic fatty liver disease via gut-liver axis. Biomed. Pharmacother. 2024, 178, 117156. [Google Scholar] [CrossRef]
- Wang, Y.; You, Y.; Tian, Y.; Sun, H.; Li, X.; Wang, X.; Wang, Y.; Liu, J. Pediococcus pentosaceus PP04 Ameliorates High-Fat Diet-Induced Hyperlipidemia by Regulating Lipid Metabolism in C57BL/6N Mice. J. Agric. Food Chem. 2020, 68, 15154–15163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, Y.; Zhang, N.; Li, X.; Wang, X.; Wang, W.; Zhang, J.; Piao, C.; Wang, Y.; Liu, J. Pediococcus pentosaceus PP04 improves high-fat diet-induced liver injury by the modulation of gut inflammation and intestinal microbiota in C57BL/6N mice. Food Funct. 2021, 12, 6851–6862. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, W.; Edirisuriya, P.; Ai, Q.; Nie, K.; Ji, X.; Zhou, K. Characterization of metabolites and biomarkers for the probiotic effects of Clostridium cochlearium on high-fat diet-induced obese C57BL/6 mice. Eur. J. Nutr. 2022, 61, 2217–2229. [Google Scholar] [CrossRef]
- Luo, Y.; Jin, Y.; Wang, H.; Wang, G.; Lin, Y.; Chen, H.; Li, X.; Wang, M. Effects of Clostridium tyrobutyricum on Lipid Metabolism, Intestinal Barrier Function, and Gut Microbiota in Obese Mice Induced by High-Fat Diet. Nutrients 2024, 16, 493. [Google Scholar] [CrossRef]
- Benvenuti, L.; D’Antongiovanni, V.; Pellegrini, C.; Fornai, M.; Bernardini, N.; Ippolito, C.; Segnani, C.; Di Salvo, C.; Colucci, R.; Martelli, A.; et al. Dietary Supplementation with the Probiotic SF68 Reinforces Intestinal Epithelial Barrier in Obese Mice by Improving Butyrate Bioavailability. Mol. Nutr. Food Res. 2023, 67, e2200442. [Google Scholar] [CrossRef]
- Xu, W.; Yu, J.; Yang, Y.; Li, Z.; Zhang, Y.; Zhang, F.; Wang, Q.; Xie, Y.; Zhao, B.; Wu, C. Strain-level screening of human gut microbes identifies Blautia producta as a new anti-hyperlipidemic probiotic. Gut Microbes 2023, 15, 2228045. [Google Scholar] [CrossRef]
- Castro-Rodríguez, D.C.; Reyes-Castro, L.A.; Vega, C.C.; Rodríguez-González, G.L.; Yáñez-Fernández, J.; Zambrano, E. Leuconostoc mesenteroides subsp. mesenteroides SD23 Prevents Metabolic Dysfunction Associated with High-Fat Diet-Induced Obesity in Male Mice. Probiotics Antimicrob. Proteins 2020, 12, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, W.; Lin, Y.; Chan, F.K.L.; Xu, Z.; Ng, S.C. Roseburia hominis improves host metabolism in diet-induced obesity. Gut Microbes 2025, 17, 2467193. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Zhou, Y.; He, L.; Li, Y.; Shahzad, M.; Li, D. Coprococcus protects against high-fat diet-induced nonalcoholic fatty liver disease in mice. J. Appl. Microbiol. 2024, 135, lxae125. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Kaicen, W.; Bian, X.; Yang, L.; Ding, S.; Li, Y.; Li, S.; Zhuge, A.; Li, L. Akkermansia muciniphila alleviates high-fat-diet-related metabolic-associated fatty liver disease by modulating gut microbiota and bile acids. Microb. Biotechnol. 2023, 16, 1924–1939. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).